Abstract
The wearable cardioverter-defibrillator (WCD) was introduced to provide protection from sudden cardiac death (SCD) in patients with transiently elevated risk or during ongoing risk stratification. Benefits and clinical characteristics of routine WCD use remain to be assessed in larger patient populations. This study aims to identify determinants of WCD compliance, therapies, and inappropriate alarms in a real-life cohort. A total of 106 cases (68.9% male) were included between 11/2010 and 04/2016. WCD therapies, automatically recorded arrhythmia episodes, inappropriate WCD alarms, patient compliance, and outcome after WCD prescription were analyzed. Median duration of WCD use was 58.5 days. Average daily wearing time was 22.7 h. Compliance was reduced in patients ≤ 50 years. Three patients received WCD therapies (2.8%). In one case ventricular fibrillation (VF) was appropriately terminated with the first shock. Two patients received inappropriate WCD therapies due to WCD algorithm activation during ventricular pacemaker stimulation. One patient died of asystole while carrying a WCD (0.9%). Additional arrhythmias detected comprised self-terminating sustained ventricular tachycardia (VT; 2.8%), non-sustained VT (2.8%), and supraventricular arrhythmias (5.7%). Inappropriate WCD alarms due to over-/undersensing occurred in 77/106 patients (72.6%), of which 41 (38.7%) experienced ≥ 10 inappropriate WCD alarms during the prescription period. Thirteen patients (12.3%) displayed a mean of > 1 inappropriate alarms/day. WCD use was associated with high compliance and provided protection from VT/VF-related SCD. The majority of patients experienced inappropriate WCD alarms. Alterations in QRS morphology during pacemaker stimulation require consideration in WCD programming to prevent inappropriate alarms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The implantable cardioverter-defibrillator (ICD) offers effective and reliable protection from sudden cardiac death (SCD) in patients at high risk of developing life-threatening ventricular arrhythmias. In everyday clinical practice, thorough risk stratification or patient re-evaluation after a defined period of optimized medical therapy may be required before an ICD indication is finally established or rejected. In addition, SCD protection may be temporarily limited due to lead dysfunction or to the need of infected device and lead explant among patients carrying ICDs. The wearable cardioverter-defibrillator (WCD) has been introduced to provide temporary SCD protection during these limited time periods. It is a non-invasive device capable of detecting and treating ventricular tachycardia (VT) and ventricular fibrillation (VF). The WCD continuously monitors the patient’s heart rhythm and follows detection algorithms specified by the prescribing physician. Previous case reports and case series demonstrated the effectiveness of the WCD in treating ventricular arrhythmias and providing SCD protection [1,2,3]. Registry analyses further point towards a benefit of temporary SCD protection by the WCD in patients after myocardial infarction [4], percutaneous coronary intervention [5], peri-partum cardiomyopathy [6], inherited structural and electrical proarrhythmic heart disease [7], and patients listed for heart transplantation [8]. The efficiency of the therapy strongly depends on patient compliance. The aim of this study was to analyze WCD use in a patient cohort of a high-volume tertiary center. Patient compliance and benefits of temporary SCD protection were evaluated in this cohort. In addition, the potential of automatically transmitted recordings to a remote monitoring network to enhance diagnostic and therapeutic decision making was assessed. Finally, inappropriate WCD alarms due to over-/undersensing were quantified, and individual patient-specific predictors of both compliance and inappropriate ECG interpretation were identified to optimize patient selection.
Materials and methods
Ethics
The study was conducted in compliance with the Declaration of Helsinki, and the research protocol was approved by the Ethics Committee of Heidelberg University, Germany (approval number S-403/2015). Written informed patient consent was not required according to the Ethics Committee.
Components and function of the wearable cardioverter-defibrillator (WCD)
The WCD consists of a garment containing three defibrillation patch electrodes (two in posterior position and one in apical position) and four electrocardiogram (ECG) electrodes, connected to a defibrillation and monitoring unit carried on a waist belt. The patient’s ECG is continuously monitored and arrhythmia is diagnosed according to specified detection algorithms [9]. Programmed WCD thresholds in our cohort are summarized in Table 1. All automatically detected episodes are stored by the device. Additionally, the patient may manually initiate ECG recording by the WCD in case of symptoms suggestive of arrhythmia. Prior to therapy delivery, the WCD initiates an alarm sequence consisting of silent vibration and audible alarms. Thus, a conscious patient may respond to the alarm by pushing two response buttons and delay or prevent unnecessary WCD shock. In case of absent patient response to the alarm and persistence of the arrhythmia, the WCD delivers up to five posterior–anterior biphasic defibrillation shocks with energy levels of up to 150 J [10]. In addition, the WCD delivers asystole alarms and records ECGs in case of severe bradycardia. Of note, the device does not offer external pacing. Patients receive detailed instructions in WCD handling and assembly, as well as in the importance of wearing the device almost 24 h/day with short interruptions for personal hygiene.
Remote monitoring network
For remote patient monitoring during the prescription period, the manufacturer provides an online platform (“LifeVest® network”) which is password-secured and can be accessed by the prescribing physician. For each patient at the prescribing center, information regarding individual WCD programming, WCD therapies, and patient compliance is accessible via this platform. Additionally, both automatically and manually initiated ECG recordings by the WCD are transmitted for evaluation.
Patient characteristics and ECG analysis
Demographic and clinical baseline characteristics were extracted from electronic patient records. Rates of ischemic and non-ischemic heart disease, as well as rates of indication for SCD protection due to primary or secondary prevention in our patient cohort were calculated. Our cohort comprised a high number of patients receiving a WCD due to temporary ICD explantation or dysfunction or with already confirmed ICD indication during preparation of complex ICD surgery. As the indication for ICD implantation was established in these patients, we may assume that with regard to current guidelines their risk profile may be considered different in comparison with patients under evaluation and yet undetermined ICD indication. Additionally, the risk for SCD in different subgroups being evaluated for primary SCD prevention, e.g. patients with dilated cardiomyopathy or myocarditis, may differ as well. To offer a more detailed overview of our patient cohort than can be produced by stratification based on underlying condition and to avoid overlapping categories, patients were divided into subgroups based on their indication of WCD prescription. These are of relevance in everyday clinical practice when evaluating a patient for a WCD and deciding whether he or she may profit from WCD therapy. Echocardiographic data were obtained from the last examination performed prior to WCD prescription. Pacemaker (PM) stimulation during WCD baseline recording was determined through review of respective episodes recorded in the LifeVest® network (ZOLL, Pittsburgh, PA, USA). Pacemaker-stimulated QRS morphology was carefully distinguished from altered QRS morphology in patients with bundle branch block. Prior diagnosis of arrhythmias other than sustained or non-sustained VT that may have triggered WCD detection was summarized as “other arrhythmias”. This category included supraventricular arrhythmias, frequent premature ventricular or supraventricular complexes, one case of intermittent bradycardia, and one case of intermittent idioventricular rhythm.
Definition of study cases
There were three patients who were prescribed a WCD at two different time points. In two of these patients, baseline parameters that were hypothesized to influence outcome or WCD recordings had changed in the meantime (left ventricular ejection fraction (LVEF) decreased and body mass index (BMI) increased in one case in which the interval between prescription periods was ~ 1.5 years; in the second case an epicardial PM-electrode had been implanted). Thus, these were counted as two separate cases. In a third patient, the WCD was prescribed again after an interval of 53 days without any alterations in patient characteristics in the meantime. These prescription periods were regarded as one case. All patients who presented for an outpatient or inpatient visit after more than 30 days following the WCD prescription period were included into the follow-up analysis.
Adjudication of recorded episodes
In addition to SCD protection, the WCD is capable of recording further arrhythmia events that do not require DC shock treatment but may influence the diagnostic workflow, risk stratification, or therapy. The WCD offers continuous rhythm monitoring, and patients may manually trigger ECG recordings in case of arrhythmia symptoms. All WCD-recorded episodes were analyzed and independently validated by a second physician experienced in the interpretation of WCD-recorded ECGs. In case of disagreement, a third experienced physician or manufacturer staff was consulted. Automatically recorded episodes were classified as triggered by true arrhythmias or as signal artifacts triggered by oversensing of myopotentials, double counting of ECG spikes, or other non-arrhythmic events. Arrhythmias in automatically recorded episodes were classified as arrhythmias of ventricular or supraventricular origin, respectively. Recorded asystole episodes were divided into “true” asystole and inappropriate ECG interpretation due to undersensing. All episodes of non-arrhythmic origin were classified as “artifacts”. All episodes which provoked an acoustic alarm but were not caused by valid detection of ventricular arrhythmia or asystole events were classified as “inappropriate alarms”. WCD therapy was classified as “appropriate” when a shock was delivered due to detection of VT or VF in the preceding ECG. “Inappropriate” WCD therapy reflects DC shock delivered due to inappropriate ECG interpretation (noise artifact, double counting) or supraventricular tachycardia. Subgroup and correlation analyses were performed to identify patient-related baseline characteristics which may influence ECG misinterpretation. Both total numbers of inappropriate alarms and alarms per day of WCD wearing time were assessed. QRS length as recorded by the WCD was determined based on the automatically recorded “baseline” episode in the remote monitoring network.
Patient compliance
The remote monitoring network provides information on patient compliance by detecting the time points of WCD activation, connection to the electrodes, and electrode-to-skin-contact. For statistical analysis, patient compliance was characterized based on WCD-recorded daily use. According to criteria specified by the monitoring network and in analogy to previous studies [11], each day with a wearing time of more than 15 min was counted as a day on which the WCD was worn. One day was subtracted for the calculation of total wearing time to compensate for partial WCD use on the first and last day of the prescription period. The number of days until return of the WCD as indicated in the LifeVest® Network by the patient was assumed as prescription duration.
Statistical analysis
Statistical analysis was performed using SPSS (Version 22; IBM, Armonk, NY, USA) algorithms. Continuous variables were described as median and 25th and 75th percentiles, and Mann–Whitney-U or Kruskal–Wallis tests were applied for between-group comparisons. Categorical data are presented as count and percentage, and compared with chi-square test. For correlation analyses the Spearman correlation coefficient rs was calculated. Calculation of regression analyses to identify predictors for inappropriate WCD alarms was not feasible due to skew data distribution with large inter-patient variability. All statistical comparisons were two-sided. P values < 0.05 were considered statistically significant.
Results
Patient characteristics
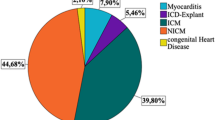
A total of 106 cases who presented at the University Hospital Heidelberg during the time from 11/2010 to 04/2016 and who were prescribed a WCD were analyzed. Median age was 52 years [P25: 37 years; P75: 66 years] and 68.9% of patients were male. Forty-one patients (38.7%) were ICD carriers and received a WCD as bridging therapy due to device-related complications. Of these, 21 patients (51.2%) had experienced ventricular arrhythmias prior to WCD prescription. In the remaining 65 patients without previous ICD-implant, the WCD was prescribed for primary prevention in 54 cases (20.4% ischemic cardiomyopathy, 79.6% non-ischemic cardiomyopathy) and for secondary prevention on 11 cases (27.3% ischemic cardiomyopathy, 72.7% non-ischemic cardiomyopathy). The majority of patients received heart failure medication (Table 2).The most common indication for WCD prescription (n = 22, 20.8%) was SCD protection during a period of preparation for complex ICD surgery, e.g. in cases of lead fractures or difficult anatomical conditions requiring preparatory imaging (Fig. 1). Patients requiring device explantation due to infection constituted an additional large subgroup. A third, significant cohort comprised patients with severely reduced ejection fraction due to dilated cardiomyopathy, myocardial infarction, or due to myocarditis during optimization of heart failure medication prior to ICD decision making.
Indications for WCD prescriptions from 2010 to 2016. Data are provided as per cent of 106 cases. BMI body mass index, CMP cardiomyopathy, DCMP dilated cardiomyopathy, HCM hypertrophic cardiomyopathy, ICD implantable cardioverter-defibrillator, LVEF left ventricular ejection fraction, MI myocardial infraction, PCI percutaneous coronary intervention, PM pacemaker, SCD sudden cardiac death
WCD use and patient compliance
The median WCD prescription time was 70.5 days (P25: 5.8 days; P75: 96.0 days). The median wear time was 58.5 days. The WCD was worn on the majority of days prescribed (median: 95.4%; P25: 87.7%; P75: 97.5%). The median daily wearing time was 22.7 h (P25: 20.7 h; P75: 23.7 h), which amounts to a daily relative use of 94.6% (P25: 86.2%; P75: 98.6%) of the recommended 24 h. The majority of patients (n = 66, 62.3%) wore the WCD between 22 and 24 h (Fig. 2a). By contrast, a subgroup of 24 patients (22.6%) exhibited daily compliance below 20 h.
Compliance was reduced when comparing patients with a prescription duration of ≥ 70 days with < 70 days (< 70 days: 97.2% [P25: 88.7%; P75: 0.5%]; ≥ 70 days: 93.7% [P25: 82.5%; P75: 97.2%]; P = 0.048; Fig. 2b) and showed a weak but statistically significant inverse correlation with prescription duration (rs = − 0.241 [95% CI = − 0.432; − 0.026], P = 0.013; Fig. 2c). We further observed lower compliance rates in younger patients (≤ 50 years: 90.4% [P25: 72.5%; P75: 96.3%]; > 50 years: 97.3% [P25: 91.1%; P75: 99.4%]; P < 0.001; Fig. 3a). There was no difference in compliance according to sex (female: 96.3% [P25: 90.0%;P75: 99.5%]; male: 93.6% [P25: 83.9%; P75: 98.4%]; P = 0.117, Fig. 3b), body mass index (BMI) (≤ 25 kg/m2: 95.6% [P25: 89.8%;P75: 98.2%]; > 25 kg/m2: 94.7% [P25: 82.5%; P75: 98.8%]; P = 0.582; n = 103; Fig. 3c, Table 3), or total number of inappropriate alarms (< 10 alarms: 94.7% [P25: 87.4%; P75: 98.7%]; ≥ 10 alarms: 94.5% [P25: 85.8%; P75: 98.3%]; P = 0.643; Fig. 3d, Table 3). Correlation analyses of compliance rates with relative incidence of inappropriate alarms per day (rs = 0.055; P = 0.578) or with number of inappropriate alarms during nighttime/days worn (rs = 0.085; P = 0.384) rendered no statistically significant results.
WCD events during the prescription period
Three patients experienced WCD shocks during the prescription period. One patient received appropriate WCD therapy for VT (cycle length 250 ms) that degenerated into VF and was successfully terminated by the first WCD shock (Fig. 4). This patient suffered from ischemic cardiomyopathy, had previously carried an ICD—initially implanted for primary prevention—and had been prescribed the WCD due to lead fracture of the previously implanted ICD and delayed lead revision due to LV thrombus. Of note, there were no signs of systemic embolism following WCD discharge despite the presence of LV-thrombus.
Appropriate arrhythmia detection and WCD therapy. a Initiation of ventricular tachycardia (VT) and detection by WCD (green symbol). b VT was validated by the WCD algorithm (red ECG-tracing), and alarms were triggered (yellow symbols). c VT degenerated into VF. The tachycardia cycle length was measured using a dedicated tool provided by the LifeVest® Network (blue lines). d DC shock delivery restored sinus rhythm. ECGs were extracted from the LifeVest® Network
Two additional WCD therapies were classified as inappropriate shocks that occurred in patients who had experienced multiple inappropriate WCD alarms prior to the event. Both patients suffered from ischemic cardiomyopathy, had been implanted with an ICD for primary prevention and had undergone ICD explantation and epicardiac pacemaker implantation due to device infection. In the first case, the ECG recorded by the WCD showed a wide QRS complex (cycle length, 240 ms) due to PM stimulation, resulting in inappropriate ECG interpretation and tachycardia detection by the WCD. In the index event, the patient was not able to manually inhibit WCD therapy in time, and a WCD shock was delivered (Fig. 5a). Re-programming of the pacemaker from VVIR to VVI mode markedly reduced the number of inappropriate WCD alarms. In the second patient with inappropriate WCD therapy, there had been no pacing activity by the epicardiac pacemaker during baseline ECG recording and WCD fitting. By contrast, during WCD wearing time when PM activity was present, 325 events of inappropriate ECG interpretation by the WCD occurred. The patient ignored the acoustic WCD alarm, which led to an inappropriate WCD shock (Fig. 5b).
Inappropriate WCD therapy. a Left panel: The WCD algorithm was triggered by inappropriate ECG interpretation during PM stimulation (green symbol), followed by tachycardia detection and alarm initiation (yellow symbols). Right panel: The patient did not inhibit WCD therapy by pressing the response buttons and, consequently, an inappropriate WCD shock was delivered. b Left panel: Baseline ECG recording by the WCD in a different patient. Right panel: Arrhythmia detection is triggered by inappropriate ECG interpretation during PM stimulation. The patient did not respond to acoustic and vibration alarms by pressing the response button to inactivate treatment. An inappropriate shock is finally delivered
It is noteworthy that asystole was correctly detected by the WCD in a 49-year-old female patient with cardiac amyloidosis. Unfortunately, this patient died while wearing the WCD as the WCD alarm occurred during nighttime and was not noted by bystanders.
Other arrhythmias detected during the prescription period
In three patients (2.8%) sustained self-terminating ventricular arrhythmia episodes were detected (Table 4) that did not require WCD therapy and were manually withheld by the patient (Table 5). Additional three (2.8%) patients displayed non-sustained VT. Furthermore, supraventricular arrhythmias were detected either recorded automatically via the tachycardia algorithms or after manual activation by the patient. These included atrial fibrillation (AF; n = 3) and other supraventricular tachycardia (n = 6) which could not be further differentiated based on the ECG recordings by the WCD (Table 4).
Inappropriate WCD alarms
The majority of patients experienced inappropriate WCD alarms (n = 77). Of these, 13 patients displayed a mean of more than 1 inappropriate alarm per day. However, numbers of inappropriate alarms varied markedly between patients (Fig. 6a). In subgroup analyses for patients with < 10 inappropriate alarms versus patients with ≥ 10 alarms, no statistically significant difference in sex, rates of previous device implantations, rates of pacing by PM/ICD at baseline, or previous diagnosis of other arrhythmias were observed (Fig. 6b–e). In addition, there were no statistically significant difference in QRS duration (< 10 alarms: 110.0 ms [P25: 92.5 ms; P75: 127.5 ms]; ≥ 10 alarms: 100 ms [P25: 90 ms; P75: 135.0 ms]; P = 0.394; Fig. 6f) and BMI (< 10 alarms: 26.7 kg/m2 [P25: 23.6 kg/m2; P75: 28.7 kg/m2]; ≥ 10 alarms: 24.4 kg/m2 [P25: 22.2 kg/m2; P75: 30.4 kg/m2]; P = 0.200; Fig. 6g). There was no significant correlation between age, BMI or QRS duration with the mean number of inappropriate WCD alarms per day (Table 6).
Inappropriate WCD alarms due to signal artifacts and subgroup analyses in patients with very low versus higher incidence of inappropriate alarms. a In the majority of patients, less than 10 signal artifacts and WCD alarms occurred during the entire WCD wearing time. b Distribution of sex in the subgroup analysis of patients with < 10 and ≥ 10 alarms during the prescription period. c Previously implanted cardiac devices in the two subgroups. d Relative proportion of patients with diagnosed other arrhythmia entities in this subgroup analysis. e Evidence of cardiac pacing via implanted devices at baseline. f QRS duration was similar in both subgroups. g The incidence of inappropriate alarms did not correlate with the patients’ body mass index (BMI). P values in b–e were calculated using the chi-square test; P values in f and g were calculated with the Mann–Whitney-U test; PM pacemaker
Remarkably, among 13 patients who experienced particularly frequent WCD alarms (i.e., mean of more than one inappropriate alarm per day; including both patients with inappropriate WCD therapy), three patients (23.1%) showed cardiac pacing by an implanted device in the WCD baseline ECG. By comparison, only 5 of 93 patients (5.4%) in the group of less than 1 alarm per day exhibited ventricular pacing at baseline. When comparing these subgroups of patients with < 1 alarm/day and ≥ 1 alarm/day, there was a tendency towards higher rates of pacing at baseline in patients with high numbers of alarms which, however, failed to reach statistical significance (P = 0.057, chi-square test). In subgroup analyses of patients with ventricular pacing versus no pacing at baseline, there was a tendency towards higher rates of inappropriate alarms per day (Table 7). However, interpretation of these results requires caution due to the different subgroup sizes.
Follow-up
WCD wearing time was prematurely discontinued in 18 patients (17.0%). The reasons for discontinuation were available in four cases and included discomfort, frequent alarms, limited re-imbursement, and non-disclosed technical defects. A subgroup of 53 patients that had not previously received an ICD was subjected to additional follow-up visits after the prescription period. Of these, 16 patients (30.2%) received an ICD immediately after WCD prescription. The most common reason for rejection of ICD indication was an improvement in LV function (n = 18; 48.6%; Table 8). Other reasons comprised low risk of SCD as indicated by further diagnostic testing (in particular negative testing for ARVC by cardiac MRI, negative genetic testing for arrhythmia syndromes, no evidence of persistent active inflammation in cardiac MRI in patients with previous myocarditis without severe LVEF reduction, heart transplantation or PCI without continuation of WCD monitoring. Seven patients (18.9%) refused ICD implantation against medical advice.
All patients who presented with sustained VT-episodes during the prescription period (n = 4; one patient treated by WCD shock, three patients who manually withheld WCD therapy) received an ICD. In three of these cases, the ICD indication had been established previously, and the WCD had been prescribed temporarily due to ICD dysfunction or infection. The fourth patient had received the WCD due to severely impaired LV-function and non-sustained VT detected by Holter monitoring. WCD alarms due to ventricular arrhythmia episodes triggered hospitalization and subsequent ICD implantation.
After a median follow-up period of 11.3 months [P25: 3.9 months; P75: 23.4 months] after WCD removal (n = 65), an ICD was implanted in three further patients who had previously declined the ICD for primary prevention at the end of WCD wearing time in spite of established indication (n = 2) or in which ICD indication had been rejected after re-evaluation of LV-function (n = 1). In two of these patients, ICD implantation was carried out after further progression of the underlying heart disease and worsening of symptoms 4 and 8 months later, respectively. In the third case, the patient required CPR due to VF 2 years after WCD wearing time and subsequently received an ICD for secondary prevention. During the follow-up period, three patients died (4.6%). Of those, two were ICD carriers and died of non-cardiovascular causes. The third patient had been recommended a subcutaneous ICD due to difficult anatomical conditions (TGA) which he had declined. He died of cardiogenic shock.
Discussion
In this study of a real-world patient population comprising different indications for WCD prescription overall compliance was high, with younger patients exhibiting reduced compliance rates. Both appropriate and inappropriate WCD therapies were infrequent events. Inappropriate WCD alarms, mainly due to ECG artifacts, were common but did not exert a statistically significant effect on compliance rates in our patient population. Prolonged QRS duration and PM stimulation were identified as sources of inappropriate ECG interpretation by the WCD in single cases, without serving as statistically significant predictors of alarm frequency. This study particularly aims to analyze inappropriate WCD alarms not leading to inappropriate shocks, triggered by signal artifacts or by non-ventricular arrhythmias, and to investigate the role of patient-specific characteristics with respect to inappropriate WCD alarms.
Comparison with previous studies
With final publication of prospective randomized WCD data still being expected, scientific evidence is primarily obtained from case reports and registries. The present patient cohort was comparable to previously published studies with regard to median age and sex distribution [12, 13]. The WCD wear time (58.5 days) was similar compared with the overall German cohort (59 days) [11] but shorter in comparison with the WEARIT-II registry (90 days) [12]. The reason may be a shorter waiting period until definite therapeutic ICD decision making. In contrast to the prospective WEARIT-II registry and other retrospective analyses, we defined patient subgroups by indication for WCD prescription rather than underlying pathology. This is of relevance for clinical decision making by the prescribing physician, and overlap between different subgroups is avoided. In the WEARIT-II registry, the rate of WCD therapies for VT/VF was comparable to the event rate in our cohort. In case of VT/VF, the WCD was able to detect the arrhythmia correctly and deliver appropriate therapy in case of unconsciousness of the patient. Another important observational single-center study analyzed a cohort of 102 patients with regard to benefit of the WCD and adverse events [14]. In comparison with our patient cohort, most patients displayed newly diagnosed heart failure and were being evaluated for an ICD for primary prevention, whereas our patient cohort represents a “high risk” population with already confirmed ICD indication in the majority of cases.
Potential factors underlying inappropriate alarms and therapy
This study aimed to identify means of therapy improvement by identifying predictors for inappropriate ECG interpretation and subgroups with frequent WCD alarms. The incidence of inappropriate therapies during WCD prescription was 1.9% in this real-world study cohort. Previously, even lower rates of inappropriate shocks were reported in WEARIT-II (0.5%) and in a German nationwide retrospective analysis (0.4%) [11, 12]. In cases of inappropriate WCD therapy, we were able to identify prolonged QRS duration, presence of a PM, and delayed response to WCD alarms as causes for inappropriate ECG interpretation and subsequent inappropriate WCD therapy. We could not identify a statistically significant relationship between these characteristics and the frequency of inappropriate alarms, possibly because of the small size of the subgroup showing ventricular PM stimulation. A recent publication [9] emphasizes the significance of the pacing mode for ECG misinterpretation by the WCD. Unipolar DDD-pacing harbors a high risk for triggering the WCD detection algorithm. Schmitt et al. tested different pacing modes under study conditions and identified ECG misinterpretation by the WCD that the patients wore solely for the purpose of the study. The WCD detection algorithm was triggered by the device due to morphology alterations, amplitude changes, and double and triple counting. Our study confirms the role of ventricular pacing-related ECG misinterpretation as a reason for inappropriate WCD therapy in clinical practice. Furthermore, Schmitt et al. observed WCD triggering in unipolar DDD-modes only, but not in bipolar stimulation modes or single-chamber devices, whereas both patients with inappropriate WCD shocks in our cohort had single-chamber pacemakers operating in bipolar VVI(R)-modes. This novel observation highlights the significance of PM stimulation independent of the pacing mode when supplying a patient carrying a PM with a WCD. Other patient-specific baseline characteristics such as BMI, sex, age, and QRS duration did not show a clear correlation with the incidence of inappropriate WCD alarms. In comparison with Erath et al., the rate of patients experiencing “false alarms” was significantly higher (73% vs. 57%) [14]. In addition to the results presented by Erath et al., we aimed to quantify rates of inappropriate alarms per patient and, on this basis, were able to perform detailed statistical analysis for correlation with patient-specific factors. In our analyses, we could not establish any relation between BMI and rate of alarms per patient, whereas Erath at el. found that artifacts were mainly observed in “skinny and/or active patients”. Furthermore, in the patient cohort described by Erath et al. no pacing-related false alarms or WCD therapies were observed. Contrastingly, these were the cause of inappropriate WCD therapies and many false WCD alarms in our patient cohort. Thus, our observations add another relevant aspect to the existing data on the use of the WCD in clinical practice. Even though, apart from pacemaker-associated ECG misinterpretations, no patient-specific predisposing factors could be defined in our study, WCD alarms due the ECG signal artifacts or to inappropriate automatic interpretation were nonetheless frequent with strong inter-individual quantitative variations that may affect the patients’ quality of life. Therefore, risk factors for frequent alarms need to be further analyzed to improve device technology or patient selection accordingly.
Determinants of WCD compliance
Despite frequent alarms, patients displayed high compliance. Importantly, even patients with a higher number of inappropriate alarms did not show a statistically significant reduction in wearing time. Overall compliance rates were similar to previously published cohorts [11, 12]. Younger patients were less compliant with the WCD. Similarly, Wäßnig et al. reported that patients in the lower quartile of daily use were younger than patients in the upper three quartiles. Lower wear times among younger patients could be explained by discomfort in everyday life during work or leisure activity. On the other hand, older patients were clearly able to handle the device properly, reflected by appropriate transmission of WCD data through the LifeVest® network. Furthermore, there was no significant correlation between age and the number of artifact-triggered alarms. Therefore, in spite of being a relatively new technology, the WCD seems to be well applicable in older patients. Benefits and safety of the WCD have not been shown to be reduced in older patients; however, to our knowledge there have been no studies specifically addressing age-related differences in the rate of inappropriate acoustic alarms. In contrast to previous studies [10, 12], compliance was reduced with increasing prescription duration, which corresponds to the role of the WCD as a temporary “bridging” therapy for a defined period of time.
Benefits of WCD treatment—impact on patient selection
In our patient cohort as well as in previous German and U.S. nationwide analyses, patients with an established ICD indication in which implantation or re-implantation after infection is delayed appear to benefit particularly with regard to appropriate therapies during WCD wearing time [10, 11]. This study primarily includes patients with an established ICD indication. These patients carry an elevated risk that qualifies for SCD protection according to current guidelines. Currently, there is no alternative, safe “bridging” therapy for these patients. The present data emphasize the effectiveness of the WCD for this purpose. In addition, potential patient-related characteristics that require consideration for therapy optimization in this subgroup are highlighted: in patients with cardiac pacing, the programming of both the implanted device and the WCD should be optimized to ensure harmonization of the two devices.
Among patients in whom an ICD indication is uncertain, the WCD offers additional diagnostic information by recording ventricular arrhythmias that are self-terminating and do not require therapy. The benefit of the WCD during ongoing risk stratification is reflected by the observation that a significant proportion of patients in our cohort did not require ICD implantation after the WCD prescription period. This observation is corroborated by the results from the PROLONG study showing that a prolonged “bridging” with a WCD during optimization of heart failure medication may allow for cardiac remodeling and, thus, prevent unnecessary ICD implantation for primary prevention [15].
Limitations
The study is affected by inherent limitations associated with its retrospective design. Follow-up data are limited to those patients who presented at our institution after WCD removal. As data from the WCD prescription sheets were not available for analysis, the days until WCD return by the patient were used as a surrogate of the prescription period that could readily be extracted from the remote monitoring network. The identification of predictors for inappropriate WCD alarms was limited by high inter-patient variability in events. In single cases, QRS-alteration due to pacemaker stimulation has contributed to inappropriate ECG interpretation, limiting statistical analysis that has to be interpreted with care due to the small subgroup size. Finally, the analysis of mechanisms triggering inappropriate WCD alarms or therapy depended on electrograms recorded through the LifeVest® network. Thus, triggers of arrhythmia detection by the WCD algorithm could be identified solely from these ECG recordings, supported by information provided by the manufacturer.
Conclusion
The WCD reliably detected ventricular arrhythmias and offered effective protection from VT/VF-related SCD. Patient compliance was influenced by patient age and by duration of WCD prescription. Remote, web-based WCD monitoring harbors added diagnostic potential through the detection of self-terminating VT/VF-episodes, supraventricular arrhythmia, or asystole. Inappropriate WCD alarms are frequent. Alterations in QRS morphology during ventricular pacemaker stimulation may lead to inappropriate alarms and WCD therapies. Careful consideration of PM-programming in WCD patients could reduce inappropriate events to further improve real-life WCD performance.
References
Sasaki S, Tomita H, Shibutani S, Izumiyama K, Higuma T, Itoh T, Sasaki K, Horiuchi D, Kimura M, Okumura K (2014) Usefulness of the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circ J 78:2987–2989
Strauss M, Kouraki K, Skarlos A, Zahn R, Kleemann T (2013) A patient with severely reduced LV function and electrical storm saved by wearable cardioverter-defibrillator: a case report. Herzschrittmacherther Elektrophysiol 24:136–138
Reek S, Geller JC, Meltendorf U, Wollbrueck A, Szymkiewicz SJ, Klein HU (2003) Clinical efficacy of a wearable defibrillator in acutely terminating episodes of ventricular fibrillation using biphasic shocks. Pacing Clin Electrophysiol 26:2016–2022
Epstein AE, Abraham WT, Bianco NR, Kern KB, Mirro M, Rao SV, Rhee EK, Solomon SD, Szymkiewicz SJ (2013) Wearable cardioverter-defibrillator use in patients perceived to be at high risk early post-myocardial infarction. J Am Coll Cardiol 62:2000–2007
Zishiri ET, Williams S, Cronin EM, Blackstone EH, Ellis SG, Roselli EE, Smedira NG, Gillinov AM, Glad JA, Tchou PJ, Szymkiewicz SJ, Chung MK (2013) Early risk of mortality after coronary artery revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ Arrhythm Electrophysiol 6:117–128
Duncker D, Haghikia A, Konig T, Hohmann S, Gutleben KJ, Westenfeld R, Oswald H, Klein H, Bauersachs J, Hilfiker-Kleiner D, Veltmann C (2014) Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function-value of the wearable cardioverter/defibrillator. Eur J Heart Fail 16:1331–1336
Rao M, Goldenberg I, Moss AJ, Klein H, Huang DT, Bianco NR, Szymkiewicz SJ, Zareba W, Brenyo A, Buber J, Barsheshet A (2011) Wearable defibrillator in congenital structural heart disease and inherited arrhythmias. Am J Cardiol 108:1632–1638
Opreanu M, Wan C, Singh V, Salehi N, Ahmad J, Szymkiewicz SJ, Thakur RK (2015) Wearable cardioverter-defibrillator as a bridge to cardiac transplantation: a national database analysis. J Heart Lung Transplant 34:1305–1309
Schmitt J, Abaci G, Johnson V, Erkapic D, Gemein C, Chasan R, Weipert K, Hamm CW, Klein HU (2017) Safety of the wearable cardioverter-defibrillator (WCD) in patients with implanted pacemakers. Pacing Clin Electrophysiol 40:271–277
Klein HU, Goldenberg I, Moss AJ (2013) Risk stratification for implantable cardioverter defibrillator therapy: the role of the wearable cardioverter-defibrillator. Eur Heart J 34:2230–2242
Wassnig NK, Gunther M, Quick S, Pfluecke C, Rottstadt F, Szymkiewicz SJ, Ringquist S, Strasser RH, Speiser U (2016) Experience with the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circulation 134:635–643
Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, Zareba W, Goldenberg I (2015) Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the prospective registry of patients using the wearable cardioverter defibrillator (WEARIT-II registry). Circulation 132:1613–1619
Chung MK, Szymkiewicz SJ, Shao M, Zishiri E, Niebauer MJ, Lindsay BD, Tchou PJ (2010) Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol 56:194–203
Erath JW, Vamos M, Sirat AS, Hohnloser SH (2017) The wearable cardioverter-defibrillator in a real-world clinical setting: experience in 102 consecutive patients. Clin Res Cardiol 106:300–306
Duncker D, König T, Hohmann S, Bauersachs J, Veltmann C (2017) Avoiding untimely implantable cardioverter/defibrillator implantation by intensified heart failure therapy optimization supported by the wearable cardioverter/defibrillator—the PROLONG study. J Am Heart Assoc 6:e004512. https://doi.org/10.1161/JAHA.116.004512
Acknowledgements
This work was supported in part by research grants from the German Center for Cardiovascular Research, DZHK (Rotation Grant to MMZ), from the Cardiology Career Program by the Department of Cardiology, University Hospital Heidelberg, to H.H., from the German Cardiac Society and the Hengstberger Foundation (Klaus-Georg and Sigrid Hengstberger Scholarship to DT), from the German Heart Foundation/German Foundation of Heart Research (project F/08/14 to DT), from the Else Kröner-Fresenius-Stiftung (2014_A242 to DT), from the Joachim Siebeneicher Foundation (to DT), and from the Ministry of Science, Research and the Arts Baden-Wuerttemberg (Sonderlinie Medizin to DT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
MMZ reports receiving travel support from Medtronic and ZOLL CMS. EZ reports receiving lecture fees/honoraria from Bayer Vital, Medtronic and St. Jude Medical. DT reports receiving lecture fees/honoraria from Bayer Vital, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer Pharma, Sanofi-Aventis, St. Jude Medical and ZOLL CMS, and research grant support from Daiichi Sankyo. The remaining authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zylla, M.M., Hillmann, H.A.K., Proctor, T. et al. Use of the wearable cardioverter-defibrillator (WCD) and WCD-based remote rhythm monitoring in a real-life patient cohort. Heart Vessels 33, 1390–1402 (2018). https://doi.org/10.1007/s00380-018-1181-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1181-x