Abstract
It has widely been acknowledged that the diversity of arbuscular mycorrhizal fungi (AMF) is greatly affected by climate, land use intensity, and soil parameters. The objective of this study was to investigate AMF diversity in multiple agricultural soils (154 sites; 92 grasslands and 62 croplands) distributed over all agricultural regions in Switzerland and differing in a number of soil parameters (e.g., land use type and intensity, and altitude). We highlighted the main factors responsible for major AMF community shifts and documented specific distribution patterns for each AMF species. AMF spores were morphologically identified and counted for each species. In total, 17,924 spores were classified and 106 AMF species were identified. In general, AMF species richness (SR) was higher in grasslands than in croplands. In croplands, SR increased with altitude but this trend was not observed in grasslands. Some species occurred at virtually all sites, while others were rarely detected, and for others, species-specific distribution patterns were revealed. Some species were affected by land use type or intensity, or related factors like soil organic matter, soil microbial biomass and respiration or nutrient availability. Other species were more affected by soil pH and related parameters like base saturation and carbonate contents, by soil texture, or by altitude, or by a combination of two to several of all these parameters. We conclude that a high number of AMF species may serve as indicator species for specific habitats and land use. These species might deliver certain ecosystem services at their habitats and deserve further investigation about their functional diversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal fungi comprise an important component of soil microorganisms (van der Heijden et al. 1998; Smith and Read 2008). They form an obligate symbiosis with 70–80% of the plant species worldwide (Brundrett 2009; van der Heijden et al. 2015) and occur in all terrestrial ecosystems where plants live, including extreme environments such as the coldest, most saline, heavy-metal contaminated and also sub-marine habitats (e.g., Hildebrandt et al. 2001, 2007; Sudová et al. 2011; Oehl and Körner 2014). They fulfill key ecosystem services such as plant growth promotion (Schlicht 1889; Gianinazzi et al. 2010; Njeru et al. 2015; Cozzolino et al. 2016) and soil erosion prevention through enhancing soil aggregate formation and water infiltration (Rillig and Mummey 2006; Rillig et al. 2015), and thus may play a major role in agricultural production sites (Jeffries et al. 2003; Avio et al. 2013).
In the last two decades, there has been an increasing interest to identify and understand the role of genetic and functional AMF diversity for ecosystem functioning and the multiple benefits, which these fungi can offer to their plant and soil environments (e.g., van der Heijden et al. 1998; Turrini et al. 2016; Montiel-Rozas et al. 2017). On the fungal side of this mycorrhizal symbiosis, this has concerned the diversity of any kind of AMF species groups, the different AMF species, and even specific AMF isolates (e.g., Munkvold et al. 2004; Maheraly and Klironomos 2007; Tchabi et al. 2010). An indispensable precondition to advance in this research field is to efficiently measure the diversity of these fungi in situ as well as ex situ (Turrini et al. 2008; Oehl et al. 2009). Morphological (e.g., Gerdemann and Trappe 1974; Schenck and Pérez 1990; Błaszkowski 2012; Säle et al. 2015) and molecular (e.g., Simon et al. 1992; Helgason et al. 1998; van Tuinen et al. 1998; Krüger et al. 2009; Öpik et al. 2014) identification tools have been developed more or less simultaneously, but often separately applied, though sometimes also combined (Wetzel et al. 2014). Both methods have their constraints (Oehl et al. 2010; Njeru et al. 2015). Some disadvantages for morphological spore identification were overcome by comprehensive identification manuals and advanced species descriptions with colored illustrations that now facilitate identification (e.g., Błaszkowski 2012). However, spores of different degradation stages found in field samples renders the spore identification sometimes difficult even for experienced experts, especially when small-spored species, such as Diversipora, Dominikia, Kamienskia, Palaeospora, and Paraglomus spp. occur (e.g., Gamper et al. 2009; Błaszkowski 2012; Błaszkowski et al. 2015; Oehl et al. 2015a, 2016), or species that might sporulate extremely rarely or only seasonally, such as Acaulospora spp. (e.g., Oehl et al. 2011b, 2012). For several, formerly called “non-sporulating” AM fungi, spores were found during the last decade of intensified sporulation surveys (e.g., Oehl et al. 2009), and such species could be described in the following through concomitant taxonomic and phylogenetic studies (e.g., Błaszkowski et al. 2015; Oehl et al. 2015a), but for other species, represented in ecological sequences or, e.g., as “virtual taxa,” spores were not yet detected or attributed. Despite being not yet fully developed, important progress has been made through both morphological and molecular identification, which can easily be deduced from the numbers of AMF taxa or virtual taxa detected about 20 years ago (e.g., Land et al. 1993; Clapp et al. 1995; Franke-Snyder et al. 2001) compared with those recovered to date (Hart et al. 2015; Horn et al. 2014; Njeru et al. 2015; Sudová et al. 2015; Pontes et al. 2017; Schläppi et al. 2016). Nevertheless, we are still far from predicting, or even approximately estimating, the complete AMF diversity in nature. Also, initial mapping of the biogeography of the most important representatives of this fungal group has been almost impossible so far (e.g., Öpik et al. 2014; Mello et al. 2013).
Several studies have shown that AMF diversity decreases due to intensified agricultural land use, especially in temperate but also in other climatic zones (Douds and Millner 1999; Oehl et al. 2005; Tchabi et al. 2008). In other studies, major shifts in the AMF community composition were recognized through agricultural intensification without reports in changes of AMF species richness (e.g., Błaszkowski 1993; Jansa et al. 2003). Surprisingly at first glance, in some studies, increased AMF diversities were detected in agricultural soils when compared to natural habitats, especially when the plant growth conditions at the study sites had been significantly improved through management practices, e.g., by efficient irrigation systems in (semi-)arid environments (e.g., Al-Yahya'ei et al. 2011). However, single diversity studies were usually restricted to about 3 to 20 sampling sites (e.g., Jansa et al. 2009; Pereira et al. 2014; Aguilera et al. 2014). More extensive sampling schemes analyzing the distribution patterns of these fungi on larger biogeographic scales have rarely been carried out so far (Hazard et al. 2013; Jansa et al. 2014; Moora et al. 2014; Valyi et al. 2015). Most likely, the lack of such studies have been due to the hitherto time consuming and/or expensive molecular analysis and identification techniques and the lack of help, knowledge, or skills for morphological identification (Wetzel et al. 2014). Fortunately, in recent years, identification and isolation techniques have become more efficient, such as the use of multiple sieves including small sieve mesh sizes (<40 μm), vibratory sieve shakers for precision wet sieving of the soils, and high-resolution and throughput sequencing techniques (e.g., Błaszkowski 2012; Horn et al. 2014; Säle et al. 2015; Schläppi et al. 2016) so that larger sampling numbers can be achieved.
In 154 Swiss agricultural soils, Jansa et al. (2014) profiled the abundance of six widespread AM fungal taxa by quantitative real-time PCR with taxon-specific primers in a well-delimited region in Central Europe, covering all agricultural areas in Switzerland between 270 and 2240 m asl. The agricultural production area in the northern front of the Swiss Alps, between St. Gallen in the east and Geneva in the west, was chosen as an ideal region for their study. Field soil samples derived from 92 grassland and 62 cropland sites of different land use intensity. The sites ranged from extensive grasslands, low-input organic and conventional crop production systems, to intensively managed croplands. The authors found that especially altitude and the geographical distance, which might have been related with different altitude levels, but also soil pH, fertility, and texture contributed to the differences in taxon distribution at the sites, while land use intensity had only minor effects on these AMF communities. The objective of the present study was to determine the AMF spore abundance and species richness at these 154 sites, to characterize the overall AMF communities and to identify AMF biogeographic distribution patterns for as many AMF species as possible. We generated a large data set of AMF species abundances and frequencies along with additional environmental data (chemical, physical, and biological soil parameters and altitude). With these data, we aimed at analyzing the specific distribution patterns for each AMF species identified and highlighting the main ecological factors responsible for the majority of AMF community shifts observed.
Material and methods
Study sites
Initial selection of potential sampling sites was done from 697 sites belonging to the Swiss-wide long-term observation network of soil quality, which is managed by several cantonal soil monitoring networks (KABOs) and the Swiss soil monitoring network (NABO). From the original pool, sites were shortlisted based upon available information including site elevation, land use type and intensity, soil pH, and several other soil characteristics (Jansa et al. 2014). The sites were selected with the goal to establish an as balanced as possible design of land use types (grasslands and croplands), elevation (lowland and highland, aiming at equal number of sites below and above the altitude of 1000 m), and soil pH (low pH and high pH, aiming at equal number of sites below and above pH 6.8 in aqueous suspension). However, as there were no high elevation croplands >750 m, this selection inevitably included more grasslands than croplands. Land use of the grasslands was classified as “very low” to “low,” if they were extensively managed without or with agricultural use, or “moderate” if they were intensively used and mown three to five times per year. Cultivated sites were classified “moderate,” when organic farming or conservation-tillage was performed, or “high” to “very high” in conventional farming and tillage systems depending on the intensity of the crop production.
Soil sampling and analyses
Soil samples were collected from the selected sites in spring and early summer (February through July) in 2010, within 2 weeks of regional snowmelt at the sites. This standardized time point was selected because the composition of indigenous AMF communities may change during the growing season (Oehl et al. 2009; Dumbrell et al. 2010). Soil cores, 3 cm in diameter and 10- (for grasslands) to 20-cm (for croplands) depth, were taken from a 10 m × 10 m plot at each site. About 50 soil cores were taken at each site, totaling approximately 5 kg of fresh soil. Overall, 154 sites were sampled, 92 grassland and 62 cropland soils. Upon collection, GPS coordinates and standing crop were recorded.
In the lab, the fresh soil was sieved (<5 mm) and divided into three pools: fresh soil for biotest and biological soil property characterization, air-dried soil for morphological spore analyses, and air-dried soil for physicochemical and AMF spore characterization. Chemical, physical, and biological soil parameters were determined as described by Jansa et al. (2009, 2014).
AMF spore isolation and identification
AMF spores were extracted from the field soils (25 g per sample) by wet precision-sieving for 5 min each, using a Fritsch Analysette 3 Pro vibratory sieve shaker for wet sieving of soils and a sieve set of 32, 125, 250, and 500 μm, and subsequent sucrose density gradient centrifugation as described by Sieverding (1991). The spores were counted as total numbers in Petri dishes and mounted on microscopic slides in polyvinyl-alcohol–lactic acid–glycerin (PVLG) and in PVLG + Melzer’s reagent. Thereafter, they were morphologically identified based on spore, spore wall, hyphal attachment, and germination characteristics (e.g., Schenck and Pérez 1990; Oehl et al. 2011c; Błaszkowski 2012) considering the most recent updates (e.g., Sieverding et al. 2014; Błaszkowski et al. 2015; Oehl et al. 2015b). Simultaneously, the identified spores were counted species-specifically on the slides.
Statistical analyses
Linear regression analyses were performed between the environmental parameters including pH, soil texture, and elevation (m asl) and the AM fungal parameters like AMF spore abundances and species richness. To ordinate AMF community profiles, i.e., species compositions, and environmental parameters, redundancy analyses (RDA; Ter Braak 1986) were performed on (a) the whole data set, (b) the data set for all grasslands, (c) the data set for all croplands, (d) and for all grasslands occurring on the lowlands only, at the elevation levels of the cropland sites. All these statistical analyses and the graphical visualizations were computed by using the R software (Ver. 3.1.0, R Core Team 2014) packages multcomp (Hothorn et al. 2008), agricolae (de Mendiburu 2014), and vegan (Oksanen et al. 2013). For analysis of the indicator species (combination of relative abundance and relative frequency of species), the Monte Carlo test was used (Dufrêne and Legendre 1997). The species with indication values IV > 25% and P values <0.05 were considered as indicative values for the specific selected environmental or soil parameters and a given AMF species.
Results
Variability of chemical, physical, and biological soil parameters
Generally, there was a high variability of the analyzed chemical, physical, and biological soil parameters reflecting well the variability of the Swiss agricultural soils (Table 1). The soil texture ranged from sandy to loamy and clay, both in grasslands and croplands. There was also a large variability for soil pH and base saturation. These values were more variable in grasslands than in croplands, reflecting for the acidic soils the “good agricultural practices” of the farmers that generally maintain a slightly higher pH in acidic cultivated soils than in grasslands by periodical soil liming. As also could be expected from the land use, organic C contents, as well as the related parameters total N, soil respiration, and soil microbial biomass, were generally higher in grasslands than in croplands, despite of the high variability between the single sites (Table 1). There was also a high variability in available nutrient and (heavy) metal contents between the single sites, but without clear general trends that would generally differentiate between grasslands or croplands.
AMF spore abundances
With a few exceptions, AMF spore abundances in the grasslands were between 6 and 33 g−1, while in the croplands between 3 and 19 g−1 (Fig. 1). There was a positive correlation between soil pH and spore abundances at the study sites (P = 0.02), and these correlations became highly significant when grasslands (P = 0.009) and croplands (P < 0.001) were separately considered (Fig. 1b, c).There was also a positive correlation between altitude and AMF spore abundances when both grasslands and croplands were considered (P = 0.001, data not shown), but this correlation was neither confirmed for the grasslands (P = 0.23) nor for the croplands (P = 0.33) when analyzed alone, and had only been obtained because of the low abundances in the croplands that are located at lower altitudes (324–750 m asl).
Overall AMF species richness
In total, 17,924 AMF spores were classified and 106 AMF species were identified at the 154 study sites. They belonged to all 5 known AMF orders, to 12 AMF families, and to 21 genera (Table 2). The majority of these, i.e., 41 species, were from the order Glomerales, while 32 were from Diversisporales, 14 of Gigasporales, 13 of Archaeosporales, and 6 of Paraglomerales. Thirty-four species were from the family Glomeraceae, 20 were from Acaulosporaceae, and 9 species were from Ambisporaceae. From all other AMF families, only a few species (1–6) were found (Table 2). On the genus level, most species belonged to Acaulospora (20), followed by the heterogeneous genus Glomus (11), by Ambispora (9), and Rhizoglomus (7). From all other genera, only 1–5 species were found (Table 2).
AMF species richness in grasslands and croplands
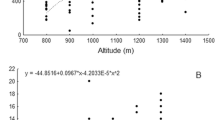
Species richness of AM fungi generally ranged from 14 to 32 species in the grasslands and between 8 and 22 species in the croplands (Fig. 2). There was a clear correlation between altitude and species richness at the study sites when both grasslands and croplands were considered (P = 2.6 × 10−7). This correlation was not confirmed for the grasslands only (P = 0.27), but for the croplands (P = 0.009). Soil pH was negatively correlated with AMF species richness at all study sites (P = 9.3 × 10−7) and in grasslands (P = 2.4 × 10−4), but not in the croplands (P = 0.33). However, the data of Fig. 2 suggest optimum species richness with soil pH between 5.3 and 6.4 in this study.
AMF species abundance and frequency
Twenty-eight AMF species were detected with relatively high total spore numbers (>100) in this study (Table 2): among these, the following species had the highest numbers (>300): Septoglomus constrictum (2048), Dominikia aurea (1901), Funneliformis geosporus (1693), Fu. mosseae (1569), Glomus diaphanum (784), Gl. badium (729), Archaeospora trappei (698), Gl. macrocarpum (665), Sclerocystis rubiformis (673), Claroideoglomus luteum (524), Sc. sinuosa (441), Cl. claroideum (398), Scutellospora calospora (369), and Acaulospora paulinae (308). Sixty-seven species were found with at least >10 spores (Table 2). On the other hand, 21 AMF species were found with less than five spores each, summarized over all 154 sites of this study (Table 1), and—among these—ten species were identified only with one spore.
Ten of the 106 AMF species detected were found very frequently, i.e., in more than 67% (>102 sites) of the 154 study sites (Table 2). Nine species belonged to the order Glomerales, and one species to Archaeosporales. These ten species were—in decreasing site numbers: Fu. geosporus (141 sites), Gl. diaphanum (138), Fu. mosseae (135), Rhizoglomus irregulare (129), Se. constrictum (128), Ar. trappei (127), Cl. luteum (120), Cl. claroideum (119), Do. aurea (119), and Rh. invermaium (104). Three other species occurred at least in 50% (>77) of the study sites: Sc. calospora (86 sites), Paraglomus turpe (84), and Gl. macrocarpum (80), and ten additional species occurred in more than 25% (>37) of the 154 sites: Gl. badium (76), Paraglomus sp. BR1 (70), Cl. etunicatum (59), Ac. paulinae (68), Ac. sieverdingii (55), Ambispora sp. CH2 (49), Rh. fasciculatum (48), Fu. caledonius (44), Entrophospora infrequens (41), and Di. epigaea (40). In total, 37 AMF species occurred in at least 10% (>15) of the sites (Table 2). On the other hand, a considerable number, i.e., 43 AMF species, were detected in less than five sites, and finally, 20 species only at one single site each. The most frequently found Gigaspora species was Gi. margarita (25 sites), while Pa. franciscana (7) was the most frequent Pacispora sp. Cetraspora armeniaca (33) and Ce. helvetica (31) were the most frequent Racocetraceae spp. in this study (Table 2).
Distribution pattern of AMF species
Several of the most frequent AMF species found had a ubiquitous distribution pattern. Some of these patterns are illustrated in Fig. 3, as for Gl. diaphanum, Rh. irregulare, Cl. claroideum, Ar. trappei, and Sc. calospora. Such patterns were also found for Fu. geosporus and Cl. luteum (data not shown). Other frequent species, however, revealed already more specific distribution patterns, such as Se. constrictum, Do. aurea, Rh. invermaium, Gl. macrocarpum, and Gl. badium, that preferentially and more abundantly occurred in the grasslands (Fig. 4). This pattern was also revealed for Rh. intraradices that was found in 29 grassland sites and in 5 croplands (data not shown). A few species, such as Sc. rubiformis, Sc. sinuosa, and Gl. heterosporum, were exclusively found in grassland sites (18, 7, and 6 sites, respectively).
Scatter plot between altitude (m asl) and soil pH, respectively, and AMF spore abundances (100 g−1 soil) for specific AMF species, a Glomus diaphanum, b Rhizoglomus irregulare, c Claroideoglomus claroideum, d Archaeospora trappei, and e Scutellospora calospora. Black spots are used for grassland sites, white spots for croplands
Scatter plot between altitude (m asl) and soil pH, respectively, and AMF spore abundances (100 g−1 soil) for specific AMF species, a Septoglomus constrictum, b Dominikia aurea, c Rhizoglomus invermaium, d Glomus macrocarpum, and e Glomus badium. Black spots are used for grassland sites, white spots for croplands
Other frequent species were not clearly affected by land use type, but had more expressed distribution patterns, such as Ac. paulinae, that did not occur in soils with pH >7.0; or Fu. mosseae that more abundantly occurred in soils with pH >6.0 and in altitudes <1000 m, while it was not found at >1600 m asl (Fig. 5). Abundance of Fu. caledonius was positively affected by soil cultivation, as it was almost exclusively found in croplands. This species was not found at elevations >1000 m asl, where crop production might not have been performed for more than 50 years. Optimum pH range for increased spore abundance of Fu. caledonius obviously was also restricted and was 5.8–6.7 (Fig.5). Pacispora franciscana was only infrequently found, however, also with a specific pattern, as it was detected preferentially in croplands, below 700 m asl, and only in soils with pH > 6.2 (Fig. 5). Distribution patterns of many AMF species were clearly affected by elevation. For instance, frequency of Ac. cavernata and all Racocetraceae spp., such as Ce. armeniaca decreased with altitude. Gigaspora spp. like Gi. margarita was never found >1100 m asl (Fig. 6). The two most frequent Paraglomus spp. (Pa. turpe and Paraglomus sp. BR1) were not found at >1500 and 1600 m asl, respectively. In contrast, Ac. punctata, Ac. pustulata, and Ac. alpina were not detected below 900, 1000, and 1500 m, respectively.
Scatter plot between altitude (m asl) and soil pH, respectively, and AMF spore abundances (100 g−1 soil) for specific AMF species, a Acaulospora paulinae, b Rhizoglomus intraradices, c Funneliformis mosseae, d Funneliformis caledonius, and e Pacispora franciscana. Black spots are used for grassland sites, white spots for croplands
Scatter plot between altitude (m asl) and soil pH, respectively, and AMF spore abundances (100 g−1 soil) for specific AMF species, a Acaulospora alpina, b Acaulospora punctata, c Acaulospora cavernata, d Cetraspora armeniaca, and e Gigaspora margarita. Black spots are used for grassland sites, white spots for croplands
Multivariate analyses on AMF spore populations
Redundancy analyses were performed on the AMF spore populations including the 17,924 spores identified from the 154 agricultural sites investigated and on the ecological parameters (Figs. 7, 8, and 9). For the environmental parameters, several clusters were recognized in the multivariate analyses. Base saturation and carbonate contents generally correlated along with pH (“pH” cluster). The microbial parameters, and also the organic matter enriched clay contents generally correlated to the Corg and Ntot contents (“Corg” cluster). The parameters land use intensity and type generally correlated to each other (“land use” cluster), sometimes showing also an association with available P (“land use and P” cluster). A few parameters were more independent such as altitude, and the silt and sand contents.
Redundancy analyses (RDA) of the AMF species compositions at a all study sites, and b all grasslands. For abbreviations of the ecological parameters, see Table 1: pH_w pH(H2O), Corg organic C, Ntot total soil N, Pav available P, Cdav available cadmium, CaCO 3 carbonate content, BS base saturation, resp basal soil respiration, SIR microbial biomass assessed by substrate-induced respiration, LUT land use type, LDI land use intensity, m_asl meters above sea level. Only the most significant parameters were included in the analysis. A species (see Table 2) is abbreviated in the figure with the first two letters of its genus name, and the first three letters of its species name, e.g., Acaulospora alpina = Ac.alp. Species that were not identified as a known species are presented with the genus abbreviation and our code for the species, e.g., Ambispora sp. CH2 = Am.CH2 (Table 2)
Redundancy analyses (RDA) of the AMF species compositions between 270 and 750 m asl, a in grasslands and croplands, b only in grasslands, and c only in croplands. For abbreviations of the ecological parameters, see Table 1: pH_w pH(H2O), Corg organic C, Ntot total soil N, Pav available P, Cdav available cadmium, CaCO 3 carbonate content, BS base saturation, resp basal soil respiration, SIR microbial biomass assessed by substrate-induced respiration, LUT land use type, LDI land use intensity, m_asl meters above sea level. Only the most significant parameters were included in the analysis. A species (see Table 2) is abbreviated in the figure with the first two letters of its genus name, and the first three letters of its species name, e.g., Acaulospora alpina = Ac.alp. Species that were not identified as a known species are presented with the genus abbreviation and our code for the species, e.g., Ambispora sp. CH2 = Am.CH2 (Table 2)
Redundancy analyses (RDA) of the AMF species compositions in the grasslands >750 m asl. For abbreviations of the ecological parameters, see Table 1: pH_w pH(H2O), Corg organic C, Ntot total soil N, Pav available P, Cdav available cadmium, CaCO 3 carbonate content, BS base saturation, resp basal soil respiration, SIR microbial biomass assessed by substrate-induced respiration, LUT land use type, LDI land use intensity, m_asl meters above sea level. Only the most significant parameters were included in the analysis. A species (see Table 2) is abbreviated in the figure with the first two letters of its genus name, and the first three letters of its species name, e.g., Acaulospora alpina = Ac.alp. Species that were not identified as a known species are presented with the genus abbreviation and our code for the species, e.g., Ambispora sp. CH2 = Am.CH2 (Table 2)
When all 154 sites were included in the RDA analysis (Fig. 7a), several remarkable results were obtained: (a) Fu. caledonius and Gl. diaphanum clustered closest with the land use and P cluster, (b) Fu. mosseae and Fu. geosporus clustered with the pH cluster, (c) Do. aurea, Gl. badium, and Se. constrictum clustered with the Corg cluster and somehow opposite of the land use and P cluster, and (d) species like Ac. alpina, Ac. cavernata, Am. gerdemannii, Am. reticulata and Sc. rubiformis clustered with the elevation (m asl), but also opposite to the land use and P cluster. For many other species, however, no clear association with specific ecological parameters or clusters was recognized.
Some of these results were confirmed when only the 92 grassland sites were included in the studies (Fig. 7b): (a) Fu. mosseae and Fu. geosporus were associated with the pH cluster, (b) Ac. alpina, Am. gerdemannii, Am. reticulata, and Sc. rubiformis were associated with the elevation (m asl). However, Do. aurea, Gl. badium, and Se. constrictum clustered between the somehow fused Corg and pH clusters, and Fu. caledonius and Gl. diaphanum disappeared within the cloud of rather indifferent AMF species, as they rarely occurred in grassland sites or with similar abundance, respectively.
When the RDA analyses focused only on the elevations with crop production (270 and 750 m asl), the analyses revealed even more details (Fig. 8a–c). Including all grassland and cropland sites of this elevation range: Fu. mosseae was again associated with the ‘pH’ cluster, but Fu. geosporus, as well as Gl. macrocarpum and Sc. sinuosa increased the number of species of the Corg cluster around the species Do. aurea, Gl. badium, and Se. constrictum, while several Acaulospora, Cetraspora, and Scutellospora species were associated with higher altitude, but more importantly opposite to the pH and land use and P clusters; and finally, Gl. diaphanum clustered between land use and P vectors. Funneliformis caledonius could not anymore be attributed clearly to land use and P, but clustered between the land use and sand content vector, and, most clearly, opposite to the pH and Corg clusters.
When only the 49 grasslands between 270 and 750 m asl were included in the RDA analysis (Fig. 8b), the pH and Corg clusters could not anymore be clearly separated. This observation is in accordance with the well-known fact that in the temperate European lowlands, higher pH grasslands usually have higher Corg contents than lower pH grasslands. Consequently, most of the species of both clusters also group together: Fu. mosseae, Fu. geosporus, Do. aurea, Gl. badium, and Se. constrictum. Another group of species, basically several Acaulospora, Ambispora, Gigaspora, Cetraspora, and Scutellospora species, associated with the higher sand content, but obviously also opposite to the pH and Corg clusters. Also remarkable, while Gl. diaphanum disappeared from the land use and P cluster and within the cloud of indifferent AMF species, now Sc. sinuosa appeared there, obviously dealing well with the higher management intensity of the lowland grasslands.
When considering only the 62 croplands in the RDA analysis (Fig. 8c), the pH and Corg clusters separated again more clearly. Some ecological parameters were in between both clusters, while the sand content, as it can be expected from the literature and very basic knowledge about soils, was opposite of both these clusters. Also, following logically, land use intensity decreased with elevation at the study sites. The following results then might be the most striking in this RDA analysis: (a) Gl. diaphanum was again associated closest with land use intensity and available P; (b) Fu. caledonius was associated with sand content, but also with elevation and land use intensity, and somehow opposite to both the pH and Corg clusters; (c) Fu. mosseae and Ar. trappei were associated with the pH cluster; (d) Fu. geosporus, Do. aurea, Gl. badium, and Se. constrictum were again associated with the Corg cluster, and (e) Ac. paulinae and Ac. sieverdingii and Ambispora sp. CH2 were associated with altitude and opposite to land use intensity and available P.
In addition, the RDA analyses of the “highland” grasslands solely (>750 m asl) revealed some already recognized characteristics for the preferred occurrence of different AMF species: Fu. mosseae, Fu. geosporus, Do. aurea, Gl. badium, and Se. constrictum clustered with the pH and Corg clusters; Ac. alpina, Am. gerdemannii, Am. reticulata, and Sc. rubiformis, but also some less conspicuous species like Ac. pustulata and Ac. punctata, were associated with the higher elevation (Fig. 9).
AMF indicator species analyses
The indicator species analyses according to Dufrêne and Legendre (1997) revealed 36 of the 106 detected AMF species with indicator values either for land use type, land use intensity, soil pH, soil organic C content, available P values, clay or sand contents, altitudes or for a combination of these parameters (Table 3). The most indicative species were Do. aurea, Gl. macrocarpum, and Se. constrictum for grasslands; Gl. diaphanum for croplands; Ac. punctata, Am. reticulata, Sc. rubiformis, and Si. hoi for acidic soils; Fu. caledonius for low Corg contents; Rh. irregulare for low clay contents; Se. constrictum for high clay contents; Se. constrictum for low sand contents; and Fu. caledonius and Gi. margarita for high sand contents. Funneliformis geosporus, Fu. mosseae, and Glomus diaphanum were most indicative for the lowlands (200–900 m). Dominikia aurea and Glomus badium were most significative for the mountainous areas (900–1600 m). Acaulospora alpina, Ac. punctata, Am. gerdemannii, Am. reticulata, and Sc. rubiformis were the most indicative species for the high mountainous to low alpine regions (1600–2300 m).
Discussion
All field soil samples of this study had been taken in 2009 at the beginning of spring (= 2 weeks after regional snowmelt, between late February to early July, depending on the mesoclimate and, especially, the altitude at the sites). In total, 17,924 AMF spores were identified on the species level from the 154 sites. More than 25 sites had rarely been analyzed in previous AMF diversity studies (e.g., Błaszkowski 1993), even when in a few studies substantially higher (e.g., Säle et al. 2015) or similar spore numbers (Oehl et al. 2003, 2010) had been identified. Our comprehensive multiple-site sampling design and the quantitative identification of thousands of AMF spores allows some discussion and general conclusions about the diversity, distribution patterns, and biogeography of these fungi in Swiss agricultural soils. Firstly, the present multiple-site study confirms single case studies (e.g., Oehl et al. 2003; Säle et al. 2015) that in Central Europe, generally higher AMF species richness is found in grasslands than in croplands. The major novelty of this study, however, is that such large number of AMF species can be used as indicators for different environmental parameters comprising land use, soil, and climatic (= elevation) parameters.
In our study, the spores were attributed to, in total, 106 AMF species, of which 77 were known species and the 29 other species were either not unequivocally attributed to known species or might represent species new to science. These numbers correspond well with the 104 AMF species recorded in all previous AMF diversity performed in Switzerland (reviewed in Oehl et al. 2010). Nevertheless, 34 AMF species detected in other studies, including also the most recent studies in this rather small country (e.g., Oehl et al. 2011b; Säle et al. 2015), were not detected in the present study. Including all results available, hitherto 140 AMF species have been identified in the country, which represents astonishing ~50% of the known AMF species worldwide. On the other hand, 43 of these 140 AMF species have so far not with certainty been attributed to known AMF species, and >20 of them might be undescribed species so far (own observations). These high numbers of non-identified species support postulations of other researchers using either morphological or molecular approaches for taxa or virtual taxa identification, respectively, that on the global level, the large majority of AMF taxa have not yet been classified and that overall AMF diversity of the Glomeromycota is several times higher than currently known (Öpik et al. 2014; Ohsowski et al. 2014; Sudová et al. 2015). Remarkably in this context, as a side product of all the AMF diversity studies performed in Switzerland within the last 15 years, 18 AMF species have already been described (Gamper et al. 2009; Oehl et al. 2012, 2015a, 2015b; Palenzuela et al. 2013). For other species, a scientific name was attributed in the meantime to formerly unidentified species, e.g., Gl. spinuliferum had preliminarily been named Glomus sp. BR7 in Oehl et al. (2003), and was originally described in the same year from neighboring southwestern Germany.
AMF species richness ranged between 14 and 32 species in the grasslands and 8–22 species in the croplands. This represent quite high species numbers and is in the range of numbers previously reported from Swiss agricultural soils in spore morphology-based studies (e.g., 20–24; 8–21 in croplands; Oehl et al. 2003, 2010). Rarely, even higher species numbers, as in soils with high clay contents (38 in a grassland; 27–32 in croplands; Säle et al. 2015) or when AMF communities were additionally propagated in greenhouse trap cultures (e.g., Oehl et al. 2009). So far, AMF diversity studies based on molecular root or soil analyses recovered less AMF species, such as 16 phylotypes from a lowland grassland (Sýkorová et al. 2007), where 24 AMF species had been recorded (Oehl et al. 2003), or from the French Alps, where in a subalpine grassland 13 “operational taxonomic units” were detected (Binet et al. 2017), while in our study, 16–24 AMF species were found at comparable altitudes (Fig. 2). Following Wetzel et al. (2014), morphological identification might currently be superior to molecular identification in terms of detecting total AMF species richness at a side. These authors found 25 AMF species based on spore morphology and nine sequence types based on molecular root analyses in winter wheat in Saxony (Germany). We assume that the currently rapidly developing high-resolution and throughput sequencing techniques (e.g., Hart et al. 2015; Schläppi et al. 2016), eventually combined with morphological spore analyses, soon can give a more complete picture about the AMF species compositions in soils and also more information about the percentage of AMF species that might regularly remain undetected by morphological approaches.
Several AMF species virtually occurred at almost all sites in the study, and sometimes even in similar spore abundances. Not surprisingly, such species were Ar. trappei, Cl. claroideum, Rh. irregulare, Gl. diaphanum, and Se. constrictum, among several others, which had frequently been found already in many other previous studies in Central Europe (Oehl et al. 2010; Maurer et al. 2014; Wetzel et al. 2014; Njeru et al. 2015; Säle et al. 2015) and also outside Europe (e.g., Higo et al. 2015), and thus might be cosmopolitan AMF fungi. This might be especially true for Ar. trappei that had been recorded from the coldest and hottest places of mycorrhizal plant life (e.g., Sieverding 1991; Oehl and Körner 2014). Some of these fungi, however, and many other species showed a preferential occurrence in different land use types, elevations or “habitats” (e.g., Turrini et al. 2016). This was a non-surprising result for some of them (e.g., Gl. diaphanum and Se. constrictum; for instance, see Błaszkowski 1993; Oehl et al. 2003, 2011a; Maurer et al. 2014), but totally surprising in some aspects for other species, e.g., for Fu. mosseae, which was thought to be a ubiquitous fungus despite some well-known preferences for higher soil pH. Remarkably, in our study, this species was among the most indicative species for low altitudes and was missing in the majority of the high mountainous to alpine sites.
Several species had a preferential occurrence, expressed by increased spore abundance and/or frequency, in grasslands that have higher organic C and soil microbiological properties when compared to croplands (e.g., Gl. macrocarpum, Gl. badium, Do. aurea, Rh. invermaium, Rh. intraradices, Ac. paulinae, and also Se. constrictum that was frequently found also in croplands, but in substantially lower abundances). This is in accordance with several single studies performed previously in Central Europe (e.g., Oehl et al. 2003, 2005, 2010; Njeru et al. 2015; Säle et al. 2015). A few species, however, were more abundantly and/or more frequently found in croplands than grasslands (e.g., Fu. caledonius and Pa. franciscana), results, which are also congruent with several other observations made before on these or related species (Błaszkowski 1993; Oehl and Sieverding 2004; Oehl et al. 2003, 2010).
Some of the just mentioned species (e.g., Se. constrictum, Gl. badium, Do. aurea, and Acaulospora paulinae) were not only affected by land use type or intensity, but also by soil pH and related soil properties (base saturation, CaCO3 contents). Several other species showed increased preferences in higher (e.g., Fu. mosseae and Fu. geosporus) or lower (e.g., Fu. caledonius, and several Acaulospora species) pH soils, confirming several single observations made in the past (e.g., Hildebrandt et al. 2001, 2007; Oehl et al. 2010).
Other, but also some of the mentioned AMF species, had a pronounced distribution pattern strongly related to the altitude levels of the sites. Some species (e.g., Ac. alpina and A. punctata) did not occur in the lowlands, but increasingly in higher mountainous, subalpine to alpine elevations. Other species were never found in the highest altitudes under study (e.g., Cetraspora armeniaca, Gigaspora margarita, and surprisingly also Fu. mosseae). Acaulospora and Ambispora species were more abundant and more frequent in high mountainous to alpine altitudes. We especially noticed that many Gigasporales species do not occur in Switzerland, while Acaulosporaceae and Ambisporaceae species numerously occur even on higher altitudes (e.g., Oehl et al. 2011b, 2012). Thus, while many Gigasporales species obviously have their major distributions in warmer climates and need longer vegetation periods to sporulate, diversity of Acaulosporaceae and Ambisporaceae is astonishingly high especially in higher European altitudes (see also Palenzuela et al. 2013). However, this deserves to be further investigated in other cold climates.
On the other hand, several Glomerales species preferentially occur at lower sites but disappear with increasing altitude (e.g., Sc. sinuosa), while others increasingly occur in higher elevations (e.g., Sc. rubiformis). However, for small-spored glomoid spores, especially Dominikia, Rhizoglomus, and Paraglomus species, we will need more sophisticated propagation techniques (e.g., Oehl et al. 2009), or molecular analyses (e.g., Lekberg et al. 2008), not only to detect such inconspicuous species (e.g., Błaszkowski et al. 2015), but also to elucidate their biogeographic patterns.
Previously, quantitative PCRs were performed with specific primers (Jansa et al. 2014) on the same soil samples that we analyzed for the present study. Their analyses aimed at studying the abundance of six selected AMF species, for which specific primers had been designed (Jansa et al. 2014). These primers, however, seem to be more genus- than species-specific, within the currently existing classification systems for Glomeromycota. These six AMF genera were analyzed: Cetraspora, Claroideoglomus, Diversispora, Funneliformis, Gigaspora, and Rhizoglomus. Detail comparison with our results on the AMF community level might not be allowed, as we do not know, how efficient the primers detected all the species of the corresponding genera in the field soil samples, and to which percentage they might have also detected species from other, related or more distant, AMF genera. In both our studies, altitude (geography) and soil fertility, soil texture and especially soil pH, were recognized as important factors affecting the AMF communities. Remarkably, however, our study on the overall AMF species richness and community composition revealed much higher impact of land use intensity and land use type on the AMF species and communities than found in Jansa et al. (2014), when they concluded, based on their data, that ‘this apparent lack of strong land management effects might be due to the rarity of highly intensive and unsustainable land management in Swiss agriculture’. We conclude that our comprehensive analyses on the overall AMF communities, based on spore morphology, led to a much higher resolution on the ecological parameters influencing the AMF community compositions than the molecular analyses performed for selected AMF species. Additionally, we could elaborate indicator species, and thus, those AMF species that might have most significantly been affected by the different environmental parameters.
Highest correlation between the abundances of the six AMF taxa in Jansa et al. (2014) and our spore abundances were obtained for the two gigasporalean genera Cetraspora and Gigaspora and the also large-spore forming glomeralean genus Funneliformis (r = 0.54, r = 0.29, and r = 0.41, respectively), while for the glomeralean genera Claroideoglomus and Rhizoglomus and the diversisporalean genus Diversispora, which all three form substantially smaller spores, these correlations were much lower to insignificant (r = 0.01, r = 0.06, and r = 0.10, respectively). The missing correlation for the smaller-spored glomoid and diversisporoid species might also be explained by the huge intraradical vesicle formation for such species, which not necessarily leads to huge sporulation of these species in the plant rhizosphere. Their spores might also be faster degraded and shorter-living than the large spores of Gigaspora, Cetraspora, and Funneliformis. On the other hand, Gigasporales do not form such vesicles, and Funneliformis species rather rarely, and thus need to sporulate in the rhizosphere to increase their survival rate at the habitats. In conclusion, the life cycle strategies, formation and persistence of mycorrhizal structures, mycelia, and especially of spores of single AMF taxa, might play a major role for correlations between concomitantly obtained molecular and morphological abundance analyses.
Conclusions
For a long time, the AM symbiosis was judged as non-specific, with only a few AMF species as fungal partners of thousands of host plants. Relatively high degree of non-specificity between plant and AM fungal symbiotic partners might be correct despite of some recently reported AMF-host preferences. In relation to land use factors, soil climate, and elevation parameters, this symbiosis is highly specific with many AMF species and only a few to multiple plant partners. It is positively or negatively affected by several to multiple environmental factors. Our comprehensive multiple-site AMF diversity study clearly suggests that quite many of the thus known AMF species have a very specific distribution pattern and might be adequate bio-indicators for many specific environmental parameters. Which functional role these and other AMF species play in the different agricultural systems has so far been poorly understood. In the future, the agricultural production and soil protection strategies will profit from a better knowledge about the biogeography of AM fungal species and their communities, especially when we better know about the ecosystem functions and specific ecosystem services of specific AMF species and species groups. Soils might better protected, water and nutrient more efficiently used, and plant growth and health further promoted by adequate measurements in the different cropland and grassland environments. In subsequent research studies, we have therefore to discover, which services can be provided by which AMF species or AMF species combinations.
References
Aguilera P, Cornejo P, Borie F, Barea JM, von Baer E, Oehl F (2014) Diversity of arbuscular mycorrhizal fungi associated to Triticum aestivum L. plants growing in an Andosol with phytotoxic aluminum levels. Agric Ecosyst Environ 186:178–184
Al-Yahya'ei M, Oehl F, Vallino M, Lumini E, Redecker D, Wiemken A, Bonfante P (2011) Uncovering arbuscular mycorrhizal fungal communities associated with date palm (Phoenix dactylifera) and its surrounding ruderal and natural vegetation in Southern Arabia. Mycorrhiza 21:195–209
Avio L, Castaldini M, Fabiani A, Bedini S, Sbrana C, Turrini A, Giovannetti M (2013) Impact of nitrogen fertilization and soil tillage on arbuscular mycorrhizal fungal communities in a Mediterranean agroecosystem. Soil Biol Biochem 67:285–294
Binet M-N, van Tuinen D, Souard F, Sage L, Périgon S, Gallet C, Legay N, Lavorel S, Mouhamadou B (2017) Responses of above- and below-ground fungal symbionts to cessation of mowing in subalpine grassland. Fungal Ecol 25:14–21
Błaszkowski J (1993) Comparative studies on the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol 28:93–140
Błaszkowski J (2012) Glomeromycota. W. Szafer Institute of Botany. Polish Academy of Sciences, Kraków, Poland, p 303
Błaszkowski J, Chwat G, Góralska A, Ryska P, Kovács GM (2015) Two new genera, Dominikia and Kamienska, and D. disticha sp. nov. in Glomeromycota. Nova Hedwigia 100:225–238
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Clapp JP, Young JPW, Merrywheather JW, Fitter AH (1995) Diversity of fungal symbionts in arbuscular mycorrhizas from a natural community. New Phytol 130:259–265
Cozzolino V, Di Meo V, Monda H, Spaccini R, Piccolo A (2016) The molecular characteristics of compost affect plant growth, arbuscular mycorrhizal fungi, and soil microbial community composition. Biol Fertil Soils 52:15–29
de Mendiburu F (2014) Agricolae: statistical procedures for agricultural research. R package version 1.2–0. http://CRAN.R-project.org/package=agricolae
Douds D, Millner P (1999) Biodiversity of arbuscular mycorrhizal fungi in agroecosystems. Agric Ecosyst Environ 74:77–93
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH (2010) Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J 4:337–345
Franke-Snyder M, Douds DD, Galvez L, Phillips JG, Wagoner P, Drinkwater L, Morton JB (2001) Diversity of communities of arbuscular mycorrhizal (AM) fungi present in conventional versus low-input agricultural sites in eastern Pennsylvania, USA. Appl Soil Ecol 16:35–48
Gamper HA, Walker C, Schüßler A (2009) Diversispora celata sp. nov.: molecular ecology and phylotaxonomy of an inconspicuous arbuscular mycorrhizal fungus. New Phytol 182:495–506
Gerdemann JW, Trappe JM (1974) The Endogonaceae in the Pacific Northwest. Mycol Memoirs 5. 76 p
Gianinazzi S, Gollote A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Hart MM, Aleklett K, Chagnon P-C, Egan C, Ghignone S, Helgason T, Lekberg Y, Öpik M, Pickles BJ, Waller L (2015) Navigating the labyrinth: a guide to sequence-based, community ecology of arbuscular mycorrhizal fungi. New Phytol 207:235–247
Hazard C, Gosling P, van der Gast CJ, Mitchell DT, Doohan FM, Bending GD (2013) The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. ISME J 7:498–508
Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPW (1998) Ploughing up the wood-wide web? Nature 394:431
Higo M, Isobe K, Kondo T, Yamaguchi M, Takeyama S, Drijber RA, Torigoe Y (2015) Temporal variation of the molecular diversity of arbuscular mycorrhizal communities in three different winter crop rotational systems. Biol Fertil Soils 51:21–32
Hildebrandt U, Janetta K, Ouziad F, Renne B, Nawrath K, Bothe H (2001) Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza 10:175–183
Hildebrandt U, Regvar M, Bothe H (2007) Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 68:139–146
Horn S, Caruso T, Verbruggen E, Rillig MC, Hempel S (2014) Arbuscular mycorrhizal fungal communities are phylogenetically clustered at small scales. ISME J 8:2231–2242
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Jansa J, Erb A, Oberholzer HR, Smilauer P, Egli S (2014) Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Mol Ecol 23:2118–2135
Jansa J, Mozafar A, Kuhn G, Anken T, Ruh R, Sanders IR, Frossard E (2003) Soil tillage affects the community structure of mycorrhizal fungi in maize roots. Ecol Appl 13:1164–1176
Jansa J, Oberholzer HR, Egli S (2009) Environmental determinants of the arbuscular mycorrhizal fungal infectivity of Swiss agricultural soils. Eur J Soil Biol 45:400–408
Jeffries P, Gianinazzi S, Perrotto S, Turnau K, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils 37:1–16
Krüger M, Stockinger H, Schüßler A (2009) DNA-based species-level detection of arbuscular mycorrhizal fungi: one PCR primer set for all AMF. New Phytol 183:212–223
Land S, von Alten H, Schönbeck F (1993) The influence of host plant, nitrogen fertilization and fungicide application on the abundance and seasonal dynamics of vesicular-arbuscular mycorrhizal fungi in arable soils of northern Germany. Mycorrhiza 2:157–166
Lekberg Y, Koide RT, Twomlow SJ (2008) Effect of agricultural management practices on arbuscular mycorrhizal fungal abundance in low-input cropping systems of southern Africa: a case study from Zimbabwe. Biol Fertil Soils 44:917–923
Maheraly H, Klironomos JN (2007) Influence of phylogeny of fungal community assembly and ecosystem functioning. Science 616:1746–1748
Maurer C, Rüdy M, Chervet A, Sturny W, Flisch R, Oehl F (2014) Diversity of arbuscular mycorrhizal fungi in field crops using no-till and conventional tillage practices. Agrarforschung Schweiz 5:398–405
Mello CMA, Silva GA, Assis DMA, Pontes JS, Ferreira ACA, Leão MPC, Vieira HEE, Maia LC, Oehl F (2013) Paraglomus pernambucanum sp. nov. and Paraglomus bolivianum com. Nov., and biogeographic distribution of Paraglomus and Pacispora. J Appl Bot Food Qual 86:113–125
Montiel-Rozas MM, López-García A, Madejón P, Madejón E (2017) Native soil organic matter as a decisive factor to determine arbuscular mycorrhizal fungal community structure in contaminated soils. Biol Fertil Soils 53:327–338
Moora M, Davison J, Öpik M, Metsis M, Saks U, Jairus T, Vasar M, Zobel M (2014) Anthropogenic land use shapes the composition and phylogenetic structure of soil arbuscular mycorrhizal fungal communities. FEMS Microbiol Ecol 90:609–621
Munkvold L, Kjoller R, Vestberg M, Rosendahl S, Jacobsen I (2004) High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol 164:357–364
Njeru E, Avio L, Bocci G, Sbrana C, Turrini A, Bàrberi P, Giovannetti M, Oehl F (2015) Contrasting effects of cover crops on 'hot spot' arbuscular mycorrhizal fungal communities in organic tomato. Biol Fertil Soils 51:151–166
Oehl F, Körner C (2014) Multiple mycorrhization at the coldest place known for angiosperm plant life. Alp Bot 124:193–198
Oehl F, Sieverding E (2004) Pacispora, a new vesicular arbuscular mycorrhizal fungal genus in the Glomeromycetes. J Appl Bot Food Qual 78:72–82
Oehl F, Jansa J, Ineichen K, Mäder P, van der Heijden MGA (2011a) Arbuscular mycorrhizal fungi as bio-indicators in Swiss agricultural soils. Agrarforschung Schweiz 2:304–311
Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, van der Heijden M, Sieverding E (2010) Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738
Oehl F, Sánchez-Castro I, Palenzuela J, Silva GA (2015a) Palaeospora spainii, a new arbuscular mycorrhizal fungus from Swiss agricultural soils. Nova Hedwigia 101:89–102
Oehl F, Sánchez-Castro I, Sousa NMF, Silva GA, Palenzuela J (2015b) Dominikia bernensis, a new arbuscular mycorrhizal fungus from a Swiss no-till farming site, and D. aurea, D. compressa, and D. indica, three new combinations in Dominikia. Nova Hedwigia 101:65–76
Oehl F, Santos VM, Palenzuela J (2016) Paraglomus turpe, a new arbuscular mycorrhizal fungal species from Central European agricultural soils. Nova Hedwigia 103:491–499
Oehl F, Schneider D, Sieverding E, Burga CA (2011b) Succession of arbuscular mycorrhizal communities in the foreland of the retreating Morteratsch glacier in the Central Alps. Pedobiologia 54:321–331
Oehl F, Sieverding E, Ineichen K, Mäder P, Boller T, Wiemken A (2003) Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl Environ Microbiol 69:2816–2824
Oehl F, Sieverding E, Ineichen K, Mäder P, Wiemken A, Boller T (2009) Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric Ecosyst Environ 134:257–268
Oehl F, Sieverding E, Ineichen K, Ris EA, Boller T, Wiemken A (2005) Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol 165:273–283
Oehl F, Sieverding E, Palenzuela J, Ineichen K, Silva GA (2011c) Advances in Glomeromycota taxonomy and classification. IMA Fungus 2:191–199
Oehl F, Palenzuela J, Sánchez-Castro I, Kuss P, Sieverding E, Silva GA (2012) Acaulospora nivalis, a new fungus in the Glomeromycetes, characteristic for high alpine and nival altitudes of the Swiss Alps. Nova Hedwigia 95:105–122
Ohsowski BM, Zaitsoff PD, Öpik M, Hart MM (2014) Where the wild things are: looking for uncultured Glomeromycota. New Phytol 204:170–179
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) vegan: Community Ecology Package. R package version 2.0–10. http://CRAN.R-project.org/package=vegan
Öpik M, Davison J, Moora M, Zobel M (2014) DNA-based detection and identificaton of Glomeromycota: the virtual taxonomy of environmental sequences. Botany 92:135–147
Palenzuela J, Azcón-Aguilar C, Barea JM, Silva GA, Oehl F (2013) Acaulospora pustulata and Acaulospora tortuosa, two new species in the Glomeromycota associated with endangered plants in sierra Nevada (southern Spain). Nova Hedwigia 97:305–319
Pereira CMR, Silva DKA, Ferreira ACA, Goto BT, Maia LC (2014) Diversity of arbuscular mycorrhizal fungi in Atlantic forest areas under different land uses. Agric Ecosyst Environ 185:245–252
Pontes JS, Oehl F, Marinho F, Coyne D, Silva DKA, Yano-Melo AM, Maia LC (2017) Diversity of arbuscular mycorrhizal fungi in Brazil's Caatinga and experimental agroecosystems. Biotropica 49:413–427
R Core Team (2014) R. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rillig MC, Aguilar-Trigueros CA, Bergmann J, Verbruggen E, Veresoglou SD, Lehmann (2015) Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol 205:1385–1388
Rillig MC, Mummey DL (2006) Mycorrhizas and soil structure. New Phytol 171:41–53
Säle V, Aguilera P, Laczko E, Mäder P, Berner A, Zihlmann U, van der Heijden MGA, Oehl F (2015) Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol Biochem 84:38–52
Schenck NC, Pérez Y (1990) Manual for the identification of VA mycorrhizal fungi, 3rd edn. Synergistic Publications, Gainesville
Schläppi K, Bender SF, Mascher F, Russo G, Patrignani A, Camenzind T, Hempel S, Rillig MC, van der Heijden MGA (2016) High-resolution community profiling of arbuscular mycorrhizal fungi. New Phytol 212:780–791
Schlicht A (1889) Beitrag zur Kenntniss der Verbreitung und der Bedeutung der Mykorrhizen. Landwirtschaftliche Jahrbücher 18:477–505
Sieverding E (1991) Vesicular-arbuscular mycorrhiza management in tropical agrosystems. Deutsche Gesellschaft für Technische Zusammenarbeit 224. Hartmut Bremer Verlag, Friedland
Sieverding E, Silva GA, Berndt R, Oehl F (2014) Rhizoglomus, a new genus in the Glomeraceae. Mycotaxon 129:373–386
Simon L, Lalonde M, Bruns TD (1992) Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl Environ Microbiol 58:291–295
Smith SE, Read D (2008) Mycorrhizal symbiosis (third edition). Academic Press, London
Sudová R, Rydlova J, Čtvrtlikova M, Havranek P, Adamec L (2011) The incidence of arbuscular mycorrhiza in two submerged Isoetes species. Aquat Bot 94:183–187
Sudová R, Sýkorová Z, Rydlová J, Čtvrtlíková M, Oehl F (2015) Rhizoglomus melanum, a new arbuscular mycorrhizal fungal species associated with submerged plants in freshwater lake Avsjøen in Norway. Mycol Prog 14:9
Sýkorová Z, Ineichen K, Wiemken A, Redecker D (2007) The cultivation bias: different communities of arbuscular mycorrhizal fungi detected in roots from the field, from bait plants transplanted to the field, and from a greenhouse trap experiment. Mycorrhiza 18:1–14
Ter Braak C (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
Tchabi A, Coyne D, Hountondji F, Lawouin L, Wiemken A, Oehl F (2008) Arbuscular mycorrhizal fungal communities in sub-Saharan savannas of Benin, West Africa, as affected by agricultural land use intensity and ecological zone. Mycorrhiza 18:181–195
Tchabi A, Coyne D, Hountondji F, Lawouin L, Wiemken A, Oehl F (2010) Efficacy of indigenous arbuscular mycorrhizal fungi for promoting white yam (Dioscorea rotundata) growth in West Africa. Appl Soil Ecol 45:92–100
Turrini A, Avio L, Bedini S, Giovannetti M (2008) In situ collection of endangered arbuscular mychorrhizal fungi in a Mediterranean UNESCO Biosphere Reserve. Biodivers Conserv 17:643–657
Turrini A, Sbrana C, Avio L, Njeru EM, Bocci G, Bàrberi P, Giovannetti M (2016) Changes in the composition of native root arbuscular mycorrhizal fungal communities during a short-term cover crop-maize succession. Biol Fertil Soils 53:643–653
Valyi K, Rillig MC, Hempel S (2015) Land-use intensity and host plant identity interactively shape communities of arbuscular mycorrhizal fungi in roots of grassland plants. New Phytol 205:1577–1586
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
van der Heijden MGA, Martin F, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present and the future. New Phytol 205:1406–1423
van Tuinen D, Jacquot E, Zhao B, Gallotte A, Gianinazzi-Pearson V (1998) Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol Ecol 7:879–887
Wetzel K, Silva GA, Matczinski U, Oehl F, Fester T (2014) Superior differentiation of arbuscular mycorrhizal fungal communities from till and no-till plots by morphological spore identification when compared to T-RFLP. Soil Biol Biochem 72:88–96
Acknowledgements
We express our gratitude to all participating farmers, as well as to Barbara Meier for her invaluable technical assistance. Angela Erb carried out most of the soil analyses. The Swiss Federal Office for the Environment and the cantons Aargau, Bern, Fribourg, Graubünden, Solothurn and St. Gallen financed this study (Projekt Mykorrhiza-Infektionspotential, Nr 09.0057.PJ). Jan Jansa was further supported by long-term development program RVO61388971. Carrie Andrew is acknowledged for language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oehl, F., Laczko, E., Oberholzer, HR. et al. Diversity and biogeography of arbuscular mycorrhizal fungi in agricultural soils. Biol Fertil Soils 53, 777–797 (2017). https://doi.org/10.1007/s00374-017-1217-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-017-1217-x