Abstract
Evaluation of in-stent restenosis (ISR) by computed tomography coronary angiography (CTCA) is less invasive but often impossible. We aimed to create a scoring model for predicting which drug-eluting stents (DES) cannot be evaluated with CTCA. We enrolled 757 consecutive implanted DES assessed with CTCA. Non-diagnostic evaluation was defined as poor/not evaluative by two different observers. These stents were randomly divided into a derivation (n = 379) and validation (n = 378) group. In the derivation group, we assessed predictors using logistic regression analysis and created a scoring model that would stratify non-diagnostic evaluation of DES-ISR. The validity of this scoring model was evaluated in the validation group using receiver-operating characteristic analysis. The percentage of non-diagnostic stents was 19/21% in the derivation/validation group (p = 0.71). Non-diagnostic evaluation was independently associated with implanted stent diameter (2.25–2.5. vs. 2.5–3 vs. > 3.0 mm), severe calcification, stent-in-stent lesion, and type of DES (stainless vs. CoCr vs. PtCr) in the derivation group. The predicting system of implanted DES non-diagnostic by CTCA (PIDENT) for non-diagnostic evaluation, including these four baseline factors, was derived (C-statistic = 0.86 in derivation group, cutoff: 8 points). The PIDENT score had a high predictive value for non-diagnostic DES in the validation model (C-statistic = 0.87, sensitivity 86%, specificity 74%, cutoff 8 points, p < 0.001). The PIDENT score, consisting of baseline characteristics including implanted stent diameter, severe calcification, stent-in-stent lesion, and type of DES, could identify non-diagnostic evaluation of DES-ISR with CTCA. The PIDENT score was valuable in reducing nonevaluable and meaningless CTCA for DES-ISR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous coronary intervention is the most common revascularization procedure performed worldwide in patients with coronary artery disease [1]. Drug-eluting stents (DES) have remarkably reduced target lesion revascularization by preventing excessive neointimal hyperplasia inside stents [2, 3]. DES are now widely used for small vessel, long chronic total occlusion lesions, and multivessel lesions [4,5,6,7]. However, even with the use of DES, lesion complexity or patient comorbidities are associated with in-stent restenosis (ISR) [8].
Invasive coronary angiography (ICA) is still considered the gold standard for detecting ISR, but it is an invasive procedure with a significant risk of complications and high cost. Recently, computed tomography coronary angiography (CTCA) has become a standard diagnostic modality without ICA-related complications in the noninvasive assessment of coronary stents [9, 10]. The stress test is also a noninvasive method, but its diagnostic performance is not very high. Previous studies indicated that CTCA has high sensitivity and specificity for detecting ISR [11,12,13].
However, thick-strut thickness and stent diameter are reported to have poor diagnostic performance for ISR [11, 14, 15]. Assessment of DES-ISR using CTCA is not necessarily applicable for all patients because imaging quality is not always guaranteed.
Newer smaller diameter DES with a flexible design and thinner struts DES are available [16,17,18]. Stent diameter has been reported to be not associated with image quality and assessment ability by CTCA in the evaluation of thin-strut (< 140 μm) DES [19]. However, the predictive ability of CTCA for ISR of these stents has not been fully elucidated. CTCA is a less invasive diagnostic tool than conventional angiography but sometimes induces contrast-induced nephropathy or life-threatening allergy. We established a scoring model to predict which implanted DES cannot be evaluated by CTCA and validated the diagnostic performance of this model to reduce unnecessary CTCA for patient safety and cost-effectiveness.
Materials and methods
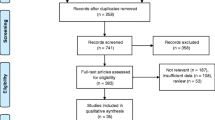
This study retrospectively included 756 DES implanted between January 2015 and August 2018 who underwent CTCA for clinical indications with an interval of > 1 month since the most recent stenting. The average time from PCI until CTCA was 45 ± 26 months. Exclusion criteria for CTCA were as follows: chronic kidney disease (serum creatinine > 1.5 mg/dL or glomerular filtration rate < 50 mL/min/1.73 m2), allergy to contrast media, inability to hold breath, or other patient status contraindicating CTCA. Based on stents, they were randomly divided into a derivation group (379 stents, 296 patients) and a validation group (378 stents, 290 patients). The study design is shown in Fig. 1. All patients provided written informed consent, and the study protocol was approved by the hospital ethics committee.
Imaging protocol
All patients were scanned with a 320-row slice scanner (INFX-8000 C/AquilionONE Vision Edition; Canon Medical Systems Corporation, Otawara, Japan). A β-blocker (oral metoprolol or intravenous propranolol 10–20 mg) was administered if the heart rate was ≥ 65 beats/min. A bolus of contrast medium was injected into the antecubital vein with an injection velocity of 3–5 mL/s. The total amount of contrast medium was estimated according to the patient’s body weight and heart rate. A test bolus tracking method was used with the following parameters: collimation width 320 × 0.5 mm, rotation time 275 ms, tube voltage 120 kV, and effective tube current 600 mA. CTCA was performed during a single breath hold with an electrocardiogram (ECG)-synchronized method at an ECG dose modulation between 30 and 100% of the RR interval.
Multidetector CT image reconstruction and analysis
The CTCA data generated were transferred to an offline workstation (Ziostation 2; Ziosoft Inc., Tokyo, Japan) for image analysis. Cross-sectional multiplanar reconstruction images of the stents and curved-planar reconstruction images through the median of the stents were reconstructed to assess the in-stent lumen and transferred to Wizard.
Definition
CTCA image quality (IQ) was graded from 0 to 3 for each stent as follows: grade 0, poor/nonevaluable/non-diagnostic image quality (severe artifacts impairing accurate evaluation, stent segment classified as nonevaluable); grade 1, adequate image quality (moderate artifacts, acceptable for routine clinical diagnosis); grade 2, good image quality (minor artifacts, good diagnostic quality); and grade 3, excellent image quality (no artifacts) [20]. Images with grade 0 were defined as non-diagnostic and those with grades 1–3 as diagnostic (Fig. 2). The IQ was evaluated by two skilled observers. If their evaluation differed from each other, they discussed and made a final decision.
Prior to the initiation of the percutaneous coronary intervention procedure, information regarding selected risk factors was obtained from hospital records made at discharge. The following data were collected to reflect baseline clinical characteristics: age, sex, hypertension (systolic blood pressure > 140 mmHg), dyslipidemia (low-density lipoprotein cholesterol > 140 mg/dL or high-density lipoprotein cholesterol < 40 mg/dL), diabetes mellitus [fasting blood glucose > 126 mg/dL, glycated hemoglobin (National Glycohemoglobin Standardization Program) > 7.0%, or casual blood glucose > 200 mg/dL], past or current smoking status, and renal function evaluated via estimated glomerular filtration rates.
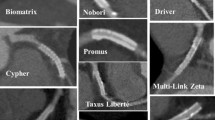
Coronary calcification was defined as obvious density within the arterial wall and at lesion sites that was readily apparent as an X-ray-absorbing mass during ICA. Severe calcification was defined as density noted without cardiac motion before contrast injection and generally involving both sides of the arterial wall [21]. Tortuous lesion is defined as three fixed bends during both systole and diastole in at least one epicardial artery, with each bend showing a 45° change in vessel direction [22]. Stent-in-stent lesions meant that the DES evaluated with CTCA was implanted to treat ISR lesions. The type of prior stent presenting with ISR was not considered. Type of DES were as follows: stainless DES included Cypher (Cordis, Warren, New Jersey), TAXUS Liberté (Boston Scientific Corporation, Natick, MA, USA), and Nobori (Terumo, Tokyo, Japan); PtCr-DES included PROMUS element and Synergy (Boston Scientific); and CoCr-DES included PROMUS (Boston Scientific), Xience series (Abbott Vascular, Santa Clara, CA, USA), Resolute Integrity (Medtronic, Santa Rosa, California), and Ultimaster (Terumo, Tokyo, Japan). An overlapping stent was defined as multiple stents implanted to treat long lesions.
Stent fracture detected with CTCA was diagnosed according to the following criteria: (1) partial or circumferential separation of the stent; (2) absence of a metallic stent strut on cross-sectional image, and (3) confirmation of radiodensity of < 300 Hounsfield units at the site of separation [23].
Development of the scoring model
Risk scores were developed using a modified Framingham Heart Study approach [24]. The predictive value of the non-diagnostic DES with CTCA was determined using logistic regression models in the derivation group. Predictors with p < 0.05 after multivariate analysis were converted to points, and a total score was calculated for each patient by summation of those points. The validity of this scoring model was evaluated in the validation group. The performance of the scoring system for non-diagnostic DES with CTCA was evaluated by receiver-operating characteristic curve analysis, and the predictive ability of this score was determined using C-statistics.
Statistical analysis
Group randomization was performed as follows: (1) all stents were numbered with a random function, (2) they were arranged in a sequence of assigned numbers, and (3) the first half of the stents were classified as the derivation group, and the latter half were classified as the validation group.
Continuous variables are expressed as mean (SD), and categorical variables are expressed as numbers and percentages. A multiple logistic regression analysis was used to identify independent predictors of the non-diagnostic DES with CTCA. To identify independent predictive factors, we selected variables with p < 0.10 in the univariate logistic regression model and included them in the multivariate models simultaneously. Predictive values of target vessel location in the left anterior descending artery or left circumflex coronary artery compared with right coronary artery (RCA) as the referent, implanted stent diameter of 2.75–3.0 or 2.25–2.5 mm compared with > 3.0 mm as the referent, and type of DES in PtCr-DES or CoCr-DES compared with stainless DES as the referent were also assessed. All statistical analyses were performed using JMP® 15(SAS Institute Inc., Cary, NC, USA). p values of < 0.05 were considered statistically significant.
Results
Non-diagnostic DES by CTCA
Non-diagnostic DES was observed in 69 stents (19%) in the derivation group and 78 stents (21%) in the derivation group. The characteristics of non-diagnostic DES and diagnostic DES in the derivation group are shown in Table 1. Age, sex, body mass index, and prevalence of cardiac comorbidities were similar between the groups. The location of target lesions differed between the two groups (p = 0.001). All DES implanted in the left main trunk (LMT) were diagnostic, and the percentage of DES implanted in the RCA was higher in the diagnostic DES group (34% vs. 16%). Severe calcification and stent-in-stent lesions were more frequently observed in the non-diagnostic DES group than in the diagnostic group (29% vs. 12%, p < 0.001). Distribution in the DES material differed between the two groups (p = 0.002). The percentage of PtCr DES was higher in the non-diagnostic DES group than in the diagnostic DES group (44% vs. 23%). The distribution of implanted stent diameter was different between the two groups, and the percentage of small stent use (2.25–2.5 mm) was significantly higher in the non-diagnostic DES group than in the diagnostic DES group (72% vs. 28%, p < 0.001). Stent diameter was also longer in the non-diagnostic DES group than in the diagnostic DES group [29 (SD 16) vs. 25 (SD 12) mm, p = 0.01]. Strut thickness was relatively greater in the non-diagnostic DES group than in the diagnostic DES group, but the difference was not statistically significant [95 (SD 23) vs. 100 (SD 24) μm, p = 0.09]. Stent fracture detected by CTCA was similarly observed between two groups (3% in non-diagnostic group vs. 2% in diagnostic group, p = 0.75).
Scoring model to predict non-diagnostic DES by CTCA
Multivariate analysis was performed including variables with a p value < 0.10 in comparison with non-diagnostic DES and diagnostic DES in the derivation group. DES implanted in LMT lesions were previously excluded from multivariate analysis because they all were diagnostic and unsuitable for analysis. As a result, implanted stent diameter [> 3.0 mm, odds ratio (OR) 1.00; 2.75–3.0 mm, (OR) 2.81, 95% confidence interval (CI) 1.81–7.25, p = 0.03; 2.25–2.5 mm, (OR) 4.96, 95% CI 2.41–12.5, p < 0.001], severe calcification (OR 5.12, 95% CI 2.33–12.3, p < 0.001), stent-in-stent lesion (OR 7.91, 95% CI 3.52–19.2, p < 0.001), type of DES [stainless, OR 1.00; PtCr, (OR) 3.11, 95% CI 1.30–6.45, p = 0.006; CoCr, OR 1.28, 95% CI 0.75–3.97, p = 0.55] were independent predictors of non-diagnostic DES by CTCA (Table 2). The predicting system of implanted DES non-diagnostic by CTCA (PIDENT) score was derived after the risk ratios were converted to risk score points (Table 3). The PIDENT score was similar between the two groups [6 (SD 3) vs. 6 (SD 3), p = 0.88]. The C-statistic for non-diagnostic DES by CTCA was 0.86 (95% CI 0.82–0.89, p < 0.001) in the derivation group. Sensitivity and specificity were 85% and 74%, respectively, with a cutoff value of 8 points (Fig. 3A). The distribution of PIDENT score and the percentage of non-diagnostic DES by CTCA is shown in Fig. 3B.
Performance of PIDENT score in the validation group
In the validation group, the c-statistic for non-diagnostic DES by CTCA was 0.87 (95% CI 0.83–0.90, p < 0.001). The sensitivity and specificity were 84% and 76%, respectively, with a cutoff value of 8 points (Fig. 4A). The distribution of PIDENT score and the percentage of non-diagnostic DES by CTCA is shown in Fig. 4B.
Discussion
The present study demonstrated that a new scoring system, PIDENT, was valuable for predicting non-diagnostic DES by CTCA and for avoiding meaningless CTCA with nonevaluable image quality. Calculation of this score is simple and derived from the following four factors: stent diameter, severity of angiographic calcification, presence or not of stent-in-stent lesion, and type of DES. The PIDENT score applied to the validation group in this study showed objectively high predictive performance.
A larger stent naturally provides fewer blooming artifacts and is more visible, which leads to more accurate assessment, whereas a stent diameter of 3 mm was likely to be a cutoff value of high or low assessment ability [15]. Although smaller stents (≤ 2.5 mm) are available, and the mid-term efficacy of these stents has also been reported [25, 26], the assessment ability of CTCA for these stents has not been clarified. In our study, small stents (≤ 2.5 mm) had a strongly negative predictive value for diagnostic performance. The PIDENT score conformed to the present day because this score differentiated stent diameter in detail compared with past studies.
A previous study reported that thick-strut stents (≥ 100 μm) had lower diagnostic accuracy than thin-strut stents, which suggested that thick-strut stents may reduce visualization of the lumen [27]. Strut thickness was not associated with the diagnostic performance of CTCA in this study. We consider the reason is that approximately 70% to 80% of DES in this study were second- or third- generation DES with strut thicknesses of < 100 μm. There is also the possibility that thin-strut DES overcame the problem of artifacts caused by the strut, which reduce the visibility of CTCA. This point should be assessed in other studies with larger sample sizes in the near future.
The appearance of blooming artifacts caused by calcification is a problem peculiar to evaluation with CTCA; especially, bulky calcification strikingly reduces diagnostic performance. A subtraction CTCA method, wherein subtraction is performed using three-dimensional datasets acquired before and after the contrast medium reaches the target coronary artery, was reported to be feasible for patients with calcified coronary arteries [28, 29]. Subtraction CTCA has also been reported to provide significantly higher diagnostic accuracy in the evaluation of ISR [30]. However, subtraction CTCA has several limitations, such as artifacts specific to the subtraction method and increased radiation dose. Routine clinical use of subtraction CTCA is difficult and not cost-effective, but appropriate use for selected patients could be feasible for evaluating patients when non-diagnostic CTCA is predicted. It should also be clarified that subtraction CTCA could evaluate implanted stents with severe calcification.
Stent-in-stent lesion is the strongest predictor of non-diagnosis with CTCA in this study. Stent-in-stent lesions were scored with 8 points and the cutoff value of the PIDENT score was also 8 points. This indicated that evaluation of stent-in-stent lesions should be performed not with CTCA but with other imaging modalities. Interestingly, overlapping stents were not predictors of non-diagnostic CTCA in this study. We considered the long overlapping length of two metals and neo-atherosclerosis causing ISR to reduce visibility of the lumen in stent-in-stent lesions.
Key improvements in design and metal alloy composition of the stent platform have been shown to affect acute stent performance and clinical outcome [31,32,33]. The combination of PtCr coronary stent platforms improved acute mechanical performance in terms of flexibility, deliverability, conformability, radial strength, and visibility under angiography [31]. PtCr-DES was associated with non-diagnostic CTCA in this study. The good angiographic visibility of the PtCr stent results from its increased radiopacity; however, in the evaluation with CTCA, increased radiopacity might cause blooming or motion artifacts and reduce the visibility inside the stents. Though, type of stents did not matter in the evaluation of large stent more than 3 mm diameter because “PtCr-DES” had only 3 points. PtCr-DES implanted for a small vessel or calcified lesion that reaches 8 points should not be evaluated with CTCA.
Late catch-up phenomena or neo-atherosclerosis of the in-stent lumen have been reported even in newer generation DES [34]. Importantly, the diagnostic performance of CTCA for DES-ISR is very high if implanted stents are clearly visualized [35, 36]. CTCA suggests that patients with stents assessed as visible without ISR can avoid ICA with its associated risks. ICA may not be suitable to assess or follow up ISR lesions on a regular basis, which is the same in the research field. When faced with patients who previously underwent DES implantation and were suspected to have angina pectoris, patients with a PIDENT score ≤ 8 could be evaluated with CTCA if there was no other contraindication for CTCA, but patients with a PIDENT score > 8 should be evaluated with other imaging modalities, including ICA. Thus, the PIDENT score was valuable in reducing nonevaluable and meaningless CTCA for DES-ISR.
Study limitations
The limitations of this study were as follows. First, only a limited number of patients in a single center were enrolled. Second, because this was a retrospective study, the selection of patients who underwent evaluation with CTCA could be biased by the physician’s discretion. We did not perform CTCA for patients with uncontrolled AF tachycardia or with known severe calcified coronary artery. A lower rate of patients with AF, on hemodialysis and using a Rotablator could suggest such discretions indirectly. However, the CTCA in the patients in this study was performed for clinical indications so that the results of this study reflected real-world clinical practice. To evaluate the diagnostic performance of CTCA for DES, especially thin-strut DES, prospective studies with all-comer patients should be planned in the near future.
Conclusion
The PIDENT score consists of the following four factors for identifying non-diagnostic evaluation of DES-ISR with CTCA: stent diameter, severity of angiographic calcification, presence or not of stent-in-stent lesion, and type of DES. The PIDENT score was helpful for the effective use of CTCA in the evaluation of DES-ISR.
References
Moschovitis A, Cook S, Meier B (2010) Percutaneous coronary interventions in Europe in 2006. EuroIntervention 6:189–194
Holmes DR Jr, Leon MB, Moses JW, Popma JJ, Cutlip D, Fitzgerald PJ, Brown C, Fischell T, Wong SC, Midei M, Snead D, Kuntz RE (2004) Analysis of 1 year clinical outcomes in the SIRIUS trial; a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation 109:634–640
Morice MC, Colombo A, Meier B, Serruys P, Tamburino C, Guagliumi G, Sousa E, Stoll HP, Trial Investigators REALITY (2006) Sirolimus- vs paclitaxel-eluting stents in de novocoronary artery lesions; the REALITY trial: a randomized controlled trial. JAMA 295:895–904
Iglesias JF, Heg D, Roffi M, Tüller D, Noble S, Muller O, Moarof I, Cook S, Weilenmann D, Kaiser C, Cuculi F, Häner J, Jüni P, Windecker S, Pilgrim T (2019) Long-term effect of ultrathin-strut versus thin-strut drug-eluting stents in patients with small vessel coronary artery disease undergoing percutaneous coronary intervention: a subgroup analysis of the BIOSCIENCE randomized trial. Circ Cardiovasc Interv 12:e008024
Lee CW, Ahn JM, Lee JY, Kim WJ, Park DW, Kang SJ, Lee SW, Kim YH, Park SW, Park SJ (2014) Long-term (8 year) outcomes and predictors of major adverse cardiac events after full metal jacket drug-eluting stent implantation. Catheter Cardiovasc Interv 84:361–365
Lee PH, Lee SW, Yun SC, Bae J, Ahn JM, Park DW, Kang SJ, Kim YH, Lee CW, Park SW, Park SJ (2017) Full metal jacket with drug-eluting stents for coronary chronic total occlusion. JACC Cardiovasc Interv 10:1405–1412
Ribichini F, Romano M, Rosiello R, La Vecchia L, Cabianca E, Caramanno G, Milazzo D, Loschiavo P, Rigattieri S, Musarò S, Pironi B, Fiscella A, Amico F, Indolfi C, Spaccarotella C, Bartorelli A, Trabattoni D, Della Rovere F, Rolandi A, Beqaraj F, Belli R, Sangiorgio P, Villani R, Berni A, Sheiban I, Lopera Quijada MJ, Cappi B, Ribaldi L, Vassanelli C (2013) A clinical and angiographic study of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with multivessel coronary artery disease: the EXECUTIVE trial (EXecutive RCT: evaluating XIENCE V in a multi vessel disease). JACC Cardiovasc Interv 6:1012–1022
Park KW, Kang J, Kang SH, Ahn HS, Lee HY, Kang HJ, Koo BK, Chae IH, Youn TJ, Oh BH, Park YB, Kandzari DE, Kim HS (2013) Usefulness of the SYNTAX and clinical SYNTAX scores in predicting clinical outcome after unrestricted use of sirolimus- and everolimus-eluting stents. Circ J 77:2912–2921
Flohr TG, McCollough CH, Bruder H, Petersilka M, Gruber K, Süss C, Grasruck M, Stierstorfer K, Krauss B, Raupach R, Primak AN, Küttner A, Achenbach S, Becker C, Kopp A, Ohnesorge BM (2006) First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol 16:256–268
Juan YH, Huang YC, Sun Z, Hsieh IC, Chan WH, Chen CC, Hung KC, Wen MS, Wan YL (2004) The evolution and investigation of native coronary arteries in patients after coronary stent implantation: a study by 320-detector CT angiography. Int J Cardiovasc Imaging 30(Suppl 1):13–24
Gaspar T, Halon DA, Lewis BS, Adawi S, Schliamser JE, Rubinshtein R, Flugelman MY, Peled N (2005) Diagnosis of coronary in-stent restenosis with multidetector row spiral computed tomography. J Am Coll Cardiol 46:1573–1579
Kong LY, Jin ZY, Zhang SY, Zhang ZH, Wang YN, Song L, Zhang XN, Zhang YQ (2009) Assessment of coronary stents by 64-slice computed tomography: in-stent lumen visibility and patency. Chin Med Sci J 24:156–160
Gilard M, Cornily JC, Pennec PY, Le Gal G, Nonent M, Mansourati J, Blanc JJ, Boschat J (2006) Assessment of coronary artery stents by 16 slice computed tomography. Heart 92:58–61
Rixe J, Achenbach S, Ropers D, Baum U, Kuettner A, Ropers U, Bautz W, Daniel WG, Anders K (2006) Assessment of coronary artery stent restenosis by 64-slice multidetector computed tomography. Eur Heart J 27:2567–2572
Mark DB, Berman DS, Budoff MJ, Carr JJ, Gerber TC, Hecht HS, Hlatky MA, Hodgson JM, Lauer MS, Miller JM, Morin RL, Mukherjee D, Poon M, Rubin GD, Schwartz RS (2010) ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 Expert consensus document on coronary computed tomographic angiography: a report of the American college of cardiology foundation task force on expert consensus documents. Circulation 121:2509–2543
Byrne RA, Stone GW, Ormiston J, Kastrati A (2017) Coronary balloon angioplasty, stents, and scaf-folds. Lancet 390:781–792
Stefanini GG, Taniwaki M, Windecker S (2014) Coronary stents: novel developments. Heart 100:1051–1061
Price MJ, Saito S, Shlofmitz RA, Spriggs DJ, Attubato M, McLaurin B, Popma Almonacid A, Brar S, Liu M, Moe E, Mehran R (2017) First report of the resolute onyx 2.0 mm zotarolimus-eluting stent for the treatment of coronary lesions with very small reference vessel diameter. JACC Cardiovasc Interv 10:1381–1388
Tsuda T, Ishii H, Ichimiya S, Kanashiro M, Watanabe J, Takefuji M, Aoyama T, Suzuki S, Tanaka A, Matsubara T, Murohara T (2015) Assessment of in-stent restenosis using high-definition computed tomography with a new gemstone detector. Circ J 79:1542–1548
Andreini D, Pontone G, Bartorelli AL, Mushtaq S, Trabattoni D, Bertella E, Cortinovis S, Annoni A, Formenti A, Ballerini G, Agostoni P, Fiorentini C, Pepi M (2011) High diagnostic accuracy of prospective ECG-gating 64-slice computed tomography coronary angiography for the detection of in-stent restenosis: in-stent restenosis assessment by low-dose MDCT. Eur Radiol 21:1430–1438
Nishida K, Kimura T, Kawai K, Miyano I, Nakaoka Y, Yamamoto S, Kaname N, Seki S, Kubokawa S, Fukatani M, Hamashige N, Morimoto T, Mitsudo K (2013) Comparison of outcomes using the sirolimus-eluting stent in calcified versus non-calcified native coronary lesions in patients on- versus not on-chronic hemodialysis (from the j-Cypher registry). Am J Cardiol 112:647–655
Turgut O, Yilmaz A, Yalta K, Yilmaz BM, Ozyol A, Kendirlioglu O, Karadas F, Tandogan I (2007) Tortuosity of coronary arteries: an indicator for impaired left ventricular relaxation? Int J Cardiovasc Imaging 23:671–677
Chung MS, Yang DH, Kim YH, Roh JH, Song J, Kang JW, Ahn JM, Park DW, Kang SJ, Lee SW, Lee CW, Park SW, Park SJ, Lim TH (2016) Stent fracture and longitudinal compression detected on coronary CT angiography in the first- and new-generation drug-eluting stents. Int J Cardiovasc Imaging 32:637–646
Sullivan LM, Massaro JM, D’Agostino RB Sr (2004) Presentation of multivariate data for clinical use: the Framingham study risk score functions. Stat Med 23:1631–1660
Kirtane AJ, Yeung AC, Ball M, Carr J, O’Shaughnessy C, Mauri L, Liu M, Leon MB (2020) Long-term (5 year) clinical evaluation of the resolute zotarolimus-eluting coronary stent: the RESOLUTE US clinical trial. Catheter Cardiovasc Interv 95:1067–1073
Yano H, Horinaka S, Ishikawa M, Ishimitsu T (2017) Efficacy of everolimus-eluting stent implantation in patients with small coronary arteries (≤ 2.5 mm): outcomes of 3 year clinical follow-up. Heart Vessels 32:796–803
Dai T, Wang J-R, Peng-Fei Hu (2018) Diagnostic performance of computed tomography angiography in the detection of coronary artery in-stent restenosis: evidence from an updated meta-analysis. Eur Radiol 28:1373–1382
Yoshioka K, Tanaka R, Muranaka K, Sasaki T, Ueda T, Chiba T, Takeda K, Sugawara T (2015) Subtraction coronary CT angiography using second generation 320-detector row CT. Int J Cardiovasc Imaging 31(Suppl 1):51–58
Amanuma M, Kondo T, Sano T, Sekine T, Takayanagi T, Matsutani H, Arai T, Morita H, Ishizaka K, Arakita K, Iwasa A, Takase S (2015) Subtraction coronary computed tomography in patients with severe calcification. Int J Cardiovasc Imaging 31:1635–1642
Amanuma M, Kondo T, Sano T, Takayanagi T, Matsutani H, Sekine T, Arai T, Morita H, Ishizaka K, Arakita K, Iwasa A, Takase S (2016) Assessment of coronary in-stent restenosis: value of subtraction coronary computed tomography angiography. Int J Cardiovasc Imaging 32:661–670
Menown IB, Noad R, Garcia EJ, Meredith I (2010) The platinum chromium element stent platform: from alloy, to design, to clinical practice. Adv Ther 27:129–141
O’Brien BJ, Stinson JS, Larsen SR, Eppihimer MJ, Carroll WM (2010) A platinum-chromium steel for cardiovascular stents. Biomaterials 31:3755–3761
Turco MA (2011) The integrity® bare-metal stent made by continuous sinusoid technology. Expert Rev Med Devices 8:303–306
Kobayashi N, Ito Y, Nakano M, Araki M, Hirano K, Yamawaki M, Takimura H, Sakamoto Y, Tsukahara R, Muramatsu T (2015) Incidence and characteristics of late catch-up phenomenon between sirolimus-eluting stent and everolimus-eluting stent: a propensity matched study. J Interv Cardiol 28:551–562
Leschka S, Alkadhi H, Plass A, Desbiolles L, Grünenfelder J, Marincek B, Wildermuth S (2005) Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J 26:1482–1487
Carrabba N, Bamoshmoosh M, Carusi LM, Parodi G, Valenti R, Migliorini A, Fanfani F, Antoniucci D (2007) Usefulness of 64-slice multidetector computed tomography for detecting drug eluting in-stent restenosis. Am J Cardiol 100:1754–1758
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Rights and permissions
About this article
Cite this article
Honda, Y., Yamawaki, M., Mori, S. et al. Scoring model to predict low image quality of drug-eluting stent evaluated by computed tomography coronary angiography. Heart Vessels 37, 229–238 (2022). https://doi.org/10.1007/s00380-021-01918-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01918-8