Abstract
Inconsistent results have been reported concerning the effect of tolvaptan treatment on long-term prognostic outcomes in patients with acute decompensated heart failure (ADHF) and data are limited on prognostic factors affecting this patient population. We investigated prognostic factors influencing long-term clinical outcomes in patients with ADHF treated with tolvaptan in a real-world setting. A total of 263 consecutive patients hospitalized for ADHF and treated with tolvaptan were retrospectively enrolled. The patients were stratified into those who developed the combined event of cardiac death or rehospitalization for worsening heart failure within 1 year (n = 108) and those who were free of this combined event within 1 year (n = 155). Adjusted multivariate Cox proportional hazards model revealed that change in serum sodium level between pre-treatment and 24 h after tolvaptan administration [hazard ratio (HR) 0.913, 95% confidence interval (CI) 0.841–0.989, p = 0.025] and the time taken for tolvaptan initiation from admission (HR 1.043, 95% CI 1.009–1.074, p = 0.015) were independent predictors of combined event occurrence within 1 year. Moreover, change in serum sodium level > 1 mEq/L between pre-treatment and 24 h after administration and initiation of tolvaptan < 5 days after admission correlated significantly with the incidence of the combined event (log-rank test p = 0.003 and p = 0.002, respectively). In conclusion, increased serum sodium level early after administration and early initiation of tolvaptan are possibly useful for assessing the long-term prognosis after tolvaptan treatment in patients with ADHF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute decompensated heart failure (ADHF) is a common cause of hospitalization, especially in individuals older than 65 years of age [1]. Over 1 million patients are hospitalized with ADHF annually in Japan alone [2]. Although reducing heart failure-associated death and rehospitalization for worsening heart failure are clinical priorities, long-term prognosis remains poor [3].
Tolvaptan is an oral vasopressin V2 receptor antagonist that increases excretion of free water without changing renal hemodynamics and it has been found to be safe and effective for the treatment of ADHF [4]. High-risk patients with ADHF with recurrent heart failure, cardiac systolic dysfunction, or renal impairment are often refractory to conventional medical therapy, such as loop diuretics, and tend to have poor long-term prognosis. To resolve this problem, tolvaptan was approved for the treatment of ADHF in high-risk or medically refractory patients. Additionally, several studies have demonstrated that tolvaptan significantly improves short-term prognosis due to factors such as a rapid reduction in body weight, amelioration of dyspnea symptoms, and reduced rates of worsening renal failure in patients with ADHF [5,6,7,8,9,10,11,12,13,14,15,16]. However, evaluation of the effect of tolvaptan on long-term prognosis, including mortality or rehospitalization for worsening heart failure, has shown inconsistent results in previous studies [17,18,19,20,21,22,23]. It remains to be clarified whether specific risk factors are associated with long-term prognosis in patients with ADHF receiving tolvaptan. Risk stratification is needed to assess long-term prognosis after tolvaptan treatment and to consider the necessity of further invasive therapies, although little is known about the association between prognostic factors involving tolvaptan treatment in patients with ADHF. The purpose of this study was to investigate prognostic factors contributing to long-term cardiac death and rehospitalization for heart failure in patients with ADHF treated with tolvaptan in a real-world setting.
Methods
Study population
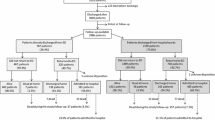
A total of 380 consecutive patients hospitalized for ADHF and treated with tolvaptan at Kansai Medical University Medical Center and Kansai Medical University between January 2012 and July 2015 were retrospectively enrolled. Eligible patients were diagnosed as having ADHF based on the Framingham criteria [24], had New York Heart Association functional class III or IV symptoms on admission, and received index tolvaptan administration during the hospitalization. Exclusion criteria were as follows: presence of a malignant tumor at admission (n = 38), non-cardiac death within 1 year (n = 32), insufficient patient data (n = 28), received tolvaptan previously (n = 14), or loss to follow-up within 1 year (n = 5); thus, 263 patients were included in the final analysis. We divided patients into two groups: those with or without combined event of cardiac death and rehospitalization with worsening heart failure within 1 year to determine the prognostic factors for the occurrence of cardiovascular events. All patients received standard therapy for ADHF consisting of loop diuretics, spironolactone, beta blocker, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, according to the physician’s discretion.
Collection of patient data
Baseline clinical characteristics were recorded including demographic information, comorbidities, laboratory parameters, oral and intravenous medications, presence of acute coronary syndrome, and transthoracic echocardiography results obtained on admission. Either B-type natriuretic peptide (BNP) or NT-pro BNP level at admission was extracted and converted to categorical variables, with the cutoff value for BNP being ≥ 350 pg/mL and that for NT-pro BNP being > 5810 pg/mL, to directly compare BNP and NT-pro BNP levels based on past reports of the prognostic value of BNP or NT-pro BNP [25,26,27]. All patients were treated with intravenous or oral treatment of loop diuretics after admission, and when further volume reduction was needed for residual fluid retention, 3.75–15 mg/day of tolvaptan was initiated during hospitalization based on the attending physician’s discretion. To investigate the prognostic factors involving tolvaptan peri-administration, serum sodium, serum creatinine, serum blood urea nitrogen (BUN) levels at pre-treatment, 24 h, 48 h, and 1 week after tolvaptan administration, the daily furosemide equivalent dose of loop diuretics [28, 29] at 24 h before tolvaptan administration (pre-treatment) and at 0 to 24 h (day 1), 24–48 h (day 2) and day 7 after tolvaptan administration was measured. In addition to the maximum tolvaptan dose, time of tolvaptan initiation from admission and the total administration period was recorded. To assess the extent of fluid reduction as a result of treatment, body weight at both admission and discharge was noted. In addition, the differences in weight between admission and discharge were identified. Responders were defined as patients with increased urine volume within 24 h after tolvaptan administration compared with the 24-h urine volume prior to tolvaptan administration. Patients treated with tolvaptan on the first day of observation were excluded from the evaluation of responders because of their unknown 24-h urine baseline volume. Acute coronary syndrome was defined as the presence of chest pain, elevation of cardiac enzymes, and treatment with primary or elective percutaneous coronary intervention during hospitalization. The study procedures were carried out in accordance with the principles laid out in the Declaration of Helsinki. We obtained consent through an opt-out procedure from all individual participants included in this study. Follow-up information was obtained using medical records from our hospitals or by sending a confirmation letter to the follow-up hospital. The study protocol was approved by the ethics committees of Kansai Medical University Medical Center and Kansai Medical University and was registered at UMIN-CTR (Unique Identifier: 000029416).
Statistical analyses
For continuous variables, all data are presented as means ± standard deviations (SDs) or as medians with interquartile ranges. Categorical variables are presented as numbers and percentages. Differences between groups were analyzed using the unpaired t test or Mann–Whitney U test for continuous variables and the Chi-square test for categorical variables. The paired t test was used to compare change in the daily dose of furosemide between pre-treatment and day 7 after administration in each group. A p value < 0.05 was considered significant. The covariates associated with tolvaptan administration were analyzed using univariate analysis. Covariates with p values < 0.05 in univariate analysis were included in a multivariate Cox proportional hazard model adjusted for clinical prognostic factors associated with congestive heart failure: age, sex, systolic blood pressure at admission, previous heart failure hospitalization, BNP ≥ 350 pg/mL or NT-pro BNP> 5810 pg/mL, and serum sodium levels at admission [30, 31]. To confirm the optimal cutoff value for change in serum sodium level between pre-treatment versus 24 h after administration and the time of tolvaptan initiation from admission, we performed receiver operating characteristic (ROC) curve analysis with these points on the curve where the products of “sensitivity × specificity” were maximized. The cumulative incidence of the combined event was analyzed using Kaplan–Meier curves and the log-rank test. Pearson’s correlation coefficient test was conducted to investigate the relationship between serum potassium level at admission and change in serum sodium level from pre-treatment to 24 h after tolvaptan administration. JMP 13.0.0 software (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyzes.
Results
Of the 263 patients, 108 patients developed combined events (34 patients with cardiac death, 74 patients with rehospitalization due to worsening heart failure) and 155 patients did not develop any combined events within 1 year.
Table 1 reports baseline characteristics of the patients. The mean age of patients in the combined event group was higher (77.0 ± 11.4 years vs. 72.1 ± 11.7 years, p < 0.001) and their mean systolic blood pressure on admission was lower (130 ± 36 mmHg vs. 146 ± 37 mmHg, p = 0.004) than patients in the free of combined event group. Patients with hospitalization for a previous heart failure and the presence of mechanical implants were significantly more common in the combined event group. Regarding the use of medication at admission, the rate of loop diuretics administration at admission and the dose of furosemide were greater in the combined event group than in the free of combined event group (64% vs. 52%, p = 0.047; 30.6 mg vs. 22.5 mg, p = 0.034, respectively). No significant difference was shown in echocardiography results including systolic function, with the exception of the presence of valvular heart disease.
Tolvaptan administration profiles showed that the mean dose of tolvaptan was smaller and earlier initiation of tolvaptan after admission was more common in the free of combined event group (Table 2). Some patients ceased taking tolvaptan less than 7 days after tolvaptan initiation (developed combined event group: 36% and free of combined event group: 41%, p = 0.458). More patients in the combined event group continued tolvaptan administration ≥ 30 days than in the free of combined event group (40% vs 25%, p = 0.012). The rate of responders to tolvaptan was not significantly different between groups (developed combined event group: 75% vs. free of combined event group: 73%, p = 0.723). Although weight at discharge was not significantly different in both groups, the proportion of weight reduction between admission to discharge was smaller in the developed combined event group than in the free of combined event group (8.4 ± 8.3% vs. 12.1 ± 7.8%, p < 0.001).
Figure 1 demonstrates the change in laboratory data pre-treatment of tolvaptan versus 24 h, 48 h, and 1 week after tolvaptan administration. Compared with pre-administration data, the change in the serum sodium levels after 24 h and 48 h was significantly different between the two groups. The free of combined event group had a significant increase in serum sodium levels compared with the developed combined event group at 24 h and 48 h (2.75 ± 3.43 mEq/mL vs. 1.53 ± 2.88 mEq/mL, p < 0.002; 3.22 ± 4.00 mEq/mL vs. 1.94 ± 3.33 mEq/mL, p < 0.005, respectively); however, this difference between the groups disappeared at 1 week after tolvaptan administration (1.90 ± 4.81 mEq/mL vs. 2.33 ± 6.82 mEq/mL, p = 0.600). Serum BUN level at 24 h was also significantly increased in the free of combined event group compared with the developed combined event group (free of combined event group 1.32 ± 5.26 mg/dL vs. developed combined event group − 0.16 ± 4.30, p = 0.016). Change in the serum creatinine level was not significantly different between the free of combined event group and the developed combined event group at 24 h, 48 h, and 1 week after administration.
The daily dose of furosemide during peri-treatment of tolvaptan is shown in Fig. 2. The combined event group was prescribed higher doses of furosemide compared with the free of combined event group in whole periods (pre-treatment: 51.9 ± 53.4 mg vs. 37.9 ± 45.2 mg, p = 0.026; day 1: 53.9 ± 49.1 mg vs. 42.0 ± 37.9 mg, p = 0.036; day 2: 48.8 ± 33.2 mg vs. 40.7 ± 37.1 mg, p = 0.031; day 7: 46.3 ± 42.3 mg vs. 34.2 ± 32.6 mg, p = 0.019). However, change in the daily dose of furosemide between pre-treatment and day 7 after administration was not significantly different in both groups (developed combined event group: 51.9 ± 53.4 mg vs. 46.3 ± 42.3 mg, p = 0.253; free of combined event group: 37.9 ± 45.2 mg vs 34.2 ± 32.6 mg, p = 0.782). To investigate prognostic factors associated with the occurrence of the combined event in tolvaptan-treated patients with ADHF, univariate and multivariate Cox proportional hazards models were constructed (Table 3). In the univariate analysis, time of initiation from admission, serum creatinine level at admission, change in serum sodium level between pre-treatment and 24 h after administration, and change in serum BUN level between pre-treatment and 24 h after administration were associated with the combined event within 1 year. No significant differences were seen in the dose of furosemide pre-treatment, and change in the daily dose of furosemide between pre-treatment and day 7 after administration. The significant variables (p < 0.05) in univariate analysis were entered into an adjusted multivariate analysis. Change in serum sodium level between pre-treatment and 24 h after tolvaptan administration [hazard ratio (HR) 0.913, 95% confidence interval (CI) 0.841–0.989, p = 0.025] and time of initiation after admission (HR 1.043, 95% CI 1.009–1.074, p = 0.015) were independent predictors of the combined event within 1 year in the adjusted multivariate analysis. In addition, ROC curve analysis revealed a cutoff value for change in serum sodium level of > 1 mEq/L pre-treatment versus 24 h after administration [area under the curve (AUC) 0.619, p = 0.003] and a cutoff value for initiation of tolvaptan of < 5 days after admission (AUC 0.612, p = 0.002). Kaplan–Meier curves demonstrated a significant difference in the free of combined event (change in serum sodium level of > 1 mEq/L (n = 143) vs. ≤ 1 mEq/L (n = 120), log-rank test p = 0.003, Fig. 3a and initiation of tolvaptan of < 5 days (n = 166) vs. ≥ 5 days (n = 97) log-rank test p = 0.002, Fig. 3b). No significant correlation was observed in serum potassium level at admission and change in serum sodium level between pre-treatment and 24 h after tolvaptan administration (R2 = 0.0012, p = 0.580), even though low serum potassium level at admission was observed in the combined event group (Supplemental Fig. 1).
Kaplan–Meier analysis for the combined event of cardiac death or rehospitalization due to worsening heart failure compared with change in serum sodium level (∆ Na)> 1 mEq/L (n = 143) and ≤ 1 mEq/L (n = 120) between pre-treatment versus 24 h after tolvaptan administration (a) and the time of tolvaptan initiation < 5 days (n = 166) versus ≥ 5 days (n = 97) after admission (b)
Discussion
In this study, we demonstrated that increasing serum sodium level early after tolvaptan administration and early initiation of tolvaptan were independent predictors of the 1-year clinical event of cardiac death or rehospitalization due to worsening heart failure. These associations were still significant after adjustment by multivariate analysis. In addition, change in serum sodium level > 1 mEq/L between pre-treatment and 24 h after administration and the initiation of tolvaptan < 5 days after admission were the optimal cut-off values to predict the incidence of the combined event.
Investigation of the prognostic impact of tolvaptan treatment is important to improve long-term outcome in patients with ADHF. Several studies have reported favorable short-term outcomes, such as rapid decongestion and improvement of dyspnea, as well as renal protection, based on a reduced incidence of worsening renal failure in patients with ADHF treated with tolvaptan. Response to tolvaptan, defined as increasing urine volume or improvement of fluid balance between pre- and post-tolvaptan administration, has been proposed as a predictive factor for short-term outcomes. However, inconsistent results have been reported concerning long-term prognostic benefits, such as mortality or rehospitalization due to worsening heart failure, in patients with ADHF treated with tolvaptan. A large clinical trial and meta-analysis demonstrated no beneficial effect of tolvaptan in terms of these long-term endpoints [5, 17, 18], although several small studies have reported improvements in long-term mortality and rehospitalization due to heart failure [20, 22, 23].
We identified increasing serum sodium level as a predictive factor associated with a reduced incidence of long-term clinical events (change in serum sodium level between pre-treatment and 24 h after administration of tolvaptan: HR 0.913, 95% CI 0.841–0.989, p = 0.025). Tolvaptan is an oral vasopressin V2 receptor antagonist that blocks water reabsorption in the renal collecting ducts, resulting in increased free water excretion (aquaresis), reflecting an increase in serum sodium concentrations. Several studies demonstrated an increase in the serum sodium level in the very early period after administration of tolvaptan in patients with ADHF [5, 8, 12, 15, 18]. The underlying mechanism is thought to be based on good responsiveness to tolvaptan leading to rapid and large volume free water excretion and subsequent elevation of intravascular sodium concentration. This alteration causes a mild increase in the serum sodium level. Therefore, increasing serum sodium level during the early phase of treatment would correlate with good response to tolvaptan in terms of the aquaretic effect and, thus, it is associated with the responsiveness of the renal collecting duct to tolvaptan.
A combination of furosemide prescription during peri-treatment using tolvaptan may change serum sodium levels. The daily doses of furosemide in the developed combined event group were significantly higher than those in the free of combined event group. However, dose of furosemide pre-treatment of tolvaptan and the change in the daily dose of furosemide between pre-treatment and day 7 after administration were not significantly associated with the incidence of combined events, as analyzed by the Cox proportional hazard model. Therefore, we believe that the influence of furosemide prescription is trivial in terms of its effect on changes in serum sodium level during peri-treatment of tolvaptan.
The rate of responders, defined as an increasing urine volume after administration, was similar in between groups, which resulted in the mismatch in the difference of elevated serum sodium levels. The following reasons were considered: first, the patients in the free of combined event group were much more likely to be excluded from a responder classification because many of these patients received tolvaptan during the first day of admission, which we were unable to compare 24 h pre-administrated urine volume. Patients who received tolvaptan on the first day of admission were more likely to be included as responders. Second, patients in the free of combined event group had a significantly large proportion of weight reduction between admission and discharge compared to the combined event group. Although the rate of responders was similar in both groups, patients in the free of combined event group obtained greater volume reduction during hospitalization. This difference would significantly affect the differences in increasing serum sodium level early after tolvaptan administration. Potential residual congestion was suspected in patients in the developed combined event group even though higher doses of furosemide and tolvaptan were used, because lesser weight reduction between admission and discharge was observed in the combined event group. Sufficient volume reduction was favorable for inhibiting the occurrence of adverse events for the treatment of heart failure. In addition, weight at admission was not significantly different in both groups but this was relatively higher in the free of combined event group. Furthermore, weight at discharge was almost similar in both groups. This implies that patients in whom the main cause was volume overload may benefit from the effects of tolvaptan as aquaresis. Elevated serum sodium level early after tolvaptan administration is associated with not only the side effect of hypernatremia but is also a predictive factor of good responsiveness to tolvaptan.
In contrast, in the current study, the significant difference in the change in serum sodium level between groups disappeared at 1 week after initiation of therapy. Some patients ceased tolvaptan treatment less 7 days in both the combined event group and the free from combined event group (36% and 41%, respectively). Although no significant differences were seen in the rate of cessation less 7 days between groups, this number is relatively large, which may cause the disappearance of the difference in serum sodium level between groups. If numerous patients continued tolvaptan administration over 7 days, the difference in serum sodium level may be observed in the long term. On the other hand, several studies reported that the patients treated with tolvaptan showed a temporary increase in serum sodium level early after tolvaptan administration compared with patients treated with conventional therapy; this significant difference disappeared 1 week after initiation of tolvaptan, while the subsequent absence of serum sodium elevation may be thought to be a common reaction to tolvaptan use [9, 12]. Elevation of the serum sodium level immediately after administration is modified by various factors (e.g., fluid balance, effect of other medications, and excretion in the urine) as patients enter the chronic phase and elevated serum sodium level subsequently resolves.
Our findings demonstrate that the initiation of tolvaptan treatment < 5 days after hospitalization correlated with improved clinical outcomes (time of tolvaptan initiation from admission, HR 1.043, 95% CI 1.009–1.074, p = 0.015). In several studies, early administration of tolvaptan was found to have clinical benefit not only in terms of short-term clinical efficacy but also reduced mid-term mortality compared to conventional diuretic therapy [10, 16, 22]. These studies reported that immediate tolvaptan therapy can prevent activation of the renin–angiotensin–aldosterone system, which suppresses deterioration of renal function [7, 22]. The suppression of the renin–angiotensin–aldosterone system may contribute to effects on mid-to long-term prognosis. In the EVEREST Outcome trial, enrolled patients received tolvaptan within 48 h of hospitalization; results showed no significant improvement in the long-term endpoint of all-cause mortality and cardiovascular death or rehospitalization for heart failure. As is evident from the above data, mid-to long-term prognostic benefits due to the early initiation of tolvaptan are uncertain and further investigations are needed to determine the most beneficial timing for initiation of tolvaptan therapy.
Increased serum sodium level early after administration and early initiation of tolvaptan are possibly useful for assessing long-term prognosis after tolvaptan treatment. When poor prognosis is considered, further invasive therapies would be needed to improve long-term outcomes.
Limitations
First, this was a retrospective, observational study and included a limited number of patients. To better evaluate prognostic factors, further study is required with a larger number of patients and with a non-tolvaptan comparison group. Second, the dose of tolvaptan, timing of treatment initiation, duration of treatment, and selection of medications were at the discretion of the attending physician. Third, our study had the potential for patient selection bias in terms of the subgroup of individuals who received tolvaptan from the attending physicians. The patients receiving tolvaptan would be expected to be more severely ill and to have failed conventional therapies. However, we also think that such patients are more likely to require tolvaptan to improve fluid retention in clinical practice. For these high-risk patients, evaluation of prognostic factors would be important to ensure optimal clinical prognosis. Fourth, the laboratory data collected at 24 h and 48 h after administration had several hours variation, which may have affected the results. Fifth, the cutoff values obtained by ROC curve analysis were based on this present study population. We do not have data regarding the validity of cutoff values and there may be better cutoff values that should be used. Sixth, we did not utilize urinalysis parameters, including urine osmolality, because many patients lacked these data during peri-treatment with tolvaptan. If we had obtained these parameters, we could have been able to evaluate responders more precisely. Finally, to assess the severity of heart failure, we converted BNP and NT-pro BNP to categorical variables based on past research, because either BNP or NT-pro BNP levels were obtained.
Conclusions
This study demonstrated that an early increase in serum sodium level after tolvaptan administration and early initiation of tolvaptan were independent predictors of the 1-year clinical event of cardiac death or rehospitalization due to worsening heart failure. In addition, changes in serum sodium level > 1 mEq/L between pre-treatment versus 24 h after administration and the initiation of tolvaptan < 5 days after admission were the optimal cut-off values to evaluate the incidence of the combined event of cardiac death or rehospitalization due to worsening heart failure. As little is known concerning the prognostic factors involving tolvaptan treatment, increased serum sodium level early after administration and early initiation of tolvaptan are possibly useful to assess long-term prognosis after tolvaptan treatment, and to consider further invasive therapies.
References
Shimokawa H, Miura M, Nochioka K, Sakata Y (2015) Heart failure as a general pandemic in Asia. Eur J Heart Fail 17:884–892
Okura Y, Ramadan MM, Ohno Y, Mitsuma W, Tanaka K, Ito M, Suzuki K, Tanabe N, Kodama M, Aizawa Y (2008) Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J 72:489–491
Metra M, Teerlink JR (2017) Heart failure. Lancet 390:1981–1995
Udelson JE, Orlandi C, Ouyang J, Krasa H, Zimmer CA, Frivold G, Haught WH, Meymandi S, Macarie C, Raef D, Wedge P, Konstam MA, Gheorghiade M (2008) Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: an international, multicenter, randomized, placebo-controlled trial. J Am Coll Cardiol 52:1540–1545
Konstam MA, Kiernan M, Chandler A, Dhingra R, Mody FV, Eisen H, Haught WH, Wagoner L, Gupta D, Patten R, Gordon P, Korr K, Fileccia R, Pressler SJ, Gregory D, Wedge P, Dowling D, Romeling M, Konstam JM, Massaro JM, Udelson JE, SECRET of CHF Investigators, Coordinators, and Committee Members (2017) Short-term effects of tolvaptan in patients with acute heart failure and volume overload. J Am Coll Cardiol 69:1409–1419
Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, Fujii H, Kitai T, Nishioka T, Sugi K, Onishi Y, Noda M, Kagiyama N, Satoh Y, Yoshida K, Goldsmith SR (2016) Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail 22:423–432
Jujo K, Saito K, Ishida I, Furuki Y, Kim A, Suzuki Y, Sekiguchi H, Yamaguchi J, Ogawa H, Hagiwara N (2016) Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail 3:177–188
Kinoshita M, Okayama H, Kosaki T, Hosokawa S, Kawamura G, Shigematsu T, Takahashi T, Kawada Y, Hiasa G, Yamada T, Matsuoka H, Kazatani Y (2018) Favorable effects of early tolvaptan administration in very elderly patients after repeat hospitalizations for acute decompensated heart failure. Heart Vessels 33:163–169
Tamaki S, Sato Y, Yamada T, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Kondo T, Ozaki T, Seo M, Ikeda I, Fukuhara E, Abe M, Nakamura J, Fukunami M (2017) Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure and preserved left ventricular ejection fraction—prospective randomized controlled study. Circ J 81:740–747
Takagi K, Sato N, Ishihara S, Sone M, Tokuyama H, Nakama K, Omote T, Kikuchi A, Ishikawa M, Amitani K, Takahashi N, Maruyama Y, Imura H, Shimizu W (2018) Effect of tolvaptan on urine output in hospitalized heart failure patients with hypoalbuminemia or proteinuria. Heart Vessels 33:413–420
Kogure T, Jujo K, Hamada K, Saito K, Hagiwara N (2018) Good response to tolvaptan shortens hospitalization in patients with congestive heart failure. Heart Vessels 33:374–383
Kimura K, Momose T, Hasegawa T, Morita T, Misawa T, Motoki H, Izawa A, Ikeda U (2016) Early administration of tolvaptan preserves renal function in elderly patients with acute decompensated heart failure. J Cardiol 67:399–405
Hanatani A, Shibata A, Kitada R, Iwata S, Matsumura Y, Doi A, Sugioka K, Takagi M, Yoshiyama M (2017) Administration of tolvaptan with reduction of loop diuretics ameliorates congestion with improving renal dysfunction in patients with congestive heart failure and renal dysfunction. Heart Vessels 32:287-294
Uemura Y, Shibata R, Takemoto K, Uchikawa T, Koyasu M, Ishikawa S, Mitsuda T, Miura A, Imai R, Iwamiya S, Ozaki Y, Kato T, Miura T, Watarai M, Murohara T (2016) Clinical benefit of tolvaptan in patients with acute decompensated heart failure and chronic kidney disease. Heart Vessels 31:1643–1649
Suzuki S, Yoshihisa A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Abe Y, Saito T, Ohwada T, Suzuki H, Saitoh S, Kubota I, Takeishi Y, AVCMA investigators (2013) Acute heart failure volume control multicenter randomized (AVCMA) trial: comparison of tolvaptan and carperitide. J Clin Pharmacol 53:1277–1285
Matsukawa R, Kubota T, Okabe M, Yamamoto Y (2016) Early use of V2 receptor antagonists is associated with a shorter hospital stay and reduction in in-hospital death in patients with decompensated heart failure. Heart Vessels 31:1650–1658
Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators (2007) Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 297:1319–1331
Wang C, Xiong B, Cai L (2017) Effects of tolvaptan in patients with acute heart failure: a systematic review and meta-analysis. BMC Cardiovasc Disord 17:164
Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, Fujii H, Kitai T, Nishioka T, Sugi K, Onishi Y, Noda M, Kagiyama N, Satoh Y, Yoshida K, Goldsmith SR (2016) Prognostic impact of early treatment with tolvaptan in patients with acute heart failure and renal dysfunction. Int J Cardiol 221:188–193
Imamura T, Kinugawa K, Fujino T, Inaba T, Maki H, Hatano M, Yao A, Komuro I (2014) Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ J 78:2240–2249
Imamura T, Kinugawa K (2016) Tolvaptan improves the long-term prognosis in patients with congestive heart failure with preserved ejection fraction as well as in those with reduced ejection fraction. Int Heart J 57:600–606
Shirakabe A, Hata N, Yamamoto M, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Shimizu W (2014) Immediate administration of tolvaptan prevents the exacerbation of acute kidney injury and improves the mid-term prognosis of patients with severely decompensated acute heart failure. Circ J 78:911–921
Udelson JE, McGrew FA, Flores E, Ibrahim H, Katz S, Koshkarian G, O'Brien T, Kronenberg MW, Zimmer C, Orlandi C, Konstam MA (2007) Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol 49:2151–2159
McKee PA, Castelli WP, McNamara PM, Kannel WB (1971) The natural history of congestive heart failure: the Framingham study. N Engl J Med 285:1441–1446
Santaguida PL, Don-Wauchope AC, Oremus M, McKelvie R, Ali U, Hill SA, Balion C, Booth RA, Brown JA, Bustamam A, Sohel N, Raina P (2014) BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev 19:453–470
Núñez J, Sanchis J, Bodí V, Fonarow GC, Núñez E, Bertomeu-González V, Miñana G, Consuegra L, Bosch MJ, Carratalá A, Chorro FJ, Llàcer A (2010) Improvement in risk stratification with the combination of the tumour marker antigen carbohydrate 125 and brain natriuretic peptide in patients with acute heart failure. Eur Heart J 31:1752–1763
Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, Pinto YM, Richards M (2006) NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 27:330–337
Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC, Hillege HL (2014) Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J 35:1284–1293
Masuyama T, Tsujino T, Origasa H, Yamamoto K, Akasaka T, Hirano Y, Ohte N, Daimon T, Nakatani S, Ito H (2012) Superiority of long-acting to short-acting loop diuretics in the treatment of congestive heart failure. Circ J 76:833–842
Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF Jr, Gheorghiade M, O'Connor CM (2004) Risk stratification after hospitalization for decompensated heart failure. J Card Fail 10:460–466
O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW (2010) Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol 55:872–878
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
380_2018_1290_MOESM1_ESM.jpg
Supplemental Fig. 1The relation between the serum potassium levels at admission and change in serum sodiumlevel from pre-treatment to 24 h after tolvaptan administration, analyzed using Pearson’s29correlation coefficient (JPG 49 KB)
Rights and permissions
About this article
Cite this article
Matsumura, K., Morishita, S., Taniguchi, N. et al. Prognostic factors for long-term outcomes in acute decompensated heart failure patients under tolvaptan treatment. Heart Vessels 34, 607–615 (2019). https://doi.org/10.1007/s00380-018-1290-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1290-6