Abstract
Yak and Tibetan sheep grazing is a common phenomenon on natural grasslands in the Qinghai-Tibetan Plateau, and large amounts of excrement are directly deposited onto alpine grasslands. However, little is known about the effects of excrement return on soil N supply and N retention capacity. This study investigated the short-term effects of yak and Tibetan sheep dung on gross N transformation rates determined by 15N tracing technique of alpine steppe (AS) and meadow (AM) soils at 60 % water holding capacity (WHC) under laboratory conditions. Cumulative gross N mineralization and NH4 + immobilization over the 21-day incubation period in AM soil were around 2.8 and 2.0 times as high as that in AS soil, respectively. Cumulative gross nitrification in AM soil was 0.96 times higher, while the value of gross NO3 − immobilization rate was 0.65 times lower than in AS soil, resulting in higher NO3 − accumulation in AM soil. Dung addition increased soil gross N mineralization and NH4 + immobilization rates by 12–35 % and 17–59 %, respectively. Amending yak and sheep dung decreased gross nitrification rates by 3–23 % but increased gross NO3 − immobilization rates by 25–190 %, which led to a decreased net NO3 − accumulation and NO3 − losses risk through leaching. The cumulative CO2 emissions over the 21 days of incubation period were enhanced by 65 and 120 % for AS and AM soil, respectively. The application of dung stimulated cumulative N2O emissions, but the stimulation was only significant in AM soil. In general, yak and sheep dung return has a positive effect on soil N supply and retention owing to the increase in NH4 + availability for plant with simultaneously decreasing NO3 − accumulation in soils.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Qinghai-Tibetan Plateau, characterized by a high mean altitude of more than 4000 m above sea level, is the largest grassland area of the Eurasian continent and also the largest area of natural grasslands in China (Yue et al. 2010; Lu et al. 2012). Alpine steppe and alpine meadow, the two dominant vegetation types on the plateau, cover 32 and 30 % of the total area of alpine grassland, respectively (Geng et al. 2012). Approximately 13 million yaks and 50 million Tibetan sheep graze on these grasslands (Lin et al. 2009; Dong et al. 2015; Sun et al. 2015), and large amounts of excrement, containing plenty of nitrogen (N), corresponding to ca. 75–90 % of the N intake of grazing animals, are thus directly deposited onto alpine grasslands. Nitrogen is a limiting factor for plant and microbial growth; soil available N mainly comes from soil N supply and atmospheric N deposition in natural grasslands in the Qinghai-Tibetan Plateau, and the return of excrement is an important N source. Consequently, understanding the soil N supply capacity and how the return of excrement affects soil N supply is important to predict N availability for plant in alpine grassland. Although it is important to understand N dynamics following the return of excrement when considering grassland degradation, climate change, and expansion of livestock, the effects of yak and Tibetan sheep dung on the N cycling remain poorly understood in alpine grassland soils in the Qinghai-Tibetan Plateau.
Net N mineralization and nitrification rates can be used to indicate the N supply capacity and N losses risk, respectively (Rennenberg et al. 2009). However, net N mineralization is controlled by both gross N mineralization and immobilization processes, and net nitrification is the result of competition between gross nitrification and gross NO3 − consumption (NO3 − immobilization and denitrification) processes. Net rates have been found to be a poor approximation of the real status of N cycling in soils (Davidson et al. 1991; Hart et al. 1994), and it is generally accepted that measuring gross N transformation rates using 15N isotope techniques is the best approach to quantify soil N dynamics. However, previous studies mainly focused on the effects of yak and Tibetan sheep dung on soil net N transformation rates and N2O, CH4, and CO2 emissions (Cai et al. 2013, 2014), and few researchers quantified gross N transformation rates after the return of yak and Tibetan sheep dung. Quantifying gross N transformation rates using 15N tracing experiments may improve our understanding of the modification of soil N cycling and N availability induced by dung input (Rennenberg et al. 2009).
Animal manure can provide ash alkalinity and enhance mineralization of organic N, and thus increase soil pH (de Boer et al. 1988; Cai et al. 2015). An increase in soil pH has been demonstrated to stimulate the oxidation of NH4 + to NO3 − via autotrophic nitrification (Ste-Marie and Pare 1999; Cheng et al. 2013). Thus, it would be expected that excrement N return may increase the conversion of NH4 + to NO3 −. Furthermore, NO3 − immobilization can occur in soils under high C availability, such as grassland and forest soils (Stark and Hart 1997; Hatch et al. 2000). It can therefore be hypothesized that excrement N deposition may promote microbial NO3 − immobilization by providing C source and increasing soil microbial biomass and activity.
N2O released from soil is primarily produced by biological processes of nitrification and denitrification (Hutchinson and Davidson 1993). The rates at which these processes promote the release of N2O depend on temperature (Lan et al. 2014), water content (Cheng et al. 2014), C availability (Cheng et al. 2015), and cultivation (Li and Lang 2014). As a type of C source, the addition of dung to soil may create the ideal conditions for denitrification (enhanced oxygen consumption and increased labile organic C and N substrate concentrations) (Granli and Bockman 1994; Lovell and Jarvis 1996). Furthermore, excrement N return may stimulate nitrification rates and thus promote N2O emission. However, few studies have examined the effects of dung return on N2O emission in alpine grassland soils in the Qinghai-Tibetan Plateau.
In this study, our primary objectives were to quantify gross N transformation rates and N2O emission in two contrasting alpine grassland (meadow vs. steppe) soils and to investigate the short-term effects of yak and Tibetan sheep dung on soil N availability and N retention capacity in the Qinghai-Tibetan Plateau grasslands.
Materials and methods
Soil and dung characteristics
The study site was located at Xainza Alpine Steppe and Wetland Ecosystem Observation Station (N 30° 57′, E 88° 42′, 4675 m above sea level (a.s.l.)) in Northern Tibet. The average annual air temperature and precipitation in this region are 0 °C and 300 mm, respectively, with most rainfall occurring from May to September. The average soil temperature at 5 cm in the growing seasons ranged from 10.8 to 18.8 °C in 2013 (Cai et al. 2014). The dominant vegetation is Stipa purpurea and Kobresia humilis in alpine steppe and alpine meadow, respectively. The alpine steppe and meadow soils are mostly equivalent to Cryic Aridisols and Gelic Cambisols according to the Chinese soil taxonomy, respectively (Cai et al. 2013). The percentages of sand, silt, and clay in the upper soil layer are 91, 7, and 2 % for alpine steppe soil and 84, 12, and 4 % for alpine meadow soil, respectively (Lu et al. 2011). In the autumn of 2012, soil was collected from a depth of 0–10 cm, air dried, crushed, and sieved (<2 mm) and then transported to the laboratory. Physical and chemical properties of soils are shown in Table 1.
Dung collection
Yak and Tibetan sheep dung were collected separately from a randomly selected subgroup of eight animals of each type on a camping area adjacent to the soil sampling sites. Grazing animals were enclosed within the camp at night and fresh dung samples were manually collected (directly under the tail of the animals) into plastic buckets the next morning. The total feces collected from each type of animal were mixed, the dung samples were frozen immediately, and samples were transported to the laboratory as soon as possible. The dung samples were dried at 60 °C and ground to less than 1 mm. The composition of the dung from yak and Tibetan sheep is shown in Table 1.
15N tracing experiment
A 15N tracing technique was used to quantify gross N transformation rates through a paired labeling experiment (15NH4NO3 and NH4 15NO3) (Mary et al. 1998). In total, 180 flasks (3 treatments × 2 labels × 2 soil types × 5 extraction times × 3 replicates) were employed. Soil samples (20-g oven-dry weight basis) were pre-incubated in 250-ml flasks at 30 % WHC and 20 °C in the dark. To prevent loss of soil water, the flasks were covered with aluminum foil with holes for aeration. After 7 days of pre-incubation, the soil samples were given one of the following treatments for alpine steppe (AS) and meadow (AM): CK, without dung addition; YD, the addition of yak dung; TSD, the addition of Tibetan sheep dung. The soil sample for each flask in the dung-treated treatments was mixed thoroughly with ground dung. The application rate of dung was equivalent to 100 mg N kg−1 and 1940 mg C kg−1soil. For all flasks, an ammonium nitrate solution containing either ammonium (15NH4NO3) or nitrate (NH4 15NO3) labeled with 15N at 10 atom% excess was applied to the soil at 20 mg N kg−1 soil (oven-dried weight). The 15N-labeled solution was added uniformly over the soil surface with a pipette, and the final soil moisture contents were adjusted to 60 % WHC using deionized water. Subsequently, all the flasks were covered with aluminum foil and incubated at 20 °C in the dark for an additional 21 days. During incubation, the samples were aerated for 30 min each day to maintain aerobic conditions inside the flasks, and any lost water was replaced every 3 days with deionized water as required.
Gas samples (three replicates) were taken from the headspace of the flasks on days 0, 3, 8, 14, and 21. Before each gas sampling event, the flasks were opened for 30 min to renew the atmosphere inside and immediately sealed for 6 h using rubber stoppers with a silicone sealant. Then, 20-mL gas sample was collected using a 25-mL gas-tight syringe with a stopcock from the headspace of each flask at the end of 6-h incubation, and was injected into pre-evacuated vials (18.5 ml) to determine the concentration of N2O and CO2. After gas sampling, the flasks were destructively sampled for analysis of NH4 +, NO3 −, and organic N. Specifically, three flasks were randomly selected from each labeling type, treatment, and soil, and the soil was extracted using 100-mL 2-M KCl solution to determine the concentration and isotopic composition of NH4 + and NO3 −. After KCl extraction, residual soil was washed with deionized water, oven-dried at 60 °C to a constant weight, and ground to pass through a 0.15-mm sieve for 15N analysis of insoluble organic N.
Soil analysis
Soil pH was measured in a slurry with a soil/water ratio of 1:2.5 (v/v) using a DMP-2 mV-pH detector (Quark Ltd., Nanjing, China). The soil organic carbon was determined by wet digestion with H2SO4–K2Cr2O7, while soil organic N was determined by semi-micro Kjeldahl digestion using Se, CuSO4, and K2SO4 as catalysts. Exchangeable NH4 + and NO3 − concentrations were determined with a continuous-flow analyzer (Skalar Analytical, Breda, the Netherlands). Klason lignin content was estimated according to TAPPI standards (T13wd-74 and T222om-88, respectively) (Tappi 2006).
The isotopic compositions of NH4 +, NO3 −, and insoluble organic N were measured using an automated C/N analyzer isotope ratio mass spectrometer (Europa Scientific Integra, Sercon 20–22, UK). Exchangeable NH4 + and NO3 − were separated for 15N measurements by distillation with magnesium oxide and Devarda’s alloy (Bremner 1996). In detail, a portion of the extract was steam-distilled with MgO to separate NH4 + on a steam distillation system. The sample in the flask was distilled again after the addition of Devarda’s alloy to separate out the NO3 −. The liberated NH3 was trapped using boric acid solution. To prevent isotopic cross-contamination between samples, 25 mL of reagent-grade ethanol was added to the distillation flasks and steam-distilled for 3 min between each distillation. Trapped N was acidified and converted to (NH4)2SO4 using 0.005 mol L−1 H2SO4 solution. The H2SO4 solution (containing NH4 +) was then evaporated to dryness at 60 °C in an oven and analyzed for 15N abundance.
N2O concentrations were determined with a gas chromatograph (Agilent 7890, Santa Clara, CA, USA) equipped with a 63Ni electron capture detector (ECD) operated at 300 °C. Separation was performed using a stainless-steel column packed with 80/100 mesh Porapak Q at 65 °C. The injection port was maintained at 100 °C. The carrier gas was argon (Ar) gas and contained 5 % CH4 at a flow rate of 40 mL min−1. CO2 concentrations, meanwhile, were determined with a gas chromatograph (Agilent 7890, Santa Clara, CA, USA) equipped with a thermal conductivity detector using a column packed with Porapak Q (80/100 mesh). The temperatures of the column oven, injector, and detector were 40, 100, and 300 °C, respectively.
Calculations and statistical analysis
Cumulative CO2 and N2O emissions were calculated using linear interpolation across sampling intervals. The FLUAZ model (based on the 15N-tracing technique) was employed to calculate gross N transformation rates (Mary et al. 1998). This model combines a numerical method (Runge–Kutta algorithm, 4th order) for solving the differential system given by the N and 15N mass equations and a nonlinear fitting program (Haus-Marquardt algorithm) for optimizing the N rate parameters by minimizing the differences between observed and simulated N and 15N data (amounts and isotopic excesses of NH4 + and NO3 −). Measurements of insoluble soil organic 15N were also considered to be much more reliable in preparing 15N balances. The optimal fit of the experimental data was calculated by minimizing the mean weighted error (MWE) criterion, which is a function of the difference between simulated and measured variables and the experimental variance of the measured variables. In this way, the measured variables with the largest experimental variability had the lowest weight in the optimization procedure (Mary et al. 1998). The main N transformations considered in FLUAZ included the following: nitrification, mineralization, and immobilization of NH4 + and NO3 −. Cumulative gross N transformations over the 21-day incubation period were estimated based on linear extrapolation between the periodically measured time points.
The difference in variables between soil types and among treatments was evaluated by paired t test and one-way ANOVA followed by a least significant difference (LSD) test, respectively. All statistical analyses were performed using SPSS 13.0. All results are reported on a soil dry weight basis.
Results and discussion
Soil inorganic N concentrations and 15N enrichments
Exchangeable NH4 + concentrations rapidly decreased during the first 8 days of incubation and then remained almost constant for AS soil, but exchangeable NH4 + concentrations in AM soil rapidly increased during the first 3 days of incubation and gradually decreased afterward (Fig. 1). In contrast, NO3 − concentrations increased during the whole incubation period for both AS and AM soils, and the values in dung-treated treatments were significantly lower than those in CK treatments for both soils at the end of incubation (P < 0.001), implying a decreasing net nitrification rate or an increasing NO3 − consumption after dung addition. In the 15NH4 +-labeled samples, the 15N enrichments of exchangeable NH4 + gradually declined (Fig. 2a), and the 15N enrichments of NO3 − increased during the whole incubation period in AM soil while increased during the first 8 days of incubation and then remained almost constant in AS soil (Fig. 2b). In the 15NO3 −-labeled samples, the 15N enrichments of exchangeable NH4 + slightly fluctuated (Fig. 2c), and the 15N enrichments of NO3 − declined over time for both soils (Fig. 2d).
Exchangeable NH4 +-N (a) and NO3 −-N (b) concentrations versus incubation time for the alpine steppe (AS) and meadow (AM) soils amended with yak (YD) and Tibetan sheep dung (TSD). SCK without dung addition in AS soil, SYD the addition of yak dung into AS soil, STSD the addition of Tibetan sheep dung into AS soil, MCK without dung addition in AM soil, MYD the addition of yak dung into AM soil, MTSD the addition of Tibetan sheep dung into AM soil. Error bars represent standard deviation for n = 3
Change in exchangeable NH4 +-N (a) and NO3 −-N (b) abundance in the 15NH4NO3 labeled samples, and NH4 +-N (c) and NO3 −-N (d) abundance in the NH4 15NO3 labeled samples. SCK, without dung addition in AS soil, SYD, the addition of yak dung into AS soil, STSD, the addition of Tibetan sheep dung into AS soil, MCK, without dung addition in AM soil, MYD, the addition of yak dung into AM soil, MTSD, the addition of Tibetan sheep dung into AM soil. Error bars represent standard deviation for n = 3
Gross N transformation rates
Gross N mineralization rates reached the highest values in the first time interval (0–3 days) and then decreased for both soils in all treatments (Table 2). The cumulative gross N mineralization over the 21-day incubation period was around 1.8 times higher in AM than in AS soil (Figs. 3 and 4). Yak and sheep dung application into AS soil increased by 30 and 22 % gross N mineralization rates, respectively (Fig. 3). In AM soil, gross N mineralization rates were enhanced by 35 % following sheep dung application and to a lesser extent (12 %) by yak dung application (Fig. 4). Since the AM and AS soils were taken from adjacent sites within 1000 m, the climatic, parent material, topography, time, and organisms could be similar, and thus the difference in gross N mineralization rates were most likely associated with the development of different soil properties under respective dominant vegetation. It has been reported that the difference in the dominant vegetation type and associated litter quality can cause different soil properties, such as soil organic C and N contents, and pH (Sotta et al. 2008; Zhang et al. 2011). Gross N mineralization rates are positively correlated with soil organic C and N contents, indicating the importance of substrate availability in controlling mineral N production (Booth et al. 2005). Thus, relatively higher soil organic C and N contents in AM soil compared with AS soil (Table 1) could induce greater gross N mineralization rates in AM soil. Likewise, the stimulation of gross N mineralization following dung addition might be attributed to organic C supply from dung, or the priming effects of dung on the decomposition of native soil organic C or the turnover of microbial biomass N (Shindo and Nishio 2005). Thus, it can be concluded that the enhanced gross N mineralization rates following amendment of grazing animals’ dung are of particular importance for increasing potential plant available N and alleviating possible N limitation in natural grasslands in the Qinghai-Tibetan Plateau.
The cumulative gross NH4 + immobilization over the whole incubation period in AM soil was approximately twice as high as that in AS soil without dung application (Figs. 3 and 4). A review by Booth et al. (2005) found that gross NH4 + immobilization rates were positively correlated with soil organic C concentrations. In this study, soil organic C and water-soluble organic carbon concentrations were significantly higher in AM than in AS soil (Table 1). As a consequence, the different amount of available C was responsible for the difference in the cumulative gross NH4 + immobilization between AM and AS soils. Yak and sheep dung application increased gross NH4 + immobilization rates by ca. 48 % in AS soil (Fig. 3). In AM soil, gross NH4 + immobilization rates were enhanced by 59 % following sheep dung application and to a lesser extent (17 %) by yak dung application (Fig. 4). Gross NH4 + immobilization rates appeared to increase by increasing C availability, as microbe needed additional inorganic N for growth under enhanced C availability (Burger and Jackson 2003). The increasing gross N mineralization and NH4 + immobilization rates due to dung application indicate more rapid turnover of NH4 + pool in dung-amended soils. Luxhøi et al. (2007) suggested that mineral N in the transition between gross N mineralization and immobilization was still available for assimilation by plants. Dung application enhanced microbial immobilization of NH4 + into soil organic N pool, but this phenomenon has been demonstrated to be temporary (Romero et al. 2015). In the long run, dung-induced N immobilization will re-mineralize with increase of inorganic N.
Gross nitrification rates decreased with incubation time in AS soil, while the tendency was reversed in AM soil (Table 2). The cumulative gross nitrification was 96 % times higher in AM than in AS soil without dung application (Figs. 3 and 4). Generally, soil nitrification rates increased by increasing soil pH (Ste-Marie and Pare 1999). However, our results showed that there was no difference in pH between AM and AS soils (Table 1). Thus, the difference in the cumulative gross nitrification between AM and AS soils might be controlled by other factors rather than soil pH. Alternatively, gross nitrification was strongly dependent on gross N mineralization, indicating that NH4 + availability played an important role in regulating nitrification rate (Booth et al. 2005). Similarly, our results also indicated that AM soil, which had greater gross N mineralization rate, displayed greater gross nitrification rate in comparison with AS soil.
Not as expected, the application of yak and sheep dung decreased gross nitrification rates for AS soil by 15 and 23 % (Fig. 3), and for AM soil by 10 and 3 %, respectively (Fig. 4). In general, mineral and organic amendments often stimulate nitrification rates (Schimel and Bennett 2004; Müller et al. 2011). Theoretically, dung application can stimulate autotrophic nitrification due to increasing soil pH by providing ash alkalinity and enhancing mineralization of organic N (Ste-Marie and Pare 1999; Cai et al. 2015). In this study, we did not determine soil pH during the incubation for all treatments. However, it could be expected that increased soil pH by dung application did not occur considering that pH of both soils were alkaline, with pH value (8.7) being significantly higher than that of both dung (Table 1). Indeed, it is generally admitted that the nitrification activity is higher in neutral or slightly alkaline conditions, and the optimum soil pH for nitrification has been reported to be from about 7.5 to 8.0 (Paul and Clark 1989; Yao et al. 2011). Thus, in both soils, nitrification might have an optimal pH value which is lower than that of soil, leading to no stimulation of nitrification by dung application. Instead, the decline in gross nitrification rates following dung application could be due to the competition for exchangeable NH4 + between heterotrophic microorganisms and nitrifiers (Booth et al. 2005). Enhanced C availability following dung application could stimulate microbial requirement for N (Figs. 3 and 4), with heterotrophic microorganisms becoming more competitive than nitrifiers for exchangeable NH4 + and this may have decreased gross nitrification rates in dung-amended soils.
Compared with NH4 + immobilization, NO3 − immobilization rates were considerably low for both soils (Table 2), consistent with the percentage of added 15N recovered in insoluble organic N pools, which was significantly higher in the 15NH4 + than in the 15NO3 −-labeled samples during the whole incubation, regardless of grassland and dung types (P < 0.001; Table 3). Gross NO3 − immobilization rates were 65 % times lower in AM than in AS soil. Thus, higher gross nitrification rates coupled with lower gross NO3 − immobilization rates was responsible for higher net nitrification and NO3 − accumulation in AM soil compared with AS soil (Fig. 1b). Heterotrophic microbes assimilated less NO3 − than NH4 + probably because the reduced energy requirements for NH4 + assimilation into microbial cells (Murphy et al. 2003), or because NH4 + even at relatively low concentrations (i.e., <1 mg N kg−1 soil), can decrease microbial NO3 − immobilization (Rice and Tiedje 1989). Microbial NO3 − immobilization occurred in grassland and forest soils (Stark and Hart 1997; Hatch et al. 2000), while it was negligible in agricultural soils (Shi and Norton 2000; Shi et al. 2004). Compared with agricultural soils, grassland and forest soils are considered N rather than C limited. It is well established that the NO3 − immobilization depends on the amount of available C (Recous et al. 1990; Bradley 2001). The addition of glucose, sucrose, and crop residue with high C/N ratio has been demonstrated to stimulate microbial NO3 − immobilization (Recous et al. 1990; Bradley 2001). Similarly, our results also showed that the application of yak and sheep dung increased gross NO3 − immobilization rates for AS soil by 1.9- and 0.9-fold, respectively (Fig. 3), and for AM soil by 0.3- and 1.5-fold, respectively (Fig. 4), consistent with our previous hypothesis that excrement N deposition may promote microbial NO3 − immobilization by providing C source and increasing soil microbial biomass and activity. Enhanced gross NO3 − immobilization rates following dung input indicate an important role of microbes in governing NO3 − concentrations and associated NO3 − losses risks.
It is generally accepted that the decomposition rate of residue and its effect on soil gross N transformations depend on residue quality, such as C/N ratio, lignin content, substrate N concentration, and lignin/N ratio (Trinsoutrot et al. 2000; Chantigny et al. 2002; Cheng et al. 2015). In this study, soil organic C and total N concentrations, C/N ratio, pH, and lignin content were not significantly different between yak and sheep dung (Table 1), indicating that the quality of both dung might be similar. Thus, it could be expected that both dung application had the consistent effects on soil gross N transformation rates. However, our results showed that the effects of yak and sheep dung application on soil gross N transformation rates varied with soil type. In AS soil, yak and sheep dung application had the similar effects on soil gross rates of N mineralization, nitrification, and NH4 + and NO3 − immobilization, whereas sheep dung application caused a greater increase in gross N mineralization-NH4 + immobilization rates compared with yak dung application in AM soil (Figs. 3 and 4). Therefore, interactive effects of soil type and dung type on soil N transformations exist in spite of the presence of similar quality of dung.
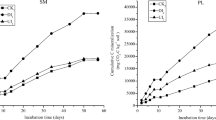
Soil CO2 and N2O emissions
The cumulative CO2 emissions, an indicator of microbial activity over the 21 days of incubation period, were significantly higher in AM than in AS soil regardless of dung application (P < 0.001; Fig. 5). The application of dung increased cumulative CO2 emissions for AS and AM soil by 65 and 120 %, respectively. Similarity, the cumulative N2O emissions over the 21 days of incubation period were significantly higher in AM than in AS soil (P < 0.05; Fig. 5). The application of dung stimulated cumulative N2O emissions, but the stimulation was significant in AM soil alone. N2O is mainly produced by nitrification and denitrification in soils (Hutchinson and Davidson 1993). Since the application of yak and sheep dung decreased gross nitrification rates for both soils (Figs. 3 and 4), nitrification may not be a measurable source of enhanced N2O emissions following dung application. On the contrary, dung addition could provide organic C as the energy source for denitrification (Chen et al. 2013). In addition, dung addition might stimulate microbial growth and activity, and thus increasing oxygen depletion with creation of temporary anaerobic microsites (Goek and Ottow 1988) with stimulation of denitrification. This interpretation was partly supported by the increased cumulative CO2 emissions following dung addition (Fig. 5).
Cumulative emissions of CO2 and N2O over the 21 days of incubation for the alpine steppe (AS) and meadow (AM) soils amended with yak (YD) and Tibetan sheep dung (TSD). SCK without dung addition in AS soil, SYD the addition of yak dung into AS soil, STSD the addition of Tibetan sheep dung into AS soil, MCK without dung addition in AM soil, MYD the addition of yak dung into AM soil, MTSD the addition of Tibetan sheep dung into AM soil. Error bars represent standard deviation for n = 3
Conclusions
Our study showed that gross N mineralization and NH4 + immobilization turnover rates and gross nitrification rates were generally greater in AM than in AS soil. In contrast, gross NO3 − immobilization rates were 65 % times lower in AM than in AS soil. Thus, higher gross nitrification rates coupled with lower gross NO3 − immobilization rates was responsible for higher net nitrification and NO3 − accumulation in AM soil compared with AS soil (Fig. 1b). Dung addition increased gross N mineralization and NH4 + immobilization turnover rates, and thus potentially increased plant N availability. The application of yak and sheep dung decreased gross nitrification rates but increased gross NO3 − immobilization rates for both soils, resulting in a decreased net NO3 − accumulation and potential NO3 − losses through leaching. In general, yak and sheep dung return has a positive effect on soil N supply and retention as a result of increasing NH4 + availability for plant, and simultaneously decreasing NO3 − accumulation in soils. However, the environmental negative effects of enhanced soil CO2 and N2O emissions following yak and sheep dung return should be carefully considered. It is noteworthy that this study was conducted in the laboratory under controlled incubation conditions, in which incubation temperature was much higher than that in situ, caution thus should be exercised when extrapolating these results to the field, and further in situ research needs to be taken into account to confirm our results. However, this study provided a process-based explanation of how yak and sheep dung return influence the internal mineralization immobilization turnover in alpine grassland soils in the Qinghai-Tibetan Plateau. Future work should be performed to investigate the microbial community patterns and the related activity in response to dung return for linking the observed results with biological function.
References
Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75:139–157
Bradley RL (2001) An alternative explanation for the post-disturbance NO3 − flush in some forest ecosystems. Ecol Lett 4:412–416
Bremner JM (1996) Nitrogen-total. In: Sparks DL (Ed) Methods of soil analysis. Part 3. Chemical methods. Soil Science Society of America, Madison, Wi, pp 1085–1121
Burger M, Jackson LE (2003) Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol Biochem 35:29–36
Cai Y, Wang X, Ding W, Tian L, Zhao H, Lu X (2013) Potential short-term effects of yak and Tibetan sheep dung on greenhouse gas emissions in two alpine grassland soils under laboratory conditions. Biol Fertil Soils 49:1215–1226
Cai Y, Wang X, Tian L, Zhao H, Lu X, Yan Y (2014) The impact of excretal returns from yak and Tibetan sheep dung on nitrous oxide emissions in an alpine steppe on the Qinghai-Tibetan Plateau. Soil Biol Biochem 76:90–99
Cai ZJ, Wang BR, Xu MG, Zhang HM, He XH, Zhang L, Gao SD (2015) Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J Soil Sediment 15:260–270
Chantigny MH, Angers DA, Rochette P (2002) Fate of carbon and nitrogen from animal manure and crop residues in wet and cold soils. Soil Biol Biochem 34:509–517
Chen HH, Li XC, Hu F, Shi W (2013) Soil nitrous oxide emissions following crop residue addition: a meta-analysis. Global Change Biol 19:2956–2964
Cheng Y, Wang J, Mary B, Zhang JB, Cai ZC, Chang SX (2013) Soil pH has contrasting effects on gross and net nitrogen mineralizations in adjacent forest and grassland soils in central Alberta, Canada. Soil Biol Biochem 57:848–857
Cheng Y, Wang J, Wang SQ, Zhang JB, Cai ZC (2014) Effects of soil moisture on gross N transformations and N2O emission in acid subtropical forest soils. Biol Fertil Soils 50:1099–1108
Cheng Y, Zhang JB, Müller C, Wang SQ (2015) 15N tracing study to understand the N supply associated with organic amendments in a vineyard soil. Biol Fertil Soils 51:983–993
Davidson EA, Hart SC, Shanks CA, Firestone MK (1991) Measuring gross nitrogen mineralization, immobilization, and nitrification by 15N isotopic pool dilution in intact soil cores. J Soil Sci 42:335–349
de Boer W, Duyts H, Laanbroek HJ (1988) Autotrophic nitrification in a fertilized heath soil. Soil Biol Biochem 20:845–850
Dong QM, Zhao XQ, Wu GL, Chang XF (2015) Optimization yak grazing stocking rate in an alpine grassland of Qinghai-Tibetan Plateau, China. Environ Earth Sci 73:2497–2503
Geng Y, Wang Y, Yang K, Wang S, Zeng H, Baumann F, Kuehn P, Scholten T, He JS (2012) Soil respiration in Tibetan alpine grasslands: belowground biomass and soil moisture, but not soil temperature, best explain the large-scale patterns. PLoS One 7:e34968
Goek M, Ottow JCG (1988) Effect of cellulose and straw incorporation in soil on total denitrification and nitrogen immobilization at initially aerobic and permanent anaerobic conditions. Biol Fertil Soils 5:317–322
Granli T, Bockman OC (1994) Nitrous oxide from agriculture. Norw J Agric Sci 12(Suppl):1–128
Hart SC, Stark JM, Davidson EA, Firestone MK (1994) Dynamics of gross nitrogen transformations in an old-growth forest: the carbon connection. Ecology 75:880–891
Hatch DJ, Jarvis SC, Parkinson RJ, Lovell RD (2000) Combining field incubation with nitrogen-15 labeling to examine nitrogen transformations in low to high intensity grassland management systems. Biol Fertil Soils 30:492–499
Hutchinson GL, Davidson EA (1993) Processes for production and consumption of gaseous nitrogen oxides in soil. In: Harper LA, Mosier AR, Duxbury JM, Rolston DE (eds) Agricultural ecosystem effects on trace gases and global climate change. ASA Special Publication, Madison, 55, pp 79–83
Lan T, Han Y, Roelcke M, Nieder R, Cai Z (2014) Temperature dependence of gross N transformation rates in two Chinese paddy soils under aerobic condition. Biol Fertil Soils 50:949–959
Li P, Lang M (2014) Gross nitrogen transformations and related N2O emissions in uncultivated and cultivated black soil. Biol Fertil Soils 50:197–206
Lin X, Wang S, Ma X, Xu G, Luo C, Li Y, Jiang G, Xie Z (2009) Fluxes of CO2, CH4, and N2O in an alpine meadow affected by yak excreta on the Qinghai-Tibetan plateau during summer grazing periods. Soil Biol Biochem 41:718–725
Lovell RD, Jarvis SC (1996) Effect of cattle dung on soil microbial biomass C and N in a permanent pasture soil. Soil Biol Biochem 28:291–299
Lu XY, Fan JH, Yan Y, Wang XD (2011) Soil water soluble organic carbon under three alpine grassland types in Northern Tibet, China. Afr J Agric Res 6:2066–2071
Lu XY, Yan Y, Fan JH, Wang XD (2012) Gross nitrification and denitrification in alpine grassland ecosystems on the Tibetan Plateau. Arct Antarct Alp Res 44:188–196
Luxhøi J, Elsgaard L, Thomsen IK, Jensen LS (2007) Effects of long-term annual inputs of straw and organic manure on plant N uptake and soil N fluxes. Soil Use Manage 23:368–373
Mary B, Recous S, Robin D (1998) A model for calculating nitrogen fluxes in soil using 15N tracing. Soil Biol Biochem 30:1963–1979
Müller C, Laughlin RJ, Christie P, Watson CJ (2011) Effects of repeated fertilizer and cattle slurry applications over 38 years on N dynamics in a temperate grassland soil. Soil Biol Biochem 43:1362–1371
Murphy DV, Recous S, Stockdale EA, Fillery IRP, Jensen LS, Hatch DJ, Goulding KWT (2003) Gross nitrogen fluxes in soil: theory, measurement and application of 15N pool dilution techniques. Adv Agron 79:69–118
Paul EA, Clark FE (1989) Soil microbiology and biochemistry. Academic Press, San Diego
Recous S, Mary B, Faurie G (1990) Microbial immobilization of ammonium and nitrate in cultivated soils. Soil Biol Biochem 22:913–922
Rennenberg H, Dannenmann M, Gessler A, Kreuzwieser J, Simon J, Papen H (2009) Nitrogen balance in forest soils: nutritional limitation of plants under climate change stresses. Plant Biol 11:4–23
Rice CW, Tiedje JM (1989) Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biol Biochem 21:597–602
Romero CM, Engel R, Chen CC, Wallander R (2015) Microbial immobilization of nitrogen-15 labelled ammonium and nitrate in an agricultural soil. Soil Sci Soc Am J 79:595–602
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Shi W, Norton JM (2000) Microbial control of nitrate concentrations in an agricultural soil treated with dairy waste compost or ammonium fertilizer. Soil Biol Biochem 32:1453–1457
Shi W, Miller BE, Stark JM, Norton JM (2004) Microbial nitrogen transformations in response to treated dairy waste in agricultural soils. Soil Sci Soc Am J 68:1867–1874
Shindo H, Nishio T (2005) Immobilization and remineralization of N following addition of wheat straw into soil: determination of gross N transformation rates by 15N-ammonium isotope dilution technique. Soil Biol Biochem 37:425–432
Sotta ED, Corre MD, Veldkamp E (2008) Differing N status and N retention processes of soils under old-growth lowland forest in Eastern Amazonia, Caxiuanã, Brazil. Soil Biol Biochem 40:740–750
Stark JM, Hart SC (1997) High rates of nitrification and nitrate turnover in undisturbed coniferous forests. Nature 385:61–64
Ste-Marie C, Pare D (1999) Soil, pH and N availability effects on net nitrification in the forest floors of a range of boreal forest stands. Soil Biol Biochem 31:1579–1589
Sun Y, Angerer JP, Hou FJ (2015) Effects of grazing systems on herbage mass and liveweight gain of Tibetan sheep in Eastern Qinghai-Tibetan Plateau, China. Rangeland J 37:181–190
Tappi (2006) 2006–2007 TAPPI test methods. TAPPI Press, Norcoss, GA 30092, USA
Trinsoutrot I, Recous S, Bentz B, Linères M, Chèneby D, Nicolardot B (2000) Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci Soc Am J 64:918–926
Yao H, Campbell CD, Qiao X (2011) Soil pH controls nitrification and carbon substrate utilization more than urea or charcoal in some highly acidic soils. Biol Fertil Soils 47:515–522
Yue G, Zhao L, Zhao Y, Li Y (2010) Research advances of grassland ecosystem CO2 flux on Qinghai-Tibetan Plateau. J Glaciol Geocryol 32:166–174
Zhang JB, Müller C, Zhu TB, Cheng Y, Cai ZC (2011) Heterotrophic nitrification is the predominant NO3 − production mechanism in coniferous but not broad-leaf acid forest soil in subtropical China. Biol Fertil Soils 47:533–542
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (41301238, 41573070), the Natural Science Foundation of Jiangsu Province (BK20131045), the Research Fund of State Key Laboratory of Soil and Sustainable Agriculture, Nanjing Institute of Soil Science, Chinese Academy of Science (Y412201421, Y412201403), and the Research Fund of Jiangsu Key Laboratory of Environmental Change and Ecological Construction.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, Y., Cai, Y. & Wang, Sq. Yak and Tibetan sheep dung return enhance soil N supply and retention in two alpine grasslands in the Qinghai-Tibetan Plateau. Biol Fertil Soils 52, 413–422 (2016). https://doi.org/10.1007/s00374-016-1088-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-016-1088-6