Abstract
Quantitative influence and underlying mechanisms of nutrient stoichiometry on mineralization of native Soil organic C (SOC) and straw C in different aggregate classes from cultivated and non-cultivated soils are still unclear. Soil samples (Mollisols) from a native woodlot and a farmland converted from woodlot were sieved into three aggregate classes (mega-aggregates (6.3-2 mm), macro-aggregates (2-0.25 mm), and micro-aggregates (< 0.25 mm)) and incubated (180 days) under different nutrient rates (nil, low, and high supplies of N and P) with or without 13C-enriched straw amendment. Significantly higher percentage of native SOC was mineralized from mega- and macro-aggregates (65.8-82.2%) compared with micro-aggregates (48.3-52.0%) in woodlot soil. Nutrient addition significantly increased aggregate-associated C in both soils with straw, and the increase was greater in farmland than in woodlot soil and in large-sized aggregates than in micro-aggregates. These results suggested that large aggregates serve as a C reservoir of labile C, while micro-aggregates that contained C was not easily mineralized even with abundant nutrients. Differences of C mineralization among aggregate size classes were significant in woodlot soil but not in farmland soil. Depletion of SOC was greater with increasing nutrient addition rates in farmland aggregates without straw, while the depletion in woodlot aggregates showed no difference among nutrient treatments, suggesting that microbial activity was nutrient-limited in farmland aggregates. The results improved our knowledge on SOC mineralization in response to residue-nutrient management in different aggregate classes from cultivated and non-cultivated soils, which have important implications for strategies to improve soil fertility or mitigate climate change via increased SOC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil organic C (SOC) sequestration depends on the increase of the stable C pool of soil to ensure that the C is not easily mineralized. The importance of SOC sequestration has been highlighted in the recent decades due to its contribution to soil fertility and, particularly, the mitigation of greenhouse effect (Lal 2004). Even minor changes of SOC storage could cause a profound influence on atmospheric CO2 concentration (Manlay et al. 2007). Studies have shown that SOC sequestration not only depended on its recalcitrance, soil type, organic inputs, climate, etc. but also was greatly affected by soil management strategies (Mack et al. 2004; Christopher and Lal 2007; Kirkby et al. 2013; Dai et al. 2018). This calls for thorough studies on strategies (land use, fertilization, residue return, etc.) to increase SOC storage in soils, especially the increase of stable SOC pool.
The assessment of C dynamics in contrasting aggregate size classes can reveal C sequestration mechanisms in agroecosystems (Wu et al. 2012; Chen et al. 2014; Bimüller et al. 2016), because different aggregate size classes contained various concentrations of SOC with different recalcitrance and microbial accessibility, and are influenced by land use systems and soil management practices (Tisdall and Oades 1982; Six et al. 2000; Gunina and Kuzyakov 2014). Organic inputs with different recalcitrance showed higher total and native SOC mineralization in the macro- than in the micro-aggregates (Tian et al. 2016; Sarker et al. 2018). There are also studies that found greater C mineralization from micro- than macro-aggregates (Drury et al. 2004). The inconsistent results could be due to the interactions of aggregates with different soil stoichiometry, straw managements, land use systems, etc. (Bimüller et al. 2016; Sarker et al. 2018).
Among the factors suggested to affect SOC sequestration, soil nutrient stoichiometry has been drawing more and more attention in recent years (Kirkby et al. 2013; Fang et al. 2019; Piaszczyk et al. 2019). Ecological stoichiometry deals with the C:N:P ratios of organisms and substrates, and it helps to gain insight into the C and nutrient cycling as well as the metabolic state of organisms (Heuck et al. 2015; de Sosa et al. 2018; Sun et al. 2019). Thus, it has important implications for SOC dynamics, microbial growth, and turnover in soil (Kirkby et al. 2013; Li et al. 2018). Kirkby et al. (2013) found that inorganic nutrient availability was critical to sequester C into stable fine fraction C pool. Butterly et al. (2018) highlighted that C mineralization was mainly determined by soil N status rather than other factors such as residue quality. Fang et al. (2019) proved that balancing the resource stoichiometry through nutrient input could enhance the mineralization of labile SOC fraction, as well as the incorporation of newly added residue C into stable C fractions in a Luvisol and a Vertisol. In addition, converting natural ecosystems to agriculture modified the nutrient dynamics and stoichiometry of C, N, and P in soil (Wang et al. 2014). However, there is a lack of knowledge on how the integrated residue-nutrient management regulates mineralization of native SOC and residue C in different classes of soil aggregates.
The conversion of natural to agricultural ecosystems has depleted the SOC pool by 60% in soils of temperate regions and 75% or more in the tropical soils (Lal 2004). Agricultural use of soil can have negative influences on soil aggregate stability and in turn SOC sequestration (Six et al. 2000; Denef et al. 2007; Qiao et al. 2019), and there was a sharp SOC decline when forests or grasslands were converted to cropland (e.g., Poeplau et al. 2011; Wei et al. 2014). Our previous study on a clay loam soil showed that aggregates provided strong protection for SOC in the native woodlot and grassland soils, whereas this protection was not evolved in arable soil (Fan et al. 2013). In Northeast China, the large-scale deforestation has caused the remarkable depletion in C storage in black soils (Mollisol-based USDA soil taxonomy) (Liang et al. 2009; Shi et al. 2012). Residue return has attracted great attention to improve SOC sequestration in farmland of this region (Zhang et al. 2018). However, understanding of the fate of native SOC and residue C in varied aggregate classes under different nutrient application regimes in these soils is still lacking.
Therefore, we hypothesized that large-sized aggregates would have higher mineralization of native SOC and residue C than micro-aggregates, likely due to higher labile C content and higher microbial activity (Six et al. 2002). We also hypothesized that the differences of SOC mineralization among aggregate size classes would be greater in woodlot than in farmland soil, due to negative influences of soil cultivation on aggregate structure (Six et al. 2000; Denef et al. 2007). Furthermore, the addition of N and P to aggregates with straw could enhance the incorporation of straw C into SOC, and the responses of SOC mineralization to nutrient addition would be different in cultivated and un-cultivated soils. The objectives of this study were 1) to investigate the effects of various C-nutrient stoichiometry and aggregate classes on mineralization of native SOC and residue C in the non-cultivated and cultivated black soils; 2) to evaluate the contributions of C-nutrient stoichiometry on increase of total SOC; and 3) to identify the interactions of C mineralization, aggregate classes, and land use (soil cultivation) on SOC mineralization and sequestration.
Materials and methods
Soil sample preparation
The soil samples were collected in September 2017 from Qianjin Village (49° 02.21′ N, 120° 58.50′ E), Yakeshi Farm, Hulun Buir City, Inner Mongolia, China, from a long-term wheat (Triticum aestivum L. cv. Tonic)-rape (Brassica campestris L.) rotation farmland and another from adjoining native woodlot. The vegetation of the native woodlot was cold-temperate coniferous forest, which mainly included Larix gmelinii (Rupr.), Pinus pumila (Pall.), and Quecus mongolica. The farmland was converted from native woodlot more than 20 years ago. Field operations for the farmland included moldboard plowing to 18- to 20-cm depth in late September after rape or wheat harvest, and secondary cultivation and harrowing in the following spring before planting. Crop residues were removed from soil after harvest. Each year, N, P, and K fertilizers were applied during plowing at 90, 45, and 80 kg ha−1, respectively, as starter fertilizers in the forms of ammonium nitrate (NH4NO3), superphosphate (CaP2H4O8), and potassium sulfate (K2SO4), respectively. Additional 50-kg N ha−1 (urea) was top dressed at the bolting stage of rape. No additional fertilizer was applied for wheat crop. The woodlot had a minimal anthropogenic disturbance with no treatment imposed. The site has a temperate continental climate with a mean annual temperature at − 2.9 °C and a mean annual precipitation at 360.8 mm. The soils were classified as Mollisols (USDA soil taxonomy). Basic soil properties (n = 3) are presented in Table 1. The pH (water) of soil of woodlot and farmland were 6.7 and 6.4, respectively. Five soil samples were taken in a random zigzag pattern from the top 0-10-cm layer of each of the two land use systems. First, a pit of 10-cm depth was dug to get a soil profile, then a 20 × 20 × 10-cm rectangular container was pushed into the soil and carefully dug out from the side of exposed profile to get an undisturbed soil sample. After sampling, the field-moist soils were sealed and transported to the lab in a portable refrigerator (4 °C) for further analysis.
In the laboratory, the field-moist soil was gently broken to pass through a 6.3-mm sieve. Any visible stones or plant residues were removed. The soil samples were then air-dried for 2-4 days at room temperature. Soil aggregates were separated by dry sieving according to Limon-Ortega et al. (2006). Briefly, 500 g of the above air-dried soils were separated using a Ro-tap sieve shaker (W.S. Tyler Inc., Mentor, OH) by shaking through 2 sieves with different-sized openings (2.0 and 0.25 mm) for 5 min at a speed of 210 cycles min−1. Aggregates remaining in each sieve were collected. In this study, the aggregate size classes of 6.3-2 mm, 2-0.25 mm, and < 0.25 mm were classified as mega-aggregates, macro-aggregates, and micro-aggregates, respectively.
Preparation of 13C-labeled straw
The 13C labeling of wheat plants started when the plant height reached 60 cm in a greenhouse following the method of Fang et al. (2016) with minor modifications. Briefly, a micro-plot was sealed with a portable polyvinyl chloride (PVC) chamber (1 m × 1 m × 1 m). The lower edge of the chamber was buried into soil to a depth of 10 cm to seal the chamber. The plants inside the chamber were pulse labeled with 13CO2 (99.0 atom% 13C) from 10:00 am and 4:00 pm. The 13C-labeled CO2 was injected into the sealed chamber through a flow meter (S325-15-170-F/M CO2, Gascon Systems, Sydney, Australia) at approximately 500 cm3 min−1. The air in the chamber was circulated by a battery-operated mini-fan. The CO2 concentration in the chamber was monitored using a portable CO2 probe (Vaisala GMP 343, Helsinki, Finland). After 3:00 am, when the air temperature increased to 40 °C, a portion of the chamber was opened for 30 min to allow the temperature to drop below 30 °C. The chamber was then resealed to capture 13CO2 respired overnight by wheat. The chamber was kept sealed until the next morning when CO2 inside the chamber decreased to < 200 ppm. Air temperature inside the chamber varied between 20 and 40 °C. The labeling process was repeated for 3 times within 30 days.
After 13C labeling, wheat straw (leaves and stems) was collected, oven dried at 65 °C, cut to 1-cm pieces, and thoroughly mixed. The wheat straw contained 465.0-g C kg−1, 9.01-g N kg−1, 0.60-g P kg−1, and 30.21-g K kg−1. The δ13C value of the thoroughly mixed straw was 839.65‰.
Experimental design and incubation
The experimental design included 36 treatments across three aggregate size classes, three nutrient rates, and two soils (woodlot, farmland) with or without wheat straw amendment. Each treatment was triplicated. Aggregate samples of 200 g (dry weight) were gently and thoroughly mixed with (or without) 3-g wheat straw, and were put in 250-mL Erlenmeyer jars fitted with gas-tight septa. All jars were pre-incubated for 3 days before treatments. Nutrients were applied to soils at three rates: no nutrient but only water (NN), a low nutrient (LN) rate at 64-mg N and 26-mg P kg−1 soils, and a high nutrient (HN) rate at 128-mg N and 52-mg P kg−1 soils in water to reach 60% water holding capacity. The nutrient solutions were prepared using ammonium nitrate and potassium dihydrogen phosphate following the method of Kirkby et al. (2013). The concentrations of nutrients were set based on the assumption that the stoichiometry of stabilized soil organic matter has a C:N:P ratio of 10,000:833:200 as suggested by Himes (1998). The availability of inorganic nutrients in the soil was ignored, since they were an order of magnitude less than those added to soil. The pH of the nutrient solutions was adjusted to 7 using a sodium hydroxide solution. The nutrient solutions were added to soil dropwise to ensure even distribution. Another set of three blank jars was used to monitor background CO2 concentration and 13C natural abundance. All 111 jars were incubated (24 °C) in the dark for 180 days.
CO2 samples were drawn from each jar daily with a 30-mL syringe on days 2, 5, 10, 16, 24, 34, 46, 58, 72, 86, 102, 118, 138, 158, and 180. Following each gas sampling, the jars were flushed with air and covered with perforated parafilm during the period between CO2 measurements to maintain an aerobic headspace and minimize water loss. The weight of each jar was checked every other day, and deionized water was added to maintain soil water concentration. Concentrations of CO2 and 13C abundance in CO2 were determined for each sample. The 13C abundance in CO2 was determined for samples collected on days 2, 16, 38, 72, 118, and 180.
Analyses
Soil microbial biomass C (MBC) and N (MBN) after incubation were determined using the fumigation extraction method (Vance et al. 1987). The total C of aggregates before and after incubation was determined using a Flash EA 1112 elemental analyzer (Thermo Finnigan, Milan, Italy). Undecomposed plant residues in soils were removed before element determination. Since the studied soils were free of carbonates, the SOC was assumed to equal the total C. Concentrations of CO2 were measured using an Agilent N6890 gas chromatograph as described by Zhang et al. (2012). The 13C abundance in CO2 and soils was analyzed using an isotope ratio mass spectrometer (IRMS) (MAT 253, Thermo Scientific, USA).
Calculations and statistical analysis
The 13C abundance in soils and CO2 samples was expressed as δ13C values (‰):
where Rsample and Rv-PDB are 13C/12C ratios of samples and the Vienna Pee Dee Belemnite (V-PDB) standard (Dawson et al. 2002), respectively.
The proportion of soil aggregate-derived CO2-C (CA (%)) in the total CO2-C was determined as:
where, δT 13C is the δ13C value of the total CO2-C released from the straw-amended aggregates, δA13C is the δ13C of CO2-C released from the aggregates incubated without straw, and δS13C is the δ13C of the added wheat straw.
The priming effect (PE) was calculated as the difference between native SOC mineralized from the aggregates incubated with and without wheat straw. The amounts of straw C or native SOC mineralized during the incubation were determined by multiplying the proportion of straw-derived C or SOC with the total C mineralized, respectively (Fang et al. 2019). The amounts of residue C and SOC mineralized from the aggregates were then calculated as per gram of added residue C and initial aggregate-associated SOC, respectively.
The statistical comparison assumed a randomized complete block design with sub-sampling. Data not meeting the assumptions were transformed logarithmically and analyzed by using the ANOVA. A significance level of P < 0.05 was used unless otherwise indicated. LSD and Duncan were chosen for the multiple comparisons between different treatments. All statistical analysis was conducted using the SigmaPlot 12.5 software (Systat Software Inc., Chicago, USA).
Results
Patterns and cumulative CO2 released from aggregates of farmland and native woodlot soils
The release pattern of total CO2 during the 180-day incubation showed distinct variation with C-nutrient stoichiometry, aggregate size class, and land use (Fig. S1). For the farmland soil, aggregates incubated without straw showed low and relatively steady CO2 emission rates (8.74-39.3 pmol g−1 s−1), which were significantly higher with higher nutrient addition rates across the aggregate size classes (Fig. S1A). CO2 release rates were significantly increased by straw amendment, especially during the first 72 days of incubation (43.3-101.1 pmol g−1 s−1) (Fig. S1A). Among the three aggregate size classes of farmland soil, CO2 release rates from macro-aggregates was generally higher than that from mega- and micro-aggregates, while the latter two classes showed no significant difference (Fig. S1A).
The CO2 emission rates from large-sized aggregates (mega- and macro-aggregates) in woodlot soil were higher than that from farmland (Fig. S1). For mega- and macro-aggregates without straw amendment, CO2 emission rates were significantly higher from woodlot (59.0-20.3 pmol g−1 s−1) than that from farmland (31.3-14.6 pmol g−1 s−1) soil. Mega-aggregates showed higher emission rates than macro-aggregates from woodlot soil without straw, especially during the first 34 days (Fig. S1B). Micro-aggregates had lower CO2 release rates than the two larger classes irrespective of C-nutrient stoichiometry rates, indicating a lack of labile C in this size class in woodlot soil.
Cumulative CO2 released from woodlot soil aggregates was higher than that from farmland soil aggregates, especially for large classes of mega- and macro-aggregates (Fig. 1). For woodlot aggregates without straw, cumulative CO2 showed no significant difference among nutrient rates and was lower from micro-aggregates than the other larger size classes; for farmland aggregates without straw, cumulative CO2 was significantly increased by nutrient addition and showed no difference among aggregate classes. In aggregates with straw amendment, nutrient addition induced higher CO2, and the increase by nutrient rates was greater for farmland aggregates than for woodlot aggregates; large-sized aggregates, especially mega-aggregates from woodlot soil, produced significantly higher amount of CO2 than micro-aggregates (Fig. 1).
Cumulative amount of total CO2 released from mega-, macro-, and micro-aggregates in farmland and native woodlot soils with different C-nutrient stoichiometry. SNN, SLN, and SHN represent incubation with straw and no, low, and high nutrient addition, respectively. Vertical bars are the standard errors (n = 3). Different uppercase letters indicate significant differences between the two soils in the same aggregate class with the same C-nutrient stoichiometry level; different lowercase letters indicate significant differences between aggregate classes with the same C-nutrient stoichiometry level at P = 0.05
Mineralization of native SOC and PE from aggregates of the two soils
The δ13C-based calculations revealed that land use, aggregate classes, and C-nutrient stoichiometry greatly influenced the percentage of native SOC mineralized in total CO2-C (Fig. 2). Significantly higher percentage of native SOC was mineralized in mega- and macro-aggregates (65.8-82.2%) compared with micro-aggregates (48.3-52.0%) in woodlot soil. By increasing nutrient addition rates, a higher percentage of native SOC was mineralized in all aggregate size classes of farmland soil. Woodlot soil aggregates also showed higher percentage of mineralized native SOC when straw was applied to the LN treatment. However, the percentage did not further increase in the HN treatment with straw. This resulted in higher percentage of mineralized native SOC in micro-aggregates of farmland soil than that of woodlot soil. In the other two large size classes, the percentage was significantly higher from woodlot than farmland soil under all three C-nutrient stoichiometry rates (Fig. 2).
Percentage of mineralized native aggregate-associated C in total CO2-C (soil C plus straw C) derived from woodlot and farmland soils incubated with straw amendment. NN, LN, and HN represent incubation with no, low, and high nutrient levels, respectively. Vertical bars are the standard errors (n = 3). Different uppercase letters indicate significant differences between the two soils in the same aggregate class with the same C-nutrient stoichiometry level; different lowercase letters indicate significant differences between aggregate classes with the same C-nutrient stoichiometry level at P = 0.05
Land use, aggregate size class, and C-nutrient stoichiometry all significantly affected the PE of aggregates, with land use being the primary factor (P < 0.0001) (Table 2). Straw addition caused stronger PE in woodlot soil aggregates than in farmland aggregates (Fig. 3). The LN treatment with straw induced higher PE in large size class (mega- and macro-) aggregates of woodlot soil than the respective control, but further nutrient addition showed no effective change. The PE in farmland aggregates increased with nutrient addition rates and was significantly higher in large-sized aggregates than micro-aggregates under HN (Fig. 3).
The priming effect of aggregates from native woodlot and farmland soils with different C-nutrient stoichiometry levels. NN, LN, and HN represent incubation with no, low, and high nutrient levels, respectively. Vertical bars are the standard errors (n = 3). Different uppercase letters indicate significant differences between the two soils in the same aggregate class with the same C-nutrient stoichiometry level; different lowercase letters indicate significant differences between aggregate classes with the same C-nutrient stoichiometry level at P = 0.05
Variation of total SOC concentrations in aggregates of farmland and native woodlot soils
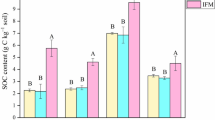
Wheat straw amendment at a rate of 15 g kg−1 increased SOC concentrations in all aggregate classes of the two soils after the incubation period of 180 days (Fig. 4). When incubated without exogenous nutrients, the increase was significantly greater in woodlot aggregates (1.15-1.80 g kg−1) than in farmland aggregates (0.77-1.26 g kg−1). The LN with straw induced 17.2-22.1% and 61.1-120.7% higher SOC increase in aggregates of woodlot and farmland soil, respectively, compared with the respective control. The HN with straw further induced a 34.7-48.0% increase of SOC in farmland aggregates compared with LN with straw, while no further increase was observed in woodlot aggregates. The SOC increase was generally greater in large size classes than micro-aggregates in woodlot soil, while no such difference was observed among farmland aggregates size classes (Fig. 4).
Variation of SOC concentration in aggregates of native woodlot and farmland soils after incubation with and without straw. SNN, SLN, and SHN represent incubation with straw and no, low, and high nutrient levels, respectively. NN, LN, and HN represent incubation with no, low, and high nutrient addition levels, respectively. Vertical bars are the standard errors (n = 3). Different uppercase letters indicate significant differences between the two soils in the same aggregate class with the same C-nutrient stoichiometry level; different lowercase letters indicate significant differences between aggregate classes with the same C-nutrient stoichiometry level at P = 0.05
Although increase of SOC in aggregates with straw amendment was low (0.77-2.79 g kg−1), the decline of SOC in aggregates without straw was non-negligible (2.28-4.71 g kg−1). The depletion of SOC in farmland aggregates increased with nutrient addition rates and showed no difference among aggregate size classes. The depletion in woodlot aggregates, however, increased with aggregate size class and showed no response to nutrient addition rates (Fig. 4). Land use was the primary factor affecting SOC variation, followed by C-nutrient stoichiometry and aggregate size class. Aggregate size class had significant interactions with land use and C-nutrient stoichiometry for SOC variation (Table 2).
Microbial properties of aggregates
Straw incorporation effectively increased concentrations of MBC and MBN across all aggregate size classes of the two soils after the incubation (Table 3). Nutrient addition also increased these concentrations, especially in aggregates of farmland soil. Larger aggregates generally had higher MBC and MBN within the same nutrient rate in woodlot soil, while farmland aggregates showed no significant differences across size classes. Both MBC and MBN were significantly lower in aggregates of farmland than woodlot. The MBC/MBN ratio was reduced by straw incorporation in aggregates of the two soils and showed a trend of decrease with increase of nutrient addition rate and decrease of aggregate size (Table 3).
Discussion
Effect of C-nutrient stoichiometry and straw amendment on SOC mineralization and sequestration
Straw amendment and nutrient addition effectively increased CO2 release from aggregates due to mineralization of straw C and native SOC, as already reported (Liu et al. 2019; Fan et al. 2020). In soils amended with straw and nutrients, Kirkby et al. (2013) found that inorganic nutrients (N, P, and S) affected C sequestration in the stable C pool of the fine fraction. This was because these nutrients were required by soil microorganisms for transforming C-rich residues into enriched fine fraction pools of soil organic matter (Christopher and Lal 2007; Kirkby et al. 2011). There are contrasting reports with positive (de Graaff et al. 2006; Fan et al. 2019), nil (Liljeroth et al. 1994), or negative (Janssens et al. 2010; Li et al. 2018) effects of N on SOC mineralization. The two hypotheses “stoichiometric decomposition” and “microbial N mining” (Craine et al. 2007) assume opposite effects of N on SOC recalcitrance, that is, “stoichiometric decomposition” suggests that high N availability promotes SOC decomposition, while according to the “microbial N mining” hypothesis low N availability likely facilitates SOC decomposition (Chen et al. 2014). In the present study, N and P nutrient inputs also increased total SOC concentration in farmland aggregates and large-sized woodlot aggregates after incubation with straw (Fig. 4). This confirms what was already reported that balancing nutrient stoichiometry with residue return stimulates incorporation of residue C into SOC pools (Chen et al. 2014; He and Dijkstra 2015; Fang et al. 2018, 2019; Yuan et al. 2019).
When aggregates were incubated without straw, however, nutrient addition increased CO2 emission and decreased the SOC content, and the depletion of SOC was greater by increasing nutrient addition rates in farmland soil. This indicated the microbial use of more stable C in soil with N and P supplies, and also a change in nutrient stoichiometry (C:N:P) (Figs. 1 and 4). The depletion in woodlot aggregates, however, showed no significant difference between LN and HN, and increased with aggregate size (Fig. 4). These results suggested that microbial activity was nutrient-limited in farmland aggregates and that microorganisms were more sensitive to nutrient addition in farmland aggregates than that in woodlot soil aggregates. This was further confirmed by the fact that the increase of SOC content was greater in woodlot than in farmland aggregates in the control treatment, while with increasing nutrient input, the difference narrowed and significantly higher amount of SOC was stored in farmland aggregates under the HN treatment. This confirmed the finding of Demoling et al. (2007) who reported that bacterial growth rates in soils with low SOC responded more to the nutrient addition than those soils with high SOC. It should be noted that SOC mineralization from farmland aggregates increased following nutrient addition without straw. This indicated that application of chemical fertilization can stimulate the depletion of SOC in farmland soil without enough crop residue return. Fortunately, increase of SOC concentration with increasing nutrient addition in straw-amended aggregates showed that with residue return, fertilization induced more newly added C into SOC, which offsets mineralization of native SOC.
Additionally, significantly higher CO2 emission from mega- and macro-aggregates of woodlot soil was observed in the HN treatment compared with the LN treatment; however, the percentage of native SOC mineralized as well as the PE showed no difference between these two nutrient rates (Fig. S1; Figs. 1, 2, and 3). This suggested that exogenous nutrient addition into woodlot soil mega- and macro-aggregates mainly promoted mineralization of the newly added straw C, which was confirmed by δ13C-based calculations. This suggested that the SOC in woodlot aggregates was more recalcitrant than straw C (Bonanomi et al. 2013).
Effect of C-nutrient stoichiometry and straw amendment on soil microbial biomass
Increased MBC and MBN in response to nutrient addition in straw-amended treatments suggested that the presence of low nutrients such as N (Mack et al. 2004; Nordin et al. 2004) or P (Ilstedt and Singh 2005) can be the most limiting factors for microbial growth. It has been reported that adding C, N, and P together induced maximum bacterial growth rate when all nutrient limitations were removed (Demoling et al. 2007). Aggregates amended with straw in this study were characterized by significantly greater microbial biomass and lower MBC/MBN compared with the un-amended aggregates (Table 3), indicating that microbial mineralization was driven by the microbial need for C when nutrients were not limiting (de Sosa et al. 2018). When straw was added, portions are transformed into microbial biomass when soil microorganisms utilize this labile C source (Herron et al. 2009), leading to a boom of MBC and MBN. The decrease of MBC/MBN ratio by straw amendments indicated a shift in microbial community composition in soil (Li et al. 2008). The straw amendment provided substances and energy for growth and reproduction of microorganisms, which in turn consumed N and P compounds from soil and decreased mineral N and P concentration (Ros et al. 2010; Begum et al. 2014; Yuan et al. 2019). Nutrient input in this circumstance eliminated nutrient restrictions and stimulated microbial growth and activity with the increase of the decomposition of native SOC through co-metabolism (Chen et al. 2014; Tian et al. 2016). Thus, the patterns of CO2 emission and the PE among treatments, i.e., increase with straw amendment and nutrient addition, were related to the variation patterns of MBC and MBN among treatments. Variation of MBC/MBN ratio by nutrient addition also indicated different responses of microbial groups to nutrient addition, due to the different stoichiometry of microbial groups in soils (e.g., fungi versus bacteria) (de Sosa et al. 2018). The significant lower MBC and MBN in aggregates of farmland than woodlot were consistent with the findings of Ding et al. (2020), who demonstrated that soil cultivation resulted in significant SOC depletion, which was closely associated with MBC loss.
Effect of aggregate size classes on SOC mineralization in cultivated and non-cultivated soils
Higher C mineralization rate was observed in the straw-amended large (mega and macro) aggregates than micro-aggregates in the two soils (especially woodlot soil) during incubation (Fig. S1; Figs. 1 and 3). This was consistent with findings that SOC mineralization was higher in the coarser than the finer aggregates, probably due to low structural stability of the former (Six et al. 2000; Bimüller et al. 2016; Sarker et al. 2018). Micro-aggregates are considered to have greater physical protected SOC compared with large-sized aggregates (Six et al. 2000; Denef et al. 2001). These results also highlighted the importance of different SOC bioavailability among the aggregate size classes besides the inherent structural stability (Six et al. 2000; Tian et al. 2015). Micro-aggregates are also characterized by a lower microbial biomass due to lower available C substrate than large-sized aggregates (Balesdent et al. 2000; Six et al. 2002). This was also observed here (Table 3). Although C mineralization was greater in large-sized aggregates, the increase of SOC after incubation with straw was also greater in these aggregates (Fig. 4), indicating that more straw-derived C was sequestrated in these aggregates relative to micro-aggregates. This was consistent with the above analysis that larger aggregates contained more microbial biomass with higher microbial activity and thus with microorganisms being capable of using the added straw C. The increase of straw-derived C in the larger aggregates relative to the micro-aggregates could also be explained by the microbial efficiency matrix stabilization mechanism (Cotrufo et al. 2015). If the micro-aggregate-associated C is primarily microbial derived, it may take longer to process than the direct physical stabilization. Since the incubation period of this study (180 days) is shorter than the reported time (18 months) needed to assess microbial contribution to SOC (Kallenbach et al. 2016), physical stabilization might be the reason for increase of C in micro-aggregates.
Effect of soil cultivation and interaction with aggregate size class on SOC mineralization and sequestration
The decrease of SOC content accounted for 6.0-8.1% of aggregate-associated SOC in woodlot soil but 13.5-18.9% of aggregate-associated SOC in farmland (data not shown). The C losses are expected be greater under field conditions than under controlled laboratory conditions (Luo et al. 2017), especially for farmland aggregates with frequent soil disturbance such as tillage and fertilization. The increase of SOC content in straw-amended aggregates was greater with nutrient addition than without in both soils, and this increase was lower in woodlot aggregates than in farmland aggregates. This might be explained by the C-saturation theory that when exposed to high C inputs, soils already rich in SOC have a limit in C sequestration capacity, while soils further away from the C saturation level will stabilize more C (Hassink and Whitmore 1997; Six et al. 2002; Chung et al. 2010; Chen et al. 2017; Wang et al. 2017). The woodlot soil may be reaching SOC saturation due to related factors such as texture, mineralogy, and high organic input, while the farmland soil still has a large capacity to store C, since more than half of its initial SOC was depleted after conversion to farmland (Table 1). More systematic research on nutrient and straw management based on long-term experiment is needed to verify this hypothesis. In addition, the change of land use type will have an impact on soil microbial community composition and their functions via diverse C:N:P ratios from exogenous organic substances, and thus might influence the PE and SOC storage (Yu et al. 2018). However, in this study, interaction between land use and C-nutrient stoichiometry and their effect on PE and SOC variation was not significant. Therefore, further research on the impacts of element stoichiometry on microbial community composition and function is needed.
Conclusions
Our incubation experiment based on 13C-enriched straw revealed that nutrient input effectively promoted mineralization of native SOC and straw C in aggregates of both cultivated and un-cultivated soils. N and P addition significantly increased aggregate-associated C with straw, and the increase was greater in larger than smaller aggregates. Depletion of SOC was greater by increasing nutrient addition rates in farmland aggregates without straw amendment, suggesting that microbial activity was nutrient-limited in farmland aggregates. It can also be inferred that application of chemical fertilizer could further promote the depletion of SOC in farmland soil without crop residue return. Fortunately, the increase of SOC concentration with increasing nutrient addition suggested that with residue return, fertilization induced more newly added C into SOC, which offsets mineralization of native SOC. Results from this study improved our knowledge on mineralization of native SOC and sequestration of residue C in different size classes of aggregates in response to different C-nutrient stoichiometry, which has important implications for strategies to restore SOC in cultivated soils.
References
Balesdent J, Chenu C, Balabane M (2000) Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res 53:215-230. https://doi.org/10.1016/s0167-1987(99)00107-5
Begum N, Guppy C, Herridge D, Schwenke G (2014) Influence of source and quality of plant residues on emissions of N2O and CO2 from a fertile, acidic black Vertisol. Biol Fertil Soils 50:499-506. https://doi.org/10.1007/s00374-013-0865-8
Bimüller C, Kreyling O, Kölbl A, von Lützow M, Kögel-Knabner I (2016) Carbon and nitrogen mineralization in hierarchically structured aggregates of different size. Soil Tillage Res 160:23-33. https://doi.org/10.1016/j.still.2015.12.011
Bonanomi G, Incerti G, Giannino F, Mingo A, Lanzotti V, Mazzoleni S (2013) Litter quality assessed by solid state 13C NMR spectroscopy predicts decay rate better than C/N and lignin/N ratios. Soil Biol Biochem 56:40-48. https://doi.org/10.1016/j.soilbio.2012.03.003
Butterly CR, Armstrong RD, Chen D, Tang CX (2018) Residue decomposition and soil carbon priming in three contrasting soils previously exposed to elevated CO2. Biol Fert Soils 55:17-29. https://doi.org/10.1007/s00374-018-1321-6
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Global Change Biol 20:2356-2367. https://doi.org/10.1111/gcb.12475
Chen H, Dong Y, Xu T, Wang Y, Wang H, Duan B (2017) Root order-dependent seasonal dynamics in the carbon and nitrogen chemistry of poplar fine roots. New For 48(5):587-607. https://doi.org/10.1007/s11056-017-9587-3
Christopher SF, Lal R (2007) Nitrogen management affects carbon sequestration in North American cropland soils. Crit Rev Plant Sci 26:45-64. https://doi.org/10.1080/07352680601174830
Chung H, Ngo KJ, Plante A, Six J (2010) Evidence for carbon saturation in a highly structured and organic-matter-rich soil. Soil Sci Soc Am J 74:130-138. https://doi.org/10.2136/sssaj2009.0097
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix M, Wall DH, Parton WJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776-779. https://doi.org/10.1038/ngeo2520
Craine JM, Morrow C, Fierer NO (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105-2113. https://doi.org/10.1890/06-1847.1
Dai W, Fu W, Jiang P, Zhao K, Li Y, Tao J (2018) Spatial pattern of carbon stocks in forest ecosystems of a typical subtropical region of southeastern China. Forest Ecol Manag 409:288-297. https://doi.org/10.1016/j.foreco.2017.11.036
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507-559. https://doi.org/10.1146/annurev.ecolsys.33.020602.095451
de Graaff MA, van Groenigen KJ, Six J, Hungate B, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Chang Biol 12:2077-2091. https://doi.org/10.1111/j.1365-2486.2006.01240.x
de Sosa LL, Glanville HC, Marshall MR, Schnefp A, Cooper DM, Hill PW, Binley A, Jones DL (2018) Stoichiometric constraints on the microbial processing of carbon with soil depth along a riparian hillslope. Biol Fert Soils 54:949-963. https://doi.org/10.1007/s00374-018-1317-2
Demoling F, Figueroa D, Bååth E (2007) Comparison of factors limiting bacterial growth in different soils. Soil Biol Biochem 39:2485-2495. https://doi.org/10.1016/j.soilbio.2007.05.002
Denef K, Six J, Bossuyt H, Frey SD, Elliott ET, Merckx R, Paustian K (2001) Influence of wet-dry cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol Biochem 33:1599-1611. https://doi.org/10.1016/S0038-0717(01)00076-1
Denef K, Zotarelli L, Boddey RM, Six J (2007) Microaggregate-associated carbon as a diagnostic fraction for management-induced changes in soil organic carbon in two Oxisols. Soil Biol Biochem 39:1165-1172. https://doi.org/10.1016/j.soilbio.2006.12.024
Ding X, Zhang B, Wei Z, He H, Filley TR (2020) Conversion of grassland into cropland affects microbial residue carbon retention in both surface and subsurface soils of a temperate agroecosystem. Biol Fert Soils 56:137-143. https://doi.org/10.1007/s00374-019-01400-8
Drury CF, Yang XM, Reynolds WD, Tan CS (2004) Influence of crop rotation and aggregate size on carbon dioxide production and denitrification. Soil Tillage Res 79:87-100. https://doi.org/10.1016/j.still.2004.03.020
Fan RQ, Yang XM, Drury CF, Guo XB, Zhang XP (2013) Distribution and stability of organic carbon in soil aggregate external and internal layers under three different land-use systems. Soil Sci Soc Am J 77:1625-1635. https://doi.org/10.2136/sssaj2013.03.0086
Fan YX, Zhong XJ, Lin TC, Lyu MK, Wang MH, Hu WF, Yang ZJ, Chen GS, Guo JF, Yang YS (2019) Effects of nitrogen addition on DOM-induced soil priming effects in a subtropical plantation forest and a natural forest. Biol Fert Soils 56:205-216. https://doi.org/10.1007/s00374-019-01416-0
Fan RQ, Zhang BH, Li JY, Zhang ZH, Liang AZ (2020) Straw-derived biochar mitigates CO2 emission through changes in soil pore structure in a wheat-rice rotation system. Chemosphere 243:125329. https://doi.org/10.1016/j.chemosphere.2019.125329
Fang YY, Singh BP, Badgery W, He X (2016) In situ assessment of new carbon and nitrogen assimilation and allocation in contrastingly managed dryland wheat crop-soil systems. Agric Ecosyst Environ 235:80-90. https://doi.org/10.1016/j.agee.2016.10.010
Fang YY, Singh BP, Collins D, Li B, Zhu J, Tavakkoli E (2018) Nutrient supply enhanced wheat residue-carbon mineralization, microbial growth, and microbial carbon-use efficiency when residues were supplied at high rate in contrasting soils. Soil Biol Biochem 126:168-178. https://doi.org/10.1016/j.soilbio.2018.09.003
Fang YY, Singh BP, Cowie A, Wang WQ, Arachchi MH, Wang HL, Tavakkoli E (2019) Balancing nutrient stoichiometry facilitates the fate of wheat residue-carbon in physically defined soil organic matter fractions. Geoderma 354:113883. https://doi.org/10.1016/j.geoderma.2019.113883
Gunina A, Kuzyakov Y (2014) Pathways of litter C by formation of aggregates and SOM density fractions: implications from 13 C natural abundance. Soil Biol Biochem 71:95-104. https://doi.org/10.1016/j.soilbio.2014.01.011
Hassink J, Whitmore AP (1997) A model of the physical protection of organic matter in soils. Soil Sci Soc Am J 61:131-139. https://doi.org/10.2136/sssaj1997.03615995006100010020x
He MZ, Dijkstra FA (2015) Phosphorus addition enhances loss of nitrogen in a phosphorus-poor soil. Soil Biol Biochem 82:99-106. https://doi.org/10.1016/j.soilbio.2014.12.015
Herron PM, Stark JM, Holt C, Hooker T, Cardon ZG (2009) Microbial growth efficiencies across a soil moisture gradient assessed using 13C-acetic acid vapour and 15N-ammonia gas. Soil Biol Biochem 41:1262-1269. https://doi.org/10.1016/j.soilbio.2009.03.010
Heuck C, Weig A, Spohn M (2015) Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol Biochem 85:119-129. https://doi.org/10.1016/j.soilbio.2015.02.029
Himes FL (1998) Nitrogen, sulfur, and phosphorus and the sequestering of carbon. In: Lal R, Kimble JM, Follett RF, Stewart BA (eds) Soil processes and the carbon cycle. CRC Press, Boca Raton, FL, pp 315-319
Ilstedt U, Singh S (2005) Nitrogen and phosphorous limitations of microbial respiration in a tropical phosphorous-fixing acrisol (ultisol) compared with organic compost. Soil Biol Biochem 37:1407-1410. https://doi.org/10.1016/j.soilbio.2005.01.002
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze ED, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315-322. https://doi.org/10.1038/ngeo844
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7:13630. https://doi.org/10.1038/ncomms13630
Kirkby CA, Kirkegaard JA, Richardson AE, Wade LJ, Blanchard C, Batten G (2011) Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils. Geoderma 163:197-208. https://doi.org/10.1016/j.geoderma.2011.04.010
Kirkby CA, Richardson AE, Wade LJ, Batten GD, Blanchard C, Kirkegaard JA (2013) Carbon-nutrient stoichiometry to increase soil carbon sequestration. Soil Biol Biochem 60:77-86. https://doi.org/10.1016/j.soilbio.2013.01.011
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304:1623-1627. https://doi.org/10.1126/science.1097396
Li J, Zhao BQ, Li XY, Hwat BS (2008) Effects of long-term combined application of organic and mineral fertilizers on soil microbiological properties and soil fertility. Sci Agric Sin 41:144-152. https://doi.org/10.3724/SP.J.1005.2008.01083
Li LJ, Xia ZB, Ye RZ, Doane TA, Horwath WR (2018) Soil microbial biomass size and soil carbon influence the priming effect from carbon inputs depending on nitrogen availability. Soil Biol Biochem 119:41-49. https://doi.org/10.1016/j.soilbio.2018.01.003
Liang AZ, Zhang XP, Yang XM, McLaughlin NB, Shen Y, Li WF (2009) Estimation of total erosion in cultivated black soils in Northeast China from vertical profiles of soil organic carbon. Eur J Soil Sci 60:223-229. https://doi.org/10.1111/j.1365-2389.2008.01100.x
Liljeroth E, Kuikman P, Veen JA (1994) Carbon translocation to the rhizosphere of maize and wheat and influence on the turnover of native soil organic matter at different soil nitrogen levels. Plant Soil 161:233-240. https://doi.org/10.1007/bf00046394
Limon-Ortega A, Govaerts B, Deckers J, Sayre KD (2006) Soil aggregate and microbial biomass in a permanent bed wheat-maize planting system after 12 years. Field Crop Res 97:302-309. https://doi.org/10.1016/j.fcr.2005.11.001
Liu SY, Fan RQ, Yang XM, Zhang ZH, Zhang XP, Liang AZ (2019) Decomposition of maize stover varies with maize type and stover management strategies: a microcosm study on a black soil (Mollisol) in Northeast China. J Environ Manag 234:226-236. https://doi.org/10.1016/j.jenvman.2019.01.008
Luo Y, Zang HD, Yu ZY, Chen ZY, Gunina A, Kuzyakov Y, Xu JM, Zhang KL, Brookes PC (2017) Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol Biochem 106:28-35. https://doi.org/10.1016/j.soilbio.2016.12.006
Mack MC, Schurr EAG, Bret-Harte MS, Shaver GR, Chapin FS III (2004) Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 431:440-443. https://doi.org/10.1038/nature02887
Manlay RJ, Feller C, Swift MJ (2007) Historical evolution of soil organic matter concepts and their relationships with the fertility and sustainability of cropping systems. Agric Ecosyst Environ 119:217-233. https://doi.org/10.1016/j.agee.2006.07.011
Nordin A, Schmidt IK, Shaver GR (2004) Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology 85:955-962. https://doi.org/10.1890/03-0084
Piaszczyk W, Błońska E, Lasota J, Lukac M (2019) A comparison of C:N:P stoichiometry in soil and deadwood at an advanced des stage. Catena 179:1-5. https://doi.org/10.1016/j.catena.2019.03.025
Poeplau C, Don A, Vesterdal L, Leifeld L (2011) Temporal dynamics of soil organic carbon after land-use change in the temperate zone-carbon response functions as a model approach. Glob Chang Biol 17:2415-2427. https://doi.org/10.1111/j.1365-2486.2011.02408.x
Qiao N, Wang J, Xu X, Shen Y, Long X, Hu Y, Schaefer D, Li SG, Wang HM, Kuzyakov Y (2019) Priming alters soil carbon dynamics during forest succession. Biol Fert Soils 55:339-350. https://doi.org/10.1007/s00374-019-01351-0
Ros GH, Hoffland E, Temminghoff EJM (2010) Dynamics of dissolved and extractable organic nitrogen upon soil amendment with crop residues. Soil Biol Biochem 42:2094-2101. https://doi.org/10.1016/j.soilbio.2010.08.004
Sarker JR, Singh BP, Cowie AL, Fang YY, Collins D, Dougherty WJ, Singh BK (2018) Carbon and nutrient mineralisation dynamics in aggregate-size classes from different tillage systems after input of canola and wheat residues. Soil Biol Biochem 116:22-38. https://doi.org/10.1016/j.soilbio.2017.09.030
Shi XH, Zhang XP, Yang XM, Drury CF, McLaughlin NB, Liang AZ, Fan RQ, Jia SX (2012) Contribution of winter soil respiration to annual soil CO2 emission in a Mollisol under different tillage practices in Northeast China. Global Biogeochem Cy 26:GB2007. https://doi.org/10.1029/2011GB004054
Six J, Elliott ET, Paustian K (2000) Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem 32:2099-2103. https://doi.org/10.1016/s0038-0717(00)00179-6
Six J, Conant RT, Paul EA, Paustian K (2002) Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241:155-176. https://doi.org/10.1023/a:1016125726789
Sun J, Gao P, Xu H, Li C, Niu X (2019) Decomposition dynamics and ecological stoichiometry of Quercus acutissima and Pinus densiflora litter in the grain to green program area of northern China. J Forestry Res:1-11. https://doi.org/10.1007/s11676-019-00981-2
Tian J, Pausch J, Yu G, Blagodatskaya E (2016) Aggregate size and glucose level affect priming sources: a three-source-partitioning study. Soil Biol Biochem 97:199-210. https://doi.org/10.1016/j.soilbio.2016.03.013
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141-163. https://doi.org/10.1111/j.1365-2389.1982.tb01755.x
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703-707. https://doi.org/10.1016/0038-0717(87)90052-6
Wang W, Sardans J, Zeng C, Zhong C, Li Y, Peñuelas J (2014) Responses of soil nutrient concentrations and stoichiometry to different human land uses in a subtropical tidal wetland. Geoderma 232-234:459-470. https://doi.org/10.1016/j.geoderma.2014.06.004
Wang GQ, Hao SS, Gao B, Chen MX, Liu YG, Yang JC, Ye NH, Zhang JH (2017) Regulation of gene expression in the remobilization of carbon reserves in rice stems during grain filling. Plant Cell Physiol 58(8):1391-1404
Wei X, Shao M, Gale W, Li L (2014) Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci Rep 4:4062. https://doi.org/10.1038/srep04062
Wu H, Wiesmeier M, Yu Q, Steffens M, Han X, Kögel-Knabner I (2012) Labile organic C and N mineralization of soil aggregate size classes in semiarid grasslands as affected by grazing management. Biol Fert Soils 48:305-313. https://doi.org/10.1007/s00374-011-0627-4
Yu Z, Chen L, Pan S, Li Y, Kuzyakov Y, Xu J, Brookes PC, Luo Y (2018) Feedstock determines biochar-induced soil priming effects by stimulating the activity of specific microorganisms. Eur J Soil Sci 69:521-534. https://doi.org/10.1111/ejss.12542
Yuan HZ, Liu SL, Razavi BS, Zhran M, Wang JR, Zhu ZK, Wu JS, Ge TD (2019) Differentiated response of plant and microbial C:N:P stoichiometries to phosphorus application in phosphorus-limited paddy soil. Eur J Soil Sci 95:103-122. https://doi.org/10.1016/j.ejsobi.2019.103122
Zhang A, Liu Y, Pan G, Hussain Q, Li L, Zheng J, Zhang X (2012) Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil 1-2:263-275. https://doi.org/10.1007/s11104-011-0957-x
Zhang Y, Li XL, Gregorich EG, McLaughlin NB, Zhang XP, Guo YF, Liang AZ, Fan RQ, Sun BJ (2018) No-tillage with continuous maize cropping enhances soil aggregation and organic carbon storage in Northeast China. Geoderma 330:204-211. https://doi.org/10.1016/j.geoderma.2018.05.037
Funding
Financial supports for this research from the National Natural Science Foundation of China (41401259), the Natural Science Foundation of Jiangsu Province (BK20161379), and the China Postdoctoral Science Foundation (2018M632253) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 251 kb)
Rights and permissions
About this article
Cite this article
Fan, R., Du, J., Liang, A. et al. Carbon sequestration in aggregates from native and cultivated soils as affected by soil stoichiometry. Biol Fertil Soils 56, 1109–1120 (2020). https://doi.org/10.1007/s00374-020-01489-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-020-01489-2