Abstract
Alpine grassland soils store large amounts of soil organic carbon (SOC) and are susceptible to rising air temperature. Soil extracellular enzymes catalyze the rate-limiting step in SOC decomposition and their catalysis, production and degradation rates are regulated by temperature. Therefore, the responses of these enzymes to warming could have a profound impact on carbon cycling in the alpine grassland ecosystems. This study was conducted to measure the responses of soil extracellular enzyme activity and temperature sensitivity (Q10) to experimental warming in samples from an alpine grassland ecosystem on the Tibetan Plateau. A free air-temperature enhancement system was set up in May 2006. We measured soil microbial biomass, nutrient availability and the activity of five extracellular enzymes in 2009 and 2010. The Q10 of each enzyme was calculated using a simple first-order exponential equation. We found that warming had no significant effects on soil microbial biomass C, the labile C or N content, or nutrient availability. Significant differences in the activity of most extracellular enzymes among sampling dates were found, with typically higher enzyme activity during the warm period of the year. The effects of warming on the activity of the five extracellular enzymes at 20 °C were not significant. Enzyme activity in vitro strongly increased with temperature up to 27 °C or over 30 °C (optimum temperature; Topt). Seasonal variations in the Q10 were found, but the effects of warming on Q10 were not significant. We conclude that soil extracellular enzymes adapted to seasonal temperature variations, but did not acclimate to the field experimental warming.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alpine grassland soils on the Tibetan Plateau, like those in high-latitude ecosystems, store large amounts of organic carbon (Yang et al. 2008; Shi et al. 2012), and the decomposition rates of these C pools may increase due to rising global temperatures (Kirschbaum 1995). In fact, the Tibetan Plateau has experienced striking warming during the last half-century (Guo and Wang 2011; Liu and Chen 2000; You et al. 2008; Niu et al. 2004) and is considered to be one of the world’s most sensitive areas to global climate change (Liu and Chen 2000; Tang et al. 2009). Therefore, the alpine grassland soils on the plateau provide a unique opportunity to explore the feedback between the soil organic carbon (SOC) stock and climate change (Yang et al. 2008).
Soil extracellular enzymes involved in the decomposition of polymeric SOC to dissolved organic carbon (DOC), have been studied to improve the understanding of the physiological underpinnings of the soil C cycle and help to refine models of the soil-C responses to global warming (Allison et al. 2010; Schimel and Weintraub 2003). However, the fundamental role of temperature in regulating enzyme activity under field conditions has been examined in relatively few studies (Wallenstein et al. 2011). In fact, changes in temperature not only affect enzyme catalysis rates but also affect enzyme production and degradation rates in the environment (Wallenstein et al. 2011). Shifts in enzyme activity can in turn lead to changes in decomposition, SOC storage, and other ecosystem processes (Sinsabaugh et al. 2002; Waldrop et al. 2004). Therefore, predicting the responses of these enzymes to elevated temperature is essential for understanding the underlying mechanisms of how climate change will affect soil and ecosystem functioning.
Several studies suggested that increasing temperature may amplify C release from soils to the atmosphere and provide a positive feedback for increasing temperature (Davidson et al. 2000; Cox et al. 2000). However, the biological effects of warming on soil microbes, which have a major influence on the net C balance of terrestrial ecosystems, remains unclear. For example, previous studies found that the warming-induced increase in soil CO2 fluxes to the atmosphere tends to decline over time and eventually results in no or small differences between soils at different temperatures (Luo et al. 2001; Melillo et al. 2002; Rustad et al. 2001; Bronson et al. 2008; Eliasson et al. 2005). This attenuation response could result from the acclimation of soil microbial physiology to increasing temperature (Allison et al. 2010; Zogg et al. 1997; Bradford et al. 2008; Bradford et al. 2010; Davidson and Janssens 2006; Luo et al. 2001). An alternative explanation for such a response is depletion of labile pools of soil organic matter (SOM) (Hartley et al. 2007; Eliasson et al. 2005; Knorr et al. 2005; Tingey et al. 2006). While differentiating between these two explanations in the field is difficult (Rinnan et al. 2011), insights into the physiological responses of microbial communities to warming may provide a mechanistic understanding of soil C flux in changing environments (Allison et al. 2010).
Changes in the temperature sensitivity of Vmax and Km [Vmax is the maximum reaction rate and Km is the Michaelis–Menten constant (Michaelis and Menten 1913)], commonly referred to as ‘temperature acclimation’, could offset temperature-induced increases in respiratory activity (Davidson and Janssens 2006). However, experimental evidence of the temperature acclimation of soil extracellular enzymes to experimental warming in the lab and/or in the field is rare. The lack of experimental evidence reduces our ability to predict feedback between the terrestrial C cycle and climate warming. Meanwhile, most of the predictions on feedback between microbial decomposition and temperature do not take into account the kinetics of extracellular enzymes (Allison et al. 2010). Yet, long-term field experiments (Bardgett et al. 2008; Davidson and Janssens 2006) and carbon cycle models (Luo 2007) must consider all possible/potential changes in soil enzyme activity (Davidson and Janssens 2006; Trasar-Cepeda et al. 2007). Therefore, more studies are needed to clarify the biological effects of warming on soil microbes through changes in soil extracellular enzymatic properties.
Determining the activity of an enzyme at different temperatures not only provides knowledge on the optimal temperature range for its activity, but also allows the estimation of the value of the temperature coefficient, or Q10 (increase in activity per 10 °C increases in temperature) (Trasar-Cepeda et al. 2007). However, existing studies of extracellular enzymes mostly focus on spatial or temporal patterns in potential activity, which is typically measured at a single reference temperature in lab assays (Henry 2012; Wallenstein et al. 2011). This approach neglects the fundamental role of temperature in regulating enzyme activity under field conditions (Wallenstein and Weintraub 2008). Therefore, it is necessary to measure enzyme activity over a range of temperatures to calculate Q10 values, which is an important property of in situ enzymatic activity (Fenner et al. 2005; Koch et al. 2007; Trasar-Cepeda et al. 2007; Wallenstein et al. 2009). To the best of our knowledge, no published studies have examined the effects of warming on the Q10 of soil extracellular enzymes in the alpine grassland ecosystem on the Tibetan Plateau. Moreover, most reports on the effects of warming on soil enzyme activity refer to hydrolases, and little is known about the temperature sensitivity of oxidoreductases (Trasar-Cepeda et al. 2007), the activity of which is often correlated with the mass loss rate of particulate organic matter at the ecosystem scale (Sinsabaugh 2010). More than 60 % of SOC is stored in the heavy fraction on the Tibetan Plateau (Tian et al. 2009), but the effects of warming on oxidoreductases in this unique ecosystem remain largely unknown.
Therefore, we set out to determine the effects of warming on soil microbial activity over the fourth and fifth years of experimental warming during a five-year field warming experiment. Specifically, we investigated the effects of warming on microbial biomass, nutrient availability and potential activity of soil extracellular enzymes, including phenol oxidase (POX), β-1,4-glucosidase (BG), β-d-cellobiohydrolase (CB), β-1,4-xylosidase (BX), and α-1,4-glucosidase (AG). These soil extracellular enzymes can be broadly divided into two groups: (1) oxidative enzymes which degrade poor-quality, chemically complex compounds such as lignin in co-metabolic acquisition of nutrients; and (2) hydrolytic enzymes responsible for the acquisition of C to support primary metabolism (Sinsabaugh and Moorhead 1994; Sylvia et al. 2004; Sinsabaugh 2010) (Table 1). Warming has the potential to affect the rapidly and slowly cycling C pools differently. For example, an increase in the relative complexity of soil C compounds could result from reduced oxidative enzyme activity (Cusack et al. 2010). In addition, over the duration of the warming experiment we expected that the relative abundance of recalcitrant soil C pools would increase, which may lead to increases in the activity of oxidative enzymes; meanwhile we expected that the relative abundance of labile soil C pools would decrease, potentially leading to decreases in the activity of hydrolytic enzymes. Therefore, we hypothesized that: (1) these two groups of enzymes would respond differently to experimental warming. In particular, we predicted that the oxidative enzyme activity would increase while the hydrolytic enzyme activity would decrease under the fourth and fifth years of experimental warming; (2) soil extracellular enzyme would acclimate to experimental warming, and thus offset temperature-induced increases in respiratory activity we previously observed at this experimental site during the growing season in 2006, 2007 and 2008 (Lin et al. 2011). More specifically, we predicted that soil extracellular enzyme would acclimate to elevated temperature with reduced Q10 in warmed soils.
Materials and methods
Experimental site
We designed a field warming manipulation experiment at the Haibei Alpine Grassland Ecosystem Research Station (37°36′N, 101°19′E, 3,215 m a.s.l.). The mean annual temperature is −1.7 °C (maximum of 27.6 °C and minimum −37.1 °C; (Zhao and Zhou 1999). The mean annual precipitation ranges from 426 to 860 mm, over 80 % of which falls in the growing season from May to September (Zhao and Zhou 1999). The mean temperature and total rainfall during the growing seasons from 1 May to 30 September in 2009 and 2010 were 7.7 and 8.3 °C, and 308.5 and 437.4 mm, respectively (He et al. unpublished data). The plant community at the experimental site is dominated by Kobresia humilis, Festuca ovina, Elymus nutans, Poa pratensis, Carex scabrirostris, Scripus distigmaticus, Gentiana straminea, Gentiana farreri, Leontop odiumnanum, Blvsmus sinocompressus, Potentilla nivea and Dasiphora fruticosa (Luo et al. 2009). The soil developed in this ecosystem is Mat-Gryic Cambisol (Chinese Soil Taxonomy Research Group 1995).
Controlled warming-grazing experiment
The infrared warming system was established in May 2006 and was described previously by Kimball et al. (2008). Eight hexagonal arrays of Mor FTE infrared heaters (1,000 W, 240 V) were deployed over the vegetation canopy, with eight dummy arrays over control plots. The heaters were controlled using the proportional integral derivative output (PID) control system to ensure constant warming in warmed and control plots. The set-point differences of the vegetation canopy temperature between warmed and corresponding control plots were 1.2 °C during the daytime and 1.7 °C at night during the growing season (from May to September). The canopy temperatures were scanned every second by the sensors, and the heaters were modulated at 1-s intervals, although the time-constant of the ceramic heating elements is ~6 min (Kimball et al. 2008). Fifteen-min averages were output by the data loggers. The power output of the heaters was manually set at 1,500 W plot−1 during winter (from October to April) because some infrared thermometers were not working.
The experiment plots used a factorial design with warming and grazing treatments and were replicated four times within each treatment combination: no-warming with no-grazing (control), no-warming with grazing, warming with no-grazing (warming), and warming with grazing. In total, 16 plots of 3-m diameter were used in a complete randomized block design in the field. Mean seasonal soil temperature increases in the warmed plots above the control plots ranged from 1.1 to 1.8 °C at 20-cm soil depth during the growing seasons in 2006, 2007 and 2008 (Luo et al. 2010; Lin et al. 2011) and by 1.9 °C at 10-cm soil depth from 2006 to 2010 (Wang et al. 2012). Soil moisture decreases by 15.6, 19.1 and 17.8 % at 10-cm soil depth in 2008, 2009 and 2010 (Wang et al. 2012). In this study, we only used soil samples from non-grazing plots.
Soil sampling
In each plot, we removed surface litter and collected three soil cores (16 mm in diameter) randomly from the surface soil (0–10 cm) and sub-layer soil (10–20 cm) on six sampling dates (Table S1). Our samples were representative of the below-ground biomass since previous studies showed that the biomass at 0–10 cm accounts for 85–95 % of the total biomass at 0–40 cm (Zhou 2001). Samples from the same depth were pooled, packed in polyethylene bags, immediately stored in a portable refrigerator, and transported to the laboratory. The composite samples were passed through a 2-mm sieve, and then all the visible plant materials were manually removed from the sieved soil. One-half of each processed sample was divided into two sub-samples, one stored at four and the other at −20 °C for no more than 1 week, and subsequently used for measurements of microbial biomass nutrients (C and N concentrations) and extracellular enzyme activity, respectively. The remaining soil samples were used to measure the gravimetric soil water content by drying at 105 °C for 48 h.
Nutrient availability measurement
In this study, we only measured soil nutrient availability during the growing season of 2010 (Table 2) by using ion-exchange resin bags (Allison et al. 2008). Ion-exchange resins have been widely used to measure soil N availability (Allison et al. 2008; Natali et al. 2012; Giblin et al. 1994). In our experiment, each nylon mesh bag was filled with 5 g anion or cation exchange resin, soaked in 0.5 M HCl for 20 min, rinsed with deionized water, and washed with 2 M NaCl. We placed two anion and two cation bags in each warmed or control plot at 5 cm below the soil surface. The bags were retrieved about 6 weeks later, rinsed in deionized water and extracted with 60 mL 2 M KCl on a shaker table for 60 min. We determined NH4 + and NO3 − by colorimetric analysis with an FIAstar 5000 flow injection analyzer (FOSS Tecator, Höganäs, Sweden). Measurements from two anion or cation bags in each plot were averaged to represent the nutrient availability in that plot. NH4 + and NO3 − availability was expressed as ng N g−1 resin day−1.
Microbial biomass measurement
We used the chloroform fumigation extraction method (Wu et al. 1990; Brookes et al. 1985; Vance et al. 1987) to measure microbial biomass C (MBC) and N (MBN) on August 5, and September 16, 2009. Six-gram soil samples were first fumigated with ethanol-free chloroform for 24 h and then extracted in 0.5 M K2SO4 for 30 min. Meanwhile, six-gram subsamples were directly extracted in a similar manner without being fumigated. Both fumigated and non-fumigated extracts were filtered and frozen until analysis of DOC and dissolved organic nitrogen (DON). DOC concentration in the extracts was measured by a TOC analyzer (Multi N/C 3100, Analytik Jena, Germany). Colorimetric analysis by a flow injection auto analyzer (AutoAnalyzer 3, Seal Analytical, German) was used to determine DON concentration. The difference in concentration between the fumigated and non-fumigated extracts is an estimate of MBC and MBN. To account for incomplete extractability, we used an extraction efficiency factor of 0.45 for microbial C (Vance et al. 1987; Wu et al. 1990) and 0.54 for microbial N (Brookes et al. 1985).
Soil extracellular enzyme assays
Phenol oxidase activity was measured on four sampling dates (May 3, June 14, August 9 and September 16 of 2010), BG and CB activity were measured on six sampling dates (August 5, September 16 of 2009 and May 3, June 14, August 9 and September 16 of 2010) and BX and AG were measured on two sampling dates (August 5, September 16 of 2009). In order to estimate potential enzyme activities in our soil samples (pH 6.4; 2 M KCl), enzyme assays were performed in soil slurries made with sodium acetate buffer (pH 5.5). We acknowledge that sodium acetate/acetic acid buffer has a pH range of 3.6–5.6 and would be inappropriate for samples with pH > 5.6 (German et al. 2011), but the questions being asked in this study were not influenced by the assay pH.
We measured POX activity (Table 1) following a modified version of the method described by German et al. (2011) and Saiya-Cork et al. (2002) using l-3,4-dihydroxyphenylalanine (DOPA) (Table 1) as the substrate. We homogenized 1 g of soil in 100 ml of 50 mM sodium acetate buffer (pH 5.5) in a Waring blender for 2 min. The slurries were then added to 96-well microplates. Fifty microliters of 5 mM DOPA was added to each well. The negative control contained 200 μl of acetate buffer and 50 μl of DOPA in each well. The blank contained 200 μl sample suspension and 50 μl of acetate buffer in each well. We used six analytical replicates for each soil sample, blank and control. The microplates were incubated in the dark at 4, 10, 15, 20, 25 or 30 °C for 18 h. Activity was quantified using a fluorescence spectrometer (Spectramax M2, Molecular Devices, USA) by measuring the absorbance at 450 nm. Enzyme activity was expressed as micromoles per gram dry weight per hour.
We measured the activity of four hydrolytic enzymes (Table 1) following a modified version of the method described by Saiya-Cork et al. (Saiya-Cork et al. 2002) and Steinweg et al. (2012) using fluorometric techniques, which included the construction of unique seven-point calibration curves for each soil sample to provide better correction for non-linear fluorescence quenching dynamics than the usual single-point correction (Weedon et al. 2012). We homogenized 2.75 g of soil in 91 ml of 50 mM sodium acetate buffer (pH 5.5) in a Waring blender for 2 min. Slurries of 200 μl were then added to 96-well microplates. Fifty microliters of 200 μM fluorometric substrate (saturating concentration) were added to each well. We used six analytical replicates for each soil sample. The microplates were incubated in the dark at 4, 10, 15, 20, 25 or 30 °C for 24 h. The amount of fluorescence was determined using a fluorescence spectrometer (Spectramax M2, Molecular Devices, USA) set to 365 nm for excitation and 450 nm for emission. Enzyme activity was expressed as nanomoles per gram dry weight per hour.
Optimum temperature, estimated as the temperature at which the maximum value of enzyme activity occurred was obtained using the Johnson and Lewin model (Johnson and Lewin 1946) (Table S2). Topt generally ranged from 27 to 30 °C, and in some cases, it was > 30 °C. Considering that the Topt of some enzymes was < 30 °C (Table S2), we limited our investigation to five temperature steps (4, 10, 15, 20 and 25 °C) for all the enzymes. This permitted the comparison of Q10 on different sampling dates with those obtained by fitting the simple first-order exponential equation,
where A is the exponential constant, or activity at 0 °C, B is the exponential parameter of the equation and T is temperature in degrees Celsius (°C). We fitted Eq. 1 by ordinary least-squares regression of log-transformed enzyme activity on log-transformed temperature. Following Wallenstein et al. (2009), regression coefficients (slopes) represent the temperature sensitivity as Q10 values according to:
Statistical analyses
Repeated measure analysis of variance (ANOVA) was used to assess the effects of warming and sampling time on soil water content, nutrient availability, soil microbial biomass, labile C and N content, enzyme activity and the apparent Q10 of enzyme activity with block as a random factor and warming as a fixed effect. If the interactive effects of warming and sampling time were significant, the effects of warming within each date were tested using a post hoc paired t test. Soil water content and soil microbial biomass, labile C and N content, nutrient availability, enzyme activity and Q10 values were log-transformed to improve normality. Two-way ANOVA was used to test the effects of treatment (warming, control), and incubation temperature (4, 10, 15, 20, 25 and 30 °C) on enzyme activity (Table S3). A significance level of < 0.05 was used for all analyses except where noted. All statistical analyses were performed in R 2.3.0 (R Development Core Team 2011).
Results
Soil water content, nutrient availability and microbial biomass
Soil water content declined significantly in the warmed plot at 0–10 cm and 10–20 cm (Fig. 1). There were no interactions between warming and sampling date at 0–10 cm (P = 0.174) and at 10–20 cm (P = 0.072). NH4 +–N was the dominant form of inorganic nitrogen in the alpine grassland ecosystem (Table 2). On average, NH4 + availability was 1.4 times higher than the NO3 − availability. The main effect of warming was not significant for either NH4 + (P = 0.468) or NO3 − availability (P = 0.876). NH4 + and NO3 − availability showed similar seasonal patterns, and were highest in the early-growing season and lowest in the late-growing season of 2010. The main effect of warming was not significant for MBC at 0–10 cm and 10–20 cm soil depth (Table 3). No significant effects of warming on MBN were detected except in August 2009 at 10–20 cm soil depth (P = 0.017). Labile C and N content showed no significant response to warming on either of the two sampling dates (Table 3).
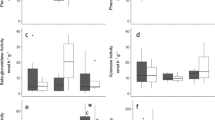
Soil water content in the warmed and control plots at 0–10 cm (A) and 10–20 cm (B). Bars show mean and 1 SE on each date (n = 4). Asterisk and double asterisk denote significance level (P < 0.05 or P < 0.001) for warming effects on the soil water content within the sampling date. A August, S September, M May, J June
Soil extracellular enzyme activity
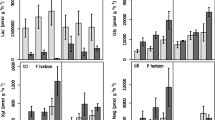
The activity of all extracellular enzymes increased with higher incubation temperature (e.g. Fig. 2). We found main effects of incubation temperature on the activity of all five enzyme throughout the sampling dates at 0–10 cm (P < 0.001) and 10–20 cm soil depth (P < 0.050) (Table S3). However, the treatment (experimental warming) effects were only significant in 12 out of 40 cases (Table S3; see Fig. 2a 10–20 cm). The interactions between incubation temperature and treatment were only significant in 1 out of 40 cases (P = 0.021), which was for CB in May, 2010 at 10–20 cm (Fig. 2b). Thus, we chose the potential activity at 20 °C to assess the effects of warming and sampling date and their interactions (Tables 4, S3, S4). In general, we found no significant effects of warming on the activity of the oxidative enzyme or the four hydrolytic enzymes (Fig. 3; Table 4). The seasonal patterns of enzyme activity differed for most enzymes (Fig. 3; Table 4). POX, BG, CB and BX showed the highest activity in June or August corresponding to the early- to mid-growing season (Table S1). No significant interactions between warming and sampling date were found for any of the five enzymes (Table 4). POX activity was much lower in the 0–10 cm surface soil than the 10–20 cm soil (Fig. 3), and this pattern was different from the other four hydrolytic enzymes.
Temperature sensitivity of soil extracellular enzymes
In general, no significant warming effect was detected in the Q10 of the oxidative enzyme and the four hydrolytic enzymes (Table 4). Only BG in May, 2010 at 10–20 cm and CB in June, 2010 at 10–20 cm were significantly lower and CB in September, 2009 at 0–10 cm was significantly higher in warmed than control plot soils (Fig. 4). Seasonal patterns of Q10 differed for most of the enzymes (Fig. 4; Table 4). There were no significant interactions between warming and sampling date for any of the enzymes (Table 4). The Q10 of POX was relatively invariant on the sampling dates at 10–20 cm (Fig. 4). This pattern was different from the seasonal pattern of the Q10 of POX at 0–10 cm.
Discussion
Soil extracellular enzyme activity
Warming had little effect on the potential activity of the five oxidative and hydrolytic enzymes. Our results are consistent with most previous field experiments showing that enzyme activity is insensitive to small increases in soil temperature (Bell et al. 2010; Bell and Henry 2011; Sardans et al. 2007). Among all of these studies, the effects of warming were too small relative to the seasonal temperature variation to elicit a response (Bell et al. 2010). Furthermore, our results did not support the primary hypothesis that oxidative and hydrolytic enzyme activity would respond differently to experimental warming. Our results suggested that experimental warming has no impacts on the relative depolymerizaion rates of soil C fractions of different chemical qualities.
This weak impact of warming on enzyme activity may be due to the reduction of soil moisture. Microbes may down-regulate enzyme production, especially substrate or other resources become limiting (Allison et al. 2011). No significant or only small effects of warming on MBC, MBN, CKSO, and NKSO may indirectly indicate the regulation of enzyme production. In addition, as field soil moisture decreases, the extant pool of functional enzymes may not be able to interact freely with substrates due to the constraints of diffusion. In this study, we found that the soil water content was significantly lower in warmed plots at all the sampling dates with reductions up to 32 % (Fig. 1). Our results are consistent with those of Allison & Treseder (2008), who reported that warming results in a 22 % decrease of soil moisture, accompanied by little effect on soil enzyme activity, except for a weak trend toward lower activity of N-acetyglucosaminidase. Thus, the reduction in soil moisture due to warming likely contributed to the absence of responses by soil extracellular enzymes.
We used the potential activity of soil extracellular enzymes under standard laboratory conditions (20 °C) to examine the long-term responses of microbial communities to experimental warming. However, the short-term temperature responses of the extant enzyme pool to warming should not be ignored (Fig. 2), because such a short-term increase of temperature might increase enzyme activity without changing the enzyme pools. Bell et al. (2010) reported that the potential activity of hydrolase enzymes is ~20 times higher in soil slurries incubated at 22 °C than those incubated at 1 °C. Baldrian et al. (2012) also reported that enzyme activity in vitro strongly increases with temperature up to the highest temperature recorded in situ (20–25 °C). Positive relationships between temperature and enzyme activity are consistently found (Wallenstein et al. 2009; Stone et al. 2012; Trasar-Cepeda et al. 2007; German et al. 2012; Koch et al. 2007; Khalili et al. 2011). It is important to consider short-term temperature responses to understand the role of temperature in regulating enzyme activity, because the potential activity measured under standard laboratory conditions might not represent the in situ experimental warming effects. Indeed, the in situ temperatures are different between the control and warmed plots under long-term experimental warming in the study; such a difference might induce an increase in the activity of soil extracellular enzyme from the warmed soils as shown in Fig. 2. Therefore, more information is required to link the potential activity of soil extracellular enzyme measured in the lab with in situ rates (Wallenstein and Weintraub 2008).
Although warming had little effect on the potential activity of the five oxidative and hydrolytic enzymes, seasonal variation in soil extracellular enzyme activity is important to understand microbial adaptation to long-term climate change. In this study, N-availability might be the key driver regulating seasonal patterns of enzyme activity in the alpine grassland ecosystem. Although the potential activity of most soil extracellular enzymes was highest in the mid-growing season of 2010, the NH4 + and NO3 − availability was lowest during the same period. Our results are consistent with some studies, such as Weintraub and Schimel (2005), who found that potential protease activity peaks as nutrient pools crash in the mid-growing season. In contrast, Wallenstein et al. (2009) showed that most of the potential enzyme activity in Arctic tundra soils are highest before the soils thaw and decline during summer, and concluded that N-limitation may explain the declines in overall enzyme activity. Substrate availability may be one of the keys to understand seasonality in enzyme activity (Boerner et al. 2005). For example, Kaiser et al. (2010) reported that soil enzyme activity reaches peak values in spring or autumn, due to root exudates or input of fresh litter. In this study, we found that the increased enzyme production was associated with decreased levels of soluble N in the environment, which could be a microbial strategy to avoid N-limitation by increasing production of C- and N-acquiring enzymes during periods of reduced soil mineral N.
In addition, seasonal variations in soil enzyme activity have been attributed to variations in temperature and/or moisture (Boerner et al. 2005; Fenner et al. 2005). At our study site, the mean annual temperature is −1.7 °C, so temperature might a major controlling factor that drive seasonal patterns of soil extracellular enzyme activity because the enzyme-producing activity of microbes is likely limited by temperature in the early- or late-growing seasons, while this limitation does not persist during the mid-growing season. For example, Wittmann et al. (2004) and Baldrian et al. (2012) reported that potential enzyme activity is predicted to account for 7–32 or 14–54 % of total annual activity in the cold period. Several studies have reported similar results that maximum microbial enzyme activity occurs in summer (Matinizadeh et al. 2008; Baldrian et al. 2012; Kang and Freeman 1999). Here, we showed that in the alpine grassland, temperature also strongly affects the seasonal patterns of enzyme activity, leading to higher activity in the warm season. However, seasonal temperature cycles are confounded with changes in plant activity, soil moisture and microbial community composition (Curiel Yuste et al. 2007; Björk et al. 2008). Therefore, temperature should not be viewed in isolation (Davidson et al. 2000) and temperature control of enzyme activity need to be further explored in other biomes (Wallenstein et al. 2011).
We found that POX activity was much lower in the 0–10 cm surface soil than the 10–20 cm soil. Within the soil profile, enzyme activity generally declines with soil depth. However, there are exceptions (Brockett et al. 2012). Qiu et al. (2005) found that POX activity showed a ‘wave’ distribution, in which activity peaked at 15 cm. In addition, in soils vegetated with gamagrass, POX activity increases with depth and with decreasing aggregate size (Jintaridth et al. 2009). The aggregate size decreased with soil depth at our study site; the proportion of aggregate <0.002 mm was 20.8 % at 0–10 cm and 25.0 % at 10–20 cm (Cao 2010). Therefore, this may account for the increase in POX activity with soil depth. In addition, the soil developed in this ecosystem is Mat-Gryic Cambisol, and most dead and live roots are distributed in the upper layer (0–8 cm) (Cao 2010). Thus, this labile C pool is likely the primary substrate used by plants and microbes to support primary metabolism, while the lignin and secondary compounds are likely the priority substrates at 8–20 cm.
Temperature sensitivity of soil extracellular enzymes
The Q10 values of individual enzymes obtained in our study ranged between 1.47 and 3.48, roughly corresponding to the values for tropical to boreal soils (1.5–3.0) (Wallenstein et al. 2009; Stone et al. 2012; German et al. 2012). The mean Q10 value of CB was highest (3.48) among the five enzymes. This indicates that there would be a larger change in the activity of CB than those of the other enzymes with the same temperature increase. Very few studies have examined the Q10 of POX (Trasar-Cepeda et al. 2007), for the methods of assaying its activity are not well resolved (Sinsabaugh 2010; German et al. 2011) and POX assays do not generally follow Michaelis–Menten kinetics (German et al. 2012). In this study, we found that POX activity followed a simple first-order exponential equation at temperatures below 25 °C. The mean Q10 of POX was 2.47, which is close to the overall mean value of 2.41 for all five enzymes. This indicates that the changes in rates of oxidative enzyme reaction would not result in disproportionally higher decomposition rates of chemically complex compounds (e.g. lignin) in this alpine grassland ecosystem. Therefore the results did not support the primary hypothesis that oxidative and hydrolytic enzyme activity would respond differently to experimental warming. In addition, our results were not consistent with those of Wang et al. (2011), who reported that the Q10 of POX is significantly higher than those of other soil enzymes in a subtropical forest, due to the quantity and quality of POX substrates (lignin). Because of the range and complexity of biological and physiochemical factors that affect enzyme temperature-sensitivity (Wallenstein et al. 2011), we do not yet have a mechanistic understanding of the drivers of Q10 of POX from the findings in this study.
We found no significant effects of warming on the Q10 of the oxidative enzyme and the four hydrolytic enzymes. Wallenstein et al. (2009) reported that the Q10 of most enzymes is greatest in frozen soils. Such adaptation of microbial extracellular enzymes to temperature has also been reported by German et al. (2012), who reported that some microbial extracellular enzymes from higher latitudes are more sensitive to temperature increases than those from lower latitudes. In fact, cold-adapted microorganisms tend to be more responsive to increasing temperature than warm-adapted microorganisms (Koch et al. 2007; Dong and Somero 2009; Brzostek and Finzi 2012), which increase the catalytic potential of enzyme (Bradford et al. 2010; Siddiqui and Cavicchioli 2006) and substrate affinity (Fenner et al. 2005) to offset the decrease in enzyme activity at lower temperatures (Georlette et al. 2004). However, our results did not support our assumption that enzymes from control soils would be more responsive to field warming. One of the reasons could be that the effects of warming were too small relative to diurnal or seasonal temperature variation. Another reason may be soil moisture limitation, which has been reported by Suseela et al. (2012), who found that warming does not affect the apparent Q10 of heterotrophic respiration due to limited soil moisture.
We found that the Q10 of soil extracellular enzymes varied among different sampling dates (Fig. 4). In fact, several studies have demonstrated that the temperature sensitivity of extracellular enzymes changes seasonally (Brzostek and Finzi 2012; Wallenstein et al. 2009; Fenner et al. 2005; Koch et al. 2007; Trasar-Cepeda et al. 2007). Changes in isoenzyme pools might be the main reason for the variation in enzyme activity on different sampling dates (Wallenstein et al. 2009, 2011), but the drivers of seasonal patterns of Q10 are not fully understood. We found that the Q10 of POX varied with soil depth (Fig. 4). At 10–20 cm, a relatively invariant Q10 of POX was found on the sampling dates. This pattern differed from the seasonal pattern of the Q10 of POX at 0–10 cm, and indicated that POX at different soil depths is likely to have different patterns of seasonality. The different seasonal patterns of POX might result from soil temperature change across the soil profile (Luo et al. 2009), such the temperature change with depth may vary from diel to seasonal timescales. Thus the choice of the soil depth used for inferring the Q10 of POX may strongly influence the shape of the temperature response curve. In addition, the seasonal patterns are more likely to result from changes in isoenzyme pools of POX at different depths, because we found the activity of POX increased over the soil profile at 0–10 and 10–20 cm.
Absence of temperature acclimation of soil extracellular enzymes to experimental warming
In our study, experimental warming did not change the Q10 of most soil extracellular enzymes, whereas soil respiration significantly decreased during the growing seasons in 2009 and 2010 (He et al., unpublished data). The decrease of soil respiration in 2009 and 2010 were not from the effects of warming on the activity or Q10 of soil extracellular enzymes. Davidson and Janssens (2006) suggested that the Km and Vmax of most enzymes increases with temperature, and the Q10 of Km and Vmax can neutralize each other, which could offset temperature-induced increases in respiratory activity. In this study, we assumed that the enzyme substrates were abundant, and thus Km becomes insignificant, and the temperature response of Vmax determines that of the respiratory activity. Our results showed that there were no significant effects of warming on the activity and Q10 of most soil extracellular enzymes, which suggest that the effects of warming on the activity of these enzymes cannot directly result in the decrease of soil respiration. Thus, our primary prediction that soil extracellular enzymes would acclimate to elevated temperature with reduced Q10 in warmed soils was not supported. However, substrate quality, temperature, and temporal and spatial differences in substrate availability can also contribute to the large variability in Q10 observed in natural ecosystems (Davidson and Janssens 2006). Hartley et al. (2007, 2008) reported that substrate availability, not microbial acclimation to the higher temperature, plays a dominant role in determining the response of heterotrophic soil respiration to warming. Thus, the changes of Km with temperature could explain the lack of warming response in this study. In addition, it is likely that reduced soil moisture played a role in the negative effects of warming on soil respiration, because reduced soil moisture may cause substrate limitation and suppress decomposition rates. Thus, the indirect effects of our experimental warming on microbial substrates and soil moisture probably contributed to the negative responses of soil respiration.
Our study used a specific experimental setup to simultaneously examine the effects of warming on soil nutrient availability, microbial biomass, and the potential activity and Q10 of soil extracellular enzymes. We found no significant warming effects on these parameters in the alpine grassland ecosystem. Soil respiration may be more strongly constrained by declining soil moisture than by temperature. Our results indicate that rising temperatures in high altitude ecosystems may cause a negative feedback on the soil C cycle, particularly in alpine grassland ecosystems with drier soils. One of the most important reasons for understanding the temperature sensitivity of soil enzymatic reactions is to improve predictions of soil respiration responses to temperature (Wallenstein et al. 2011). Although absence of microbial acclimation to experimental warming at the level of soil extracellular enzymes was found in our study, the drivers of soil respiration at the molecular level need further investigation. In addition, because of the uniqueness in geography, climate, and soil characteristics, concurrent investigation of the effects of warming on enzyme activity and temperature sensitivity will generate further insights into the effects of global warming on terrestrial C balance and will improve our ability to predict how soil biogeochemical cycles will respond to changes in the environment. Therefore, additional studies of soil extracellular enzymes responses to long-term temperature change and climate gradients are essential for accurately predicting soil C turnover in a changing climate.
References
Allison SD, Treseder KK (2008) Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob Change Biol 14(12):2898–2909. doi:10.1111/j.1365-2486.2008.01716.x
Allison SD, Czimczik CI, Treseder KK (2008) Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Change Biol 14(5):1156–1168. doi:10.1111/j.1365-2486.2008.01549.x
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3(5):336–340. doi:10.1038/Ngeo846
Allison SD, Weintraub MN, Gartner TB, Waldrop MP (2011) Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In: Shukla G, Varma A (eds) Soil Enzymology. Springer, Berlin, pp 229–243
Baldrian P, Šnajdr J, Merhautová V, Dobiášová P, Cajthaml T, Valášková V (2012) Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol Biochem. doi:10.1016/j.soilbio.2012.01.020
Bardgett RD, Freeman C, Ostle NJ (2008) Microbial contributions to climate change through carbon cycle feedbacks. ISME J 2(8):805–814. doi:10.1038/ismej.2008.58
Bell TH, Henry HAL (2011) Fine scale variability in soil extracellular enzyme activity is insensitive to rain events and temperature in a mesic system. Pedobiologia 54(2):141–146. doi:10.1016/j.pedobi.2010.12.003
Bell TH, Klironomos JN, Henry HAL (2010) Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen addition. Soil Sci Soc Am J 74(3):820. doi:10.2136/sssaj2009.0036
Björk RG, Björkman MP, Andersson MX, Klemedtsson L (2008) Temporal variation in soil microbial communities in alpine tundra. Soil Biol Biochem 40(1):266–268. doi:10.1016/j.soilbio.2007.07.017
Boerner REJ, Brinkman JA, Smith A (2005) Seasonal variations in enzyme activity and organic carbon in soil of a burned and unburned hardwood forest. Soil Biol Biochem 37(8):1419–1426. doi:10.1016/j.soilbio.2004.12.012
Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Treseder KK, Wallenstein MD (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11(12):1316–1327. doi:10.1111/j.1461-0248.2008.01251.x
Bradford MA, Watts BW, Davies CA (2010) Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Glob Change Biol 16(5):1576–1588. doi:10.1111/j.1365-2486.2009.02040.x
Brockett BFT, Prescott CE, Grayston SJ (2012) Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol Biochem 44(1):9–20. doi:10.1016/j.soilbio.2011.09.003
Bronson DR, Gower ST, Tanner M, Linder S, Van Herk I (2008) Response of soil surface CO2 flux in a boreal forest to ecosystem warming. Glob Change Biol 14(4):856–867. doi:10.1111/j.1365-2486.2007.01508.x
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842
Brzostek ER, Finzi AC (2012) Seasonal variation in the temperature sensitivity of proteolytic enzyme activity in temperate forest soils. J Geophys Res 117:G01024. doi:10.1029/2011jg001688
Cao G (2010) Ecosystem observation and research data set: grassland and desert ecosystems volume (Qinghai Haibei Station 2001–2006). China Agriculture Press, Beijing
Chinese Soil Taxonomy Research Group (1995) Chinese Soil Taxonomy. Science Press, Beijing
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408(6809):184–187. doi:10.1038/35041539
Curiel Yuste J, Baldocchi D, Gershenson A, Goldstein A, Misson L, Wong S (2007) Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob Change Biol 13(9):2018–2035. doi:10.1111/j.1365-2486.2007.01415.x
Cusack DF, Torn MS, McDowell WH, Silver WL (2010) The response of heterotrophic activity and carbon cycling to nitrogen additions and warming in two tropical soils. Glob Change Biol 16:2555–2572. doi:10.1111/j.1365-2486.2009.02131.x
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440(7081):165–173. doi:10.1038/nature04514
Davidson EA, Trumbore SE, Amundson R (2000) Soil warming and organic carbon content. Nature 408(6814):789–790. doi:10.1038/35048672
Dong Y, Somero GN (2009) Temperature adaptation of cytosolic malate dehydrogenases of limpets (genus Lottia): differences in stability and function due to minor changes in sequence correlate with biogeographic and vertical distributions. J Exp Biol 212(2):169–177. doi:10.1242/jeb.024505
Eliasson PE, McMurtrie RE, Pepper DA, Stromgren M, Linder S, Agren GI (2005) The response of heterotrophic CO2 flux to soil warming. Glob Change Biol 11(1):167–181. doi:10.1111/j.1365-2486.2004.00878.x
Fenner N, Freeman C, Reynolds B (2005) Observations of a seasonally shifting thermal optimum in peatland carbon-cycling processes; implications for the global carbon cycle and soil enzyme methodologies. Soil Biol Biochem 37(10):1814–1821. doi:10.1016/j.soilbio.2005.02.032
Georlette D, Blaise V, Collins T, D’Amico S, Gratia E, Hoyoux A, Marx JC, Sonan G, Feller G, Gerday C (2004) Some like it cold: biocatalysis at low temperatures. FEMS Microbiol Rev 28(1):25–42. doi:10.1016/j.femsre.2003.07.003
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem. doi:10.1016/j.soilbio.2011.03.017
German DP, Marcelo KRB, Stone MM, Allison SD (2012) The Michaelis–Menten kinetics of soil extracellular enzymes in response to temperature: a cross-latitudinal study. Glob Change Biol 18:1468–1479. doi:10.1111/j.1365-2486.2011.02615.x
Giblin A, Laundre J, Nadelhoffer K, Shaver G (1994) Measuring nutrient availability in arctic soils using ion exchange resins: a field test. Soil Sci Soc Am J 58(4):1154–1162
Guo D, Wang H (2011) The significant climate warming in the northern Tibetan Plateau and its possible causes. Int J Climatol 31:1257–1413. doi:10.1002/joc.2388
Hartley IP, Heinemeyer A, Ineson P (2007) Effects of three years of soil warming and shading on the rate of soil respiration: substrate availability and not thermal acclimation mediates observed response. Glob Change Biol 13(8):1761–1770. doi:10.1111/j.1365-2486.2007.01373.x
Hartley IP, Hopkins DW, Garnett MH, Sommerkorn M, Wookey PA (2008) Soil microbial respiration in arctic soil does not acclimate to temperature. Ecol Lett 11(10):1092–1100. doi:10.1111/j.1461-0248.2008.01223.x
Henry HAL (2012) Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 47:53–59. doi:10.1016/j.soilbio.2011.12.026
Jintaridth B, Coyne MS, Matocha CJ, Workman S (2009) Vegetation and soil depth affect phenol oxidase activity in a model riparian site. J World Assoc Soil Water Conserv J4:1–15
Johnson FH, Lewin I (1946) The growth rate of E. coli in relation to temperature, quinine and coenzyme. J Cell Physiol 28(1):47–75
Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Rasche F, Zechmeister-Boltenstern S, Sessitsch A, Richter A (2010) Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol 187(3):843–858. doi:10.1111/j.1469-8137.2010.03321.x
Kang HJ, Freeman C (1999) Phosphatase and arylsulphatase activities in wetland soils: annual variation and controlling factors. Soil Biol Biochem 31(3):449–454
Khalili B, Nourbakhsh F, Nili N, Khademi H, Sharifnabi B (2011) Diversity of soil cellulase isoenzymes is associated with soil cellulase kinetic and thermodynamic parameters. Soil Biol Biochem 43(8):1639–1648. doi:10.1016/j.soilbio.2011.03.019
Kimball BA, Conley MM, Wang S, Lin X, Luo C, Morgan J, Smith D (2008) Infrared heater arrays for warming ecosystem field plots. Glob Change Biol 14(2):309–320. doi:10.1111/j.1365-2486.2007.01486.x
Kirschbaum MUF (1995) The temperature-dependence of soil organic-matter decomposition, and the effect of global warming on soil organic-C storage. Soil Biol Biochem 27(6):753–760
Knorr W, Prentice IC, House JI, Holland EA (2005) Long-term sensitivity of soil carbon turnover to warming. Nature 433(7023):298–301. doi:10.1038/nature03226
Koch O, Tscherko D, Kandeler E (2007) Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Global Biogeochem Cycles 21(4):4017. doi:10.1029/2007gb002983
Lin X, Zhang Z, Wang S, Hu Y, Xu G, Luo C, Chang X, Duan J, Lin Q, Xu B, Wang Y, Zhao X, Xie Z (2011) Response of ecosystem respiration to warming and grazing during the growing seasons in the alpine meadow on the Tibetan plateau. Agric For Meteorol 151(7):792–802. doi:10.1016/j.agrformet.2011.01.009
Liu X, Chen B (2000) Climatic warming in the Tibetan Plateau during recent decades. Int J Climatol 20(14):1729–1742
Luo Y (2007) Terrestrial carbon-cycle feedback to climate warming. Annu Rev Ecol Evol Syst 38:683–712. doi:10.1146/annurev.ecolsys.38.091206.095808
Luo Y, Wan S, Hui D, Wallace LL (2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413(6856):622–625. doi:10.1038/35098065
Luo C, Xu G, Wang Y, Wang S, Lin X, Hu Y, Zhang Z, Chang X, Duan J, Su A (2009) Effects of grazing and experimental warming on DOC concentrations in the soil solution on the Qinghai-Tibet plateau. Soil Biol Biochem 41(12):2493–2500. doi:10.1016/j.soilbio.2009.09.006
Luo C, Xu G, Chao Z, Wang S, Lin X, Hu Y, Zhang Z, Duan J, Chang X, Su A (2010) Effect of warming and grazing on litter mass loss and temperature sensitivity of litter and dung mass loss on the Tibetan Plateau. Glob Change Biol 16(5):1606–1617. doi:10.1111/j.1365-2486.2009.02026.x
Matinizadeh M, Korori S, Teimouri M, Praznik W (2008) Enzyme activities in undisturbed and disturbed forest soils under oak (Quercus brantii var. persica) as affected by soil depth and seasonal variation. Asian J Plant Sci 7:368–374. doi:10.3923/ajps.2008.368.374
Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP, Catricala C, Magill A, Ahrens T, Morrisseau S (2002) Soil warming and carbon-cycle feedbacks to the climate system. Science 298(5601):2173–2176. doi:10.1126/science.1074153
Michaelis L, Menten ML (1913) Die kinetik der invertin wirkung. Biochem Z 49(333–369):352
Natali SM, Schuur EAG, Rubin RL (2012) Increased plant productivity in Alaskan tundra as a result of experimental warming of soil and permafrost. J Ecol 100(2):488–498. doi:10.1111/j.1365-2745.2011.01925.x
Niu T, Chen L, Zhou Z (2004) The characteristics of climate change over the Tibetan Plateau in the last 40 years and the detection of climatic jumps. Adv Atmos Sci 21(2):193–203. doi:10.1007/BF02915705
Qiu L, Liu J, Wang Y, Zhang X (2005) Profile distribution of enzyme activity of long-term fertilization soil and its dynamic characteristics. Plant Nutr Fertil Sci 11(6):737–741
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing Vienna Austria, Austria
Rinnan R, Michelsen A, Bååth E (2011) Long-term warming of a subarctic heath decreases soil bacterial community growth but has no effects on its temperature adaptation. Appl Soil Ecol 47(3):217–220. doi:10.1016/j.apsoil.2010.12.011
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) GCTE-NEWS. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126(4):543–562. doi:10.1007/s004420000544
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34(9):1309–1315. doi:10.1016/S0038-0717(02)00074-3
Sardans J, Peñuelas J, Estiarte M (2007) Seasonal patterns of root-surface phosphatase activities in a Mediterranean shrubland: responses to experimental warming and drought. Biol Fertil Soils 43(6):779–786. doi:10.1007/s00374-007-0166-1
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35(4):549–563. doi:10.1016/S0038-0717(03)00015-4
Shi Y, Baumann F, Ma Y, Song C, Kühn P, Scholten T, He J (2012) Organic and inorganic carbon in the topsoil of the Mongolian and Tibetan grasslands: pattern, control and implications. Biogeosciences 9:1869–1898. doi:10.5194/bg-9-2287-2012
Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 75:403–433. doi:10.1146/annurev.biochem.75.103004.142723
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42(3):391–404. doi:10.1016/j.soilbio.2009.10.014
Sinsabaugh R, Moorhead D (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26(10):1305–1311
Sinsabaugh R, Carreiro M, Repert D (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60(1):1–24. doi:10.1023/A:1016541114786
Steinweg JM, Dukes JS, Wallenstein MD (2012) Modeling the effects of temperature and moisture on soil enzyme activity: linking laboratory assays to continuous field data. Soil Biol Biochem 55:85–92. doi:10.1016/j.soilbio.2012.06.015
Stone MM, Weiss MS, Goodale CL, Adams MB, Fernandez IJ, German DP, Allison SD (2012) Temperature sensitivity of soil enzyme kinetics under N-fertilization in two temperate forests. Glob Change Biol 18:1173–1184. doi:10.1111/j.1365-2486.2011.02545.x
Suseela V, Conant RT, Wallenstein MD, Dukes JS (2012) Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob Change Biol 18(1):336–348. doi:10.1111/j.1365-2486.2011.02516.x
Sylvia D, Fuhrmann J, Hartel P, Zuberer D (2004) Principles and applications of soil microbiology. Prentice Hall, Upper Saddle River
Tang Y, Wan S, He J, Zhao X (2009) Foreword to the special issue: looking into the impacts of global warming from the roof of the world. J Plant Ecol 2(4):169–171. doi:10.1093/jpe/rtp026
Tian Y, Xu X, Song M, Zhou C, Gao Q, Ouyang H (2009) Carbon sequestration in two alpine soils on the Tibetan Plateau. J Integr Plant Biol 51(9):900–905. doi:10.1111/j.1744-7909.2009.00852.x
Tingey DT, Lee EH, Waschmann R, Johnson MG, Rygiewicz PT (2006) Does soil CO2 efflux acclimatize to elevated temperature and CO2 during long-term treatment of Douglas-fir seedlings? New Phytol 170(1):107–118. doi:10.1111/j.1469-8137.2006.01646.x
Trasar-Cepeda C, Gil-Sotres F, Leiros MC (2007) Thermodynamic parameters of enzymes in grassland soils from Galicia, NW Spain. Soil Biol Biochem 39(1):311–319. doi:10.1016/j.soilbio.2006.08.002
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19(6):703–707
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14(4):1172–1177. doi:10.1890/03-5120
Wallenstein MD, Weintraub MN (2008) Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol Biochem 40(9):2098–2106. doi:10.1016/j.soilbio.2008.01.024
Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Change Biol 15(7):1631–1639. doi:10.1111/j.1365-2486.2008.01819.x
Wallenstein MD, Allison S, Ernakovich J, Steinweg JM, Sinsabaugh R (2011) Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates. In: Shukla G, Varma A (eds) Soil enzymology, vol 22. Springer, Berlin, pp 245–258
Wang C, Han G, Jia Y, Feng X, Tian X (2011) Insight into the temperature sensitivity of forest litter decomposition and soil enzymes in subtropical forest in China. J Plant Ecol 5(3):279–286. doi:10.1093/jpe/rtr013
Wang S, Duan J, Xu G, Wang Y, Zhang Z, Rui Y, Luo C, Xu B, Zhu X, Chang X, Cui X, Niu H, Zhao X, Wang W (2012) Effects of warming and grazing on soil N availability, species composition and ANPP in alpine meadow. Ecology. doi:10.1890/11-1408.1
Weedon JT, Kowalchuk GA, Aerts R, van Hal J, van Logtestijn R, Taş N, Röling WFM, van Bodegom PM (2012) Summer warming accelerates sub-arctic peatland nitrogen cycling without changing enzyme pools or microbial community structure. Glob Change Biol 18(1):138–150. doi:10.1111/j.1365-2486.2011.02548.x
Weintraub MN, Schimel JP (2005) Seasonal protein dynamics in Alaskan arctic tundra soils. Soil Biol Biochem 37(8):1469–1475. doi:10.1016/j.soilbio.2005.01.005
Wittmann C, Kähkönen MA, Ilvesniemi H, Kurola J, Salkinoja-Salonen MS (2004) Areal activities and stratification of hydrolytic enzymes involved in the biochemical cycles of carbon, nitrogen, sulphur and phosphorus in podsolized boreal forest soils. Soil Biol Biochem 36(3):425–433. doi:10.1016/j.soilbio.2003.10.019
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation extraction—an automated procedure. Soil Biol Biochem 22(8):1167–1169
Yang Y, Fang J, Tang Y, Ji C, Zheng C, He J, Zhu B (2008) Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob Change Biol 14(7):1592–1599. doi:10.1111/j.1365-2486.2008.01591.x
You Q, Kang S, Aguilar E, Yan Y (2008) Changes in daily climate extremes in the eastern and central Tibetan Plateau during 1961–2005. J Geophys Res 113(D7):101. doi:10.1029/2007jd009389
Zhao XQ, Zhou XM (1999) Ecological basis of alpine meadow ecosystem management in Tibet: Haibei alpine meadow ecosystem research station. Ambio 28(8):642–647
Zhou XM (2001) Alpine meadow in China. Science Press, Beijing
Zogg GP, Zak DR, Ringelberg DB, MacDonald NW, Pregitzer KS, White DC (1997) Compositional and functional shifts in microbial communities due to soil warming. Soil Sci Soc Am J 61(2):475–481
Acknowledgments
We thank Donovan German, Steven Allison, Matthew Wallenstein, Meg Steinweg and Shawna McMahon for help with lab protocols and advice, Lei Liu for access of microplate reader, Wenjing Chang and Zhengxia Chen for assistance in soil microbial biomass analysis, Xuemei Zhao and Jianjing Ma for assistance with enzyme assays, and Nan Cong and Samraat Pawar for statistical analysis. The authors are grateful to Biao Zhu, Chao Song, Yan Geng, Yue Shi, Iain Bruce and three anonymous referees for valuable and constructive comments that substantially improved this manuscript. This study was supported by the National Program on Key Basic Research Project (Grant No. 2010CB950602), the National Natural Science Foundation of China (Grants No. 31025005 and 31021001), and the Program of “One Hundred Talented People” of the Chinese Academy of Sciences (Grant No. KSCX2-YW-Z-0806). HC is supported by the Research Fellowship for International Young Scientists of Chinese Academy of Sciences. SW is partially supported by the National Natural Science Foundation of China (Grant No. 41230750) and the National Basic Research Program (2010CB833502). We thank USDA Grant to SSI (2010) at Colorado State University that allowed XJ to learn enzyme assays.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jing, X., Wang, Y., Chung, H. et al. No temperature acclimation of soil extracellular enzymes to experimental warming in an alpine grassland ecosystem on the Tibetan Plateau. Biogeochemistry 117, 39–54 (2014). https://doi.org/10.1007/s10533-013-9844-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9844-2