Abstract
We studied the responses of soil microbial biomass, respiration and enzyme activities to temperature in three Mexican soils. Soils were incubated at temperature range of 15–550 °C at 50 (wet) and 10 % (dry) of their water holding capacity. Soils were assayed for their adenosine triphosphate (ATP) content, CO2-C evolution and acid and alkaline phosphomonoesterase, phosphodiesterase, β-glucosidase, urease, and protease activities. Thermal responses of soil enzyme activities were fitted to both the equilibrium model (EM) and the classical Q10 model to describe the effects of temperature on enzyme activity. Total organic C, Fe speciation, available P, and inorganic N contents were also determined in all soils and at all temperatures. The results showed that the ATP content, CO2-C evolution, and enzyme activities of soil increased within the temperature range 15–65 °C, and at higher temperatures, soil enzyme activities were more resistant than the ATP content and CO2-C evolution. The effects of temperature were more drastic in wet than in dry soil, although the two soil series displayed similar trends. The enzyme activities showed a good fit to the EM, making this model suitable for determining activation energy, enthalpy of inactivation, and equilibrium inactivation temperatures of soil enzymatic reactions. The results of the EM indicated that soil chemical parameters may not affect the activation energy of enzyme reactions, but may influence the enzyme resistance to inactivation at higher temperature. The results also indicated that the EM model can be used for predicting the impact of high temperatures on soil enzyme activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial activity and enzyme activities of soil are responsible for nutrients cycling in the terrestrial ecosystems, their availability to plants and microorganisms, and both are key factors of soil fertility. Microbial activity and soil enzyme activity are sensitive to several environmental factors such as soil organic matter (SOM) content and quality (Sinsabaugh et al. 2002; Waldrop et al. 2004; Zornoza et al. 2009), soil management (Bandick and Dick 1999), soil pollution and remediation (D’Ascoli et al. 2006; Kumpiene et al. 2009), composition of microbial communities (Kamolmanit et al. 2013; Moreno et al. 2013; Nannipieri et al. 2012), and soil warming (Schimel and Weintraub 2003; Wallenstein et al. 2011). Decomposition of plant litter and SOM by soil microorganisms requires the coordinated synthesis of various enzymes hydrolyzing the plant polymers and mineralizing N, P, and S from organic compounds to meet the microbial metabolic nutrient demand, with elemental stoichiometric relationships in soils of different ecosystems (Sinsabaugh et al. 2009). Generally, the synthesis of enzymes responds to nutrient availability in soil (Allison and Vitousek 2005; Renella et al. 2007a, b; Nannipieri et al. 2012). Temperature is one of the main environmental factors influencing the soil processes and soil enzyme activity. Soils can reach high temperatures at their surfaces depending on the sunlight irradiation intensity, their color, on land use, and vegetation cover (Doran et al. 1984). In particular, tropical surface of soils can reach temperatures up to 50 °C, especially when they are subjected to cropping regimes (Lal et al. 1980), mulching and ridging the soil surface, or to solarization to suppress soil-borne diseases. In tropical environments, fire is also used to clear forest areas to gain arable lands, increase nutrient availability, control weeds, and reduce fire hazard in forests. Soil temperature under fire can range between 170 °C and 410 °C depending on the soil characteristics and the fire event (Biederbeck et al. 1980).
High thermal resistance of clay- and humic-enzyme complexes to thermal denaturation at temperatures higher than 400 °C has been reported (Nannipieri et al. 1996; Ciardi 1998).
We hypothesize that enzyme activity of soils from different location and under different management have a different thermal response. A differential sensitivity of soil enzymes may alter soil organic matter quality and soil fertility on the long term in soils that experience high temperatures, and therefore, determination of the thermodynamic parameters of important soil enzyme activities is crucial to assess the impact of increasing temperature on soil biochemical activity and fertility. Most of the previous studies on the effects of increased temperature on soil microbial activity and enzyme activity have involved single soils and single soil management or the measurement of one or a few enzyme activities. Most of the modeling on the thermal sensitivity of soil enzyme activity has concerned the impact of the climate change on soil enzyme activity and the responses to increased temperatures (Burns et al. 2013). Differently, resistance of the different soil enzyme activities to thermal denaturation in soils at higher temperatures than those of the environment has generally not been modeled, and information about the thermal parameters of soil enzymes is still scarce (Steinweg et al. 2013). Responses of enzyme activity to temperature has often been described by a two state model (hereafter referred as classical model) that considers changes in the maximum reaction rate (Vmax) and enzyme substrate affinity (Km) until enzymes are irreversibly inactivated by thermal denaturation. Peterson et al. (2007) proposed the three state equilibrium model (EM) to describe the effects of temperature on enzyme activity in terms of a reversible equilibrium between the active and inactive state, but not necessarily denatured forms of enzymes. In vitro studies demonstrated that the EM better represented the responses of enzyme activity to temperature by accounting for the thermodynamic terms of the enzyme’s substrate affinity and inactivation than classical models (Daniel et al. 2010). The use of the EM is also interesting because by considering both the ascending and descending parts of the thermal response curves, instead of only the ascending parts like the Q10 models, it allows for a much better determination of the parameters.
Soil moisture is an important soil co-variate parameter when assessing the temperature effects on soil microbial biomass, respiration and enzyme activity (Brockett et al. 2012), and stronger dependence of soil respiration and enzyme activity on warming moist than dry soils have been reported from either laboratory or field studies (Conant et al. 2004; Steinweg et al. 2012). Because tropical soils undergo to prolonged water limitation periods during the dry season followed by intense rain period, the study of interactive effects of temperature and soil moisture on soil microbial biomass and biochemical activity can be important as irregular rainfall due to global climate changes is expected also in nontropical areas in the future (Planton et al. 2008).
The aim of the present work was to evaluate the thermal sensitivity of acid and alkaline phosphatase, phosphodiesterase, β-glucosidase, urease, and protease activities in three tropical soils under forest and coffee crop management, located in areas with different mean temperature by using the EM (Daniel and Danson 2010). In particular, we determined the activation energy of soil enzyme activities, the change in enthalpy between the activated and inactivated forms of soil enzymes, and the equilibrium temperature between the activated and inactivated forms of enzymes in soils at two soil moisture levels. To our knowledge, this is the first application of the EM to describe the thermal sensitivity of soil enzyme activity. The thermodynamic parameters determined by the EM were compared to the Q10 values. Because soil enzyme activity depends on both intracellular and soil-stabilized extracellular enzyme activities which may differ for their thermal catalytic and stability optima (Nannipieri et al. 2012; Rao and Gianfreda 2000), microbial biomass and respiration of the three soils were measured and their relationship with enzyme activity and specific soil main properties was discussed. Iron speciation at different temperatures was also monitored because Fe forms can stabilize extracellular enzymes in tropical soils.
Materials and methods
Soil sampling and analysis of the main chemical properties
Three Mexican soils, two from the Oaxaca region and one from the Puebla region, were studied. Two soils were sampled from Sierra Norte of Oaxaca, in the Talea de Castro District (17° 20′ LN 96° 24′ LW) and one from the Puebla district (Ahuatamimilotl, Municipio Tlatauquitepec 97° 25′ LW 20° 00′ LN). One of the two Oaxaca soils (Anthropic Kandihumults) was under coffee crop (Coffea arabica L.) intercalated by scattered natural vegetation, whereas the other Oaxaca soil (Typic Kandihumults) and the Puebla soil (Andic Kandihumults) were under a tropical semideciduous forest dominated by Pinus spp. and Quercus spp. All soils were sampled at 0–20 cm depth and their main characteristics are reported in Table 1. Climate in the Oaxaca area is warm humid with annual precipitation of ca. 2,000 mm and mean annual temperature of 22 °C, with rain events mainly concentrated in the period June-November followed by a dry season in the December-May period. Climate of the Puebla area is humid-temperate with ca. 2,000 mm year precipitation and mean annual temperature of 15 °C, with a wet period between June and November and a dry winter period.
For the analysis of the main chemical properties, soils were sieved (<2 mm) and air dried. Soil pH was measured at each temperature step by using a soil water mixture (1:2.5 soil to water ratio). Total Fe (Fetot) was determined after digestion of soil with a mixture of concentrated acids (HNO3, HF) and H2O2 in a microwave oven (Lim and Jackson 1982). Iron pools were characterised as it follows: poorly crystalline Fe oxides were extracted with ammonium oxalate (Feo; Schwertmann 1964), crystalline Fe (Fedcb) with dithionite-citrate-bicarbonate as extractant (Mehra and Jackson 1960), and organic Fe (Fepyr) with sodium pyrophosphate as an extractant (Bascomb 1968). Inorganic N (NH4 +-N plus NO3 −-N) was extracted by shaking 5 g fresh soil for 1 h with 1 M KCl (ratio of 1:5 soil solution) (Keeney and Nelson 1982). Soil suspensions were filtered through glass fibre filter (Whatman GF/A) and analysed for NH4 +-N and NO3 −-N content by a flow injection analyser system (FIAS 300 Perkin-Elmer) coupled to a spectrophotometer (Lambda 2 Perkin-Elmer). The soil available P was determined according to Bray and Kurtz (1945) and the extractable C (Ext-C) was determined after soil extraction with 0.5 M K2SO4 followed by C dichromate wet oxidation (Walkley and Black 1934).
Incubation experiments and microbial and biochemical analyses
Soils were sieved (<2 mm) at field moisture, moistened at the 10 or 50 % water holding capacity (WHC), and soil portions (50 g) were incubated into 1 L air tight glass jars each provided with a 3-way valve for gas sampling. All jars were incubated for 7 days at 15 °C, 20 °C, 25 °C, 30 °C, 37 °C, 45 °C, 55 °C, 65 °C, and 80 °C. Other portions (50 g) of both soils moistened at the 10 or 50 % WHC were placed into closed glass or ceramic containers and placed for 24 h at 110 °C and for 6 h at 250 °C and 550 ° C. All treatments were replicated three times for each incubation time.
During the incubation period, all jars were regularly opened to refresh the headspace gas and closed for 24 h before new headspace was sampled by an air-tight syringe for measuring the CO2-C evolution by gas-chromatography, according to Blackmer and Bremner (1977); the gas sample was injected into a gas-chromatograph (Hewlett-Packard 6890) equipped with a gas-sampling valve, a packed column (Poropack Q), and a thermal conductivity detector, and run in isothermal mode.
Soil microbial biomass was measured by quantifying the adenosine triphosphate (ATP) content of soils according to Ciardi and Nannipieri (1990). Acid and alkaline phosphomonoesterase activities were measured according to Tabatabai and Bremner (1969). The phosphodiesterase activity was measured according to Browman and Tabatabai 1978. The β-glucosidase activity was measured according to Tabatabai (1982). Protease activity was determined by hydrolysis of N-benzoylargininamide (BAA) according to Ladd and Butler (1972), and the urease activity was measured as reported by Nannipieri et al. (1974), using 0.5 g of soil and 2 ml of 0.1 M phosphate. Concentration of p-nitrophenol (p-NP) produced in the assays of acid and alkaline phosphomonoesterase, phosphodiesterase and β-glucosidase activities was calculated from a p-NP calibration curve after subtraction of the absorbance of the controls at 400 nm wavelength. The NH4 +-N produced by urease and BAA hydrolyzing activities was determined by a Flow Injection Analyzer (FIAS 300-Perkin Elmer) coupled with a spectrophotometer Lambda 2 (Perkin Elmer) as for the inorganic N measurements. Potential NH4 +-fixation by the studied soils was evaluated by measuring the NH4 +-N recovery from solutions with NH4 +-N concentrations in the same range as product concentrations of both urease and protease activities; all soils were shaken with the NH4 +-N solution for 1 h at 37 °C and then extracted with 2 M KCl. The NH4 +-N recovery from the test solutions was greater than 95 % for all soils.
Data analysis and modeling
Data analysis was performed using the R software (R Development Core Team 2011). Correlations were assessed by Pearson’s correlation coefficient. Optimal temperatures (Topt) were calculated by fitting the data with a local polynomial regression (Cleveland et al. 1992) and then selecting the temperature at which the considered parameter was at its maximum on the regressed function.
The thermodynamic parameters of soil enzymes were determined according to the EM (Daniel and Danson 2010) using the entire temperature range:
This model considers the inactivation of the enzyme due to temperature as a reversible reaction described by an equilibrium constant, and it allows for a higher precision compared to a model based on the idea of an irreversible activation. Full details on the model can be found in Peterson et al. (2007), Daniel and Danson (2010), and Daniel et al. (2010).
Because in the used soil enzyme assay the period of time is fixed and substrate concentration is not limiting, we simplified the model (Eq. 2) to consider thermal enzyme inactivation as the only limiting factor and the measured enzyme activity as constant during the assay.
This simplification does not affect the accuracy of the model in estimating the thermodynamic parameters of enzymes as shown by Peterson et al. (2007). In Eq. 2, Vmax is the activity at time t and it is therefore a known term, and E0 represents the total concentration of enzyme in the essay. The term kcat in Eq. 3 represents the enzyme catalytic rate constant according to the Eyring-Polanyi equation and is calculated as:
where kb is the Boltzmann’s constant, h is the Planck’s constant, ΔGcat represents the Gibbs’ free energy of activation for the enzyme catalyzing the reaction, and T is the temperature (°K) at which the reaction takes place and it is a known term. The term kcat in Eq. 3 describes the increase of the reaction rate by increasing the temperature.
The term Keq in Eq. 4 represents the equilibrium constant describing the ratio between the inactive and active forms of the enzyme and is calculated as:
where ΔHeq is the change in enthalpy for the transition from the active to the inactive form of the enzyme, R is the gas constant (known term), and Teq is the temperature at which the equilibrium between the active and inactive forms of the enzyme is at its middle point. The term Keq in Eq. 4 describes the decrease in the reaction rate at high temperature caused by the inactivation of the enzyme due to modification of the protein structure induced by heat.
Equation 2 was calibrated for each site, condition and enzyme by a Hooke–Jeeves optimization algorithm (Quarteroni et al. 2007) through minimization of the root mean squared errors. In order to estimate the error of the calibrated parameters, we applied the Monte Carlo Markov Chain (MCMC) approach. The model was again calibrated by a Metropolis-Hastings search algorithm, implemented in R through the JAGS sampler (Plummer 2003), and considering the results of the previous calibration a the prior knowledge, which for each parameter was described with the normal distribution with mean corresponding to the previously calibrated value and error to 0.8 times of such value. Each MCMC was based on 100,000 runs. The resulting error estimate associated with each parameter is calculated as the standard deviation of the whole MCMC. When two values of the same parameter, including their error, do not overlap, it means that they are significantly different. The procedure for model calibration considering all replicates in a Bayesian framework allows a precise uncertainty estimate (Kruschke 2013). For the comparison of values, we rely, therefore, on the deviation of the calibrated parameters. Please note that such deviation is that of the whole Markov Chain and not that of the original samples.
Soil enzyme activity was also modelled with the Q10 function considered as the classical model:
where V1 and V2 are enzyme activities at T1 and T2 temperatures, respectively. The Q10 calculation was performed at 15 °C and 30 °C and the Q10 function was calibrated with the MCMC method based on the same number of runs to have an error estimate comparable with Eq. 2.
Results
Changes in soil chemical properties induced by temperature
The effects of temperature on soil chemical properties are reported in Table S-1. Because there were no significant differences in the chemical properties of wet or dry soils, values in Table S-1 were presented as average values of the wet and dry soils. The pH value markedly increased in all soils for temperatures higher than 110 °C. The crystalline Fe content (Feo) did not change in the Oaxaca coffee soil whereas it increased in the Oaxaca soil under forest and slightly decreased in the Puebla soil (Table S-1). The total (Fetot) and crystalline (Fedcb) Fe contents markedly increased at 110 °C and at higher temperatures than 110 °C in all soils, particularly in the Oaxaca soil under coffee crop (Table S-1). Phosphorus availability was higher in the Oaxaca soil under coffee crop than in the Oaxaca and Puebla forest soils (Table S-1). The P availability increased in all soils by increasing temperature, markedly for temperature higher than 250 °C (Table S-1) due to ash formation. The Ext-C content of the two Oaxaca soils was similar, whereas the Puebla soil contained 2.7 and 3.3 times more Ext-C than the Oaxaca cropped and forest soils, respectively, and decreased in all soils by increasing temperature, with two breakpoints at 250 and 550 °C (Table S-1). Inorganic N (exchangeable NH4 +-N, NO3 −-N) concentrations of the two Oaxaca soils were similar whereas the Puebla soil contained less inorganic N, particularly exchangeable NH4 +-N than Oxaca soils (Table S-1). The exchangeable NH4 +-N concentration showed a slight increase in all soils up to 45 °C for the Oaxaca coffee soil, 55 °C for the Oaxaca forest soil, and 37 °C for the Puebla forest soil (Table S-1). The NO3 −-N content increased more than the exchangeable NH4 +-N content up to 45 °C for the Oaxaca coffee soil and 37 °C for the Oaxaca forest soil and for the Puebla forest soil (Table S-1).
Changes in soil ATP content, respiration, and enzyme activity by increasing temperature
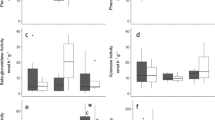
The ATP content, soil respiration, and enzyme activities increased upon temperature increase up to 30–55 °C, then decreased in all soils after exposure at higher temperatures (Fig. 1). The peak values varied depending on the enzyme activity and soil; but in general, the enzyme activity peaks were reached at lower temperatures in the Puebla than in the two Oaxaca soils, whereas no definite trends in relation to the management of the two Oaxaca soils (Fig. 1). By considering the temperature of 25 °C, commonly used for studying soil respiration under laboratory conditions, the Puebla soil had approximately 3 and 5 times higher ATP content than the Oaxaca forest and coffee soils, respectively (Fig. 1). At 25 °C, also respiration values were significantly higher than the those of Oaxaca forest and coffee soils (Fig. 1). The Oaxaca forest soil had a significantly higher ATP content than the Oaxaca cropped soil (Fig. 1). Enzyme activities showed no significantly different values in the two Oaxaca soils, with the exception of urease and protease activities, which were significantly higher in the Oaxaca forest soil (Fig. 1). The Puebla soil had much higher enzyme activities than the Oaxaca soils, particularly the acid phosphomonoesterase activity (Fig. 1).
The soil ATP content and respiration were significantly correlated with the measured enzyme activities, with the strongest correlation values found for ATP content with acid phosphomonoesterase activity (r = 0.91, P < 0.01), soil respiration with protease (r = 0.89, P < 0.01), and β-glucosidase (r = 0.82, P < 0.01) activities.
Modelling of thermal responses of soil ATP content, respiration, and enzyme activity
The optimal temperature (Topt) of the soil biochemical parameters in °C is reported in Table 2. The Topt for the ATP content was in the range 20.7–26.0 °C, the Topt for of CO2 evolution was in the range 28.7–41.3 °C, whereas optimal temperature for soil enzyme activities, except for the alkaline phosphomonoesterase activity of the Oaxaca dry soil, showed higher optimal temperatures than those of ATP content and CO2 evolution.
The Q10 values, calculated according to Eq. 5 (Table 3), ranged from 0.79 to 1.52.
Values of activation energy required by the catalytic reaction (ΔGcat) were in the 44.1–68.8 kJ range, with the highest values of urease and protease activities (Table 4). The ΔGcat values were generally higher in the dry than the wet soils with the exceptions of phosphodiesterase activity of the Oaxaca coffee soil, β-glucosidase activity of the Puebla soil, and urease activity of the Puebla and Oaxaca forest soils (Table 4).
Values of enthalpy of the equilibrium between activated and inactivated enzymes (ΔHeq) did not show a specific trend (Table 4). In the Puebla soil, the acid and alkaline phosphomonoesterase, β-glucosidase, and urease activities had lower values in dry than in wet soil and the opposite was observed for the phosphodiesterase and protease activities (Table 4). In the Oaxaca forest soil, all the measured enzyme activities except the urease activity showed lower ΔHeq values (Table 4) in the wet than dry soil; whereas in the Oaxaca coffee soil, the acid and alkaline phosphomonoesterase and urease activities showed lower ΔHeq values in the wet than dry soil, and the phosphodiesterase, β-glucosidase, and protease activities showed lower ΔHeq values in the dry than wet soil (Table 4).
The equilibrium temperature values (Teq) for the measured enzyme activities varied depending on the soil type and moisture content (Table 4). In the Puebla forest soil, the acid phosphomonoesterase and the phosphodiesterase showed lower Teq values in the wet than dry soil whereas the alkaline phosphomonoesterase, β-glucosidase urease, and protease activities had lower Teq values in dry than in wet soil (Table 4). In the Oaxaca forest soil, the acid phosphomonoesterase, phosphodiesterase, and protease activities had lower Teq values in the wet than the dry soil; whereas, the alkaline phosphodiesterase, β-glucosidase, and urease activities had lower Teq values in the dry than wet soil (Table 4). In the Oaxaca coffee soil, the acid phosphomonoesterase and β-glucosidase activities showed lower Teq values in the wet than dry soil; whereas, the alkaline phosphomonoesterase, phosphodiesterase, urease, and protease activities showed lower Teq values in the dry than in the wet soil (Table 4).
In general, a good fit of the measured values with those predicted by the EM model was observed for both wet and dry soils (Fig. 2); whereas, the Q10 values, calculated according to Eq. 5, were relatively low and characterized by high deviation of the calibrated values (Table 3).
Measured versus predicted values by Eq. 3 for each enzyme activity the studied soils. The x axis reports the temperature (°K) while the y axis the enzyme activity
Discussion
The ATP content, soil respiration, and enzyme activity were markedly changed at 65 °C, a temperature which can ben reached in the Mexican soils during the hot periods. The peak values of the ATP content of the wet soils decreased more in the Oaxaca and Puebla forest soils (28 and 36 %, respectively) than in the Oaxaca coffee soil (17 %); whereas, decreases in the ATP content of the dry soils were less pronounced. Ciardi (1998) observed a marked decrease in the ATP content of fresh soils heated at temperature higher than 60 ° C; whereas, the decrease in the ATP content in air-dried soils was slower than in fresh soils even at temperatures higher than 100 °C. Lower impact of heating on the ATP content of the Oaxaca coffee soil may depend on the presence of an higher abundance of heat-resistant microorganisms than in forest soils. Our results also confirmed the previous finding that heating of wet soils is more effective in destroying soils mircoorganisms than heating of dry soils (Speir and Ross 1978), probably due to the better conduction of heat by water in filled pores then by gas.
Soil respiration was the most sensitive process responding to soil drying and heating. Impact of moisture on soil respiration is well established (Tang and Baldocchi 2005; Moyano et al. 2012) and is related to both cellular activity and better SOM solubility and better movement of available organic C in pores of moist soils (Kätterer et al. 1998). Temperature impacted the microbial utilization of SOM possibly due to changes in the composition of the active microbial communities and/or physiological stress induced by high temperature, whereas soil drying smoothed the temperature effects, particularly in the Puebla soil, thus confirming the interactive effects of temperature and moisture on soil respiration (Moyano et al. 2012). The observed progressive increase up to peak values and the sudden decay of soil respiration by increasing temperature could be due to the activation of viable but dormant soil microorganisms or bacterial and fungal spores followed by their inactivation or death at high temperatures, as reported for the spore forming bacterium Bacillus subtilis extracted from soils and exposed to different temperatures and moisture regimes (Funke and Harris 1968).
The three studied soils showed very high acid phosphomonoesterase activity, probably related to extracellular enzyme release by soil microorganisms in response to low P availability in the three soils (Nannipieri et al. 2011, 2012). Differently, the alkaline phosphomonoesterase activity was relatively low in all soils, likely due to the acidic soil pH values (Nannipieri et al. 2011). Predominance of acidic over alkaline phosphomonoesterase activity in acidic soils has been previously reported (Dick et al. 2000). Such differences could depend on the greater molecular stability and catalytic activity of the acid phosphomonoesterases in the acid soils but also on the specific composition of soil microbial communities of acidic soils (Renella et al. 2006). In fact, it has been proposed that the acid phosphomonoesterase is mainly produced by free-living and michorrizal fungi roots whereas the alkaline phosphomonoesterases is mainly synthesized by bacteria (Taylor et al. 2002). Fungi should be predominant and more active than bacteria in acidic soils (Killham 1994), but a better relationship between soil enzyme activity and microbial community composition requires determination of both composition and expression of phosphomonoesterase encoding genes (Renella et al. 2006; Nannipieri et al. 2012). From in vitro studies, it was shown that different soil microbial species synthesize different types of phosphomonoesterase with different optimal temperatures and stability, some of them being still active at 70 °C (Wyss et al. 1998). Soil enzyme activities persisted at higher temperatures than ATP and respiration, with residual but still measureable values after soil exposure at 550 °C (Fig. 1). Even if it is well established that organo-mineral phosphomonoesterase complexes can protect enzymes from thermal inactivation (Nannipieri et al. 2011; Rao and Gianfreda 2000), it is unlikely that these enzyme complexes are still present at temperatures as high as 500 °C, and such activity can be due to inorganic catalysts (Ruggero et al. 1996). Unfortunately, the present enzyme assays do not discriminate among intracellular enzyme activities, stabilized extracellular enzyme activities and enzyme-like activities (Nannipieri et al. 2012).
The ATP content and CO2 production peaked at lower temperature than enzyme activities although they were correlated; such correlation may depend on the contribution of the stabilized extracellular enzyme activities to the respective measured enzyme activity. If this hypothesis is true, it confirms that the stabilized extracellular enzymes are active under conditions limiting the microbial activity in soil.
The classical Q10 model (Eq. 5) presented some limitations, not being able to consider anything above or equal to the optimal temperature and thus discarding many relevant information. The chosen limit of 35 °C ensures that all data points were below the optimal value and in the growing part of the curve (Table 3).
The calibration of the EM over the broad temperature range we used allowed us to estimate the enzymes thermodynamic parameters with a high accuracy, as showed by the low errors associated with each parameter. This increases in precision is one of the main advantages over calibrating an exponential Q10 model over the ascending part of the curve.
The measurement of soil enzyme activity over a broad temperature range demonstrated that the EM could well describe the behavior of enzyme activities across a wide temperature range for both wet and dry soils (Fig. 2). Differently, the Q10 model can only be applied in the range of increasing enzyme activities providing only a partial picture of the behaviour of enzyme activities in soils under broad temperature variations (Silverstein 2012).
The calculated values of ΔGcat (Table 4), indicating the activation energy required by the catalytic reaction, were inversely proportional to the measured enzyme activity as expected, and in the same order of magnitude of those previously reported (Peterson et al. 2007; Daniel and Danson 2010). Among the wet soils, the ΔGcat values were higher in the Oaxaca coffee than in the Oaxaca and Puebla forest soils (Table 4). This could be explained by the fact that the coffee cropped soil is subjected to higher mean temperatures and hosts microbial communities synthesizing more thermal resistant enzyme isoforms than microbial communities of the forest soils, while subjected to lower mean temperatures. Only the acid phosphomonoesterase activity displayed slightly lower activation energies and seemed less temperature-resistant in the Puebla soil, whose area has colder annual mean temperatures than the Oaxaca area (Table 4). The acid phosphomonoesterase activity seemed to be more resistant to thermal inactivation in the two Oaxaca soils than that of Puebla (Table 4), although the initial enzyme activity of the latter was about three times higher than those of the former soils. Differences in the activation energy among enzyme in different soils could be calculated by the EM thanks to its greater accuracy than the Q10 model, the latter showing large deviations in values and this makes impossible to understand differences between different enzymes in a soil and for a specific enzyme in the studied soils.
The ΔHeq values, indicating the enthalpy of the equilibrium between activated and inactivated enzyme forms, can be related to the enzyme protection from inactivation (Daniel and Danson 2010). By considering these values, we hypothesize that both intracellular and extracellular enzyme activities contributed to the overall measured enzyme activity of wet soils; whereas probably in dry soils, the extracellular stabilized enzyme activities were those mainly contributing to the overall measured enzyme activity. The lack of a precise pattern in the ΔGcat and ΔHeq parameters between wet and dry soils could be attributed to the fact that different enzymes were contributing to the enzyme activity of the wet and dry soils. These differences could be due to microbial physiological adaptation or changes in the nutrient availability to soil microorganisms, as hypothesized by Allison et al. (2010).
The equilibrium temperature (Teq) indicates the upper activity limit of the enzyme that can be reached before enzyme inactivation, and in our case represent site-specific differences. Among the wet soils, the Oaxaca coffee soils displayed the highest Teq values for the phosphatase and urease activities; whereas, all forest soils displayed the highest Teq values for the β-glucosidase and protease activities as compared to the cropped soil. Dry soils behaved in a different way (Table 4), as the phosphodiesterase activity presented both a high Teq and a high ΔHeq in the Puebla dry soil, suggesting a high stability of the enzyme probably related to its higher SOM content.
Overall, the complex pattern of thermodinamic values of the measured enzyme activities determined by the EM indicated that the stability of enzymes of the three soils depended on soil type, management, and moisture contents, and probably, on different enzymes catalyzing the same reaction and different enzyme synthesis rates by different microbial communities of the three soils. Differently, the response of the activation energy over temperature was not particularly influenced by soil type. Edaphic factors seemed, therefore, affecting soil enzyme stability as much as moisture. It has already been noted (Burns et al. 2013) that drought can slow down enzyme turnover rates; but in this experiment, this was only true for some enzyme activities. These results provide a deeper understanding for the lack of acclimation of soil enzyme activity to increased temperature as previously reported (Jing et al. 2013). The complex interactions between soil parameters and enzyme activities revealed by our study evidentiate the necessity to account for soil parameters in future models assessing the effects of global warming on soil biological and biochemical activity.
Conclusions
Our results showed that enzyme activities of tropical soils have different thermal sensitivity reflecting their location and use, with soils under warmer climate and agricultural use generally showing higher optimal temperatures and greater resistance to thermal denaturation than in soils from forest ecosystems and colder climate. Enzyme activity in soil persists at higher temperature than microbial biomass and more than soil respiration likely due to stabilization of extracellular enzyme activities and presence of enzyme-like activities. Our results also prove that the EM was suitable for modelling the thermal response of soil enzyme activity over a wider temperature interval than that usually considered for global warming studies and was also more accurate than the classical Q10 model. We suggest that the EM can be used for further studies on the effects of increased temperature on soil enzyme activity, especially when the soil is subjected to temperatures beyond the optimal value. The EM model showed that the activation energy of enzymatic reactions was less influenced by soil types or land use than the enzyme protection against inactivation. This result suggests that the response of soil enzyme activities to increased temperatures, considered here as a change in the activation energy of the reaction, is more influenced by soil moisture and land soil management than by soil physico-chemical properties. In this sense, the equilibrium temperature (Teq) and the enthalpy of inactivation ΔHeq could be used as indices of site-specific thermal sensitivity of soil enzyme activity.
References
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Bandick AK, Dick RP (1999) Field management effects on soil enzyme activities. Soil Biol Biochem 31:1471–1479
Bascomb CL (1968) Distribution of pyrophosphate-extractable iron and organic carbon in soils of various groups. J Soil Sci 19:251–258
Biederbeck UO, Campbell CA, Bowren KE, Schnitzer M, McIver RN (1980) Effects or burning cereal straw on soil properties and grain yields in Saskatchewan. Soil Sci Soc Am J 44:103–111
Blackmer AM, Bremner JM (1977) Gas chromatographic analysis of soil atmosphere. Soil Sci Soc Am J 41:908–912
Bray RH, Kurtz LT (1945) Determination of total, organic and available form of phosphorus in soils. Soil Sci 59:39–45
Brockett BFT, Prescott CE, Grayston SJ (2012) Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol Biochem 44:9–20
Browman MG, Tabatabai MA (1978) Phosphodiesterase activity of soils. Soil Sci Soc Am J 42:284–290
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234
Ciardi C (1998) Soil heating to distinguish the contribution of abiotic and extracellular activities to the overall enzyme activity in soil. Agrochimica 42:104–117
Ciardi C, Nannipieri P (1990) A comparison of methods for measuring ATP in soil. Soil Biol Biochem 22:725–727
Cleveland WS, Grosse E, Shyu WM (1992) Local regression models. In: Chambers JM, Hastie TJ (eds) Statistical Models in S. Wadsworth, Pacific Grove, pp 249–308
Conant RT, Dalla-Betta P, Klopatek CC, Klopatekd JM (2004) Controls on soil respiration in semiarid soils. Soil Biol Biochem 36:945–951
D’Ascoli R, Rao MA, Adamo P, Renella G, Landi L, Rutigliano FA, Terribile F, Gianfreda L (2006) Impact of river overflowing on trace element contamination of volcanic soils in south Italy: Part II. Soil biological and biochemical properties in relation to trace element speciation. Environ Poll 144:317–326
Daniel RM, Danson MJ (2010) A new understanding of how temperature affects the catalytic activity of enzymes. Tr Biochem Sci 35:584–591
Daniel RM, Peterson ME, Danson MJ, Price NC, Kelly SM, Monk CR, Weinberg CS, Oudshoorn ML, Lee CK (2010) The molecular basis of the effect of temperature on enzyme activity. Biochem J 425:353–360
Dick WA, Cheng L, Wang P (2000) Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol Biochem 32:1915–1919
Doran JW, Wilhelm WW, Power JF (1984) Crop residue removal and soil productivity with no-till corn, sorgum and soy bean. Soil Sci Soc Am J 48:640–645
Funke BR, Harris JO (1968) Early respiration responses of soil treated by heat or drying. Pl Soil 28:38–48
Jing X, Wang Y, Chung H, Mi Z, Wang S, Zeng H, He J-S (2013) No temperature acclimation of soil extracellular enzymes to experimental warming in an alpine grassland ecosystem on the Tibetan Plateau. Biogeochem 117:39–54
Kamolmanit B, Vityakon P, Kaewprdit W, Cadish G, Rasche F (2013) Soil fungal communities and enzyme activities in a sandy soil, highly weathered tropical soil treated with biochemically contrasting organic inputs. Biol Fertil Soils 49:905–917
Kätterer T, Reichstein M, Andren O, Lomander A (1998) Temperature dependence of organic matter decomposition: a critical review using literature data analyzed with different models. Biol Fertil Soils 27:258–262
Keeney DR, Nelson DW (1982) Nitrogen-Inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Agronomy monograph 9, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 643–698
Killham K (1994) Soil ecology. Cambridge University Press, Cambridge, 242 pp
Kruschke JK (2013) Bayesian estimation supersedes the t test. J Exp Psychol: Gen 142:573–603
Kumpiene J, Guerri G, Landi L, Pietramellara G, Nannipieri P, Renella G (2009) Microbial biomass, respiration and enzyme activities after in situ aided phytostabilization of a Pb- and Cu-contaminated soil. Ecotoxcol Environ Saf 72:115–119
Ladd JN, Butler JHA (1972) Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol Biochem 4:19–30
Lal R, De Vleeschenwer D, Malafa-Nganje R (1980) Changes in properties of a newly cleared tropical Alfisol as affected by mulching. Soil Sci Soc Am J 40:827–833
Lim CH, Jackson JL (1982) Dissolution for total element analysis. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Agronomy monograph 9, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 1–12
Mehra OP, Jackson ML (1960) Iron oxide removal from soils and clays by a dithionite-citrate buffered with sodium bicarbonate. Clays Clay Min 7:317–327
Moreno B, Canizares R, Nunez R, Benitez E (2013) Genetic diversity of bacterial B-glucosidase-encoding genes as a function of soil management. Biol Fertil Soils 49:735–746
Moyano F, Vasilyeva N, Bouckaert L, Cook F, Craine J, Curiel Yuste J, Don A, Epron D, Formanek P, Franzluebbers A, Ilsted U, Kätterer T, Orchard V, Reichstein M, Rey A, Ruamps L, Subke J-A, Chenu C (2012) The moisture response of soil heterotrophic respiration: interaction with soil properties. Biogeosciences 9:1173–1182
Nannipieri P, Ceccanti B, Cervelli S, Sequi P (1974) Use of 0.1 M pyrophosphate to extract urease from a podzol. Soil Biol Biochem 6:359–362
Nannipieri P, Sequi P, Fusi P (1996) Humus and enzyme activity. In Piccolo A (Ed) Humic Substances in Terrestrial Ecosystems. Elsevier, pp. 293–328
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bünemann EK, Oberson A, Frossard E (eds) Phosphorus in action. Soil biology 26. Springer, Berlin, pp 215–243
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Marinari S (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762
Peterson ME, Daniel RM, Danson MJ, Eisenthal R (2007) The dependence of enzyme activity on temperature: determination and validation of parameters. Biochem J 402:331–337
Planton S, Déqué M, Chauvin F, Terray L (2008) Expected impacts of climate change on extreme climate events. Compt Rendus Geosci 340:564–574
Plummer M (2003) JAGS: A program for analysis of bayesian graphical models using Gibbs sampling. In Hornik K, Leisch F, Zeileis A (Eds.) Proceedings of the 3rd International Workshop on Distributed Statistical Computing, Vienna, Austria. ISSN 1609-395X
Quarteroni A, Sacco R, Salieri F (2007) Numerical mathematics, 2nd edn. Springer, Berlin, 654 pp
R Development Core Team, 2011. R: A language and environment for statistical computing. (R-Foundation-for-Statistical-Computing, Ed.). Vienna (http://www.r-project.org/)
Rao MA, Gianfreda L (2000) Properties of acid phosphatase-tannic acid complexes formed in the presence of the Fe and Mn. Soil Biol Biochem 32:1921–1926
Renella G, Landi L, Ascher J, Ceccherini MT, Pietramellara G, Nannipieri P (2006) Phosphomonoesterase production and persistence and composition of bacterial communities during plant material decomposition in soils with different pH values. Soil Biol Biochem 98:795–802
Renella G, Landi L, Valori F, Nannipieri P (2007a) Microbial and hydrolase activity after release of low molecular weight organic compounds by a model root surface in a clayey and a sandy soil. Appl Soil Ecol 36:124–129
Renella G, Szukics U, Landi L, Nannipieri P (2007b) Quantitative assessment of hydrolase production and persistence in soil. Biol Fertil Soils 44:321–329
Ruggero P, Dec J, Bollag J-M (1996) Soil as a catalytic system. In: Stotzky G, Bollag J-M (eds) Soil biochemistry Vol. 9. Marcel Dekker, New York, pp 79–122
Schimel J, Weintraub M (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schwertmann U (1964) Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Zeitschrift für Pflanzenernährung, Düng, Bodenkd 105:194–202
Silverstein TP (2012) Falling enzyme activity as temperature rises: negative activation energy or denaturation? J Chem Educ 89:1097–1099
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochem 60:1–24
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 46:795–798
Speir TW, Ross DJ (1978) Soil phosphatase and sulphatase. In: Burns RG (ed) Soil enzymes. Academic, London, pp 198–250
Steinweg JM, Dukes JS, Wallenstein MD (2012) Modelling the effects of temperature and moisture on soil enzyme activity: linking laboratory assays to continuous field data. Soil Biol Biochem 55:85–92
Steinweg JM, Jagadamma S, Frerichs J, Mayes M (2013) Activation energy of extracellular enzymes in soils from different biomes. PLoS One 8:e59943
Tabatabai MA (1982) Soil Enzymes. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Agronomy monograph 9, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 907–943
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tang JW, Baldocchi DD (2005) Spatial-temporal variation in soil respiration in an oak-grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochem 73:183–207
Taylor JP, Wilson B, Mills MS, Burns RG (2002) Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol Biochem 34:387–401
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14:1172
Walkley A, Black IA (1934) An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:497–505
Wallenstein M, Allison SD, Ernakovich J, Steinweg JM, Sinsabaugh RL (2011) Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates. In: Shukla G, Varma A (eds) Soil enzymology. Springer, Berlin, pp 245–258
Wyss M, Pasamontes L, Rémy R, Kholer J, Kusznir E, Gadient MF, van Loon APGM (1998) Comparison of the thermostability properties of three acid phosphatases from molds: Aspergillus fumigatus phytase, A. niger phytase and A. niger pH 2.5 acid phosphatase. Appl Environ Microbiol 64:4446–4451
Zornoza R, Landi L, Nannipieri P, Renella G (2009) A protocol for the assay of arylesterase activity in soil. Soil Biol Biochem 41:659–662
Acknowledgments
The DISPAA thanks the Ente Cassa di Risparmio di Firenze for the acquisition of new instruments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 68 kb)
Rights and permissions
About this article
Cite this article
Menichetti, L., Reyes Ortigoza, A.L., García, N. et al. Thermal sensitivity of enzyme activity in tropical soils assessed by the Q10 and equilibrium model. Biol Fertil Soils 51, 299–310 (2015). https://doi.org/10.1007/s00374-014-0976-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0976-x