Abstract
We have explored the possible mechanisms by which mineralocorticoid (MR) and glucocorticoid (GR) receptors regulate the response to freshwater transfer in the gills of the euryhaline killifish Fundulus heteroclitus. Killifish were implanted with RU486 (GR antagonist) or spironolactone (MR antagonist) at doses of 0.1–1.0 mg g−1, and subsequently transferred from 10‰ brackish water to freshwater. Compared to brackish water sham fish, mRNA expression of CFTR and NKCC1 decreased in the gills of sham fish transferred to freshwater, whereas Na+,K+–ATPase α1a mRNA expression and α protein abundance, as well as cell proliferation (detected using BrdU) increased. Spironolactone inhibited the normal increase in cell proliferation and Na+,K+-ATPase expression after freshwater transfer. RU486 increased plasma cortisol levels and may have slightly inhibited Na+,K+–ATPase activity, but did not change α 1a expression. RU486 had no effect on cell proliferation in the non-lamellar region of the gills, but increased proliferation in the lamellar region. Neither antagonist inhibited the suppression of CFTR or NKCC1 expression after freshwater transfer. Glucocorticoid receptor expression was reduced in all sham and antagonist treatments compared to untreated controls, but no other consistent differences were observed. The effects of spironolactone suggest that MR is important for regulating ion transport in killifish gills after freshwater transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among teleost fish, some euryhaline species are exceptionally tolerant of environmental salinity change. These animals modulate ion transport rates in response to salinity change to offset the passive movement of ions and maintain ion balance, doing so through several coordinated molecular and cellular adjustments (reviewed by Sakamoto et al. 2001). Of these adjustments, changes in ion transporter gene expression seem particularly important when salinity change persists. For example, we have recently demonstrated in killifish (Fundulus heteroclitus) that Na+,K+–ATPase expression increases after transfer from brackish water to freshwater (Scott et al. 2004a). Expression tends to increase transiently, and is followed by changes in Na+ absorption (Scott et al. 2004b). The probable role of Na+,K+–ATPase in Na+ uptake across the gills of this species in freshwater (Patrick and Wood 1999; Katoh et al. 2003) suggests that changes in the expression of this gene are important for activating ion absorption after freshwater transfer.
Changes in gene expression after salinity transfer could be mediated by several hormones, including cortisol and prolactin, which are traditionally thought to be important for freshwater acclimation in fish. Cortisol has been shown to play an important role in the regulation of ionic homeostasis in fish, particularly after environmental salinity change (see reviews by Foskett et al. 1983; McCormick 2001). For example, cortisol benefits ion retention in freshwater by increasing transepithelial resistance of the gills and reducing passive ion loss, and can also stimulate ion uptake (Kelly and Wood 2002; Zhou et al. 2003). The effects of cortisol are primarily mediated through binding to intracellular receptors. Upon binding, the hormone–receptor complex enters the nucleus and influences transcription of cortisol responsive genes (Wendelaar Bonga 1997; Nishi et al. 2001).
Historically, cortisol has been thought to primarily act through glucocorticoid receptors (GR) in fish. However, the identification of mineralocorticoid receptors (MR) in several fish species, coupled with the apparent absence of the mineralocorticoid hormone aldosterone (Baker 2003), and the observation that MR binds cortisol with high affinity (Colombe et al. 2000; Greenwood et al. 2003), suggests that cortisol may act through this additional receptor type. Indeed, cortisol can induce transcription of MR responsive genes in fish in vitro (Greenwood et al. 2003). Conversely, some recent evidence demonstrated that 11-deoxycorticosterone (DOC) is a more potent agonist for fish MR than cortisol (Sturm et al. 2005), suggesting that cortisol may not be the primary agonist for MR in vivo. These findings imply that the regulation of hydromineral balance in fish could be more complicated than previously thought, and warrant a further examination of the roles of MR and GR in regulating ion transport after salinity transfer.
Spironolactone and RU486 are used routinely in fish to antagonize MR and GR, respectively (e.g., Veillette et al. 1995; Sloman et al. 2001; Bury et al. 2003; McDonald et al. 2004). Although their specificity has not yet been definitively assessed in fish, both antagonists have been shown to influence ionoregulatory physiology. For example, RU486 inhibits intestinal fluid absorption in Atlantic salmon (Salmo salar) during the parr-smolt transformation (Veillette et al. 1995), and impairs Cl− secretion by the opercular epithelium of killifish after seawater transfer (Marshall et al. 2005). Spironolactone inhibits some of the normal responses of rainbow trout gills (Oncorhynchus mykiss) to ion-poor water (Sloman et al. 2001), which suggests that MR may be important for freshwater acclimation. Both GR and MR may therefore be involved in fish osmoregulation, but their exact roles remain unclear.
In this study we examine how spironolactone and RU486 influence the molecular and cellular responses to freshwater transfer in the gills of the common killifish Fundulus heteroclitus, to provide insight into the roles of mineralocorticoid and glucocorticoid receptors in mediating these responses. Gene expression and cell proliferation were assessed in the gills of fish transferred from near-isosmotic (10‰) brackish water to freshwater. Near-isosmotic brackish water is the preferred salinity for F. heteroclitus (Fritz and Garside 1974), and transfer from brackish water to freshwater may be more environmentally representative of the conditions killifish naturally encounter in estuaries. Because the effects of antagonists may be dose-dependent (Sturm et al. 2005), multiple doses of spironolactone and RU486 were used. By antagonizing MR or GR signalling after freshwater transfer, the effects of spironolactone and RU486 will help elucidate the roles of these receptors in freshwater acclimation.
Materials and methods
Experimental animals
Adult killifish (Fundulus heteroclitusL.) were captured from estuaries in Hampton, New Hampshire, and were held in static filtered indoor holding facilities containing 10‰ synthetic brackish water (Deep Ocean, Energy Savers, Carson, CA, USA) made up in dechlorinated Vancouver city tap water ([Na+], 0.17 mmol l−1; [Cl−], 0.21 mmol l−1; hardness, 30 mg l−1 as CaCO3; pH 5.8–6.4). Fish were kept at room temperature (18–21°C) and a 14L:10D photoperiod, and were fed commercial trout chow (PMI Nutrition International, Brentwood, MO, USA: 2.2% calcium, 0.8% chloride, 0.5% sodium, 0.5% potassium, 0.2% magnesium) at an approximate daily ration of 1–2% (food mass/body mass).
In all experiments, fish were acclimated to 10‰ brackish water for at least 1 month before sampling or treatment. For sampling, fish were rapidly collected and stunned by cephalic blow. Blood was collected from the severed caudal peduncle in heparinized capillary tubes, and fish were then killed by rapid decapitation. Blood was centrifuged at 13,000 × g for 10 min and plasma was frozen in liquid nitrogen. Second and third gill arches were isolated and either immediately frozen in liquid nitrogen (experiment 1 below), or fixed for 24 h in 0.1 mol l−1 phosphate–buffered saline (PBS, pH 7.4) containing 4% paraformaldehyde (experiment 2 below). Frozen tissue and plasma were stored at −80°C until analysed. Treatment of animals was conducted according to University of British Columbia animal care protocol #A01-0180.
Experimental protocols
In experiment 1, the effects of RU486 and spironolactone on gene expression and protein abundance/activity after freshwater transfer were assessed. Killifish held in brackish water were anaesthetized and given intraperitoneal injections of the glucocorticoid receptor antagonist RU486 (Sigma–Aldrich), the mineralocorticoid receptor antagonist spironolactone (Sigma–Aldrich), or coconut oil alone at a volume of 10 μl g−1. RU486 and spironolactone were administered at low and high doses of 0.1 and 1.0 mg g−1 in experiment 1. Fish were then returned to brackish water and were allowed to recover from sham or antagonist injections for 5 days. By 5 days after injection the rate of release from implants generally stabilizes and endogenous stress-induced cortisol release is minimal (DeKoning et al. 2004). After recovery, fish were transferred to either brackish water (sham only) or freshwater. As large changes in gene expression and cell proliferation are known to occur 3–4 days after freshwater transfer (Katoh and Kaneko 2003; Scott et al. 2004a, 2005), plasma and gill samples were collected 4 days post-transfer. The plasma and gills of an additional group of untreated killifish were also sampled.
In experiment 2, the effects of RU486 and spironolactone on cell proliferation after freshwater transfer were assessed. Because of the results obtained in experiment 1, antagonists were administered at only one intermediate dose of 0.5 mg g−1, but fish were otherwise injected and transferred as described in experiment 1. Four days after transfer, fish were anaesthetized and given intraperitoneal injections of 5-bromo-2′-deoxyuridine (BrdU; 200 μg g−1 fish weight; Sigma–Aldrich) dissolved in Cortland’s saline (143 mmol l−1 NaCl, 5.0 mmol l−1 KCl, 1.5 mmol l−1 CaCl2, 1.0 mmol l−1 MgSO4, 5.0 mmol l−1 NaHCO3, 3.0 mmol l−1 NaH2PO4, pH 7.4) at a volume of 10 μl saline solution g−1 fish weight. BrdU is a thymidine analogue that selectively incorporated into DNA during the synthesis phase of the cell cycle, and can therefore be used to identify proliferating cells (e.g., Laurent et al. 1994). The gills from BrdU-injected animals were sampled 4 h later, fixed for 24 h in phosphate–buffered saline (PBS) containing 4% paraformaldehyde (wt/vol), and then processed as described in Immunohistochemistry below.
Immunohistochemistry
Immediately after gill arches were fixed, they were cryoprotected in PBS containing 24% sucrose (wt/vol) and then frozen in embedding medium (Tissue Tek, VWR International, Mississauga, ON, Canada). Arches were sectioned 5 μm thick in the sagittal plane (parallel to the long axis of the filament and perpendicular to the secondary lamellae) and mounted on Superfrost slides (VWR International). Slides were stored at −80°C until proceeding with immunolabelling.
For immunocytochemical detection of cell proliferation, slides were first incubated in 2 mol l−1 HCl for 0.5 h (to denature the DNA and make the incorporated BrdU available for antibody detection), and then incubated for 1 h in 10% normal donkey serum. Slides were then incubated overnight in anti-BrdU antibody (1:250 dilution), a mouse monoclonal antibody (Sigma–Aldrich) that recognizes BrdU and thus labels proliferating gill cells. Slides then incubated in Alexa Fluor 568 goat anti-mouse secondary antibody (1:200; Molecular Probes) for 2 h. Slides were rinsed thoroughly after each incubation, and both antibodies were diluted in PBS containing 0.2% Triton X-100 and 0.1% sodium azide. Finally, immunomount (Fisher Scientific, Nepean, ON, Canada) was added to each slide, which were then covered with a coverslip. Fish not injected with BrdU, as well as slides incubated without primary antibody, showed only background staining (no positively labelled nuclei, data not shown).
Digital images were captured with an Axioplan 2 microscope (Zeiss, Jena, Germany), a digital camera (Q Imaging, Burnaby, BC, Canada), and Northern Eclipse software (Empix Imaging, Mississauga, ON, Canada). Images were collected from randomly selected sections throughout the gills, at random locations on both the non-lamellar trailing edge and the lamellar region in the middle of the primary gill filaments. At least 30 images were collected per fish. The number of cells immunoreactive to the anti-BrdU antibody was quantified per mm of primary filament (measured along the long axis). The average number of immunoreactive cells was then determined for each fish.
Real-time PCR analysis of gene expression
For analysis of gene expression, total RNA was first extracted from gill samples (approximately 20 mg of tissue) using Tripure isolation reagent (Roche Diagnostics, Montreal, QC, Canada) following the manufacturer’s instructions. RNA concentrations were determined spectrophotometrically and RNA integrity was verified by agarose gel electrophoresis (∼1% [wt/vol] agarose:Tris–acetate EDTA). Extracted RNA samples were stored at −80°C following isolation. First strand cDNA was synthesized by reverse transcribing 3 μg total RNA using 10 pmol oligo(dT18) primer and 20 U RevertAid H Minus M-MuLV reverse transcriptase (MBI Fermentas Inc., Burlington, ON, Canada) following the manufacturer’s instructions.
Primers for killifish Na+,K+–ATPase α1a (Acc. No. AY057072), cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel (Acc. No. AF000271), Na+,K+,2Cl− cotransporter 1 (NKCC1; Acc. No. AY533706), glucocorticoid receptor (GR; Acc. No. AY430088), and elongation factor 1α (EF1α, expression control; Acc. No. AY430091) were designed using Primer Express software (version 2.0.0, Applied Biosystems Inc., Foster City, CA, USA). The sequences for all primers are reported in Scott et al. (2004a). Gene expression was quantified using quantitative real-time PCR (qRT-PCR) on an ABI Prism 7000 sequence analysis system (Applied Biosystems). PCR reactions contained 1 μl of cDNA, 4 pmol of each primer and Universal SYBR green master mix (Applied Biosystems) in a total volume of 21 μl. All qRT-PCRs were performed as follows: 1 cycle of 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. PCR products were subjected to melt curve analysis to confirm the presence of a single amplicon and representative samples were electrophoresed to verify that only a single band was present. Control reactions were conducted with no cDNA template or with non-reverse transcribed RNA to determine the level of background or genomic DNA contamination, respectively. Genomic contamination was below 1:147 starting cDNA copies for all templates. A randomly selected control sample was used to develop a standard curve for each primer set, and all results were expressed relative to these standard curves. Results were then standardized to EF1α. Expression of this gene does not change in killifish gills at any time following salinity transfer when expression is normalized to total RNA concentration (data not shown), demonstrating that EF1α is an appropriate control gene. All samples were run in duplicate (coefficients of variation were ≤10%).
Na+,K+–ATPase activity and Western blotting
Na+,K+–ATPase activity was determined by coupling ouabain-sensitive ATP hydrolysis to pyruvate kinase- and lactate dehydrogenase-mediated NADH oxidation as outlined by McCormick (1993). For this assay, second and third gill arches were homogenized in 500 μl of SEI buffer (150 mmol l−1 sucrose, 10 mmol l−1 EDTA, 50 mmol l−1 imidazole, pH 7.3) containing 0.1% Na-deoxycholate and centrifuged at 5,000 × g for 30 s at 4°C. Supernatants were immediately frozen in liquid nitrogen and stored at −80°C until analysed. ATPase activity was determined in the presence or absence of 0.5 mmol l−1 ouabain using 10 μl supernatant thawed on ice and was normalized to total protein content (measured using the bicinchoninic acid method, Sigma-Aldrich). All samples were run in triplicate (coefficients of variation were always ≤10%). Na+,K+–ATPase activity, measured as ouabain-sensitive ATPase activity is expressed as μmol ADP mg protein−1 h−1.
Na+,K+–ATPase protein abundance was measured by Western immunoblotting as in Scott et al. (2004a). Gill homogenates were prepared as outlined above and denatured for 3 min in boiling SDS-sample buffer (Laemmli 1970). Eight percent SDS-polyacrylamide gels were loaded with total gill homogenates (20 μg protein/lane) and protein was transferred to nitrocellulose membranes (Bio-Rad) using a Trans-Blot semi-dry transfer cell (Bio-Rad). Blots were first incubated for 1 h with NAK121 primary antibody diluted 1:400 in TTBS buffer (17.4 mmol l−1 Tris–HCl, 2.6 mmol l−1 Tris base, 500 mmol l−1 NaCl, 2.0 mmol l−1 sodium azide, 0.05% Tween-20, pH 7.5) containing 2% skim milk powder (wt/vol). Blots were then incubated for 1 h with goat anti-rabbit IgG secondary antibody (alkaline phosphatase conjugated; Sigma-Aldrich) diluted 1:5,000 in TTBS. Membranes were developed in alkaline phosphatase buffer containing 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitro-blue tetrazolium (NBT) (Sigma–Aldrich). Band intensity was quantified using a FluorChem 8800 imager (Alpha Innotech) assisted by AlphaEaseFC software (v.3.1.2; Alpha Innotech). Samples are expressed relative to a randomly chosen protein standard (included on each gel to control for transfer efficiency) and normalized to pre-transfer brackish water control samples. The sensitivity of Western immunoblotting was verified using dose-response analysis.
Plasma variables
Plasma sodium was determined using flame atomic absorption spectrophotometry (SpectrAA-220FS, Varian, Mulgrave, VC, Australia) with Fisher Scientific certified standards. Plasma chloride concentrations were measured colorimetrically (Zall et al. 1956) with Radiometer (Copenhagen, Denmark) certified standards. Plasma cortisol was determined by enzyme-linked immunosorbant assay following the manufacturer’s instructions (Neogen, Lansing, MI, USA).
Statistical analyses
Data are expressed as means ± SEM. All data passed tests of normality and homogeneity of variance. ANOVA was therefore used to determine overall treatment effects. The effects of each treatment were assessed by comparison with untreated, sham-injected brackish water transferred fish, and sham-injected freshwater transferred fish, using Tukey post-hoc comparisons. Additional Tukey comparisons were made between freshwater sham fish and each antagonist treatment where data from both doses were pooled. We interpreted significant differences between brackish water and freshwater shams as a specific effect of freshwater transfer. When these differences did exist between sham groups, an effect of antagonist treatment was assumed when a significant difference existed between freshwater sham and antagonist groups, or when there was a lack of significance between brackish water sham and antagonist groups. Statistical analyses were conducted using Sigmastat version 3.0 and a significance level of P<0.05 was used throughout.
Results
Effects of spironolactone and RU486 on gene expression
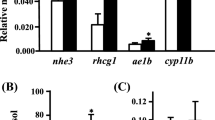
Spironolactone and RU486 significantly affected some of the normal molecular responses of killifish gills to freshwater transfer. Four days after transfer of sham-injected fish to freshwater, Na+,K+–ATPase α1a expression increased nearly 3-fold above sham-injected brackish water controls (Fig. 1a). RU486 had no significant effect on this increase in expression after freshwater transfer, as levels were similar in RU486- and sham-injected fish gills. The mineralocorticoid receptor antagonist spironolactone did, however, reduce Na+,K+–ATPase α1a expression in the gills after freshwater transfer to levels intermediate between brackish water and freshwater shams. Expression of this gene was 30% lower in spironolactone-injected fish compared to sham-injected freshwater fish, and levels were not significantly different from sham-injected brackish water controls. Furthermore, Na+,K+–ATPase α1a expression was significantly reduced compared to freshwater sham fish when data from both spironolactone doses were pooled.
Na+,K+–ATPase α1a mRNA expression (a), activity (b), and α-subunit protein abundance (c) in the gills of killifish (n≥7). Treatments were untreated (white hatched bar), sham-injected (coconut oil) brackish water (BW) transfer (white bar), sham-injected freshwater (FW) transfer (black), RU486-injected freshwater transfer, and spironolactone-injected freshwater transfer. Antagonists were injected at low (0.1 mg g−1 fish weight; dark grey) or high (1.0 mg g−1 fish weight; light grey) doses. (d) Representative western blots of the Na+,K+–ATPase α-subunit in brackish water (left) and freshwater (right) controls, illustrating an immunoreactive band at approximately 100 kDa. Expression data are standardized to elongation factor 1α, and all data are expressed as means ± SE. *: Significant difference from sham-injected brackish water control. #: Significant difference from untreated control. +: Significant difference from sham-injected freshwater control (P<0.05). In (a) Na+,K+–ATPase α 1a mRNA expression was significantly reduced compared to freshwater shams when data from both spironolactone doses were pooled
Na+,K+–ATPase activity increased after freshwater transfer in sham- and spironolactone-injected fish when compared to untreated controls (1.5- to 1.8-fold) (Fig. 1b). However, only fish injected with 1.0 mg g−1 spironolactone were higher than brackish water controls. In contrast, Na+,K+–ATPase activity in RU486-injected fish was not significantly different from untreated fish. The lack of a significant difference in activity between untreated and RU486-treated fish was likely not due to differences in protein abundance, at least for the 1.0 mg g−1 dose. Freshwater transfer increased α-subunit protein levels from 1.9- to 2.7-fold above brackish water controls in all treatment groups. Furthermore, levels in all freshwater-transferred fish were significantly higher than untreated controls, except for those treated with the 0.1 mg g−1 RU486 dose (Fig. 1c, d).
Expression of the seawater gill ion transporters, CFTR and NKCC1, decreased after freshwater transfer for all treatments compared to untreated and brackish water controls (Fig. 2a, b). GR expression was reduced in brackish water sham-, freshwater sham-, RU486-, and spironolactone-injected fish compared to untreated controls, but only 0.1 mg g−1 spironolactone reduced expression compared to sham-injected brackish water controls (Fig. 2c).
CFTR (a), NKCC1 (b), and GR mRNA expression in the gills of killifish (n≥7). Treatments were untreated (white hatched bar), sham-injected (coconut oil) brackish water (BW) transfer (white bar), sham-injected freshwater (FW) transfer (black), RU486-injected freshwater transfer, and spironolactone-injected freshwater transfer. Antagonists were injected at low (0.1 mg g−1 fish weight; dark grey) or high (1.0 mg g−1 fish weight; light grey) doses. Expression data are standardized to elongation factor 1α, and all data are expressed as means ±SE. *: Significant difference from sham-injected brackish water control. #: Significant difference from untreated control. +: Significant difference from sham-injected freshwater control (P<0.05)
Plasma ions were disrupted after freshwater transfer in several treatment groups, as levels were reduced up to approximately 20% (Table 1). Plasma Cl− levels were maintained after freshwater transfer in sham-, RU486-, and spironolactone-injected fish when compared to sham-injected brackish water controls, but were reduced compared to untreated controls in freshwater sham, 0.1 mg g−1 RU486, and 0.1 and 1.0 mg g−1 spironolactone treated fish. In contrast, no significant differences in plasma Na+ were observed due to any treatment. However, the variance of both plasma Cl− and plasma Na+ data tended to be higher in some of the freshwater transferred groups, suggesting that there may be inter-individual differences (as well as inter-population differences, Scott et al. 2004b) in freshwater tolerance. Interestingly, a high dose of RU486 increased plasma cortisol levels, compared to untreated fish, brackish water sham fish, and freshwater sham fish.
For all measurements in experiment 1, namely gene expression, Na+,K+–ATPase activity and abundance, plasma ions and cortisol, untreated fish were statistically indistinguishable from sham-injected brackish water controls. For all treatments, plasma cortisol was significantly influenced by the serial sampling protocol: there was a significant correlation between sample order and cortisol, such that cortisol levels were higher in fish sampled later.
Effects spironolactone and RU486 on cell proliferation
Spironolactone and RU486 significantly influenced cell proliferation in the gills of killifish after freshwater transfer (Figs. 3, 4). Proliferation (indicated by the number of BrdU-labelled nuclei) was observed primarily in cells at the base of secondary lamellae, as well as in cells near the central venous sinus. In the non-lamellar region of the gills in sham-injected killifish, transfer to freshwater increased the number of BrdU-labelled nuclei by 5-fold above brackish water controls, and this was unaffected by RU486 treatment (Fig. 4a). Cell proliferation was reduced by spironolactone treatment, however, which reduced the number of BrdU-labelled nuclei by nearly 50%, such that levels were significantly reduced compared to sham-injected fish transferred to freshwater. In the lamellar region of the gills, freshwater transfer also increased cell proliferation by 5-fold (Fig. 4b). Spironolactone treatment had similar effects in this region, as it reduced cell proliferation compared to freshwater sham fish to levels that were not significantly different from brackish water sham fish. In contrast, RU486 increased cell proliferation in the lamellar region 1.5-fold above that observed in freshwater sham fish gills.
Immunohistochemical labelling of BrdU in the lamellar portion of the gill epithelium of killifish. Treatments were sham-injected (coconut oil) brackish water transfer (a), sham-injected freshwater transfer (b), spironolactone-injected (0.5 mg g−1 fish weight) freshwater transfer (c), and RU486-injected (0.5 mg g−1 fish weight) freshwater transfer (d). BrdU was injected intraperitoneally 4 h before sampling, and the subsequent incorporation into nuclei of proliferating gill cells was detected (arrow). Scale bar is 100 μm
The number of BrdU-labelled nuclei (as an index of cell proliferation) per mm filament length in the non-lamellar (a) and lamellar (b) regions of the killifish gill epithelium (n≥ 4). Treatments were sham-injected (coconut oil) brackish water (BW) transfer (white bar), sham-injected freshwater (FW) transfer (black), RU486-injected (0.5 mg g−1 fish weight) freshwater transfer (grey), and spironolactone-injected (0.5 mg g−1 fish weight) freshwater transfer (grey). All data are expressed as means ± SE. *: Significant difference from sham-injected brackish water control. +: Significant difference from sham-injected freshwater control (P<0.05)
Discussion
The common killifish can experience both daily and seasonal fluctuations in environmental salinity in their natural habitats. In order to maintain ion balance under these conditions, killifish modulate ion flux rates across the gills. For example, when killifish move into hyposmotic environments, ion secretion is suppressed (Marshall 2003) and ion absorption increases (Wood and Laurent 2003; Scott et al. 2004b). In the current study, we have observed both spironolactone and RU486 to have effects on the molecular and cellular responses of killifish gills to freshwater transfer. In particular, mineralocorticoid receptor antagonism with spironolactone reduced the normal increases in Na+,K+–ATPase expression and cell proliferation. Whereas the glucocorticoid receptor appears to be important for seawater transfer in this species (Scott et al. 2004a; Marshall et al. 2005), the results of this study support a role for the mineralocorticoid receptor in freshwater acclimation in killifish.
Responses of killifish to freshwater transfer
Previous studies have identified several molecular and cellular responses to freshwater transfer in killifish gills that contribute to the euryhalinity of this species. These responses are involved in both reducing ion loss and activating ion uptake. Passive routes of ion secretion are minimized primarily through decreases in paracellular permeability (Karnaky 1992; Scott et al. 2004b). Active routes of ion secretion are suppressed through inactivation of secretory ion transporters, which has been shown to occur by protein internalization (Marshall et al. 2002), activation of signal cascades responsible for protein phosphorylation (Kültz et al. 2001), and suppression of ion transporter expression (Scott et al. 2004a, 2005). Ion uptake is activated in part by increasing Na+,K+–ATPase mRNA expression and activity (Scott et al. 2004a, 2005); indeed, changes in Na+,K+–ATPase expression in freshwater are followed by progressive increases in Na+ influx (Scott et al. 2004b). Cell proliferation (Katoh and Kaneko 2003) and differentiation (hypertrophy and apical surface morphology, Marshall et al. 1999; Daborn et al. 2001; Katoh et al. 2001) are likely also involved in ion uptake. Together, the responses of killifish to freshwater transfer allow these animals to rapidly re-establish ion balance after freshwater transfer (Jacob and Taylor 1983; Katoh and Kaneko 2003; Scott et al. 2004a).
Maintenance of ionic homeostasis after freshwater transfer probably involves a transformation from ion secretion to ion absorption, as expression of the seawater transporters CFTR and NKCC1 decreases in the gills after transfer, while expression and activity of Na+,K+–ATPase increases (as in some other euryhaline species, Deane and Woo 2004). Furthermore, we observed the rate of cell proliferation in killifish gills to increase after freshwater transfer. This has also been observed in rainbow trout gills during acclimation to ion-poor water (Laurent et al. 1994) and is likely responsible for increasing the abundance of new mitochondria-rich cells, as previously demonstrated in killifish after transfer from seawater to freshwater (Katoh and Kaneko 2003).
In addition to the molecular and cellular changes that occur in the gills of killifish and other fish species in freshwater, transient increases in plasma cortisol occur within a few hours of transfer (McCormick 2001). Elevating plasma cortisol early after freshwater transfer may increase the expression and activity of Na+,K+–ATPase in the gills, as may also be the case after seawater transfer (e.g., Madsen et al. 1995; Seidelin et al. 1999). These changes likely occurred in the present study, except much earlier and distinct from the changes observed in this study due to serial sampling (see Results). Changes in plasma cortisol early after freshwater transfer could regulate ion transporters through both glucocorticoid and mineralocorticoid receptors, as could changes in the plasma levels of other GR or MR agonists (Sturm et al. 2005). Both GR and MR are expressed in the gills of fish at the same absolute levels (Greenwood et al. 2003), and GR is known to be expressed in cells expressing high levels of Na+,K+–ATPase (Uchida et al. 1998). Therefore, it is perhaps not surprising that the GR and MR antagonists RU486 and spironolactone altered some of the normal molecular and cellular responses to freshwater transfer, as discussed below.
Evaluation of antagonist treatment
Many previous studies have used RU486 as an antagonist of glucocorticoid receptors, and antagonism of several cortisol-mediated functions has been demonstrated at doses comparable to those used in this study. Examples include reduction of pulsatile urea excretion in gulf toadfish (Opsanus beta, 0.05 mg g−1) (McDonald et al. 2004), prevention of cortisol-induced changes in hepatocyte metabolism (0.1 mg g−1) (Vijayan et al. 1994), and inhibition of the normal increase in fluid absorption during the parr-smolt transformation in Atlantic salmon (1.0 mg g−1) (Veillette et al. 1995). Therefore, in the range of doses used in this study (0.1 to 1.0 mg g−1), RU486 is likely antagonizing GR. Interestingly, 1.0 mg g−1RU486 treatment increased plasma cortisol levels in this study. By blocking glucocorticoid receptors in the hypothalamus or pituitary, RU486 could increase cortisol secretion by inhibiting negative feedback that is normally present. Elevated plasma cortisol levels after RU486 treatment has been demonstrated in several fish species (Veillette et al. 1995), including killifish (Marshall et al. 2005), which further suggests that RU486 treatment successfully antagonized GR in this study.
Unlike RU486, few previous studies have employed spironolactone as an antagonist of mineralocorticoid receptors in fish. Spironolactone is a commonly used antagonist of the mineralocorticoid receptor in mammals, and is known to inhibit nuclear translocation of agonist-bound MR (Fejes-Tóth et al. 1998). Recently, spironolactone was reported to antagonize transcription of rainbow trout MR in the presence of aldosterone at some doses in vitro (in a mammalian COS cell culture model), but in the absence of aldosterone was observed to have an agonistic effect at other doses (Sturm et al. 2005). It is unclear how concentrations of spironolactone in culture relate to those injected in vivo, but 0.1 mg spironolactone g−1 inhibited the normal increase in cell proliferation in the gills of trout after transfer to ion-poor water (Sloman et al. 2001), and approximately 0.05 mg spironolactone g−1 has been shown to have no effects on cortisol-mediated regulation of urea excretion in toadfish (McDonald et al. 2004). Therefore, in the range of doses used in this study (0.1 to 1.0 mg g−1), spironolactone is likely antagonizing MR.
In addition to specifically antagonizing GR and MR, respectively, RU486 and spironolactone may also antagonize other corticosteroid receptors when administered at high doses. For example, at very high doses, RU486 inhibits transactivation of trout MR by aldosterone (Sturm et al. 2005). It is similarly possible that high doses of spironolactone inhibits GR signalling. To address these possibilities, we administered multiple doses (0.1–1.0 mg g−1) of each antagonist in this study. Spironolactone generally had consistent effects at all doses, and inhibited some of the normal changes associated with freshwater transfer, as discussed below. There was a trend for high doses of RU486 (1.0 mg g−1) to have a similar, albeit statistically insignificant, effect on Na+,K+–ATPase expression as spironolactone. A plausible explanation for these observations is that spironolactone (and possibly RU486 at high dose) antagonized signalling via MR, and thus inhibited the normal changes associated with freshwater transfer. However, GR antagonism (at least in the hypothalamus or pituitary), as suggested by elevated plasma cortisol levels, appeared to occur only after RU486 treatment. Furthermore, GR antagonism by an intermediate dose of RU486 had an opposite effect to spironolactone on cell proliferation, providing added support that spironolactone is specifically antagonizing MR.
Effects of RU486 on the responses to freshwater transfer
Intracellular signalling through the glucocorticoid receptor could help maintain ion balance after freshwater transfer by either suppressing pathways involved in ion loss or activating those involved in ion absorption. Our results and those of others (Wilson et al. 2004) are not consistent with an effect on ion loss, as the normal suppression of the seawater transporters CFTR and NKCC1 in freshwater was unaffected by RU486. In contrast, GR antagonism with RU486 may have affected the pathways responsible for activating ion absorption after freshwater transfer. Compared to untreated animals in brackish water, Na+,K+–ATPase activity increased after freshwater transfer in the gills of control killifish, but in killifish subjected to RU486 activity of this enzyme did not increase significantly. It is likely that this effect on Na+,K+–ATPase was post-translational, as RU486 had very little effect on α1a mRNA expression and α protein abundance. Spironolactone did not appear to alter activity, suggesting that this effect of RU486 was specific to GR. GR antagonism may have therefore inhibited the normal post-translational increase in Na+,K+–ATPase activity in the gills of killifish after freshwater transfer. Cortisol is thought to increase Na+,K+–ATPase activity via GR (Shrimpton and McCormick 1999), consistent with the observed inhibition of the activity of this enzyme by RU486; however, the effects of RU486 were modest and did not impair plasma Na+ levels, so the role of GR in freshwater acclimation is likely minor. Indeed, unlike seawater transfer, which causes GR expression to increase (Scott et al. 2004a), neither freshwater transfer nor RU486 treatment altered GR expression in this study.
Curiously, cell proliferation was higher in RU486-treated fish transferred to freshwater than in freshwater-transferred controls in the lamellar region of the gills. This compensation may have enhanced the absorptive capacity of their gills, and compensated for reduced Na+,K+–ATPase activity. In this regard, it is notable that rainbow trout do not increase Na+,K+–ATPase activity in their gills after transfer to ion-poor water (Sloman et al. 2001). Because activity does not normally change in this species, there was no effect for 0.5 mg RU486 g−1 to antagonize, so it is perhaps not surprising that compensatory changes in cell proliferation in response to RU486 were unnecessary (Sloman et al. 2001). Additionally, higher plasma cortisol levels in RU486 treated fish may explain the further increase in cell proliferation after freshwater transfer (Laurent et al. 1994; van der Salm et al. 2002), possibly through enhanced signalling via MR (see the spironolactone discussion below). Furthermore, the increase in plasma cortisol induced by RU486 may have led to a compensatory increase in prolactin secretion, which is known to be important for freshwater acclimation in killifish (Grau et al. 1984), and could have stimulated cell proliferation in the gills (Manzon 2002). Alternatively, some studies have suggested that RU486 acts as an agonist to GR under some experimental conditions (see review by Mommsen et al. 1999), but this possibility seems less convincing, as RU486 has been shown repeatedly at the doses used in this study to be either antagonistic or have no effect (Veillette et al. 1995; Kelly and Wood 2002; van der Salm et al. 2002). Cell proliferation was unaffected by RU486 in the non-lamellar region of the gills, where mitochondria-rich cell abundance is greatest (Katoh and Kaneko 2003), so the contribution of this increased proliferation in the lamellar region to total gill function is unclear. Overall, the results in RU486 treated fish suggest that GR plays only a minor role in freshwater acclimation in killifish.
Effects of spironolactone on the responses to freshwater transfer
Spironolactone did not inhibit the normal decrease of CFTR and NKCC1 expression after transfer, suggesting that the normal suppression of ion loss in freshwater was unaffected. Instead, the normal increase in ion uptake after freshwater transfer may have been affected by MR antagonism in two interacting ways. Firstly, killifish treated with spironolactone did not significantly increase Na+,K+–ATPase α1a mRNA expression, in contrast to the significant increase observed in freshwater-transferred sham fish. Secondly, MR antagonism with spironolactone inhibited the normal increase in cell proliferation in the gills after freshwater transfer (see also Sloman et al. 2001). However, because spironolactone treated fish suffered no additional ion imbalance compared to freshwater transferred controls, these animals may have compensated in other ways.
It is possible that reduced cell proliferation was responsible for the reduced Na+,K+–ATPase mRNA abundance, as lower cell proliferation could reduce the number of cells in killifish whole gills expressing high levels of Na+,K+–ATPase (potential mitochondria-rich cells). However, no such effect was observed for protein abundance. Alternatively, or perhaps additionally, MR antagonism could inhibit transcription of the Na+,K+–ATPase α1a gene after freshwater transfer within individual mitochondria-rich cells. Whatever the cause for altered Na+,K+–ATPase α 1a expression, it is likely that this inhibition had physiological effects: previous studies have suggested a strong relationship between Na+,K+–ATPase expression and unidirectional Na+ influx rate (Scott et al. 2004b). If indeed the case, reduced Na+,K+–ATPase expression and cell proliferation by spironolactone treatment could have affected the normal increase in Na+ uptake after freshwater transfer, possibly resulting in compensatory changes to maintain ion balance.
Recent evidence suggests that MR-mediated signalling may involve multiple ligands in fish. Cortisol has previously been suggested to act as both glucocorticoid and mineralocorticoid in fish (Mommsen et al. 1999), possibly interacting with both GR and MR in vivo (Colombe et al. 2000; Sloman et al. 2001), because cortisol has a higher affinity for MR than GR (Colombe et al. 2000; Greenwood et al. 2003) and can induce transcription via MR in vitro (Greenwood et al. 2003). In addition, it has recently been shown that 11-deoxycorticosterone (DOC) has potent agonistic effects on MR, and enhances transcription via MR at lower concentrations than does cortisol (Sturm et al. 2005). Fish are able to synthesize DOC (Li et al. 2003), but whether this hormone responds to osmotic stress is unknown (Gilmour 2005). Furthermore, 11β-hydroxysteroid dehydrogenase (11HSD), which converts cortisol to inactive metabolites, has recently been identified in fish (Kusakabe et al. 2003). This suggests that access of cortisol to MR can be regulated by 11HSD, which might allow DOC and other hormones to bind MR in tissues involved in regulating hydromineral balance. It is therefore likely that MR antagonism with spironolactone is inhibiting the effects of both cortisol and other hormones that are mediated by MR.
In summary, we have demonstrated that both spironolactone and RU486 influence the molecular and cellular responses of killifish to freshwater transfer. In particular, mineralocorticoid receptor antagonism with spironolactone inhibited the normal increases in Na+,K+–ATPase α1a mRNA expression and cell proliferation in the gills, which supports a role for MR in freshwater acclimation. The effects of RU486 were less clear, as treatment had small effects on the normal increase in Na+,K+–ATPase activity but increased cell proliferation in the lamellar region of the gills. By mediating many aspects of the molecular and cellular responses to freshwater transfer, mineralocorticoid receptors may be exceedingly important for regulating ion transport in euryhaline fish.
Abbreviations
- BrdU:
-
5-Bromo-2′-deoxyuridine
- BW:
-
Brackish water
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator Cl− channel
- DOC:
-
11-Deoxycorticosterone
- EF1α:
-
Elongation factor 1α
- FW:
-
Freshwater
- GR:
-
Glucocorticoid receptor
- MR:
-
Mineralocorticoid receptor
- NKCC1:
-
Na+,K+,2Cl− cotransporter 1
- qRT-PCR:
-
Quantitative real-time PCR
References
Baker ME (2003) Evolution of glucocorticoid and mineralocorticoid responses: go fish. Endocrinology 144:4223–4225
Bury N, Sturm A, Le Rouzic P, Lethimonier C, Ducouret B, Guiguen Y, Robinson-Rechavi M, Laudet V, Rafestin-Oblin ME, Prunet P (2003) Evidence for two distinct functional glucocorticoid receptors in teleost fish. J Mol Endocrinol 31:141–156
Colombe L, Fostier A, Bury N, Pakdel F, Guiguen Y (2000) A mineralocorticoid-like receptor in the rainbow trout, Oncorhynchus mykiss: cloning and characterization of its steroid binding domain. Steroids 65:319–328
Daborn K, Cozzi RRF, Marshall WS (2001) Dynamics of pavement cell-chloride cell interactions during abrupt salinity change in Fundulus heteroclitus. J Exp Biol 204:1889–1899
Deane EE, Woo NYS (2004) Differential gene expression associated with euryhalinity in sea bream (Sparus sarba). Am J Physiol Regul Integr Comp Physiol 287:R1054-R1063
DeKoning ABL, Picard DJ, Bond SR, Schulte PM (2004) Stress and interpopulation variation in glycolytic enzyme activity and expression in a teleost fish Fundulus heteroclitus. Physiol Biochem Zool 77:18–26
Fejes-Tóth G, Pearce D, Náray-Fejes-Tóth A (1998) Subcellular localization of mineralocorticoid receptors in living cells: effects of receptor agonists and antagonists. Proc Natl Acad Sci USA 95:2973–2978
Foskett JK, Bern HA, Machen TE, Conner M (1983) Chloride cells and the hormonal control of teleost fish osmoregulation. J Exp Biol 106:255–281
Fritz ES, Garside ET (1974) Salinity preferences of Fundulus heteroclitusand F. diaphanus(Pisces: Cyprinodontidae): their role in geographic distribution. Can J Zool 52:997–1003
Gilmour KM (2005) Mineralocorticoid receptors and hormones: fishing for answers. Endocrinology 146:44–46
Grau EG, Prunet P, Gross T, Nishioka RS, Bern HA (1984) Bioassay for salmon prolactin using hypophysectomized Fundulus heteroclitus. Gen Comp Endocrinol 53:78–85
Greenwood AK, Butler PC, White RB, DeMarco U, Pearce D, Fernald RD (2003) Multiple corticosteroid receptors in a teleost fish: distinct sequences, expression patterns, and transcriptional activities. Endocrinology 144:4226–4236
Jacob WF, Taylor MH (1983) The time course of seawater acclimation in Fundulus heteroclitus L. J Exp Zool 228:33–39
Karnaky KJ (1992) Teleost osmoregulation: changes in the tight junction in response to the salinity of the environment. In: Cereijido M (ed) Tight Junctions. CRC, Boca Raton, USA, pp 175–185
Katoh F, Kaneko T (2003) Short-term transformation and long-term replacement of branchial chloride cells in killifish transferred from seawater to freshwater, revealed by morphofunctional observations and a newly established ‘time-differential double fluorescent staining’ technique. J Exp Biol 206:4113–4123
Katoh F, Hasegawa S, Kita J, Takagi Y, Kaneko T (2001) Distinct seawater and freshwater types of chloride cells in killifish, Fundulus heteroclitus. Can J Zool 79:822–829
Katoh F, Hyodo S, Kaneko T (2003) Vacuolar-type proton pump in the basolateral plasma membrane energizes ion uptake in branchial mitochondria-rich cells of killifish Fundulus heteroclitus, adapted to a low ion environment. J Exp Biol 206:793–803
Kelly SP, Wood CM (2002) Cultured gill epithelia from freshwater tilapia (Oreochromis niloticus): effect of cortisol and homologous serum supplements from stressed and unstressed fish. J Membr Biol 190:29–42
Kültz D, Chakravarty D, Adilakshmi T (2001) A novel 14-3-3 gene is osmoregulated in gill epithelium of the euryhaline teleost Fundulus heteroclitus. J Exp Biol 204:2975–2985
Kusakabe M, Nakamura I, Young G (2003) 11β-hydroxysteroid dehydrogenase complementary deoxyribonucleic acid in rainbow trout: cloning, sites of expression, and seasonal changes in gonads. Endocrinology 144:2534–2545
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head bacteriophage T4. Nature 227:680–685
Laurent P, Dunel-Erb S, Chevalier C, Lignon J (1994) Gill epithelial cells kinetics in a freshwater teleost, Oncorhynchus mykiss during adaptation to ion-poor water and hormonal treatments. Fish Physiol Biochem 13:353–370
Li YY, Inoue K, Takei Y (2003) Interrenal steroid 21-hydroxylase in eels: primary structure, progesterone-specific activity and enhanced expression by ACTH. J Mol Endocrinol 31:327–340
Madsen SS, Jensen MK, Nøhr J, Kristiansen K (1995) Expression of Na+–K+–ATPase in the brown trout, Salmo trutta: in vivo modulation by hormones and seawater. Am J Physiol Regul Integr Comp Physiol 38:R1339-R1345
Manzon LA (2002) The role of prolactin in fish osmoregulation: a review. Gen Comp Endocrinol 125:291–310
Marshall WS (2003) Rapid regulation of NaCl secretion by estuarine teleost fish: coping strategies for short-duration freshwater exposures. Biochim Biophys Acta 1618:95–105
Marshall WS, Emberley TR, Singer TD, Bryson SE, McCormick SD (1999) Time course of salinity adaptation in a strongly euryhaline estuarine teleost, Fundulus heteroclitus: a multivariable approach. J Exp Biol 202:1535–1544
Marshall WS, Lynch EA, Cozzi RRF (2002) Redistribution of immunofluorescence of CFTR anion channel and NKCC cotransporter in chloride cells during adaptation of the killifish Fundulus heteroclitusto sea water. J Exp Biol 205:1265–1273
Marshall WS, Cozzi RRF, Pelis RM, McCormick SD (2005) Cortisol receptor blockade and seawater adaptation in the euryhaline teleost Fundulus heteroclitus. J Exp Zool 303A:132–142
McCormick SD (1993) Methods for nonlethal gill biopsy and measurement of Na+,K+–ATPase activity. Can J Fish Aquat Sci 50:656–658
McCormick SD (2001) Endocrine control of osmoregulation in teleost fish. Am Zool 41:781–794
McDonald DG, Wood CM, Grosell M, Walsh PJ (2004) Glucocorticoid receptors are involved in the regulation of pulsatile urea excretion in toadfish. J Comp Physiol [B] 174:649–658
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Nishi M, Ogawa H, Ito T, Matsuda K-I, Kawata M (2001) Dynamic changes in subcellular localization of mineralocorticoid receptor in living cells: in comparison with glucocorticoid receptor using dual-color labeling with green fluorescent protein spectral variants. Mol Endocrinol 15:1077–1092
Patrick ML, Wood CM (1999) Ion and acid–base regulation in the freshwater mummichog (Fundulus heteroclitus): a departure from the standard model for freshwater teleosts. Comp Biochem Physiol A 122:445–456
Sakamoto T, Uchida K, Yokota S (2001) Regulation of the ion-transporting mitochondrion-rich cell during adaptation of teleost fishes to different salinities. Zool Sci 18:1163–1174
van der Salm AL, Nolan DT, Wendelaar Bonga SE (2002) In vitro evidence that cortisol directly modulates stress-related responses in the skin epidermis of the rainbow trout (Oncorhynchus mykissWalbaum). Fish Physiol Biochem 27:9–18
Scott GR, Claiborne JB, Edwards SL, Schulte PM, Wood CM (2005) Gene expression after freshwater transfer in gills and opercular epithelia of killifish: insight into divergent mechanisms of ion transport. J Exp Biol 208:2719-2729
Scott GR, Richards JG, Forbush B, Isenring P, Schulte PM (2004a) Changes in gene expression in gills of the euryhaline killifish Fundulus heteroclitus after abrupt salinity transfer. Am J Physiol Cell Physiol 287:C300-C309
Scott GR, Rogers JT, Richards JG, Wood CM, Schulte PM (2004b) Intraspecific divergence of ionoregulatory physiology in the euryhaline teleost Fundulus heteroclitus: possible mechanisms of freshwater adaptation. J Exp Biol 207:3399–3410
Seidelin M, Madsen SS, Byrialsen A, Kristiansen K (1999) Effects of insulin-like growth factor-I and cortisol on Na+,K+–ATPase expression in osmoregulatory tissues of brown trout (Salmo trutta). Gen Comp Endocrinol 113:331–342
Shrimpton JM, McCormick SD (1999) Responsiveness of gill Na+/K+–ATPase to cortisol is related to gill corticosteroid receptor concentration in juvenile rainbow trout. J Exp Biol 202:987–995
Sloman KA, Desforges PR, Gilmour KM (2001) Evidence for a mineralocorticoid-like receptor linked to branchial chloride cell proliferation in freshwater rainbow trout. J Exp Biol 204:3953–3961
Sturm A, Bury N, Dengreville L, Fagart J, Flouriot G, Rafestin-Oblin ME, Prunet P (2005) 11-deoxycorticosterone is a potent agonist of the rainbow trout (Oncorhynchus mykiss) mineralocorticoid receptor. Endocrinology 146:47–55
Uchida K, Kaneko T, Tagawa M, Hirano T (1998) Localization of cortisol receptor in branchial chloride cells in chum salmon fry. Gen Comp Endocrinol 109:175–185
Veillette PA, Sundell K, Specker JL (1995) Cortisol mediates the increase in intestinal fluid absorption in Atlantic salmon during parr-smolt transformation. Gen Comp Endocrinol 97:250–258
Vijayan MM, Reddy PK, Leatherland JF, Moon TW (1994) The effects of cortisol on hepatocyte metabolism in rainbow trout: a study using the steroid analogue RU486. Gen Comp Endocrinol 96:75–84
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Wilson JM, Antunes JC, Bouça PD, Coimbra J (2004) Osmoregulatory plasticity of the glass eel of Anguilla anguilla: freshwater entry and changes in branchial ion-transport protein expression. Can J Fish Aquat Sci 61:432–442
Wood CM, Laurent P (2003) Na+ versus Cl− transport in the intact killifish after rapid salinity transfer. Biochim Biophys Acta 1618:106–119
Zall DM, Fisher MD, Garner QM (1956) Photometric determination of chlorides in water. Anal Chem 28:1665–1678
Zhou BS, Kelly SP, Ianowski JP, Wood CM (2003) Effects of cortisol and prolactin on Na+ and Cl− transport in cultured branchial epithelia from FW rainbow trout. Am J Physiol Regul Integr Comp Physiol 285:R1305–R1316
Acknowledgements
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to P.M. Schulte and a NSERC Post Graduate Scholarship to G.R. Scott. The authors would like to thank Dr Chris Wood, Dr Matt Ramer, Angela Scott, and Joe Rogers for invaluable technical support, as well as Dr Toyoji Kaneko for generously providing antibodies. The authors would also like to thank Dr Jeff Richards and Anne Dalziel for comments on a previous version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey
Rights and permissions
About this article
Cite this article
Scott, G.R., Keir, K.R. & Schulte, P.M. Effects of spironolactone and RU486 on gene expression and cell proliferation after freshwater transfer in the euryhaline killifish. J Comp Physiol B 175, 499–510 (2005). https://doi.org/10.1007/s00360-005-0014-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-005-0014-2