Abstract
Background
The second-generation cryoballoon (CB2) provides effective and durable pulmonary vein isolation (PVI) associated with encouraging clinical outcome data. The novel third-generation cryoballoon (CB3) incorporates a 40 % shorter distal tip. This design change may translate into an increased rate of PVI real-time signal recording, facilitating an individualized ablation strategy using the time to effect (TTE).
Methods and results
Thirty consecutive patients with paroxysmal or short-standing persistent atrial fibrillation underwent CB3-based PVI and were compared to 30 patients treated with the CB2. Individual freeze-cycle duration was set to TTE + 120 s for both groups. A total of 118 (CB3) and 119 (CB2) pulmonary veins (PV) were identified and all PVs successfully isolated utilizing the CB3 and CB2, respectively. The real-time PVI visualization rate was 74 % (CB3) and 40 % (CB2; p = 0.001) and the mean freeze-cycle duration 204 ± 88 s (CB3) and 215 ± 90 s (CB2; p = 0.15). Per individual PV, a shorter mean freeze-duration was found for the CB3 and the right superior PVs (188 ± 92 vs. 211 ± 124 s, p = 0.04) and right inferior PVs (192 ± 75 vs. 200 ± 37 s, p = 0.02). No differences were found for the left-sided PVs.
Conclusions
A higher rate of real-time electrical PV recordings is seen using the novel CB3 as compared to CB2, which may facilitate an individualized ablation strategy using the TTE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Use of the second-generation cryoballoon (CB2, Arctic Front Advance, Medtronic, Inc., Minneapolis, MN, USA) for pulmonary vein isolation (PVI) has demonstrated high procedural success rates and encouraging 1- and 2-year clinical outcome for patients with paroxysmal (PAF) and persistent atrial fibrillation (PersAF) [1–7]. Superior clinical outcome is based on a high rate of durable PVI ranging between 69 and 77 % for patients undergoing repeat procedures due to AF recurrence [8, 9]. Current ablation strategies commonly deploy a fixed freeze-cycle duration of 180–240 s. Use of a spiral mapping catheter (Achieve, Medtronic, Inc., Minneapolis, MN, USA) for real-time verification of PVI may allow for an individualized ablation strategy based on the time-to-effect (TTE). However, the long distal tip of the CB2 may limit proper visualization of PV signals. The recently launched third-generation cryoballoon (CB3, Arctic Front Advance ST, Medtronic) incorporates a 40 % shorter tip facilitating a more proximal Achieve catheter position. Whether these modifications maintain the favorable freezing characteristics seen with the CB2, while concurrently allowing for a higher rate of real-time PV recordings demands further evaluation. To the best of our knowledge the present study reports the first-in-man experience and acute procedural efficacy using the novel CB3 for PVI.
Methods
Inclusion and exclusion criteria
Consecutive patients with symptomatic, drug-refractory PAF or short standing PersAF (duration ≤3 months) were consented for CB-based PVI. From 05/2015 to 09/2015, 30 consecutive patients were treated with the second-generation cryoballoon (CB2 group, control), followed by 30 patients consecutively treated with the third-generation cryoballoon (CB3 group). The patients were not randomized. Exclusion criteria were prior left atrial (LA) ablation attempts, a LA diameter >60 mm, severe valvular heart disease or contraindications to postinterventional oral anticoagulation. Transesophageal echocardiography was performed in all patients prior to PVI to rule out intracardiac thrombi and to assess the LA diameter. No further pre-procedural imaging was performed. In patients on vitamin K antagonists the procedure was performed under therapeutic INR values of 2–3. New oral anticoagulants were stopped on the day before the procedure and continued 6 h post ablation. All patients gave written informed consent and all patient information was anonymized. The study was approved by the local ethic’s board (ethical review board number: WF-028/15) and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Intraprocedural management
The intraprocedural management has been described in detail in previous studies from our group [1, 2]. In brief, the procedure was performed under deep sedation using midazolam, sufentanil and propofol. Prior to transseptal puncture one diagnostic catheter was introduced via the right femoral vein and positioned within the coronary sinus (7F, Webster TM, Biosense Webster, Inc. Diamond Bar, CA, USA). Single transseptal puncture was performed under fluoroscopic guidance using a modified Brockenbrough technique and an 8.5 F transseptal sheath (SL1, St. Jude Medical, Inc., St. Paul, MN, USA). Selective PV angiography was performed to identify the pulmonary vein (PV) ostia. The transseptal sheath was exchanged over a guidewire for a 12 F steerable sheath (Flexcath Advance, Medtronic). After transseptal puncture heparin boluses were administered targeting an activated clotting time of >300 s.
Cryoballoon-based PVI
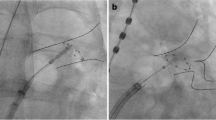
The 28 mm CB was advanced into the LA via the 12F steerable sheath using the Achieve catheter (20 mm diameter, Medtronic, Inc.) for guidance. The CB was inflated proximal to the PV ostium followed by gentle push aiming for complete PV sealing. Occlusion of the PV ostium was verified by contrast dye injections. Standard freeze-cycle duration was TTE + 120 s. If the TTE could not be visualized, the freeze-cycle duration was set to 240 s. No additional bonus freeze-cycle was applied [2]. The procedural endpoint was defined as persistent PVI verified by Achieve catheter (Medtronic, Inc.) recordings after the last CB application.
A spiral temperature probe (Sensitherm, St. Jude Medical) was advanced into the esophagus to monitor esophageal temperatures during each energy application. The intraluminal esophageal temperature cut-off was set at 15 °C [10, 11]. During energy delivery along the septal PVs, continuous phrenic nerve (PN) pacing was performed using a diagnostic catheter introduced into the superior vena cava (7F, Webster TM, Biosense Webster, Inc.). Pacing was set at maximum output and pulse width (12 mA, 2.9 ms) and a cycle length of 1200 ms. PN capture was monitored by tactile feedback of diaphragmatic contraction and assessment of the right diaphragmatic compound motor action potential (CMAP). Energy delivery was interrupted immediately if weakening or loss of diaphragmatic contraction was noted or a decrease of the CMAP amplitude of ≥30 % was seen. In case of persistent PN palsy, no further cryoenergy was delivered to the septal PVs [12, 13].
Postprocedural care
Following ablation, all patients underwent transthoracic echocardiography to rule out a pericardial effusion. Low molecular-weight heparin was administered in patients on vitamin K antagonists and an INR <2.0 until a therapeutic INR of 2–3 was achieved. New oral anticoagulants were re-initiated 6 h post ablation. Anticoagulation was continued for at least 3 months and continued thereafter based on the individual CHA2DS2-VASc score. Previously ineffective antiarrhythmic drugs were continued for 3 months post ablation [1, 2].
All patients were treated with proton-pump inhibitors for 6 weeks. After a 3-month blanking period outpatient clinic visits or visits at the referring physician are performed at 3, 6, and 12 months post ablation including 24-h Holter-ECGs.
Statistical analysis
Differences of metric variables between the two groups were analyzed with t test if the data were normally distributed, and with Wilcoxon-Mann–Whitney test otherwise. Differences between categorical variables were evaluated using the Chi square test or the Fisher’s exact test in case of small expected cell frequencies. PVI data were analyzed using mixed models. Linear mixed models were used for continuous data. Generalized linear mixed models were used for binary or count data. A hierarchical logistic regression model was applied for binary data. A Poisson distribution was assumed for count data. All p values are two-sided and a p value <0.05 was considered significant. All calculations were performed with the statistical analysis software SAS (SAS Institute Inc., version 9.3, Cary, NC, USA) [2].
Results
Patient characteristics
A total of 60 consecutive patients underwent 28 mm CB-based PVI utilizing the CB2 (n = 30) or CB3 (n = 30). Patient baseline characteristics are depicted in Table 1.
Acute ablation results
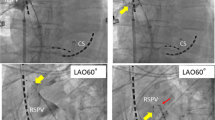
In 60 patients a total of 237 PVs were identified and targeted for ablation [60 right superior PVs (RSPV), 60 right inferior PVs (RIPV), 57 left superior PVs (LSPV), 57 left inferior PVs (LIPV) and 3 left common PVs (LCPV)]. All PVs were successfully isolated using the CB2 (n = 119) or CB3 (n = 118). Real-time PVI was visualized in 74 % of patients using the CB3 and 40 % of patients using the CB2, respectively (p < 0.001) (Fig. 1). There was a significant difference with regard to the mean minimal CB temperatures reached using the CB2 and CB3 (−48.4 vs. −44.6 °C, p = 0.0002). However, no difference was found for the mean total number of CB freeze-cycles per PV until isolation, the mean number of successful PVI after the first CB freeze-cycle, the mean TTE, the total freeze time applied, the mean CB temperature after 30 s of freeze-time, or the mean minimal esophageal temperature (Table 2). Furthermore, no difference was observed for the mean procedure time, mean fluoroscopy time and amount of contrast dye utilized. In a small portion of targeted PVs [(13 % (CB3), 6 % (CB2)] artifacts on the achieve-catheter occurred during the freeze-cycles due to ice formation along the balloon tip. However, these artifacts usually developed after isolation of the respective PV was recorded and no differences were found when comparing both groups. All procedures were performed by two operators, both highly experienced in cryoballoon ablation procedures. No differences have been observed between CB2 and CB3 concerning catheter maneuverability and catheter stability along the targeted PVs.
Rate of real-time PVI recordings: The figure presents the rate of real-time pulmonary vein isolation recordings for the total number of pulmonary veins as well as for individual pulmonary veins using the CB3 and the CB2, respectively. PV(s) pulmonary vein(s), RSPV right superior pulmonary vein, RIPV right inferior pulmonary vein, LSPV left superior pulmonary vein, LIPV left inferior pulmonary vein
Acute ablation results per individual PV
Ablation data per individual PV is summarized in Table 3. Applying a TTE-guided ablation strategy, shorter mean freeze-cycle durations were noted for the CB3 targeting the RSPVs (188 ± 92 vs. 211 ± 124 s, p = 0.04) and the RIPVs (192 ± 75 vs. 200 ± 37 s, p = 0.02), while no difference was found for the left-sided PVs.
Peri- and postprocedural complications
Transient PN palsy occurred in 1/30 (3.3 %) patients in the CB3 group during energy delivery targeting the RSPV with full recovery of nerve function after 15 min. No pericardial effusion, pericardial tamponade, symptomatic PV stenosis, TIA/stroke, or atrioesophageal fistula was observed.
Discussion
The current study set out to compare the procedural efficacy and ablation characteristics of the novel 28 mm CB3 to the CB2 for PVI. The major findings are (1) the CB3 provides an identical rate of acute PVI during the index freeze-cycle as the CB2, (2) the rate of real-time PV-recordings was significantly higher in the CB3 group, and (3) a higher rate of real-time PV recordings translates into shorter overall freeze time targeting the right-sided PVs when applying a TTE-guided ablation protocol, (4) no differences were observed between CB2 and CB3 concerning catheter maneuverability and catheter stability.
Recent studies focusing on second-generation CB-based PVI demonstrated a high rate of isolation (84–90 %) during the index freeze cycle [2, 14, 15], superior 1- and 2-year clinical outcome even if a “no-bonus” freeze protocol or a shorter freeze cycle duration was utilized [2–4], and a high rate of durable PVI in patients undergoing repeat procedures for recurrent atrial tachyarrhythmias [8, 9]. Ablation strategies that account for the TTE are currently under investigation. The TTE can only be measured in the presence of real-time PV potential recordings. Incorporating the TTE into ablation protocols may reduce the time of total energy delivery per PV, which may in turn improve the safety-profile of CB-based PVI.
The present study confirms that the high rate of PVI seen during the initial freeze-cycle applies to both the CB3 and CB2 (86 vs. 92 %, p = 0.14). Although the mean minimal balloon-temperature reached with the CB2 targeting the RSPV (−50.0 ± 6 vs. −45.6 ± 6, p = 0.01) and LIPV (−46.6 ± 6 vs. 40.9 ± 5, p < 0.001) was significantly lower than with the CB3, the acute efficacy was similar for both CB generations. Interestingly, no significant difference was identified for the balloon temperature reached at 30 s [16]. There was also no difference when comparing the lowest intraluminal esophageal temperature.
Characteristic complications of CB-based PVI such as PN palsy or a significant decline in esophageal temperature, which may result in esophageal thermal lesion formation typically occur at the later stage of the freeze-cycle [10, 12]. To improve the safety profile of CB-based PVI, several ablation protocols aim at reducing the number of freeze-cycles per vein or the duration of the individual freeze-cycle [16]. The Achieve catheter provides online visualization of PV signals. The longer distal tip of the CB2 may prevent proper positioning of the Achieve catheter at the proximal PV ostium, limiting real-time signal recording in a considerable number of PVs [15, 17]. The novel CB3 was modified to incorporate a 40 % shorter distal tip, facilitating proximal positioning of the Achieve-catheter and an increased rate of PV signal recordings. Accordingly, the current study found a significantly higher percentage of real-time PVI recordings for the CB3 group (74 vs. 40 %). Utilizing a TTE-guided ablation protocol (TTE + 120 s) a higher rate of real-time PVI recordings translated into a non-significant decrease in total freeze-cycle duration in the CB3 group. However, a significantly shorter total freeze-cycle duration could be demonstrated for the RSPV and RIPV while no difference was observed for the LSPV or LIPV. Since PN palsy occurs during ablation along the septal PVs, the incidence of these complications might be further reduced by using the CB3 in conjunction with individualized ablation strategies implementing the TTE. The impact of CB3-based PVI in conjunction with individualized ablation protocols implementing the TTE on long-term clinical outcome needs further evaluation.
Limitations
The current study is based on a single-center experience in a limited number of patients. Because of the recent launch of CB3, only acute efficacy and safety data are provided, while long-term clinical outcome will need future assessment.
Conclusions
To the best of our knowledge this is the first study reporting on the acute results of CB3-based PVI as compared to CB2. While demonstrating an identical acute efficacy for PVI, the CB3 provides a significantly increased rate of real-time PV-recordings as compared to CB2 and thus facilitates individual ablation strategies considering the TTE.
References
Metzner A, Reissmann B, Rausch P, Mathew S, Wohlmuth P, Tilz R, Rillig A, Lemes C, Deiss S, Heeger C, Kamioka M, Lin T, Ouyang F, Kuck KH, Wissner E (2014) One-year clinical outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 7:288–292
Wissner E, Heeger CH, Grahn H, Reissmann B, Wohlmuth P, Lemes C, Rausch P, Mathew S, Rillig A, Deiss S, Maurer T, Lin T, Tilz RR, Ouyang F, Kuck KH, Metzner A (2015) One-year clinical success of a ‘no-bonus’ freeze protocol using the second-generation 28 mm cryoballoon for pulmonary vein isolation. Europace 17:1236–1240
Metzner A, Heeger CH, Wohlmuth P, Reissmann B, Rillig A, Tilz RR, Mathew S, Lemes C, Deiss S, Maurer T, Saguner A, Ouyang F, Kuck KH, Wissner E (2015) Two-year outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon: lessons from the bonus freeze protocol. Clin Res Cardiol (Epub ahead of print)
Ciconte G, de Asmundis C, Sieira J, Conte G, Di Giovanni G, Mugnai G, Saitoh Y, Baltogiannis G, Irfan G, Coutino-Moreno HE, Hunuk B, Velagic V, Brugada P, Chierchia GB (2015) Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm 12:673–680
Furnkranz A, Bordignon S, Dugo D, Perotta L, Gunawardene M, Schulte-Hahn B, Nowak B, Schmidt B, Chun JK (2014) Improved 1-year clinical success rate of pulmonary vein isolation with the second-generation cryoballoon in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 25:840–844
Lemes C, Wissner E, Lin T, Mathew S, Deiss S, Rillig A, Heeger C, Wohlmuth P, Reissmann B, Tilz R, Ouyang F, Kuck KH, Metzner A (2015) One-year clinical outcome after pulmonary vein isolation in persistent atrial fibrillation using the second-generation 28 mm cryoballoon: a retrospective analysis. Europace (Epub ahead of print)
Ciconte G, Ottaviano L, de Asmundis C, Baltogiannis G, Conte G, Sieira J, Di Giovanni G, Saitoh Y, Irfan G, Mugnai G, Storti C, Montenero AS, Chierchia GB, Brugada P (2015) Pulmonary vein isolation as index procedure for persistent atrial fibrillation: one-year clinical outcome after ablation using the second-generation cryoballoon. Heart Rhythm 12:60–66
Heeger CH, Wissner E, Mathew S, Deiss S, Lemes C, Rillig A, Wohlmuth P, Reissmann B, Tilz RR, Ouyang F, Kuck KH, Metzner A (2015) Once isolated, always isolated? Incidence and characteristics of pulmonary vein reconduction after second-generation cryoballoon-based pulmonary vein isolation. Circ Arrhythm Electrophysiol 8:1088–1094
Bordignon S, Furnkranz A, Perrotta L, Dugo D, Konstantinou A, Nowak B, Schulte-Hahn B, Schmidt B, Chun KR (2015) High rate of durable pulmonary vein isolation after second-generation cryoballoon ablation: analysis of repeat procedures. Europace 17:725–731
Metzner A, Burchard A, Wohlmuth P, Rausch P, Bardyszewski A, Gienapp C, Tilz RR, Rillig A, Mathew S, Deiss S, Makimoto H, Ouyang F, Kuck KH, Wissner E (2013) Increased incidence of esophageal thermal lesions using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 6:769–775
Furnkranz A, Bordignon S, Bohmig M, Konstantinou A, Dugo D, Perrotta L, Klopffleisch T, Nowak B, Dignass AU, Schmidt B, Chun JK (2015) Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm 12:268–274
Metzner A, Rausch P, Lemes C, Reissmann B, Bardyszewski A, Tilz R, Rillig A, Mathew S, Deiss S, Kamioka M, Toennis T, Lin T, Ouyang F, Kuck KH, Wissner E (2014) The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J Cardiovasc Electrophysiol 25:466–470
Casado-Arroyo R, Chierchia GB, Conte G, Levinstein M, Sieira J, Rodriguez-Manero M, di Giovanni G, Baltogiannis Y, Wauters K, de Asmundis C, Sarkozy A, Brugada P (2013) Phrenic nerve paralysis during cryoballoon ablation for atrial fibrillation: a comparison between the first- and second-generation balloon. Heart Rhythm 10:1318–1324
Martins RP, Hamon D, Cesari O, Behaghel A, Behar N, Sellal JM, Daubert JC, Mabo P, Pavin D (2014) Safety and efficacy of a second-generation cryoballoon in the ablation of paroxysmal atrial fibrillation. Heart Rhythm 11:386–393
Furnkranz A, Bordignon S, Schmidt B, Gunawardene M, Schulte-Hahn B, Urban V, Bode F, Nowak B, Chun JK (2013) Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J Cardiovasc Electrophysiol 24:492–497
Su W, Kowal R, Kowalski M, Metzner A, Svinarich JT, Wheelan K, Wang P (2015) Best practice guide for cryoballoon ablation in atrial fibrillation: the compilation experience of more than 3000 procedures. Heart Rhythm 12:1658–1666
Straube F, Dorwarth U, Vogt J, Kuniss M, Heinz Kuck K, Tebbenjohanns J, Garcia Alberola A, Chun KR, Souza JJ, Ouarrak T, Senges J, Brachmann J, Lewalter T, Hoffmann E (2014) Differences of two cryoballoon generations: insights from the prospective multicentre, multinational FREEZE Cohort Substudy. Europace 16:1434–1442
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CH Heeger received travel grants by St. Jude Medical, Biotronik and Medtronic. KH Kuck received travel grants and research grants from Biosense Webster, Stereotaxis, Prorhythm, Medtronic, Edwards, Cryocath, and is a consultant to St. Jude Medical, Biosense Webster, Prorhythm, and Stereotaxis. He received speaker’s honoraria from Medtronic. E Wissner received speaker’s honoraria from Medtronic and is member of Medtronic’s advisory board. A Metzner received speaker’s honoraria and travel grants from Medtronic, Biosense Webster and Cardiofocus. All other authors have no relevant disclosures.
Additional information
C.-H. Heeger and E. Wissner contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Heeger, CH., Wissner, E., Mathew, S. et al. Short tip–big difference? First-in-man experience and procedural efficacy of pulmonary vein isolation using the third-generation cryoballoon. Clin Res Cardiol 105, 482–488 (2016). https://doi.org/10.1007/s00392-015-0944-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0944-y