Abstract
Device migration is one of serious complications in neonates and infants undergoing transcatheter closure of the patent ductus arteriosus (PDA). We hypothesize that neonates and young infants possess the distensibility of the ductus, which may be related to device migration. We retrospectively reviewed angiographic findings in 41 neonates and infants who underwent transcatheter closure of PDA. We measured diameters of the ductus at the pulmonary (PA) side, the center, and the aortic (AO) side before PDA closure, and the device center diameter after device closure. The distensibility index was defined as the ratio of the device center diameter after device deployment to the diameter at the center of the ductus before PDA closure. Age and weight at the procedure were 168 (117–260) days and 5.3 (4.3–6.9) kg, respectively. Thirty-seven subjects accomplished the successful device closure, and four subjects were declined because of the device instability or migration. Implanted devices included Amplatzer Duct Occluders in 33 subjects and Amplatzer Vascular Plug-2 in 8 subjects. The PDA diameters at PA side, at the center, AO side, and the device center diameter were 3.2 (2.2–4.3) mm, 4.7 (3.6–5.7) mm, 7.7 (6.3–9.4) mm, and 5.8 (4.2–6.9) mm, respectively. The PDA diameter before closure was not correlated age and weight. The distensibility index was 1.28 (1.06–1.64), which was significantly correlated to age (r = − 0.49, P = 0.001) and weight (r = − 0.53, P < 0.001). Infants with the younger age and the lower weight have the more distensible PDA, which may be a risk for device migration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transcatheter closure of patent ductus arteriosus (PDA) has become a standard procedure alternative to surgical closure among neonates and young infants who present in congestive heart failure and/or pulmonary hypertension [1]. Despite the widespread use of the Amplatzer Duct Occluder (ADO) family or Amplatzer Vascular Plug (AVP), there are still concerns for infection, hemolysis, protrusion of a device into the aorta or the pulmonary artery, and device migration [2, 3]. Previous reports have shown that transcatheter PDA closure is technically feasible among neonates and young infants with weight less than 6 kg; however, younger age (< 30 days) is a greater risk for major adverse events (risk ratio 3.3) and composite failure (risk ratio 3.0). It has been supposed that low weight babies are at a higher risk for device migration [3]. Usually, the size of a device depends on the diameter of the ductus, rather than age. Therefore, we hypothesize that neonates and young infants may have more distensibility of the ductus which may be result in device migration. We aimed to explore our hypothesis in neonates and infants with PDA who underwent transcatheter closure.
Materials and methods
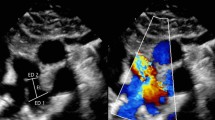
This retrospective cohort study was approved by the Institutional Ethics Committee of Kyushu Hospital, Japan Community Healthcare Organization. Informed consent was obtained from all patients’ guardians. We retrospectively reviewed neonates and infants (aged < 12 months) with PDA who underwent transcatheter closure in our hospital between 2013 and 2020. Transcatheter PDA closure was indicated when a patient presented with symptoms related to heart failure, the presence of pulmonary hypertension, or left heart overload. There was no patient who underwent coil embolization during the study period. We performed PDA closure using Amplatzer device family according to the previously described method [4]. Descriptions of the procedure in detail were omitted. At first, we performed right heart catheterization to measure the pulmonary arterial pressure and to estimate the ratio of pulmonary to systemic blood flow based on the Fick principle. Orthogonal biplane aortograms were obtained to measure the diameter of PDA at the following three sites: at the pulmonary arterial site, at the aortic site, and at the center of PDA, between the pulmonary and aortic ends (the left panel in Fig. 1). Each measurement was obtained at the maximum diameter during systolic phase. We choose either ADO™ (Abott Medical, Chicago, IL) or AVP-2™ (Abott Medical, Chicago, IL) based on the angiographic findings. Basically, we preferred ADO and AVP-2 in patients with Krichenko classification type A and type C, respectively. We chose a suitable device according to the configuration of PDA in patients with other types PDA. After the deployment of the device, we measured the device center diameter adjacent to the center site of the ductus, which is usually at the tracheal borders. We calculated the distensibility index which was defined as the ratio of the device center diameter after device deployment to the ductus diameter at the center before PDA closure (the right panel in Fig. 1). Two examiners (K.N and H.E) independently validated angiographic measurements.
Measurements points were shown before and after transcatheter closure of patent ductus arteriosus (PDA). We measure at the pulmonary arterial side (A), the center of the ductus (B), and the aortic side (C) before PDA closure, and measure the device center diameter (D) after PDA closure. The distensibility index is defined as the ratio of D to B
Statistical analysis was performed using the analysis toolpack in Microsoft Office Excel add-in software. Continuous values are described as median following the interquartile range, and categorized values are described as the number of subjects following the percentage. We compared the correlation between age, weight, pulmonary arterial pressure, the ratio of pulmonary to systemic blood flow (Qp/Qs), the diameters of PDA, the device center diameter, and the distensibility index in the studied subjects using Spearman correlation analysis. Adjusting for factors including age, prematurity, and the ductus diameter are likely to have complex patterns of covariance; however, these are absolutely necessary to establish the correlations. Correlations > 0.4 was considered significant.
Results
A total of 41 subjects were studied. Patients’ baseline data were shown in Table 1. There were 28 females (68%). Median gestational age and birth weight were 38 (37–39) weeks and 2.71 (2.40–3.03) kg, respectively. There were four preterm infants. Median age and weight at the procedure were 168 (117–260) days and 5.32 (4.33–6.93) kg, respectively.
Hemodynamic evaluation showed mean pulmonary arterial pressure of 28 (16–60) mmHg and Qp/Qs of 2.19 (1.53–3.13). Krichenko classification included as follows: type A, N = 29; type B, N = 1; type C, N = 8; type D, N = 1; and type E, N = 3. The diameters of PDA at PA side, at the center, and AO side were 3.2 (2.2–4.3) mm, 4.7 (3.6–5.7) mm, and 7.7 (6.3–9.4) mm, respectively. Interobserver correlation was 0.87. Thirty-seven subjects (93%) accomplished successful device closure; however, there were four subjects who declined transcatheter PDA closure due to device instability in three subjects and device migration in one subject. Implanted devices included ADO in 33, ADO-2 in 1, and AVP-2 in 8 subjects. After the deployment of a device, the device center diameter was 5.8 (4.2–6.9) mm. The distensibility index was 1.28 (1.04–1.64). The diameters of the ductus before PDA closure did not correlate with age and weight. Although the device center diameter did not correlate with age or weight, the distensibility index inversely correlated to age (r = − 0.49, P = 0.001) and weight (r = − 0.53, P < 0.001) (Fig. 2). These findings suggest that the ductus can be stretched during the device deployment in younger patients.
Discussion
The major finding of our present study is that the younger and lower weighed patients had more distensible ductus. This finding may be explained by differences in histological features of PDA according to age. The narrowest site of the ductus is located between the distal disc and the center drum in patients who underwent PDA closure using ADO-2 or AVP-2. Therefore, it is reasonable to measure the center diameter of the ductus to assess the distensibility of the ductus. When we further analyzed the narrowest pulmonary site of the ductus and the corresponding device diameter after PDA closure in 32 patients who underwent PDA closure using ADO, the ratio of the narrowest ductus diameter to the corresponding device diameter was not significantly correlated to age and weight (data are not shown). As device migration is concerned in patients with type C PDA who underwent closure using AVP-2, it is meaningful to measure the distensibility index.
In the present study, some patients presented the distensibility index less than 1.0. We speculated the reason why the distensibility index was calculated less than 1.0 was owing to spastic constriction of the ductus. As we usually determine the device size according to the diameter at the pulmonary side of the ductus, it will be possible that the diameter at the device center diameter after device deployment become less than the diameter before PDA closure.
Usually, spontaneous closure of the ductus arteriosus occurs in two phases; functional closure within the first hours after birth due to smooth muscle constriction, and anatomical closure during the next several days due to extensive neointimal thickening. Anatomical closure of the ductus is associated with the formation of intimal thickening, which are characterized by subendothelial deposition of extracellular matrix, the disassembly of the internal elastic lamina and loss of elastic fiber in the medial layer, and migration into the subendothelial space of undifferentiated medial smooth muscle cells [5, 6]. Histological findings of PDA are characterized by two distinct elastic laminae; (1) normal internal elastic lamina located between intima and media, and (2) subendothelial elastic lamina beneath intimal proliferation. Subendothelial elastic lamina sometimes lacks among infants younger than 3 months [7, 8]. Therefore, less subendothelial elastic lamina may be associated with the distensibility of the ductus arteriosus in neonates and young infants.
ADO and AVP-2 are made of nitinol fiber, and characterized by being self-expandable and repositionable devices, which allows for easy delivery though a guiding sheath or catheter. The restoring force may expand the ductus wall, especially in neonates and young infants with fragile wall layers of the ductus. Therefore, it is supposed that a softer device will be suitable for PDA closure among them.
The present study has several limitations. First, it was susceptible to information bias owing to its retrospective nature, and our cohort consisted of a limited number of patients. Second, the configuration of the ductus could be altered after the deployment of the device. Therefore, the device center diameter after device closure was not always identical to the center of the ductus before device closure. Other modalities such as contrasted computed tomography would be ideal to measure the diameter of the ductus before and after device closure. However, according to the principle “as low as reasonably achievable”, it is considered to avoid further radiation exposure. Finally, in the present study, we mainly used ADO and AVP-2 to close PDA. As characteristics in rigidity and radial strength are different according to the size and type of the devices, the device center diameter can be altered according to the different implanted device, and not always be measured in constant.
Conclusions
Infants with the younger age and the lower weight have the more distensible PDA, which may be a risk for device migration.
References
O’Byrne ML, Millenson ME, Grady CB, Huang J, Bamat NA, Munson DA, Song L, Dori Y, Gillespie MJ, Rome JJ, Glatz AC (2019) Trends in transcatheter and operative closure of patent ductus arteriosus in neonatal intensive care units: analysis of data from the Pediatric Health Information Systems Database. Am Heart J 217:121–130
Baruteau AE, Hascoët S, Baruteau J, Boudjemline Y, Lambert V, Angel CY, Belli E, Petit J, Pass R (2014) Transcatheter closure of patent ductus arteriosus: past, present and future. Arch Cardiovasc Dis 107:122–132
Backes CH, Kennedy KF, Locke M, Cua CL, Ball MK, Fick TA, Rivera BK, Smith CV, Holzer RJ, Boe BA, Berman DP, Bergersen L, Armstrong AK (2017) Transcatheter occlusion of the patent ductus arteriosus in 747 infants <6 kg. JACC Cardiovasc Interv 10:1729–1737
Mullins CE (2006) Transcatheter occlusion of the patent ductus arteriosus (PDA). In: Mullins CE (ed) Cardiac catherization in congenital heart disease: pediatric and adult. Blackwell Futura, Massachusetts, pp 693–727
Smith GC (1998) The pharmacology of the ductus arteriosus. Pharmacol Rev 50:35–58
Yokoyama U, Minamisawa S, Ishikawa Y (2010) Regulation of vascular tone and remodeling of the ductus arteriosus. J Smooth Muscle Res 46:77–87
Gittenberger-de Groot AC (1977) Persistent ductus arteriosus: most probably a primary congenital malformation. Br Heart J 39:610–618
Chuaqui B, Piwonka G, Farrú O (1977) Uber den Wandbau des persistierenden Ductus arteriosus [The wall in persistent ductus arteriosus (author’s transl)]. Virchows Arch A Pathol Anat Histol 372:315–324
Funding
This research received no grant from any funding agency in the public, commercial of not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagasawa, K., Muneuchi, J., Sugitani, Y. et al. Distensibility of the ductus arteriosus in neonates and young infants undergoing transcatheter closure. Heart Vessels 37, 513–516 (2022). https://doi.org/10.1007/s00380-021-01925-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01925-9