Abstract
The association between low birth weight and premature cardiovascular disease has led to the “prenatal origin of adult disease-hypothesis”. We postulated that fetal growth restriction is associated with cardiovascular changes detectable at birth and in early infancy. Fifty-two appropriately grown fetuses (AGA) and 60 growth-restricted fetuses (FGR) with (n = 20) or without (n = 40) absent or reversed end-diastolic umbilical artery blood flow were prospectively examined by echocardiography before birth, at 1 week and 6 months of life. The impact of growth restriction on postnatal blood pressure, heart rate, cardiovascular dimensions, and function, as well as on vascular morphology of umbilical cord vessels was studied. FGR fetuses displayed significant blood flow redistribution and were delivered earlier with lower birth weights than AGA fetuses. After adjustment for gender, gestational age, and weight at birth, there were no intergroup differences in blood pressure, heart rate, left ventricular morphology, mass, and performance, and in cord vessel morphology. During the first 6 months of life brachioradial pulse wave velocity increased more in FGR fetuses, while other parameters describing vascular stiffness remained comparable between the groups. Fetal growth restriction had no detectable adverse impact on cardiovascular dimensions and function at birth. Cardiovascular findings also remained comparable during the first 6 months of life between the groups except a higher increase in brachioradial pulse wave velocity in the FGR group. Our observations suggest that abnormalities that link reduced intrauterine growth with premature cardiovascular diseases may commence later in childhood, indicating a potential window for screening and prevention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A direct association between fetal growth restriction (FGR) and premature cardiovascular disease forms the basis of “fetal origins of adult disease” hypothesis by Barker [1]. Physiologic adaptations that enable a fetus to survive intrauterine deprivation are thought to cause permanent reprogramming of developing key organs with pathological consequences in later life, including a higher risk for cardiovascular morbidity. Indeed, in children and adults, low birth weight has been associated with increased blood pressure (BP) [2–5], faster heart rates (HRs) [6], stiffer, smaller and less reactive systemic arteries [6–9], and with atherosclerosis and coronary artery disease in later life [2, 10]. Several pathways that initiate or predispose FGR to arterial disease have been proposed. The fetus responds to decreased transplacental nutrient transfer and hypoxemia with a preferential blood supply to the most essential organs, while reducing the perfusion of other organs. Impaired growth of the abdominal aorta and kidneys into adulthood [6, 11, 12] suggest that intrauterine blood flow redistribution may permanently reprogram the development and function of post-ductal organs. Malnutrition and premature iatrogenic delivery [13] may also have adverse effects on defense mechanisms against oxidative stress and arterial elastin synthesis, which may lead to the early development of atherosclerosis and thrombosis and permanent reduction in arterial compliance [10, 14]. Finally, other factors have been suggested to play roles in the development of cardiovascular diseases, such as excessive catch-up growth due to improved nutrition after birth [15], hereditary factors [16], smoking, and socioeconomic status.
Despite the accumulating evidence on Barker’s hypothesis on children and adults, early postnatal data on cardiovascular function in human FGR is scarce. Logically, Barker’s concept would imply a direct correlation between the severity of fetal deprivation and cardiovascular consequences that might already be obvious at birth and early infancy. To test this hypothesis, we performed a comprehensive longitudinal analysis of the relationship between FGR and cardiovascular properties prenatally, at 1 week and 6 months of life.
Materials and methods
This prospective study was approved by the Research Ethics Boards of the Hospital for Sick Children and Mount Sinai, Toronto (File No. 1000006155 and 08-0057-C). Study participation required written maternal consent. All clinical investigations were conducted according to the declaration of Helsinki.
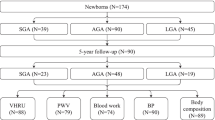
Sixty growth-restricted (FGR) and 52 appropriately grown (AGA) fetuses with normal umbilical artery blood flow velocimetry were recruited to this study from the High-Risk Pregnancy and the Fetal Cardiac Programs at our institutions. FGR was defined as birth weight <10th percentile for gestational age and/or an umbilical artery pulsatility index (UA PI) >2 SD [17, 18]. Of the 60 FGR fetuses, 40 showed continuous antegrade UA flow through the cardiac cycle while 20 had absent/reversed end-diastolic UA flow [19]. Other inclusion criteria included the confirmation of fetal age <20 gestational weeks, absence of major congenital anomalies, delivery ≥26 weeks, and ≥1 complete postnatal exam. Excluded from analyses were studies of critically ill newborns (e.g., mechanical ventilation or vasoactive medication) or with persistent arterial duct patency.
Study protocol
Eligible subjects were invited to undergo a series of evaluations before birth to 6 months of life (Fig. 1). This included
-
(a)
A maternal questionnaire for pregnancy history and cardiovascular risk factors,
-
(b)
A fetal echocardiogram by a single sonographer (HQ) blinded to gestational age and using a Philips iU22 ultrasound system (Philips Medical Systems, Andover, MA, USA) with 5–9 MHz transducers. Fetal weight was estimated by Hadlock formula [20]. Color imaging was used to optimize placement of the Doppler gate with the insonation angle <20°. Pulsatility indices were measured in the UA, ductus venosus and middle cerebral artery (MCA) [21]. Doppler inflow velocities and the left ventricular myocardial performance index (LV MPI) were obtained from the cardiac 4-chamber view [21]. Doppler flow velocity–time integrals (VTIs) and the inner diameters (Ds) of the great arteries during systole were obtained from cardiac 5-chamber and short-axis views. Left (LCO) and right cardiac output (RCO) was calculated as π × (D/2)2 × VTI × heart rate and the indexed combined CO as (LCO+RCO)/estimated fetal weight [21]. Tissue Doppler velocity curves of the longitudinal wall motion during diastole (E′; A′) and systole (S′) were obtained at the lateral base of the mitral and tricuspid annulus in the 4-chamber view. Tissue Doppler insonation angle was kept <10°, the frame rate >100 MHz and the 1.5 mm sample volume within the center of the myocardial wall [22]. LV dimensions and shortening fraction were measured by M-mode [21]. The mean of 3 measurements was used for each variable. z score values were adjusted for gestational age [23].
-
(c)
Assessment at birth UA hemoglobin and arterial blood gas values were measured. A 2-cm specimen of the umbilical cord was obtained from the fetal end. After fixation in buffered formalin, it was cut in cross sections of 4 mm, embedded in paraffin, sectioned, and stained with Elastic (Verhoeff’s) Trichrome. Stained slides were analyzed with 20–30 times magnification using a Leica EZ4D stereomicroscope (Spectronic Analytical Instruments) and a standard ruler for calibration. Measurements of the umbilical cord compartments including walls and lumens of blood vessels and the area of Wharton’s jelly were performed offline from stored images with the Leica statistical software (Visiopharm Integrator System for digital image analysis, 3.0.8.0).
-
(d)
Postnatal exams were obtained at a median age of 9 days (Exam 1 3–17 days; n = 99) and 202 days (Exam 2 165–263 days; n = 65) of life. Height was measured with a stadiometer to the nearest 0.1 cm and the weight with an electronic balance to the nearest 0.1 kg. BSA was calculated by Mosteller formula. BP of both arms and the left thigh was measured by oscillometry using appropriate sized cuffs (Dinamap, Criticon Inc, Tampa, FL, USA) in the supine position, after ≥30 min of rest and immediately after imaging of the aorta. Three recordings were obtained from each site and the mean of the lowest two was used in the analyses. Postnatal echocardiograms with a Vivid-7 ultrasound system (GE Medical Systems, Wauwatosa, WI, USA) and 4–10 MHz transducers were performed by a single sonographer (CS) who was blinded to the pregnancy history. Pulsed Doppler was used to measure LV E/A ratios and MPI. Longitudinal color tissue Doppler velocities from the lateral mitral and tricuspid annulus were analyzed offline (EchoPAC version 7, GE Medical Systems). Cardiovascular measurements including ventricular dimensions, LV ejection fraction, mass and sphericity index, aortic wall distensibility and stiffness index, and pulse wave velocity (PWV) were obtained using previously described methods [24–29]. PWV is inversely related to the square root of arterial distensibility, and the stiffer the arterial wall, the faster the pulse wave will be. Central PWV was obtained in the suprasternal echocardiographic view from the aortic arch by dividing the distance between the two measurement sites in the ascending and descending aorta by the transit time [28]. Peripheral PWV of the right brachioradial artery was measured by photoplethysmography that uses 2 infrared emitting and sampling probes placed over the brachial and radial arterial sites to simultaneously record the local arterial pressure waveforms to offline measure the transit time (Pulse Arterial Analysis version 97.01, L.M. Styles, London, UK) [29].
Data on the accuracy, precision, and reproducibility of the different study methods have been reported by our group [22, 26, 29, 30]. Intra- and inter-observer coefficients of variation of postnatal measurements determined in 10 randomly selected study subjects were 2.2–5.4 % and 2.8–6.1 % for different cardiovascular dimensions; 2.2 and 2.6 % for flow velocities; 1.8–4.2 % and 5.5–7.4 % for Doppler-derived time intervals; and 4 and 17 % for central PWV. The repeatability coefficient of peripheral PWV was 0.28 m/s with a coefficient of variation of 11.2 %.
Statistical analysis
Results are presented as mean with standard deviation, median with interquartile range, and frequencies as appropriate. Direct comparisons between study groups were performed using a Student’s t test assuming unequal variance between samples and Fisher’s exact test as appropriate. A multivariable linear regression model was created to compare the change in outcomes between 1 week and 6 months follow-up between the study groups. This regression model included all observations and was adjusted for repeated measures per patients through a compound symmetry covariance structure, gestational age at birth, birth weight percentile, and gender. General estimating equations (GEE) were used to determine the within-patient effect, overall group effects, and the interaction between change over time and study groups. A two-tailed p value <0.05 was selected as the level of statistical significance. Analyses were performed with SAS Statistical Software v9.2 (The SAS Institute, Cary NC, USA).
Results
Pregnancy and delivery
Maternal characteristics did not differ between the FGR and AGA groups, apart from a higher proportion of hypertensive mothers in FGR (Table 1). Compared with AGA, FGR fetuses displayed decreased MCA/UA PI ratios suggestive of blood flow redistribution towards the brain and were delivered earlier with smaller weight percentiles and increased placenta/birth weight ratios (Table 1). UA blood gas and hemoglobin values at birth were comparable between the groups (Table 1). Table 2 shows the fetal echocardiographic findings. FGR was associated with smaller LV and aortic diameter compared to AGA. Other fetal cardiac findings did not differ among the cohorts.
Neonatal and infant data
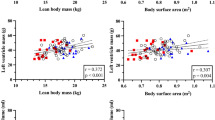
Table 3 describes the evolution of clinical and echocardiographic parameters from the neonatal to the infant period. The study cohorts differed significantly in weight z scores and body surface areas. At 1 week, FGR was associated with lower mitral A′ and tricuspid S′ velocities, and smaller descending aorta z scores compared with AGA. Other cardiovascular parameters were largely comparable between AGA and FGR infants with no intergroup differences in left ventricular (LV) morphology, shape, mass, and myocardial performance. Table 3 also illustrates the results of the multivariable regression model, assessing the interactions between change over time and study groups, adjusted for gestational age at delivery, birth weight percentile and gender. After these adjustments, the FGR group continued to display a higher increase in mitral A′ and tricuspid S′ velocities. Brachioradial PWV increased in both groups between 1 week and 6 months, but this increase was greater in the FGR group compared to AGA. Other parameters describing vascular function did not differ between the study groups.
Discussion
In this study, we prospectively evaluated the effect of FGR on a comprehensive portfolio of cardiovascular outcomes from fetal life to infancy. Our findings demonstrate no significant changes in cardiovascular performance at birth that can be correlated with FGR, suggesting that later postnatal changes play an important role in the development of early cardiovascular morbidity in adulthood. Indeed, during the follow-up period to the age of 6 months, the increase in brachioradial PWV was higher in FGR infants, but no other differences in cardiovascular properties between AGA and FGR groups were detected.
Our unexpected findings cannot be explained on the basis of the severity of FGR or its hemodynamic consequences in our study population. The FGR cohort presented obvious signs of blood flow redistribution related to placental malfunction (increased UA PI; decreased MCA/UA ratio) and also included a severely affected subgroup with absent/reversed UA flow, cardiomegaly and elevated ductus venosus pulsatility [21, 31–33]. Similar to the observation by Kiserud [34], we found that ventricular outputs were maintained, indicating that FGR was not associated with ventricular failure at the time of fetal investigation.
Despite significant differences in fetal circulatory responses between the groups, we detected no pathologic vascular abnormalities immediately after birth. Highly distensible arterial walls are important determinants of a normal cardiac output and ventricular-vascular coupling [35]. It is well established that increased arterial pulse pressure and circumferential wall stress promotes collagen synthesis by arterial smooth muscle cells and leads to thickening and stiffening of the arterial wall, which in turn promotes atherosclerosis and LV hypertrophy. Previously, cardiovascular abnormalities, including increased abdominal aortic thickness [36], myocardial hypertrophy, and abnormal LV shape were observed in younger FGR children [24, 37, 38]. In a recent small study on 20 term-born FGR and 20 AGA babies, FGR was associated with increased intima media thickness and stiffness in the abdominal aorta and lower cardiac function parameters between 2 and 5 days of life [39]. We elected to not quantify arterial wall dimensions in this study, as the resolution of conventional vascular ultrasound imaging is insufficient to obtain accurate measurements in young children [26]. However, our data showing no significant cardiovascular functional alterations in FGR infants are supported by the microscopic analysis of the cord vessels, which showed no increase in arterial wall thickness between FGR and AGA. Umbilical arteries represent fetal muscular arteries that are accessible at birth, and therefore offer a unique opportunity to study pregnancy-related vascular remodeling.
The ventricular wall dimensions, mass/shape and most other cardiac function parameters, including rates of changes to 6 months of life, were largely comparable between the study groups. These findings as well as counter-intuitive findings of higher myocardial mitral A′ and tricuspid S′ velocities are contradictory to previous reports that suggested cardiac dysfunction, myocardial hypertrophy, and abnormally shaped LVs in FGR children [24, 37, 38]. However, children in earlier studies were significantly older (≥5 years) than our subjects. During the postnatal 6-month follow-up period, with the exception of a faster increase in brachioradial pulse wave velocities in FGR, all parameters describing vascular stiffness showed no significant differences between FGR and AGA groups.
Previous studies [37, 40] showing no inverse effect of FGR on neonatal BP are in line with our results. Reports on BP of children and adolescents with FGR are variable. Crispi [24] reported increased systole-diastolic BP in 2-year old, while Brodski [6] did not detect BP differences in adolescents born growth-restricted. Moreover, 24-h BP and renal function tests did not differ between 8-year old AGA and FGR cases [41]. A large American study on 30,641 mothers and 3145 FGR offspring suggested that it is not intrauterine growth restriction per se but rather childhood weight and body mass index trajectory that may influence BP [42]. Indeed, associations between FGR, catch-up growth during infancy and risk of adult cardiovascular disease have been reported [43], while individuals with normal postnatal growth had comparable cardiovascular findings to adults without FGR [44]. We did not observe a more rapid growth/weight trajectory in our infant cohort with FGR and thus are unable to comment on the potential effect of later catch-up growth.
The relatively small study cohorts and unexpectedly high loss in postnatal follow-up at 6 months are acknowledged as study limitations. However, the loss-rate was similar in AGA and FGR groups (1 week: 6 vs. 10 %; 6 months: 32 vs. 35 %). The prospective longitudinal study design from fetal period to infancy did not permit matching for gestational age, as preterm delivery to avoid fetal demise is a common outcome of severe FGR. However, statistical adjustments for gestational age and weight were used where appropriate. Due to the small sample size of FGR fetuses with severe placental insufficiency, the results of FGR fetuses with abnormal and normal umbilical artery Doppler findings are presented as one group. However, no statistical differences in postnatal outcome parameters were observed between these two FGR groups (data not shown). Longitudinal follow-up of this cohort to later childhood might reveal the role of postnatal changes in the development of cardiovascular morbidity.
In conclusion, we were unable to demonstrate significant cardiovascular consequences of FGR in neonates and young infants. This not only implies that the later postnatal adaptation plays a significant role in the development of cardiovascular morbidity in FGR but also suggests that there may be a therapeutic window prior to the emergence of overt abnormalities, during which time interventions could modify cardiovascular risk factors.
References
Barker DJ (1992) The fetal origins of adult hypertension. J Hypertens Suppl 10(7):S39–S44
Barker DJ (1995) Fetal origins of coronary heart disease. BMJ 311(6998):171–174
Launer LJ, Hofman A, Grobbee DE (1993) Relation between birth weight and blood pressure: longitudinal study of infants and children. BMJ 307(6917):1451–1454
Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S (2005) Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation 112(22):3430–3436
Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Barker DJ, Cruddas AM, Fall CH (2002) Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation 105(9):1088–1092
Brodszki J, Lanne T, Marsal K, Ley D (2005) Impaired vascular growth in late adolescence after intrauterine growth restriction. Circulation 111(20):2623–2628
Leeson CP, Kattenhorn M, Morley R, Lucas A, Deanfield JE (2001) Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation 103(9):1264–1268
Martin H, Hu J, Gennser G, Norman M (2000) Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation 102(22):2739–2744
Cosmi E, Fanelli T, Mautone AJ, Zanardo V (2009) Aortic intima media thickness in fetuses and children with intrauterine growth restriction. Obstet Gynecol 114(5):1109–1114
Martyn CN, Gale CR, Jespersen S, Sherriff SB (1998) Impaired fetal growth and atherosclerosis of carotid and peripheral arteries. Lancet 352(9123):173–178
Silver LE, Decamps PJ, Korst LM, Platt LD, Castro L (2003) Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am J Obstet Gynecol 188(5):1320–1325
Zanardo V, Fanelli T, Weiner G, Fanos V, Zaninotto M, Visentin S, Cavallin F, Trevisanuto D, Cosmi E (2011) Intrauterine growth restriction is associated with persistent aortic wall thickening and glomerular proteinuria during infancy. Kidney Int 80(1):119–123
Keijzer-Veen MG, Dulger A, Dekker FW, Nauta J, van der Heijden BJ (2010) Very preterm birth is a risk factor for increased systolic blood pressure at a young adult age. Pediatr Nephrol 25(3):509–516
Beckman JS, Koppenol WH (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271(5 Pt 1):C1424–C1437
Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ (1999) Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 318(7181):427–431
Walker BR, McConnachie A, Noon JP, Webb DJ, Watt GC (1998) Contribution of parental blood pressures to association between low birth weight and adult high blood pressure: cross sectional study. BMJ 316(7134):834–837
Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, Blondel B, Breart G (2001) A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 108(2):E35
Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T (2005) Reference ranges for serial measurements of blood velocity and pulsatility index at the intra-abdominal portion, and fetal and placental ends of the umbilical artery. Ultrasound Obstet Gynecol 26(2):162–169
Giles WB, Trudinger BJ, Baird PJ (1985) Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol 92(1):31–38
Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK (1984) Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 150(2):535–540
Makikallio K, Vuolteenaho O, Jouppila P, Rasanen J (2002) Ultrasonographic and biochemical markers of human fetal cardiac dysfunction in placental insufficiency. Circulation 105(17):2058–2063
Nii M, Roman KS, Kingdom J, Redington AN, Jaeggi ET (2006) Assessment of the evolution of normal fetal diastolic function during mid and late gestation by spectral Doppler tissue echocardiography. J Am Soc Echocardiogr 19(12):1431–1437
Schneider C, McCrindle BW, Carvalho JS, Hornberger LK, McCarthy KP, Daubeney PE (2005) Development of z scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol 26(6):599–605
Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, Ahmed A, Gratacos E (2010) Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 121(22):2427–2436
Pettersen MD, Du W, Skeens ME, Humes RA (2008) Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr 21(8):922–934
Sarkola T, Manlhiot C, Slorach C, Bradley TJ, Hui W, Mertens L, Redington A, Jaeggi E (2012) Evolution of the arterial structure and function from infancy to adolescence is related to anthropometric and blood pressure changes. Arterioscler Thromb Vascular Biol 32(10):2516–2524
O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE (2002) Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 15(5):426–444
Sandor GG, Hishitani T, Petty RE, Potts MT, Desouza A, Desouza E, Potts JE (2003) A novel Doppler echocardiographic method of measuring the biophysical properties of the aorta in pediatric patients. J Am Soc Echocardiogr 16(7):745–750
Cheung YF, Taylor MJ, Fisk NM, Redington AN, Gardiner HM (2000) Fetal origins of reduced arterial distensibility in the donor twin in twin-twin transfusion syndrome. Lancet 355(9210):1157–1158
Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Friedberg MK, Mertens L, Jaeggi ET (2010) Myocardial tissue Doppler velocity imaging in children: comparative study between two ultrasound systems. J Am Soc Echocardiogr 23(9):929–937
Wladimiroff JW, vd Wijngaard JA, Degani S, Noordam MJ, van Eyck J, Tonge HM (1987) Cerebral and umbilical arterial blood flow velocity waveforms in normal and growth-retarded pregnancies. Obstet Gynecol 69(5):705–709
Hecher K, Bilardo CM, Stigter RH, Ville Y, Hackeloer BJ, Kok HJ, Senat MV, Visser GH (2001) Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound Obstet Gynecol 18(6):564–570
Ferrazzi E, Bozzo M, Rigano S, Bellotti M, Morabito A, Pardi G, Battaglia FC, Galan HL (2002) Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol 19(2):140–146
Kiserud T, Ebbing C, Kessler J, Rasmussen S (2006) Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet Gynecol 28(2):126–136
Senzaki H, Akagi M, Hishi T, Ishizawa A, Yanagisawa M, Masutani S, Kobayashi T, Awa S (2002) Age-associated changes in arterial elastic properties in children. Eur J Pediatr 161(10):547–551
Skilton MR, Evans N, Griffiths KA, Harmer JA, Celermajer DS (2005) Aortic wall thickness in newborns with intrauterine growth restriction. Lancet 365(9469):1484–1486
Leipala JA, Boldt T, Turpeinen U, Vuolteenaho O, Fellman V (2003) Cardiac hypertrophy and altered hemodynamic adaptation in growth-restricted preterm infants. Pediatr Res 53(6):989–993
Mikkola K, Leipala J, Boldt T, Fellman V (2007) Fetal growth restriction in preterm infants and cardiovascular function at five years of age. J Pediatr 151(5):494–499
Sehgal A, Doctor T, Menahem S (2013) Cardiac function and arterial biophysical properties in small for gestational age infants: postnatal manifestations of fetal programming. J Pediatr 163(5):1296–1300
Robel-Tillig E, Knupfer M, Vogtmann C (2003) Cardiac adaptation in small for gestational age neonates after prenatal hemodynamic disturbances. Early Hum Dev 72(2):123–129
Bilge I, Poyrazoglu S, Bas F, Emre S, Sirin A, Gokalp S, Eryilmaz S, Hekim N, Darendeliler F (2011) Ambulatory blood pressure monitoring and renal functions in term small-for-gestational age children. Pediatr Nephrol 26(1):119–126
Wen X, Triche EW, Hogan JW, Shenassa ED, Buka SL (2011) Prenatal factors for childhood blood pressure mediated by intrauterine and/or childhood growth? Pediatrics 127(3):e713–e721
Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB (2000) Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 320(7240):967–971
Gomes FM, Subramanian SV, Escobar AM, Valente MH, Grisi SJ, Brentani A, Fink G (2013) No association between low birth weight and cardiovascular risk factors in early adulthood: evidence from Sao Paulo, Brazil. PLoS ONE 8(6):e66554
Acknowledgments
Financial support by Heart and Stroke Foundation of Ontario Grant (#NA 5922, EJ), The Finnish Cardiovascular Research Foundation (KM), and The Instrumentarium Research Foundation (KM) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mäkikallio, K., Shah, J., Slorach, C. et al. Fetal growth restriction and cardiovascular outcome in early human infancy: a prospective longitudinal study. Heart Vessels 31, 1504–1513 (2016). https://doi.org/10.1007/s00380-015-0742-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-015-0742-5