Abstract
Microcosms were set up to evaluate the effect of nitrification inhibitors (DCD, c-PTiO, and NaClO3) on the abundance and expression of ammonia-oxidizing bacteria (AOB) and archaea (AOA), as well as the nitrite-oxidizing bacteria (NOB) Nitrospira and Nitrobacter. Both DCD and NaClO3 inhibited the net nitrification rate, while c-PTiO had no significant effects, and NaClO3 had a much greater inhibitory effect (> 60%) in all soils than DCD. No significant changes in total microbial abundance were observed with DCD and NaClO3. DCD limited only the growth of AOB; however, NaClO3 inhibited growth of both AOA and Nitrospira-NOB with no significant effects on AOB and Nitrobacter-NOB. Probably NaClO3 inhibited both ammonia oxidation and nitrite oxidation. This is the first report to reveal the inhibitory effects of NaClO3 on a specific nitrification process, helping to clarify the ecological niche of nitrifiers and the potential of nitrification inhibitors applied to soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrification, a key process of the biogeochemical nitrogen (N) cycle, was always considered to be a two-step process, including ammonia oxidation and nitrite oxidation, which is driven by ammonia oxidizers and nitrite oxidizers, respectively.
Ammonia oxidizers, including autotrophic ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA), involve the ammonia oxidation process—the rate-limiting step of nitrification (Könneke et al. 2005; Prosser and Nicol 2008). Both AOB and AOA contain the key enzyme ammonia monooxygenase (AMO), but the sequence of their amoA, amoB, and amoC genes is distinctly different. Dicyandiamide (DCD), a nitrification inhibitor used in agriculture worldwide for decreasing nitrogen (N) losses, inhibits nitrification by deactivating the enzyme AMO (Amberger 1989). Shen et al. (2013) reported that AOB Nitrosopira multiformis was completely inhibited by 100 μmol L−1 DCD whereas AOA Ca. Nitrososphaera viennensis continued activity even at 500 μmol L−1 DCD. Soil microcosm-based studies found that 50 mg kg−1 DCD effectively reduced the abundance of AOA or AOB in different soils (Di et al. 2009; Zhang et al. 2012). However, Dai et al. (2013) found that when applying urea (50 kg N hm−2) and DCD (10 kg hm−2) simultaneously in a grazed pasture soil nitrification, abundance and community composition of AOA or AOB were not affected. Contradictory results of these studies might be attributable to both physiological differences of AOA and AOB, communities, and/or differences in soil types. Both AOA and AOB produce hydroxylamine from ammonia (Vajrala et al. 2013), but their possible downstream pathways are different, with only AOA producing nitric oxide (NO) as an intermediate (Stahl and de la Torre 2012). Carboxy-PTiO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, c-PTiO), a NO scavenger, could inhibit the nitrification by interrupting the NO-based electron shuttling in the hydroxylamine pathway (Jung et al. 2014). Shen et al. (2013) reported that AOA were completely inhibited by c-PTiO at 200 μmol L−1 whereas the AOB were not affected. In addition, c-PTiO has been used to inhibit ammonia oxidation in environmental samples with relatively high AOA abundances (Martens-Habbena et al. 2015). Although c-PTiO is not used as a nitrification inhibitor in agriculture, it could be a selective inhibitor for AOA to further elucidate the contribution of AOA and AOB to nitrification in soils (Jung et al. 2014; Martens-Habbena et al. 2015).
Nitrite-oxidizing bacteria (NOB) catalyze the second step of nitrification, nitrite oxidation to nitrate, and nitrite oxidoreductase (nxr) genes of NOB comprise NxrA, NxrB, and NxrC subunits in two widespread species Nitrobacter and Nitrospira (Pester et al. 2014). PCR primers targeting the nxrA or nxrB of Nitrobacter were developed and tested in pure cultures and soil samples (Vanparys et al. 2007; Poly et al. 2008). In addition, genome sequencing of a Nitrospira has enabled the development of PCR primers targeting the nxrB of Nitrospira (Pester et al. 2014), but primers for nxrA in Nitrospira have yet to be reported. Compared to the ammonia oxidizers, the ecology of NOB has received surprisingly little attention in nitrification research due in part to the absence of molecular diagnostic tools. The development of specific PCR primers for the nxr genes should enable a new focus on NOB. Sodium chlorate (NaClO3) can prevent nitrite oxidation by Nitrobacter (Belser and Mays 1980; Hynes and Knowles 1983), but its effects on Nitrospira are not known. Chlorate was also found to inhibit AMO (Zhang et al. 2009; Tong and Xu 2012) and heterotrophic nitrification in acid forest soil (Wang et al. 2014). Therefore, the inhibition of nitrification by NaClO3 is very complicated, especially because of the diverse ecological functions of NOB in nitrogen cycling (Daims et al. 2016) and the recent discovery of complete ammonia oxidation in the NOB genus Nitrospira.
In this study, three soils under contrasting land management for more than 50 years in the Highfield Experiment at Rothamsted Research (Johnston 1994) were used to analyze short-term effects of different nitrification inhibitors (DCD, c-PTiO, and NaClO3) on nitrification rate, abundance, and transcriptional activity of AOA, AOB, and NOB. Differences in AOA and AOB abundance had previously been found in these soils (Hirsch et al. 2017), and new primers were designed based on the metagenome of these soils (Neal et al. 2017) to analyze the nxrA of Nitrobacter and Nitrospira. The objective was to clarify the effects of nitrification inhibitors (DCD, c-PTiO, and NaClO3) on ammonia oxidizers (AOA and AOB) and nitrite oxidizers (Nitrobacter and Nitrospira) in these soils.
Materials and methods
Soils
Soils were collected from the three permanent treatments in the Highfield Experiment: arable soil (AS, established 1949 with winter wheat), grassland soil (GS, since 1850s), and bare fallow soil (BFS, since 1959). The details of the treatments are given by Johnston (1994).
Surface soils were sampled with a 2-cm-diameter auger to the plow depth (0–23 cm) in December 2014, and 30 cores were collected randomly from three field replicates of each soil and pooled together to form composite samples. Plant roots, fauna, and debris were removed, and the field moist soils were sieved (< 2 mm) then stored at 4 °C. Subsamples were air-dried, ground to < 160 μm mesh, then analyzed for total C and N in triplicate using a combustion analyzer (LECO CN628, Stockport, UK). The concentration of NO3 −-N and exchangeable NH4 +-N extracted in 2 mol L−1 KCl was measured by a Skalar colorimetric continuous flow analyzer. Soil pH (soil/water ratio of 1:2.5) was also measured in triplicate. The basic physicochemical properties of tested soils are listed in Table 1.
Experimental design and microcosm incubation
Dicyandiamide (DCD), carboxy-PTiO potassium salt (c-PTiO), and sodium chlorate (NaClO3) (Sigma Aldrich, UK) were chosen as nitrification inhibitors for a non-disruptive laboratory incubation experiment with six treatments and five replicates per treatment. The treatments were (1) control (CK)—soil with no amendments, (2) N only—soil with NH4 +, (3) DCD only—soil with DCD, (4) DCD + N—soil with NH4 + and DCD, (5) c-PTiO + N—soil with NH4 + and c-PTiO, and (6) NaClO3 + N—soil with NH4 + and NaClO3; in total, 90 incubation samples were set up (3 soils × 6 treatments × 5 replicates). The fresh soils were given a conditioning incubation at room temperature for 7 days to permit soil metabolism to stabilize before use, and 45 g dry-weight equivalent of each fresh soil was used for the incubation in each pot. Nitrogen was applied as (NH4)2SO4 at the rate of 100 mg NH4 +-N kg−1 dry soil, DCD was applied at the rate of 200 μmol L−1 pore water, c-PTiO was applied at the rate of 400 μmol L−1 pore water, and NaClO3 was applied at the rate of 100 mg kg−1 dry soil (equal to 9.2 mmol L−1 pore water for arable soil, 8.7 mmol L−1 pore water for grassland soil, and 9.6 mmol L−1 pore water for bare fallow soil); the amount of inhibitors applied in this experiment was based on previous works (Shen et al. 2013; Wang et al. 2014). The soil mixtures were incubated at 60% water holding capacity in the dark at 21 °C for 7 days, and subsamples were collected at days 0 and 7. A portion of each subsample was immediately frozen at − 80 °C and stored for molecular analysis until required; another part was extracted using 2 M KCl to measure the concentration of NO3 −-N and exchangeable NH4 +-N. Nitrite (NO2 −-N) concentrations were too low to infer any significant effects in these experiments. The net nitrification rate was calculated from the average nitrate difference during 7 days of incubation (Shi et al. 2016).
Nucleic acid extraction
RNA and DNA were extracted from 2 g of each soil sample using an RNA PowerSoil® Total RNA Isolation Kit and RNA PowerSoil® DNA Elution Accessory Kit (Mo Bio, Carlsbad, CA, USA), respectively, following a modification to the manufacturer’s instructions, whereby the 15-min shaking on a flat bed vortex was replaced by a 30-s bead beating step (5.5 m s−1, Fastprep). DNA and RNA concentrations were determined using the Qubit quantification platform with Quant-iT™ dsDNA high-sensitivity assay kit and Quant-iT™ RNA assay kit (Invitrogen, NZ). DNA and RNA were diluted to 5 ng μL−1 and stored in a − 20 °C freezer for the following molecular applications.
Primer design
The abundance of the bacterial 16S rRNA gene, bacterial amoA, archaeal amoA, Nitrospira nxrA, and Nitrobacter nxrA in all the soil samples were estimated using quantitative real-time PCR (qPCR). Primers for Nitrospira nxrA and Nitrobacter nxrA gene were designed based on metagenome sequence data of Highfield soils (Neal et al. 2017), and 14-pair primers for Nitrospira nxrA gene and 8-pair primers for Nitrobacter nxrA gene were designed. On the basis of gradient PCR results, the best primers for Nitrospira nxrA gene were nxr-spira-for5 and nxr-spira-rev6, corresponding to position 3143–3417 of metagenome, and the best candidates for Nitrobacter nxrA gene were nxr-bacter-for1 and nxr-bacter-rev3 which were located in the position 761–925 of metagenome. Other primers have been described previously and are detailed in Supplementary Table 1.
Quantitative real-time PCR
All qPCR reactions were performed in triplicate by using an LineGene 9600 (Bioer) in a 20-μL reaction mixture consisting of 20 ng DNA template, 10 μL QuantiTect SYBR Green Master Mix (QIAGEN), and 1 μL 2 μmol L−1 forward and reverse primers. Programs used for real-time amplification are shown in Supplementary Table 1. A melting curve analysis was performed to confirm PCR product specificity after amplification by measuring fluorescence continuously as the temperature increased to 95 °C. The specificity of the PCR was further evaluated by running on an agarose gel. Standards were generated from PCR products that had been obtained from soil DNA extracts, gel purified, and quantified using a Qubit™ fluorometer (Invitrogen, NZ). Then, the standards were diluted accordingly to give a concentration range from 0 to 109 gene copies μL−1. The copy numbers of these genes per gram of dry soil were calculated.
cDNA synthesis and quantitative real-time reverse transcription PCR
Reverse transcription (RT) was carried out using QuantiTect Reverse Transcription Kit (QIAGEN) on the previously extracted RNA. In order to eliminate genomic DNA, 12 μL RNA was added to 2 μL gDNA Wipeout Buffer DNase to a final volume of 14 μL. The suspension was heated for 2 min at 42 °C and then placed immediately on ice. An RT Master Mix of 1 μL reverse transcriptase containing RNase inhibitor, 4 μL Quantiscript RT buffer, and 1 μL RT Primer Mix (supplied), to a final volume of 20 μL, was prepared. RNA free of contaminating DNA obtained previously was added on ice to each tube containing RT Master Mix. The suspension was incubated for 15 min at 42 °C and then heated for 3 min at 95 °C to stop the reaction.
The abundance of bacterial 16S rRNA, amoA mRNA, and nxrA mRNA in all the soil samples was quantified using a two-step quantitative real-time reverse transcription (RT-PCR). The cDNA was synthesized as described above and the RT-PCR programs were the same as those used in real-time amplification assays.
Statistical analysis
The copy numbers of gene transcripts were log-transformed prior to statistical analysis, and all statistical analyses were performed using SPSS version 18.0. Significant differences (P < 0.05) between treatments were determined using analysis of variance followed by a post hoc Tukey test of the means. Net nitrification was calculated as the difference in NO3 − generated between the initial and incubated samples.
Results
Soil exchangeable ammonium and nitrate concentrations
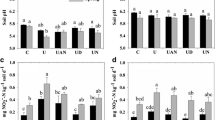
The concentration of NO3 −-N and exchangeable NH4 +-N in four treatments with (NH4)2SO4 (N only, DCD + N, c-PTiO + N, and NaClO3 + N) were significantly higher than that of the other two treatments (CK and DCD) after 7 days of incubation (Figs. 1 and 2). The control treatments without (NH4)2SO4 application showed no significant change in the concentration of NO3 −-N and exchangeable NH4 +-N in arable and bare fallow soil, but a small and significant increase of NO3 −-N was seen in grassland soil, presumably due to conversion of the original organic N, and this was not affected by DCD. However, when mineral N was added (N only treatment), the addition of DCD (DCD + N) significantly decreased the concentration of NO3 −-N in all soils. This indicates that the application of DCD strongly inhibited the conversion of exogenous N from NH4 +-N to NO3 −-N. Meanwhile, the NaClO3 + N treatment also decreased the concentration of NO3 −-N in soils, to a much greater extent than DCD. The influence of DCD and NaClO3 is also apparent by the retention of exchangeable NH4 +-N in arable and grassland soils, shown in Fig. 2a and b. In contrast, the c-PTiO + N treatment had no negative effects on the conversion of mineral N in the grassland soil (Figs. 1 and 2), and a small but significant effect on the bare fallow soil apparent in Fig. 1c, although this was not supported by changes in exchangeable NH4 +-N, shown in Fig. 2c. The basal net nitrification rate with no added N (CK treatments), as shown in Fig. 3, was not significantly different in the three soils despite more than 60 years of different management, but differences became apparent after adding N fertilizer (N only treatments), specifically, arable soil > grassland soil > bare fallow soil. The results indicated that the long-term regular application of N fertilizer in arable soil influenced short-term nitrification rates but not the basal level of net nitrification. The application of DCD decreased net nitrification rates by 47, 35.8, and 42.1% in arable soil, grassland soil, and bare fallow soil, respectively, showing significant inhibition. The inhibition of NaClO3 was even greater than DCD, more than 60% in all three soils, but c-PTiO showed no significant effects.
Effects of nitrification inhibitors on the concentration of nitrate in Highfield soils. a Arable soil, b grassland soil, c bare fallow soil. Similar letters above bars indicate no significant difference (P > 0.05), whereas different letters represent a significant difference (P < 0.05), and the error bars stand for the standard error of means. CK, soil with no amendments; DCD, soil with DCD; N only, soil with NH4 +; DCD + N, soil with NH4 + and DCD; c-PTiO + N, soil with NH4 + and c-PTiO; NaClO3 + N, soil with NH4 + and NaClO3, and the same are in the rest of the figures
Abundance of bacterial 16S rRNA, ammonia oxidation, and nitrite oxidation genes
As mentioned, the c-PTiO had no effects on the nitrification for three tested soils, thus their effects on the bacterial 16S rRNA, AOA, AOB, and NOB were not measured in this experiment.
There were no significant changes in bacterial 16S rRNA gene abundance compared to the control (CK) after 7 days of incubation in all three soils, indicating that over this short time, the addition of N and nitrification inhibitors (DCD and NaClO3) had no overall effects on the abundance of bacterial and archaea. However, Fig. 4a shows significant difference in bacterial 16S rRNA gene copy number between the three tested soils, in the order grassland soil > arable soil > bare fallow soil, as previously reported (Hirsch et al. 2009, 2017). This demonstrates that long-term land management can change the abundance of soil bacteria significantly.
In the control soils, there were no significant differences in abundance of archaeal amoA (AOA), whereas bacterial amoA copy number (AOB) was in the order arable soil > grassland soil > bare fallow soil, shown in Fig. 4b and c, respectively. AOA gene abundance were not significant different in arable soil and grassland soil when compared to their N treatments after the 7-day incubation with DCD and N (Fig. 4b). However, the AOB amoA abundance increased significantly after the 7-day incubation with N when compared to the corresponding control (CK), whereas it was decreased by the addition of DCD in all three soils, significantly so in the grassland and bare fallow soils (Fig. 4c). Probably DCD inhibited the growth of AOB but not AOA in the soils tested. Meanwhile, NaClO3 decreased the abundance of AOB and AOA in all three soils, but the inhibition was not significant (Fig. 4b, c) except for AOA in bare fallow soil.
The abundance of Nitrospira nxrA in grassland soil was 2.47 × 108 copies g−1 dry soil in the control soils and decreased significantly after the 7-day incubation with N or/and NaClO3, whereas the decrease in DCD + N treatment was not significant (Fig. 4e, f). The abundance of Nitrospira nxrA in arable and bare fallow soil was 6.92 × 107 and 4.95 × 107 copies g−1 dry soil in the control soils, much lower than that in grassland soil, and both showed a similar decrease after the 7-day incubation with N, DCD, and NaClO3. Therefore, the nitrification inhibitors DCD and NaClO3 also affected nitrite oxidization, inhibiting the growth of Nitrospira, and that the effect of NaClO3 was greater than that of DCD. The Nitrobacter nxrA in arable soil increased significantly after adding N, but showed no obvious changes in grassland and bare fallow soils. However, the nitrification inhibitors had no significant effects on the abundance of Nitrobacter nxrA.
Transcriptional activity of ammonia oxidizer and nitrite oxidizer
Figure 5a shows 16S rRNA abundance in the order grassland soil > arable soil > bare fallow soil, with no significant changes after the 7-day incubation with N, DCD, and NaClO3. Therefore, DCD and NaClO3 had no effects on the overall activity of the soil bacterial community and that abundance estimated by 16S rRNA gene copy number corresponds to expression of these ribosomal genes in all three soils.
The gene and mRNA copies for AOA were much higher than for AOB (Figs. 4 and 5), which suggests that AOA are the most numerous ammonia oxidizer in all three soils. Changes in the amoA mRNA level of AOA and AOB were not significant after the 7-day incubation with DCD in arable soil and grassland soil, and no AOB amoA was detected in the bare fallow soil.
The Nitrospira and Nitrobacter nxrA mRNA in all three soils was less abundant than in the control after the 7-day incubation with N, DCD, and NaClO3, but this was significant only for Nitrobacter nxrA, for the DCD + N treatment in bare fallow soil. The negative effects were more pronounced for Nitrospira nxrA mRNA than for Nitrobacter nxrA, and the inhibition by NaClO3 was greater than by DCD on Nitrospira nxrA mRNA.
Discussion
Nitrification relates to both the biological availability of soil N, emissions of the greenhouse gas N2O and leaching of NO3 −. Nitrification inhibitors can slow the nitrification process and reduce the loss of soil N (Chen et al. 2010; Lan et al. 2013; Liu et al. 2013; Florio et al. 2014; Hill et al. 2015). Our results showed that more than 60% of net nitrification rate can be inhibited by NaClO3, and the inhibition of the three soils tested were in order arable soil > grassland soil > bare fallow soil. In these three long-term treatments, only the arable soil received regular applications of mineral N fertilizer and the permanent grassland retains much higher soil organic matter than arable of bare fallow soil, factors that have had differential influences on their microbial biomass and diversity (Hirsch et al. 2017). Shi et al. (2016) reported that soil clay fractions and organic matter content could be critical factors influencing nitrification inhibitor (DMPP) efficacy. Therefore, further studies involving more soil types under field conditions should be considered in the future research. Belser and Mays (1980) reported that chlorate was a specific inhibitor of nitrite oxidation by Nitrobacter winogradskyi in pure culture, but in our experimental soils, NaClO3 showed the greatest inhibition of the abundance and transcription of nxrA in Nitrospira, and had little influence on Nitrobacter. Nitrospira was the prevalent NOB in the tested soils, according to nxrA gene and mRNA abundance. Some Nitrospira species involved in NO2 − oxidation also take part in complete ammonia oxidation (Santoro 2016; Stein and Klotz 2016); therefore, the inhibition of NaClO3 might involve in complete ammonia oxidation which needs further study to confirm. Our results also indicated that NaClO3 inhibited the growth of AOA, consistent with reports that chlorate inhibits ammonia monooxygenase (Zhang et al. 2009; Tong and Xu 2012). Some researchers also indicated that NaClO3 inhibited heterotrophic nitrification in acid forest soil (Wang et al. 2014). Therefore, inhibition of nitrification through Nitrospira by NaClO3 may involve multiple nitrification processes: ammonia oxidation (which determines supply of nitrite to NOB), nitrite oxidation, and complete ammonia oxidation in comammox bacteria (Fig. 6), although at present it is not known if this latter group constitutes a significant proportion of soil Nitrospira. Ours is the first report on the effects of NaClO3 on Nitrospira communities in soil, but the inhibition of nitrification by NaClO3 is complex and care is needed in the interpretation of pathways inhibited by NaClO3.
DCD (C2H4N4) is one of the most widely used nitrification inhibitors (Di et al. 2007; Monaghan et al. 2009). With the –NH2 and =NH groups, DCD has a similar molecular structure to NH3 causing DCD to interfere with substrate utilization by ammonium-oxidizing bacteria (Zacherl and Amberger 1990). Our results confirmed that DCD inhibited the nitrification rate, and we conclude that this reduction in energy supply to AOB caused a drop in cell numbers and the expression of the amoA gene. There are reports that DCD inhibits the abundance of AOA (Di et al. 2009; Zhang et al. 2012), and also that DCD does not inhibit nitrification (Dai et al. 2013). These contradictory findings indicate that changes in the relative abundance of AOA and AOB as the dominant functional microorganisms in different soils will influence their responses to DCD.
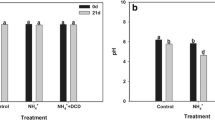
The specific NO-scavenger c-PTiO (C14H16KN2O4) inhibits nitrification in the AOA Ca. N. maritimus (Yan et al. 2012). Nitrification by AOA was completely inhibited by c-PTiO at 200 μmol L−1 (Shen et al. 2013). But in soil, our results suggested that the inhibition of c-PTiO on nitrification was limited, even when the effective concentration of c-PTiO in pore water was increased to 800 μmol L−1 (Supplementary Fig. 1). This may be due to the diversity of soil microorganisms and the immobilization of c-PTiO by soil minerals or organic matter. The small effect seen in bare fallow soil could be due to the lower SOM concentration, the lower microbial biomass, or differences in the microbial community. In fact, our results also indicated that c-PTiO slightly promoted the nitrification rate in grassland soil without exogenous N addition, possibly because the presence of c-PTiO promoted the mineralization of organic N as the concentration of exchangeable NH4 + increased slightly on the first day of incubation (Supplementary Fig. 2). Therefore, our results suggest that c-PTiO is not suitable as a selective inhibitor for AOA in soils. We do not propose that c-PTiO, which is expensive and ineffective, or chlorate, which is toxic and hazardous, are useful as nitrification inhibitors in agricultural settings and could replace DCD and other commercially available products. But the commercial usage of DCD in the field should consider the uptake of DCD in plant (Pal et al. 2016). Rather, our results show that both DCD and chlorate are useful to discriminate different steps in experimental setting.
Conclusion
The abundance and transcriptional activities of AOA in all three tested soils were higher than those of AOB, and those of Nitrospira were higher than those of Nitrobacter, indicating that the AOA and Nitrospira are the most numerous nitrifiers present in the Highfield soil. However, the AOB-specific nitrification inhibitor DCD caused a significant reduction in nitrification in conjunction with added N, especially in the arable soil where AOB are the most abundant compared to the other treatments, indicating their selection by long-term regular mineral N application. This also demonstrates the importance of AOB nitrification after N fertilizer application to agricultural soil.
DCD and NaClO3 decreased net nitrification rate, and the inhibition by NaClO3 was greater than DCD. Whereas DCD inhibited only AOB, NaClO3 inhibited the abundance of AOA and Nitrospira, responsible for multiple steps in the N cycle. Elucidation of the role of comammox Nitrospira in soil will require further investigation.
References
Amberger A (1989) Research on dicyandiamide as a nitrification inhibitor and future outlook. Commun Soil Sci Plant Anal 20:1933–1955
Belser LW, Mays EL (1980) Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl Environ Microbiol 39:505–510
Chen D, Suter HC, Islam A, Edis R (2010) Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biol Biochem 42:660–664
Dai Y, Di HJ, Cameron KC, He JZ (2013) Effects of nitrogen application rate and a nitrification inhibitor dicyandiamide on ammonia oxidizers and N2O emissions in a grazed pasture soil. Sci Total Environ 465:125–135
Daims H, Lücker S, Wagner M (2016) A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol 24:699–712
Di HJ, Cameron KC, Sherlock RR (2007) Comparison of the effectiveness of a nitrification inhibitor, dicyandiamide, in reducing nitrous oxide emissions in four different soils under different climatic and management conditions. Soil Use Manag 23:1–9
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624
Florio A, Clark IM, Hirsch PR, Jhurreea D, Benedetti A (2014) Effects of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on abundance and activity of ammonia oxidizers in soil. Biol Fertil Soils 50:795–807
Hill AM, Di HJ, Cameron K, Podolyan A (2015) The effect of animal trampling and DCD on ammonia oxidisers, nitrification, and nitrate leaching under simulated winter forage grazing conditions. J Soils Sediments 15:972–981
Hirsch PR, Gilliam LM, Sohi SP, Williams JK, Clark IM, Murray PJ (2009) Starving the soil of plant inputs for 50 years reduces abundance but not diversity of soil bacterial communities. Soil Biol Biochem 41:2021–2024
Hirsch PR, Jhurreea D, Williams JK, Murray PJ, Scott T, Misselbrook TH, Goulding KWT, Clark IM (2017) Soil resilience and recovery: rapid community responses to management changes. Plant Soil 412:283–297
Hynes RK, Knowles R (1983) Inhibition of chemoautotrophic nitrification by sodium chlorate and sodium chlorite: a reexamination. Appl Environ Microbiol 45:1178–1182
Johnston AE (1994) The Rothamsted classical experiments. In: Leigh RA, Johnston AE (eds) Long-term experiments in agricultural and ecological sciences. CAB international, Wallingford, UK, pp 9–37
Jung MY, Well R, Min D, Giesemann A, Park SJ, Kim JG, Kim SJ, Rhee SK (2014) Isotopic signatures of N2O produced by ammonia-oxidizing archaea from soils. ISME J 8:1115–1125
Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546
Lan T, Han Y, Roelcke M, Nieder R, Cai Z (2013) Effects of the nitrification inhibitor dicyandiamide (DCD) on gross N transformation rates and mitigating N2O emission in paddy soils. Soil Biol Biochem 67:174–182
Liu C, Wang K, Zheng X (2013) Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat-maize cropping system. Biogeosciences 10:2427–2437
Martens-Habbena W, Qin W, Horak REA, Urakawa H, Schauer AJ, Moffett JW, Armbrust EV, Ingalls AE, Devol AH, Stahl DA (2015) The production of nitric oxide by marine ammonia-oxidizing archaea and inhibition of archaeal ammonia oxidation by a nitric oxide scavenger. Environ Microbiol 17:2261–2274
Monaghan RM, Smith LC, Ledgard SF (2009) The effectiveness of a granular formulation of dicyandiamide (DCD) in limiting nitrate leaching from a grazed dairy pasture. N Z J Agric Res 52:145–159
Neal AL, Rossmann M, Brearley C, Akkari E, Guyomar C, Clark IM, Allen E, Hirsch PR (2017) Land-use influences phosphatase microdiversity in soils. Environ Microbiol 19:2740–2753
Pal P, McMillan AMS, Saggar S (2016) Pathways of dicyandiamide uptake in pasture plants: a laboratory study. Biol Fertil Soils 52:539–546
Pester M, Maixner F, Berry D, Rattei T, Koch H, Lücker S, Nowka B, Richter A, Spieck E, Lebedeva E, Loy A, Wagner M, Daims H (2014) NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ Microbiol 16:3055–3071
Poly F, Wertz S, Brothier E, Degrange V (2008) First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol Ecol 63:132–140
Prosser JI, Nicol GW (2008) Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 10:2931–2941
Santoro AE (2016) The do-it-all nitrifier. Science 351:342–343
Shen TL, Stieglmeier M, Dai JL, Urich T, Schleper C (2013) Responses of the terrestrial ammonia-oxidizing archaeon ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett 344:121–129
Shi XZ, Hu HW, He JZ, Chen DL, Suter HC (2016) Effects of 3,4-dimethylpyrazole phosphate (DMPP) on nitrification and the abundance and community composition of soil ammonia oxidizers in three land uses. Biol Fertil Soils 52:927–939
Stahl DA, de la Torre JR (2012) Physiology and diversity of ammonia-oxidizing archaea. Annu Rev Microbiol 66:83–101
Stein LY, Klotz MG (2016) The nitrogen cycle. Curr Biol 26:R94–R98
Tong DL, Xu RK (2012) Effects of urea and (NH4)2SO4 on nitrification and acidification of ultisols from southern China. J Environ Sci 24:682–689
Vajrala N, Martens-Habbena W, Sayavedra-Soto LA, Schauer A, Bottomley PJ, Stahl DA, Arp DJ (2013) Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci U S A 110:1006–1011
Vanparys B, Spieck E, Heylen K, Wittebolle L, Geets J, Boon N, De Vos P (2007) The phylogeny of the genus Nitrobacter based on comparative rep-PCR, 16S rRNA and nitrite oxidoreductase gene sequence analysis. Syst Appl Microbiol 30:297–308
Wang CH, Dannenmann M, Meier R, Butterbach-Bah K (2014) Inhibitory and side effects of acetylene (C2H2) and sodium chlorate (NaClO3) on gross nitrification, gross ammonification and soil atmosphere exchange of N2O and CH4 in acidic to neutral montane grassland soil. Eur J Soil Biol 65:7–14
Yan J, Haaijer SC, Op den Camp HJ, van Niftrik L, Stahl DA, Könneke M, Rush D, Sinninghe Damsté JS, Hu YY, Jetten MSM (2012) Mimicking the oxygen minimum zones: stimulating interaction of aerobic archaeal and anaerobic bacterial ammonia oxidizers in a laboratory-scale model system. Environ Microbiol 14:3146–3158
Zacherl B, Amberger A (1990) Effect of the nitrification inhibitors dicyandiamide, nitrapyrin and thiourea on Nitrosomonas europaea. Fert Res 22:37–44
Zhang TY, Xu XK, Luo XB, Han L, Wang YH, Pan GX (2009) Effects of acetylene at low concentrations on nitrification, mineralization and microbial biomass nitrogen concentration in forest soils. Chin Sci Bull 54:296–303
Zhang LM, Hu HW, Shen JP, He JZ (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J 6:1032–1045
Acknowledgements
This work was supported by Chinese Scholarship Council (no. 201406765033) and Natural Science Foundation of Hubei Province (2015CFB481). Rothamsted Research receives strategic funding from the Biotechnology and Biological Research Council of the UK. This work was supported by BBS/E/C/00005196 and BB/P01268X/1.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 161 kb)
Rights and permissions
About this article
Cite this article
Fu, Q., Clark, I.M., Zhu, J. et al. The short-term effects of nitrification inhibitors on the abundance and expression of ammonia and nitrite oxidizers in a long-term field experiment comparing land management. Biol Fertil Soils 54, 163–172 (2018). https://doi.org/10.1007/s00374-017-1249-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-017-1249-2