Abstract
Incomplete neointimal coverage and malapposed struts after stenting are associated with increased risk of stent thrombosis. We aimed to evaluate neointimal coverage early after Resolute zotarolimus-eluting stent (R-ZES) implantation using optical coherence tomography (OCT). A total of 20 patients with de novo native coronary lesions with R-ZES were enrolled. Among these patients, 20 stented lesions in 19 patients were evaluated at 1, 2, and 3 months after R-ZES implantation. The strut apposition and neointimal coverage were evaluated by OCT. Neointimal hyperplasia (NIH) thickness and percentage of covered struts and the proportion of incompletely apposed struts were measured at 1-mm intervals. The mean percentages of covered stent struts were over 85 % within 3 months (88.4 ± 6.3 % at 1 month, 95.5 ± 5.5 % at 2 months, 93.6 ± 3.5 % at 3 months). The percentages of incompletely apposed struts were not significantly different among the groups (4.4 ± 4.2 % at 1 month, 1.9 ± 1.9 % at 2 months, 3.1 ± 2.2 % at 3 months, p = 0.51). Mean NIH thickness (38.9 ± 8.1 μm at 1 month, 70.6 ± 18.8 μm at 2 months, 54.1 ± 5.9 at 3 months, p = 0.0016) was thickest in the 2 months group. Most of all OCT findings within 2 months demonstrated neointimal coverage with low signal intensity. The neointimal coverage of ZES-R was over 85 % within 3 months. These data may support shorter requirement of dual antiplatelet therapy duration with R-ZES.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drug-eluting stents (DES) provide reduced in-stent restenosis and the subsequent need for repeated revascularization compared with bare metal stents (BMS) [1]. However, the required duration of dual antiplatelet therapy (DAPT) after DES implantation has been considered longer than that of BMS to avoid the risk of stent thrombosis (ST). Resolute zotarolimus-eluting stent (R-ZES) (Resolute Integrity™; Medtronic, Santa Rosa, California) was CE marked in May 2010 and received FDA approval in February 2012. R-ZES has a thin strut, cobalt chromium alloy, and release zotarolimus, over a period of approximately 180 days, from Biolinx™ tri-polymer coating system [2]. In patients with various types of lesion, such as acute coronary syndrome, stable angina, and small vessel disease, 1 or 2-year outcomes of R-ZES had been reported to be safe and effective [3, 4]. A recent all-comers randomized clinical trial of the R-ZES also demonstrated comparable clinical outcomes to the everolimus-eluting stents up to 4 years [5]. Interestingly, the analysis from four prospective multicenter clinical trials showed that DAPT interruptions between 1 and 12 months after R-ZES implantation were not associated with increased risk of ST and cardiac outcomes [6]. The European agency gave a CE mark for 1-month DAPT duration after treatment with R-ZES, although there has been only this clinical evidence to support the shorter requirement of DAPT. Incomplete neointimal coverage after stenting is associated with increased risk of ST [7, 8]. Optimal coherence tomography (OCT) provides an axial resolution of 10–15 μm, thus enabling accurate evaluation of tissue coverage after stenting. OCT has now become a standard exploratory tool for the evaluation of neointimal studies comparing various types of stent [9, 10]. The aim of this study was to evaluate neointimal coverage at early phase after R-ZES implantation using OCT.
Methods
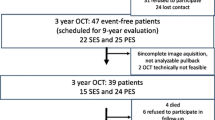
This study was a prospective, single-center study to evaluate the early vessel responses of R-ZES using OCT. From December 2012 to April 2014, a total of 20 consecutive patients who had multivessel coronary lesion and consented to this study protocol were randomized in a 1:1:1 fashion to observe by OCT either the 1-, 2-, or 3-month group. All patients had multivessel lesions; therefore, we performed staged PCI at months 1–3 after the index PCI with R-ZES. Time-course OCT was conducted concurrently with staged PCI at months 1, 2, and 3 after R-ZES implantation to determine the percentage of stent strut coverage (Fig. 1).
Inclusion criteria for this study were as follows: (1) de novo multivessel lesions with ≥75 % diameter stenosis by visual estimation and (2) objective evidence of myocardial ischemia in at least 2 lesions. Exclusion criteria were as follows: (1) ST-segment elevation myocardial infarction, (2) lesions with chronic total occlusion, (3) significant left main or bifurcation lesions, (4) poor view of OCT findings, (5) in-stent restenosis lesions, (6) more than two lesions with acute coronary syndrome, (7) patients on hemodialysis, and (8) patients with single-vessel lesion.
The study protocol was approved by the institutional review board and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients received oral and written information about the study and signed an informed consent before participation in the study.
In all patients who signed informed consent, stents were evaluated using OCT imaging after the index procedure and each month after stent implantation. Coronary angioplasty was performed according to standard techniques. Before the 1st intervention, all patients with acute coronary syndrome received 200 mg loading of aspirin and 300 mg loading of clopidogrel, and patients with stable angina pectoris started receiving DAPT (aspirin 100 mg and clopidogrel 75 mg) at least 5 days prior to the 1st intervention. After the intervention, all patients continued to receive DAPT during the entire study period.
OCT pullbacks were obtained with a frequency domain C7-XR OCT intravascular imaging system (LightLab Imaging, Inc., Westford, Massachusetts) allowing acquisition at 100 frames per second with non-occlusive technique. A standard guide wire was advanced distally in the target vessel and the OCT companion C7 Dragonfly™ catheter was advanced over the wire. OCT image acquisition was performed as previously described [11]. OCT images were analyzed by two physicians at our institution, who were given no information about clinical presentations, lesion characteristics, and time points for taking OCT images [12]. All OCT cross-sectional images were analyzed at 1-mm intervals (every 5 frames) to evaluate neointimal coverage and apposition of the struts. Apposition was assessed strut by strut by measuring the distance between the strut marker and the lumen contour. The marker of each strut was placed at the endoluminal leading edge in the midpoint of its long axis, and the distance was measured following a straight line connecting this marker with the center of gravity of the vessel [13]. Struts with distance to lumen contour larger than the sum of strut + polymer thickness were considered malapposed (>97 μm for ZES-R: stent strut thickness 91 μm + abluminal polymer thickness 6 μm). Struts were classified as uncovered if any part of the strut was visibly exposed to the lumen, or covered if a layer of tissue was visible over all reflecting surfaces. The stent struts found at the level of bifurcation or branching were excluded from the analysis of malapposition [9].
In covered struts, the neointimal hyperplasia (NIH) thickness was measured from the strut marker to the endoluminal edge of the tissue coverage, following a straight line connecting the strut marker with the center of gravity of the vessel [9]. Neointimal coverage of the stent struts were assessed based on the following two variables of OCT: the percentage of covered struts and mean thickness of NIH. The stent and lumen areas were measured on the 1-month interval OCT images to calculate the percentage of NIH according to the following formula: (stent area − lumen area)/stent area × 100 [14]. The numbers of the strut cross sections covered or uncovered with the neointima were counted for each R-ZES. A thrombus was defined as an irregular mass with a solitary free shadow on an OCT image [15]. To evaluate the quantification of strut covering tissue, computer-assisted densitometric analysis was performed as described previously [16]. We compared the optical intensity of stent struts with that of the surrounding facing the intraluminal side of the strut in the cross-section exist in thickest NIH for each lesion by using Geneotools software (Syngene, Cambridge, UK). In the present study, the intensity of intraluminal side/that of stent struts area, <0.44, was divided to a low intensity, and >0.44 to a high intensity [16].
Quantitative coronary angiography parameters were measured using a computerized edge-detection quantitative coronary angiographic analysis system (CASS system, Pie Medical Instruments, Maastricht, The Netherlands) by a single individual blinded to the patient information. Values of in-stent minimal lumen diameter, % diameter stenosis, and mean reference diameter were obtained at three points: before PCI (pre-intervention), immediately after PCI (post-intervention), and at 1-month-interval early phase (follow-up). In addition, we calculated acute gain (post-minus pre-intervention minimal lumen diameter) and late lumen loss (post-intervention minus follow-up minimal lumen diameter).
Categorical variables are presented as numbers and frequencies and were compared by the Chi square test. Continuous variables are expressed as mean ± SD and categorical variables as number (percentage). Comparisons of continuous variables were made using Student’s t tests, and the Wilcoxon rank sum test was applied if the distributions were non-parametric. All analyses were performed using JMP 9.0 software for Windows (SAS Institute, Cary, North Carolina). A p value <0.05 was considered to indicate statistical significance.
Results
A total of 20 patients were enrolled in the study and underwent PCI with 24 R-ZES implantations to treat 21 de novo native coronary arteries. Among them, 1 patient (with 2 stents in 2 lesions) in the 3-month group was excluded from the study due to poor OCT image acquisition (Fig. 1). Patient and lesion characteristics at baseline are shown in Tables 1 and 2. There were no significant differences among the groups. Target lesions were located in the right coronary (n = 3), left anterior descending (n = 12), and left circumflex (n = 4) arteries. Angiographic findings are included also in Table 2. The ACC/AHA type B2/C was found in 74 % of lesions.
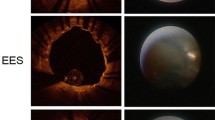
OCT procedures revealed no serious complications during or after the implantation of R-ZES: in total, 523 cross-sectional OCT images, in which 5927 stent struts were analyzed after excluding 17 cross-sectional images that had overlapping. Follow-up OCT finding are shown in Table 3. In the present study, adjunct balloon dilations after post-stenting were performed in lesions at the physician’s discretion. The percentages of malapposed struts were not significantly different among the groups (4.4 ± 4.2, 1.9 ± 1.9, 3.1 ± 2.2 %, 1-, 2- and 3-month group, respectively, p = 0.51). The percentages of stent strut coverage in 1-, 2-, and 3-month groups were 88.4 ± 6.3, 95.5 ± 5.5, and 93.6 ± 3.5 %, respectively. Representative post-intervention and 1, 2, and 3 months’ follow-up OCT images after R-ZES implantation are shown in Fig. 2. The mean NIH thickness in the 2-month group was greatest among the groups (38.9 ± 8.1, 70.6 ± 18.8, and 54.1 ± 5.9 μm in the 1-, 2-, and 3-month groups, respectively, p = 0.0016). The percentages of NIH were also highest in the 2-month group (5.2 ± 1.0, 9.0 ± 3.8, and 7.1 ± 1.6 % in the 1-, 2-, and 3-month groups, respectively, p = 0.015). OCT findings often demonstrated the low signal intensity around struts within 2 months (10/12 cases) (Fig. 2; Table 3). Thrombus was detected in 2 stents [1 (17 %) in the 1-month group and 1 (14 %) in the 3-month group]. No clinical events were observed in the patients during the study period.
Discussion
This was a prospective randomized study to evaluate the early neointimal coverage within 3 months after R-ZES implantation using a frequency domain OCT. The major findings of this study were as follows: (1) the percentages of stent strut coverage were over 85 % within 3 months, (2) mean NIH thickness was greatest at 2 months after R-ZES implantation, and (3) most of all the OCT findings in the 1- and 2-month groups demonstrated neointimal coverage with low signal intensity. The analysis from four prospective multicenter clinical studies showed that DAPT interruptions beyond 30 days were associated with a low risk of ST and less increased risk of cardiac death and target vessel myocardial infarction [6]. It has been reported that lack of neointimal coverage after stenting and stent incomplete apposition have an important risk of ST [7]. Kim et al. [9], Kim et al. [17], and Gutierrez-Chico et al. [18] conducted observational clinical studies of R-ZES by serial OCT and reported that the percentages of uncovered strut were 6.2 % at 3 months, 3.3 % at 9 months, and 7.4 % at 13 months after R-ZES implantation, and the percentages of malapposed struts were 0.7 % at 3 months, 0.7 % at 9 months, and 1.8 % at 13 months, respectively. In the present study, the percentages of uncovered struts were 7.2 % at 1 month, 2.6 % at 2 months, and 3.2 % at 3 months, and the percentages of malapposed struts were 4.4 % at 1 month, 1.9 % at 2 months, and 3.1 % at 3 months, respectively. The percentage of uncovered struts at 3 months with R-ZES is similar between COVER OCT II trial [16] and the present study, although there is discrepancy in the percentages of malapposed struts. It might have been due to the differentiation of definition of malapposition, patients, and lesion characteristics between the present study and COVER OCT II trial. In the present study, struts with distance to lumen contour >97 μm were considered malapposed; on the other hand, >117 μm in COVER OCT II trial including OCT resolution limit was 20 μm. In addition, the age of patients and the ratio of type of B2/C lesion were higher in the 3-month group of the present study than that of the COVER OCT II trial in R-ZES.
This early stent strut coverage within 3 months after R-ZES implantation might be attributable to its product profile. DES has been designed with the intent to target intimal thickening and hyperplasia without interfering with the process of functional endothelial regrowth. Our group previously reported the percentages of uncovered struts after the Endeavor zotarolimus-eluting stent (E-ZES) were 29.1 % at 1 month, 13.1 % at 2 months, and 0.8 % at 10 weeks, respectively [19]. The percentages of stent strut coverage after R-ZES implantation are higher than that of E-ZES within 2 months. The key difference between R-ZES in comparison with E-ZES is its biocompatibility polymer coating. The biocompatibility polymer may favor early endothelialization of the vessel and strut surface.
Most of all OCT findings demonstrated smooth layer with low signal intensity regardless of with or without statin therapy within 2 months. On the other hand, 3-month follow-up OCT demonstrated thin neointima that exhibited a homogenous pattern except for 1 case. While high-intensity signal, smooth and homogenous coverage mostly correspond to well-organized neointimal tissue mainly composed of smooth muscle cells, lower-intensity signal strut coverage might correspond to acellular material such as proteoglycan or fibrin [20, 21]. It had been reported that the optical intensity of (intraluminal side/stent struts area) <0.44 was considered as the cutoff value, with the sensitivity of detecting neointimal or fibrin coverage over 80 % [16]. This time course of strut covering tissue may suggest the change from fibrin or proteoglycan to neointimal coverage of stent struts within 3 months after R-ZES implantation.
This study has several limitations. First, 1-, 2-, and 3-month analysis was not performed on the same patient; therefore, it is still unclear whether the results reflect the time course of neointimal accumulation in each patient. We are fully aware that the optimal duration of DAPT after R-ZES implantation was still unclear in the present study; however, our date at least may still support the shorter requirement of DAPT duration, because the lack of neointimal coverage after stenting is associated with increased risk of ST [7]. More investigations are necessary to prove if shorter DAPT is sufficient in these stents. Second, the number of enrolled patients was small, and the number of patients who were categorized into each of three 1-month-interval group was too small to permit any statistical analysis. Third, there is a possible selection bias because only patients with multivessel coronary lesions were enrolled. Fourth, because of its nature of an observational single-center prospective study, selection bias cannot be ruled out.
References
Stefanini GG, Holmes DR Jr (2013) Drug-eluting coronary-artery stents. N Engl J Med 368:254–265
Udipi K, Chen M, Cheng P, Jiang K, Judd D, Caceres A, Melder RJ, Wilcox JN (2008) Development of a novel biocompatible polymer system for extended drug release in a next-generation drug-eluting stent. J Biomed Mater Res A 85:1064–1071
Widimsky P, Motovska Z, Belardi J, Serruys P, Silber S, Windecker S, Neumann FJ (2013) Long-term outcomes of patients receiving zotarolimus-eluting stents in ST elevation myocardial infarction, non-ST elevation acute coronary syndrome, and stable angina: data from the Resolute program. Int J Cardiol 168:3522–3526
Jim MH, Yiu KH, Fung RC, Ho HH, Ng AK, Siu CW, Chow WH (2014) Zotarolimus-eluting stent utilization in small-vessel coronary artery disease (ZEUS). Heart Vessels 29:29–34
Taniwaki M, Stefanini GG, Silber S, Richardt G, Vranckx P, Serruys PW, Buszman PE, Kelbaek H, Windecker S (2014) Four year clinical outcomes and predictors of repeat revascularization in patients treated with new generation drug-eluting stents in the RESOLUTE all comers randomized trial. J Am Coll Cardiol 63:1617–1625
Silber S, Kirtane AJ, Belardi JA, Liu M, Brar S, Rothman M, Windecker S (2014) Lack of association between dual antiplatelet therapy use and stent thrombosis between 1 and 12 months following resolute zotarolimus-eluting stent implantation. Eur Heart J 35:1949–1956
Guagliumi G, Sirbu V, Musumeci G, Gerber R, Biondi-Zoccai G, Ikejima H, Ladich E, Lortkipanidze N, Matiashvili A, Valsecchi O, Virmani R, Stone GW (2012) Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv 5:12–20
Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R (2007) Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115:2435–2441
Gutierrez-Chico JL, van Geuns RJ, Regar E, van der Giessen WJ, Kelbaek H, Saunamaki K, Escaned J, Gonzalo N, di Mario C, Borgia F, Nuesch E, Garcia-Garcia HM, Silber S, Windecker S, Serruys PW (2011) Tissue coverage of a hydrophilic polymer-coated zotarolimus-eluting stent vs. a fluoropolymer-coated everolimus-eluting stent at 13-month follow-up: an optical coherence tomography substudy from the RESOLUTE all comers trial. Eur Heart J 32:2454–2463
Ikuta S, Kobuke K, Iwanaga Y, Nakauchi Y, Yamaji K, Miyazaki S (2014) Difference in neointimal coverage at chronic stage between bare metal stent and sirolimus-eluting stent evaluated at stent-strut level by optical coherence tomography. Heart Vessels 29:320–327
Tada T, Byrne RA, Schuster T, Cuni R, Kitabata H, Tiroch K, Dirninger A, Gratze F, Kaspar K, Zenker G, Joner M, Schomig A, Kastrati A (2013) Early vascular healing with rapid breakdown biodegradable polymer sirolimus-eluting versus durable polymer everolimus-eluting stents assessed by optical coherence tomography. Cardiovasc Revasc Med 14:84–89
Takano M, Inami S, Jang IK, Yamamoto M, Murakami D, Seimiya K, Ohba T, Mizuno K (2007) Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent 3 months after implantation. Am J Cardiol 99:1033–1038
Guagliumi G, Musumeci G, Sirbu V, Bezerra HG, Suzuki N, Fiocca L, Matiashvili A, Lortkipanidze N, Trivisonno A, Valsecchi O, Biondi-Zoccai G, Costa MA (2010) Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. JACC Cardiovasc Interv 3:531–539
Kim JS, Jang IK, Kim JS, Kim TH, Takano M, Kume T, Hur NW, Ko YG, Choi D, Hong MK, Jang Y (2009) Optical coherence tomography evaluation of zotarolimus-eluting stents at 9-month follow-up: comparison with sirolimus-eluting stents. Heart 95:1907–1912
Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K (2006) Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol 97:1713–1717
Templin C, Meyer M, Muller MF, Djonov V, Hlushchuk R, Dimova I, Flueckiger S, Kronen P, Sidler M, Klein K, Nicholls F, Ghadri JR, Weber K, Paunovic D, Corti R, Hoerstrup SP, Luscher TF, Landmesser U (2010) Coronary optical frequency domain imaging (OFDI) for in vivo evaluation of stent healing: comparison with light and electron microscopy. Eur Heart J 31:1792–1801
Kim S, Kim JS, Shin DH, Kim BK, Ko YG, Choi D, Cho YK, Nam CW, Hur SH, Jang Y, Hong MK (2013) Comparison of early strut coverage between zotarolimus- and everolimus-eluting stents using optical coherence tomography. Am J Cardiol 111:1–5
Kim JS, Kim BK, Jang IK, Shin DH, Ko YG, Choi D, Hong MK, Cho YK, Nam CW, Hur SH, Choi JH, Song YB, Hahn JY, Choi SH, Gwon HC, Jang Y (2012) ComparisOn of neointimal coVerage betwEen zotaRolimus-eluting stent and everolimus-eluting stent using Optical Coherence Tomography (COVER OCT). Am Heart J 163:601–607
Nishinari M, Shimohama T, Tojo T, Shiono T, Shinagawa H, Kameda R, Aoyama N, Izumi T (2013) Two-week interval optical coherence tomography: imaging evidence on neointimal coverage completion after implantation of the endeavor zotarolimus-eluting stent. Catheter Cardiovasc Interv 82:E871–E878
Teramoto T, Ikeno F, Otake H, Lyons JK, van Beusekom HM, Fearon WF, Yeung AC (2010) Intriguing peri-strut low-intensity area detected by optical coherence tomography after coronary stent deployment. Circ J 74:1257–1259
Gonzalo N, Serruys PW, Okamura T, van Beusekom HM, Garcia-Garcia HM, van Soest G, van der Giessen W, Regar E (2009) Optical coherence tomography patterns of stent restenosis. Am Heart J 158(284–293):1
Acknowledgments
The authors have nothing to disclose and are solely responsible for the design and conduct of this study, drafting and editing of the manuscript and its final contents.
Conflict of interest
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors. Dr. Junya Ako received speaking honorarium from Medtronic. Other authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashikata, T., Tojo, T., Namba, S. et al. Neointimal coverage of zotarolimus-eluting stent at 1, 2, and 3 months’ follow-up: an optical coherence tomography study. Heart Vessels 31, 206–211 (2016). https://doi.org/10.1007/s00380-014-0598-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-014-0598-0