Abstract
The association between circulatory dynamics changes during cryoballoon applications and a successful pulmonary vein isolation (PVI) is unknown. Seventy atrial fibrillation patients who underwent PVI with 28-mm second-generation cryoballoons and single 3-min freezes were included. Intra-procedural parameters including circulatory dynamics changes during cryoapplications, were compared between 113 successful applications (30 left superior PVs[LSPVs], 30 left inferior PVs[LIPVs], 25 right superior PVs[RSPVs], and 28 right inferior PVs[RIPVs]) and 47 failed applications (10 LSPVs, 9 LIPVs, 8 RSPVs, and 20 RIPVs). In all individual PVs, lower nadir balloon temperatures (MinTemps) and longer thawing times (ThawTimes) significantly predicted a successful PVI. In addition, greater systolic blood pressure drops following releasing the PV occlusion (SBP-drops) significantly predicted a successful right PV PVI, and longer elapse times during SBP-drops significantly predicted a successful RIPV PVI. Composite parameters incorporating MinTemps and ThawTimes, SBP-drops, and ThawTimes showed the highest area under the curve to predict a successful left PV (0.876 for LSPVs, 0.851 for LIPVs) and right PV (0.927 for RSPVs, 0.980 for RIPVs) PVI, respectively. If the ThawTime (≥ 30 s) and SBP-drop (≤ − 21 mmHg) cutoff values were achieved for the RIPVs, the positive predictive value was 100%. In contrast, if both criteria were not achieved for the RIPVs, the negative predictive value was 100%. In the second-generation cryoballoon PVI, the MinTemp and ThawTime were significantly associated with acute success for all four PVs. In addition, SBP-drops further improved the accuracy of predicting a successful right PV PVI, especially of the RIPV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary vein isolation (PVI) is a fundamental curative therapy for paroxysmal atrial fibrillation (AF) [1, 2]. Although radiofrequency (RF) catheter ablation have been established as a standard procedure, the cryoballoon (CB) technology is becoming a major alternative thanks to a less-complicated technique, shorter procedure time, and higher durability of the PVI than RF-PVI [3,4,5,6]. There have been multiple studies reporting the non-inferiority of the mid-term outcome after the CB-PVI when compared to RF-PVI [5].
One of the vital factors for a successful CB-PVI is the complete occlusion of the entire ostium of the PV, and freezing with a complete occlusion results in a significant impact on the balloon temperature and circulatory parameters [7,8,9]. In brief, the nadir balloon temperature is lower with successful than failed freezes [7, 8]. The blood pressure (BP) rises during freezing and sharply drops after releasing the PV occlusion during completely occluded cryoapplications [9]. This is presumably because freezing of atrial tissue might result in a BP elevation, and the peripheral circulation of dammed chilled blood in the distal PV might cause a sharp BP drop [9]. However, those intra-procedural parameters significantly differ among the 4 individual PVs due to the different PV anatomies and CB position during the applications. The purpose of the present study was to explore the intra-procedural parameters, including the circulatory dynamics changes during the cryoapplication, predicting a successful CB-PVI of all 4 individual PVs.
Methods
Study population
We retrospectively enrolled 70 AF patients who underwent CB-PVI using second-generation CBs (Arctic Front Advance, Medtronic, Minneapolis, MN) in our institute between March and December 2016. The CB-PVI was performed with a single 3-min freeze strategy using exclusively 28-mm CBs. To calculate the optimal cutoff values for a successful CB-PVI, the study included 113 successful applications in 32 patients in whom all 4 PVs were successfully isolated by a single application (group A), and 47 failed applications in 38 patients who had at least one failed freezing (group B). Cryoapplications that were discontinued before 180 s were excluded from the analysis. All the patients gave written informed consent prior to the procedure. The study protocol was approved by the hospital’s institutional review board. The study complied with the Declaration of Helsinki.
Ablation procedure
All antiarrhythmic drugs were discontinued for at least five half-lives prior to the procedure. The surface electrocardiogram, bipolar intracardiac electrograms, and femoral intra-arterial BP were continuously monitored and stored on a computer-based digital recording system (LabSystem PRO, Bard Electrophysiology, Lowell, MA). The bipolar electrograms were filtered from 30 to 500 Hz. The procedure was performed under moderate sedation obtained with dexmedetomidine. A 100 IU/kg body weight of heparin was administered immediately following the venous access, and heparinized saline was additionally infused to maintain the activated clotting times at 300–350 s. A single transseptal puncture was performed using an RF needle (Baylis Medical., Montreal, QC) and 8-Fr long sheath (SL0, AF Division, SJM, Minneapolis, MN). The transseptal sheath was exchanged over a guidewire for a 15-Fr steerable sheath (Flexcath Advance, Medtronic). A 20-mm circular mapping catheter (Lasso, Biosense-Webster, Diamond Bar, CA) was used for mapping all PVs before and after the cryoablation to confirm an electrical isolation. A spiral mapping catheter (Achieve, Medtronic) was used to advance the CB into the PV for support and mapping the PV potentials. Following sealing at the PV antrum, a complete occlusion was confirmed by injecting contrast medium. This was followed by a freeze cycle of 180 s. No additional applications were performed after the isolation. Touch-up radiofrequency ablation was applied in cases with residual PV potentials after a maximum of 3 cryoapplications. To avoid bilateral phrenic nerve injury, all CB applications were applied under diaphragmatic electromyography monitoring [10]. When the balloon nadir temperatures exceeded − 60 °C or phrenic nerve injury was suspected, the application was interrupted [11]. As the standard deflation technique, the intraballoon shaft was manually straightened when the intraballoon temperature reached 15 °C to rewrap the balloon prior to the balloon deflation. The procedural endpoint was defined as an electrical PVI.
Evaluation of the circulatory dynamics
The changes in the circulatory parameters were evaluated by comparing the systolic BP (SBP) at specific time points: (1) the beginning and the end of the 3-min freezing phase (T0 min and T3 min), (2) at a balloon temperature of 15 °C during the thawing phase (T15 °C), and (3) at the nadir of the BP after balloon deflation (Tnadir). An interval thaw time at 15 °C was selected since that was generally the CB temperature limit at which the balloon was manually stretched by the operator on termination of the cryoballoon application. The nadir balloon temperature (MinTemp), rise in the SBP from T0 min to T3 min (SBP-rise), drop in the SBP after the T15 °C to Tnadir (SBP-drop), and elapsed time from T3 min to T15 °C (ThawTime) and T15 °C to Tnadir (DropTime) were compared between the successful and failed freezes (Fig. 1). The parameters were also compared between the successful cryoapplications during AF and sinus rhythm. All the analyses were performed individually in the 4 PVs, including the left superior (LSPVs), left inferior (LIPVs), right superior (RSPVs), and right inferior PVs (RIPVs).
Transition of the SBP and in-balloon temperature during the freezing and thawing phases of the cryoapplications. In-procedural parameters were defined as follows: MinTemp: nadir in-balloon temperature; SBP-rise: rise in the systolic blood pressure during the freezing phase; SBP-drop; sharp drop of the systolic blood pressure after releasing the PV occlusion; ThawTime: thawing time (from the end of the freezing to a 15 °C balloon temperature); DropTime: elapsed time during the SBP-drop
Statistics
All statistical analyses were performed using R version 3.4.1 software [12]. The continuous variables are reported as mean values ± standard deviations and were compared using a Student’s t test. Differences between proportions were compared using Fisher’s exact tests. Differences in the mean values between three or more groups were evaluated by a Welch’s ANOVA. The changes in the circulatory parameters and intra-PV analyses of each predictor were compared by a paired t test. A receiver-operator characteristics (ROC) curve of each parameter was calculated to quantify the area under the curve (AUC). The ROC curves were exclusively calculated for the parameters that significantly differed between the successful and failed freezes. As for the composite predictors incorporating multiple parameters, the AUC of the ROC curve was also calculated. The numbers of the pretest probability of an acute success for all 4 PVs were quoted from the previous report [7]. All p values were two-sided, and statistical significance was established at a p < 0.05.
Results
Procedural results
The patient characteristics are shown in Table 1. In group A, among a total of 128 successful CB applications, 15 were interrupted during the 3-min freeze. The remaining 113 applications (30 LSPVs, 30 LIPVs, 25 RSPVs, and 28 RIPVs) in which a 3-min freeze was applied were further analyzed. In group B, a total of 47 failed freezes (10 LSPVs, 9 LIPVs, 8 RSPVs, 20 RIPVs) were analyzed.
Nadir balloon temperature and thawing time
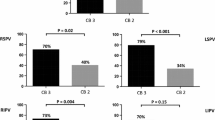
In group A, the mean MinTemp was − 52.1 ± 5.4 °C, which significantly differed among the 4 PVs (p < 0.001) (Table 2). In group B, the mean MinTemp was − 44.0 ± 6.0 °C, and it was similar among the 4 PVs (p = 0.251). The mean MinTemp was significantly lower in group A than group B (p < 0.0001), and the results were consistent for the 4 individual PVs. The AUCs of the ROC curves and best cutoff values are noted in Table 3.
As for the time course, both the ThawTime (40.6 ± 11.8 vs. 23.4 ± 9.8 s, p < 0.001) and DropTime (21.5 ± 7.5 vs. 15.7 ± 9.2 s, p < 0.001) were significantly longer in group A than group B. The ThawTime significantly differed among the 4 PVs both in group A (p < 0.001) and group B (p = 0.013). The DropTime significantly differed among the 4 PVs in group B (p = 0.042) but not in group A (p = 0.060). The difference in the ThawTime between group A and group B was still significant for all 4 PVs (Table 2). The AUC of the ThawTime is shown in Table 3. In contrast, the difference in the DropTime between group A and group B was significant only for the RIPVs (Table 2), and the AUC was 0.910 (Table 3).
Circulatory dynamics during the freezing and thawing phases
The SBP significantly increased during the 3-min freeze phase from 138.7 ± 28.8 to 148.0 ± 28.3 mmHg in group A (p < 0.001) and from 144.9 ± 33.0 to 152.0 ± 31.2 mmHg in group B (p < 0.001). The magnitude of the SBP rise differed among the 4 PVs in group A (11.5 ± 18.7 in the LSPV, 14.5 ± 13.0 in the LIPV, 6.3 ± 11.0 in the RSPV, and 3.8 ± 12.6 in the RIPV, p = 0.013) but not in group B (p = 0.132). The magnitude of the SBP rise was similar between group A and group B (9.2 ± 14.7 vs. 7.1 ± 13.6, p = 0.403).
After the balloon temperature reached 15 °C, the SBP significantly dropped from 136.4 ± 26.8 to 94.6 ± 18.5 mmHg in group A (p < 0.001) and from 138.1 ± 27.7 to 102.2 ± 27.9 mmHg in group B (p < 0.001). The magnitude of the SBP-drop was significantly greater in group A than group B (− 44.1 ± 19.1 vs. − 26.5 ± 19.4, p < 0.001). The difference was significant for the right PVs but not the left PVs (Table 2). The AUC of the SBP-drop for a successful CB-PVI of the RSPVs and RIPVs was 0.755 and 0.931, respectively (Table 3).
Impact of the rhythm on the procedural parameters
The MinTemp did not significantly differ during sinus rhythm and AF in the LSPVs (− 52.5 ± 4.6 vs. − 51.0 ± 4.5, p = 0.481), LIPVs (− 48.4 ± 3.9 vs. − 45.7 ± 2.9, p = 0.119), and RIPVs (− 53.2 ± 5.7 vs. − 55.3 ± 6.1, p = 0.427), but significantly differed in the RSPVs (− 56.2 ± 3.1 vs. − 50.5 ± 7.2, p = 0.013). The ThawTime did not significantly differ in the LSPVs (43.7 ± 13.8 vs. 45.5 ± 12.2, p = 0.778), LIPVs (33.9 ± 9.1 vs. 33.0 ± 4.8, p = 0.830), RSPVs (48.6 ± 9.8 vs. 45.3 ± 1.2, p = 0.574), and RIPVs (36.1 ± 10.3 vs. 42.1 ± 9.7, p = 0.212). The DropTime did not significantly differ in the LSPVs (24.7 ± 7.8 vs. 17.7 ± 8.0, p = 0.060), LIPVs (19.5 ± 4.5 vs. 20.8 ± 8.3, p = 0.591), RSPVs (19.9 ± 6.9 vs. 18.5 ± 22.9, p = 0.814), and RIPVs (22.3 ± 4.9 vs. 25.1 ± 4.7, p = 0.191). The SBP-drop did not significantly differ in the LSPVs (− 48.9 ± 23.5 vs. 38.0 ± 16.1, p = 0.296), LIPVs (− 42.5 ± 18.1 vs. − 27.3 ± 10.3, p = 0.062), RSPVs (− 45.3 ± 20.6 vs. − 34.0 ± 19.9, p = 0.324), and RIPVs (− 48.0 ± 13.4 vs. − 43.6 ± 19.3, p = 0.500).
Comparison of the parameters predicting a successful PVI
Table 3 summarizes the significant predictors and their best cutoff values with the sensitivities, specificities, positive predictive values (PPVs), and negative predictive values (NPVs). The MinTemp and ThawTime were both significant predictors for all PVs. These 2 parameters were significantly correlated (R = − 0.715, p < 0.001), while the per-PV analyses still remained significant for the LSPVs (R = − 0.755, p < 0.001), LIPVs (R = − 0.638, p < 0.001), RSPVs (R = − 0.768, p < 0.001), and RIPVs (R = − 0.815, p < 0.001). The AUC of each significant parameter was statistically similar for each PV (LSPV: p = 0.339, LIPV: p = 0.578, RSPV: p = 0.100, RIPV: p = 0.324).
For the individual PVs, we tried to build a composite predictor incorporating 2 different factors. In the univariate analysis for the left PVs, only the MinTemp and ThawTime were significant predictors. For the LSPVs and LIPVs, the AUC of the composite predictor was 0.876 and 0.851, respectively, which was not significantly better than the univariate models. As for the RSPVs, we calculated the composite predictors including any 2 of the MinTemp, ThawTime, and SBP-drop. The AUC of the composite predictor calculated from the MinTemp + ThawTime, MinTemp + SBP-drop, and ThawTime + SBP-drop was 0.917, 0.915, and 0.927, respectively.
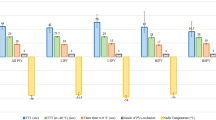
As for the RIPVs, the AUC of the 4 parameters was equivalent over 0.9. The correlation coefficients between every 2 factors as well as the AUC of the composite predictors for the RIPVs are shown in Fig. 2. The highest correlation was observed between the MinTemp and ThawTime. The lowest correlation was between the MinTemp and DropTime. As for the ROC curve analyses, the highest AUC was obtained from the combination of the SBP-drop and ThawTime, while the lowest was the combination of the SBP-drop and DropTime. If the cutoff values of the ThawTime (≥ 30 s) and SBP-drop (≤ − 21 mmHg) were achieved for the RIPVs, the positive predictive value was 100%. In contrast, if both the criteria were not achieved, the negative predictive value was 100% (Fig. 3).
Correlation coefficient and AUC of the composite predictors incorporating every two parameters during freezes of the RIPVs. The highest and lowest correlations were observed with the combination of the MinTemp/ThawTime and MinTemp/DropTime, respectively. The highest and the lowest AUCs were obtained from the ThawTime/SBP-drop and SBP-drop/DropTime, respectively. AUC: area under the receiver operating characteristics curve, R: correlation coefficient. The other abbreviations are the same as in Fig. 1
Discussion
To the best of our knowledge, this is the first report to explore the parameters, including the circulatory dynamics changes predicting a successful PVI in each individual PV, in the CB-PVI. We found that (1) both the MinTemp and ThawTime were significant parameters predicting a successful PVI of all 4 PVs, and (2) the SBP-drop was a significant parameter predicting a successful PVI of the right PVs, and a composite parameter incorporating the ThawTime and SBP-drop improved the diagnostic accuracy for the right PVs, especially the RIPV.
Predictors of a successful PVI
When analyzing all PVs altogether, the MinTemp and ThawTime are already known to be predictors of successful cryoapplications for both the 1st-generation [13] and 2nd-generation CB ablation [7, 8]. Our study confirmed the results in an analysis of the 4 individual PVs, and also there was a strong correlation between the MinTemp and ThawTime. Moreover, we revealed that the magnitude of the SBP-drop could be an additional predictor of a successful freeze of the right PVs. A previous study showed a gradual rise in the SBP during the freezing phase and a recovery of the elevated SBP during the initial thawing phase followed by a sharp drop of the SBP after releasing the PV occlusion [9]. The present study clarified that the magnitude of the SBP rise was similar between a successful and failed application, while the magnitude of the SBP drop was more evident in a successful application. That was because a sharp SBP drop is provoked by peripheral circulation of dammed chilled blood [9], and the amount might be greater in a complete occlusion than an incomplete occlusion.
Although the magnitude of the SBP-drop was greater for successful than failed freezes of all 4 PVs, the utility of the SBP-drop for predicting a successful freeze was most prominent for the RIPV. We speculated that the greatest number of failed applications in the RIPV ablation increased the statistical power to prove the difference. For the left PVs, it might also be possible that inter-PV connections had an influence on the study results. The difference might also be explained by the (1) different anatomical location and surrounding tissue in each PV, which lead to a different atrial tissue distensibility and tissue contact area when the PVs were occluded, (2) different alignment from the trans-septal hole to each PV, which lead to a different balloon pushability and anatomical distortion, (3) different PV size and ostial area, which lead to a different amount of dammed blood, and (4) different occlusion technique (a complete occlusion is required in the LSPV, but a small amount of contrast leakage is acceptable in the RSPV). In the RIPV ablation, the combination of the SBP-drop and ThawTime showed the highest AUC for predicting a successful application. Generally, RIPVs are the most challenging target among the 4 PVs during the CB-PVI due to the anatomical factors such as the proximity of the ostium to the interatrial septum, and frequent early branching with large oval ostia. Furthermore, the capability of real-time PV potential monitoring is the lowest for the RIPVs due to the short PV sleeve and procedural difficulty. Therefore, the knowledge of the factors predicting a successful RIPV application seems to be particularly important and useful.
Limitations
First, this study was a single center, retrospective study concerning the outcome in the acute phase. Further investigation is needed to evaluate the durability of the PVI in the mid-term or long term. Second, the number of subjects was limited. Third, the operators cannot control the ThawTime and SBP-drop during freezing; however, that information might aid in evaluating the electrical isolation of the RIPV especially when solely using an Achieve mapping catheter. Fourth, the balloon temperature is not the tissue temperature and it is a measurement of the return gas temperature, which is influenced by several factors including the balloon–tissue contact area and collateral blood flow. Fifth, the BP might be influenced by the sedation level and alternation of the autonomic nervous system. However, we previously reported that the administration of atropine did not have an impact on the BP change, suggesting that the impact of the autonomic nervous system on the change in the BP seemed to be limited [9].
Conclusions
In the second-generation CB-PVI, the longer thawing time and lower nadir in-balloon temperature significantly predicted a successful application for all 4 individual PVs. In addition, a greater magnitude of the SBP drop following releasing the PV occlusion significantly predicted a successful application for the right PVs, and a composite parameter incorporating the SBP drop and thawing time showed the highest accuracy in predicting a successful RIPV ablation.
References
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339:659–666
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D, Heart Rhythm Society Task Force on Catheter, and Surgical Ablation of Atrial Fibrillation (2012) 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 9(632–696):e21
Kojodjojo P, O'Neill MD, Lim PB, Malcolm-Lawes L, Whinnett ZI, Salukhe TV, Linton NW, Lefroy D, Mason A, Wright I, Peters NS, Kanagaratnam P, Davies DW (2010) Pulmonary venous isolation by antral ablation with a large cryoballoon for treatment of paroxysmal and persistent atrial fibrillation: medium-term outcomes and non-randomised comparison with pulmonary venous isolation by radiofrequency ablation. Heart 96:1379–1384
Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, Lehmann JW, Ruskin JN, Cryoablation Investigators STOPAF (2013) Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 61:1713–1723
Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albenque JP, Tondo C, FIRE AND ICE investigators (2016) Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 374:2235–2245
Coulombe N, Paulin J, Su W (2013) Improved in vivo performance of second-generation cryoballoon for pulmonary vein isolation. J Cardiovasc Electrophysiol 24:919–925
Kajiyama T, Miyazaki S, Matsuda J, Watanabe T, Niida T, Takagi T, Nakamura H, Taniguchi H, Hachiya H, Iesaka Y (2017) Anatomic parameters predicting procedural difficulty and balloon temperature predicting successful applications in individual pulmonary veins during 28-mm second-generation cryoballoon ablation. JACC Clin Electrophysiol 3:580–588
Deubner N, Greiss H, Akkaya E, Zaltsberg S, Hain A, Berkowitsch A, Guttler N, Kuniss M, Neumann T (2017) The slope of the initial temperature drop predicts acute pulmonary vein isolation using the second-generation cryoballoon. Europace 19:1470–1477
Kajiyama T, Miyazaki S, Watanabe T, Yamao K, Kusa S, Igarashi M, Nakamura H, Hachiya H, Iesaka Y (2017) Circulatory dynamics during pulmonary vein isolation using the second-generation cryoballoon. J Am Heart Assoc 6:e006559
Miyazaki S, Hachiya H, Taniguchi H, Nakamura H, Ichihara N, Usui E, Kuroi A, Takagi T, Iwasawa J, Hirao K, Iesaka Y (2015) Prospective evaluation of bilateral diaphragmatic electromyograms during cryoballoon ablation of atrial fibrillation. J Cardiovasc Electrophysiol 26:622–628
Su W, Kowal R, Kowalski M, Metzner A, Svinarich JT, Wheelan K, Wang P (2015) Best practice guide for cryoballoon ablation in atrial fibrillation: the compilation experience of more than 3000 procedures. Heart Rhythm 12:1658–1666
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Furnkranz A, Koster I, Chun KR, Metzner A, Mathew S, Konstantinidou M, Ouyang F, Kuck KH (2011) Cryoballoon temperature predicts acute pulmonary vein isolation. Heart Rhythm 8:821–825
Acknowledgements
We would like to thank Mr. John Martin for his help in the preparation of the manuscript.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kajiyama, T., Miyazaki, S., Hamaya, R. et al. Circulatory dynamics changes are an additional predictor of successful pulmonary vein isolation during cryoballoon ablation. Heart Vessels 35, 125–131 (2020). https://doi.org/10.1007/s00380-019-01467-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01467-1