Abstract
Several species of passerines leave their nest with unfinished feather growth, resulting in lower feather insulation and increased thermoregulatory demands compared to adults. However, feather insulation is essential for avian species breeding at northern latitudes, where cold conditions or even snowstorms can occur during the breeding season. In altricial arctic species, increased heat loss caused by poor feather insulation during growth could be counter-adaptative as it creates additional energy demands for thermoregulation. Using flow-through respirometry, we compared resting metabolic rate at thermoneutrality (RMRt), summit metabolic rate (Msum) and heat loss (conductance) in adult and juvenile snow buntings on their summer and winter grounds. In summer, when buntings are in the Arctic, juveniles had a 12% higher RMRt, likely due to unfinished growth, and lost 14% more heat to the environment than adults. This pattern may result from juveniles fledging early to avoid predation at the cost of lower feather insulation. Surprisingly, an opposite pattern was observed at lower latitudes on their wintering grounds. Although they showed no difference in RMRt and Msum, adults were losing 12% more heat than juveniles. We suggest that this difference is due to poorer insulative property of plumage in adults stemming from energetic and time constraints encountered during their post-breeding molt. High plumage insulation in first-winter juvenile buntings could be adaptive to reduce thermoregulatory demands and maximize survival in the first winter of life, while adults could use behavioral strategies to compensate for their greater rate of heat loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For altricial bird species, rapid nestling growth limits vulnerability to nest predation (e.g. Cheng and Martin 2012; Martin 1995; Naef-Daenzer and Grüebler 2016; Remeš and Matysioková 2016). A direct consequence of this trade-off is that nestlings must invest large amounts of energy into body development to fledge at a younger relative age, often before they reach adult body mass (Cheng and Martin 2012; Naef-Daenzer and Grüebler 2016; Remeš and Matysioková 2016). Indeed, nestlings frequently leave the nest before completing growth and continue being fed by adults (Russell 2000). For example, Naef-Daenzer and Grüebler (2016) showed that among 65 ground and cavity-nesting passerine species, nestlings fledged at approximately 60% and 93% of adult body mass, respectively. Other studies reported nestlings leaving the nest with structural body growth 50–70% complete, although some traits such as tarsus length may be comparable to that of adults (e.g. Cheng and Martin 2012; Portelli 2016; Stienen and Brenninkmeijer 2002). As growth is energetically demanding (Drent and Daan 1980; Vézina et al. 2009b; Ton and Martin 2016), recently fledged juveniles thus need to invest a certain proportion of their energy budget in the last stages of development.
Feather growth is typically incomplete immediately after fledging in birds. Cheng and Martin (2012) found wing length at fledging to be 60–90% of adult length in all 12 passerine species they studied. Contour feathers have also been reported to differ between juveniles and adults birds (Butler et al. 2008; Callan et al. 2019). For example, Rintamäki et al. (1983) reported higher thermal conductance (i.e., the rate at which heat is transferred from the body to the environment (McNab 1980)) in juvenile black grouse (Lyrurus tetrix) compared to adults in autumn, an indication of lower insulative property of contour plumage (Walsberg 1988; Wolf and Walsberg 2000). Higher thermal conductance was also reported during the first winter of life in blue tits (Cyanistes caeruleus) (Andreasson et al. 2020) and in Svalbard ptarmigan (Lagopus muta hyperborean) (Nord and Folkow 2018).
Lower insulation in juveniles likely adds to the energetic cost of post-fledging growth as increased heat loss creates additional energy demands for thermoregulation. This is important for species breeding at northern latitudes such as the Arctic, where relatively cold conditions or even snow storms can occur during the breeding season (Meltofte 1983; Hahn et al. 1995; Astheimer et al. 1995; Serreze and Barry 2014). Furthermore, some of these species, like snow buntings (Plectrophenax nivalis) and hoary redpolls (Acanthis hornemanni) winter in cold snowy environment (Brooks 1968; Montgomerie and Lyon 2020) and experience high thermoregulatory demands for most of the year. Comparing data for ten passerine species, Robinson et al. (2007) showed that adverse weather on temperate wintering grounds had a negative impact on survival, with greater effects on first-year individuals than adults. As juveniles may be less efficient at foraging (Weathers and Sullivan 1989; Wunderle 1991), their plumage insulation is therefore paramount as greater heat loss (Stettenheim 1972; Walsberg 1988; Wolf and Walsberg 2000) may also contribute to reduced survival during the first winter of life.
Arctic passerines are known to have remarkable adaptations for life in the cold (Meltofte 1983; Ryzhanovsky 2015). For example, Le Pogam et al. (2020) recently reported impressive cold endurance in wintering snow buntings with adult birds reaching their maximal shivering heat production at air temperatures estimated at − 94 ℃. These birds also appear to maintain a cold-acclimated phenotype through migration and summer months as a potential adaptation to face unexpected cold spikes (Le Pogam et al. 2021a, b). With such adaptations for life in the cold, one would expect juveniles of Arctic species to be as well prepared as adults for these conditions since leaving the nest early with suboptimal insulation and concomitantly having to invest energy in growth (Rintamäki et al. 1983; Callan et al. 2019) could be counter-adaptive. Juvenile snow buntings are known to fledge at 80–84% of adult body mass with primary and rectrix feathers shorter than that of adults (Maher 1964; Hussell 1972). They also have a rapid growth period with nestlings leaving the nest at 13 days on average (Maher 1964). It is unknown, however, whether juvenile buntings experience higher levels of heat loss than adults after fledging and whether this persists over the first winter of life.
Working with a snow bunting population at the northern boundary of their breeding range (Alert, NU, Canada 82°N), we examined morphological traits and metabolic variation in juvenile buntings in the weeks following fledging and compared it to that of adults. Given that buntings fledge before achieving adult size and mass, we expected higher maintenance energy consumption (basal metabolic rate (BMR), Swanson et al. 2017) in juveniles than in adults as young birds should still be investing energy in the last stages of growth (Rintamäki et al. 1983). We also expected one of two potential scenarios regarding juvenile plumage insulation. Snow buntings could show a pattern similar to that of other species (Rintamäki et al. 1983; Nord and Folkow 2018; Andreasson et al. 2020) with juveniles experiencing greater heat loss (i.e., higher thermal conductance) than adults, indicating elevated thermoregulation costs after fledging. Alternatively, given the species adaptations for life in the cold (e.g. Le Pogam et al. 2020, 2021a), snow buntings could fledge with insulation levels comparable to that of adults, which would suggest that this Arctic species is adapted for minimizing post-fledging energy demands for thermoregulation. High insulation would promote better energy efficiency for finalizing growth and for surviving their first winter. We also included a comparison of phenotypes between adults and first-winter juveniles captured on their wintering grounds in Québec, Canada, to investigate whether potential differences in traits remain over the first winter of life in snow buntings.
Material and methods
Comparing phenotypes on the breeding and wintering grounds
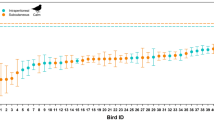
We captured forty-nine snow buntings (31 adults and 18 juveniles) at the Canadian Forces Station Alert, Nunavut (82°30′05″ N, 62°20′20″ W) in the Canadian High Arctic between May and August 2019 (Fig. 1). Adults were captured using either walk-in traps baited with mixed seeds before breeding, double potter traps with a live male decoy and territorial playback on their breeding territory or at their nest while provisioning nestlings. Juvenile birds were captured using walk-in and potter traps baited with seeds between 19 July and 18 August. Although it was not possible to determine the time since fledging for juveniles, the last observed fledging event recorded in 2019 took place on July 24th. Recaptures of fledged juveniles in 2015, 2018 and 2019 also suggest that our captures and measurements were conducted during a period where structural growth was not completed as indicated by the length of the head plus beak (Fig. 2). All juveniles were in their first plumage and had not begun their postjuvenal molt (Pyle 1997; K. G. Young personal observation, 2020). Immediately after capture, we weighed birds (± 0.01 g) and took morphometric measurements on the head plus beak (± 0.01 mm), tarsus (± 0.01 mm), wing (± 0.1 cm) and tail (± 0.1 cm). We then brought the birds back to the field laboratory for respirometry trials (RMRt, see the metabolic performance below, n = 49; conductance, n = 35) and ultrasonography. Birds were kept in captivity for an average of 0.5 ± 0.5 days before RMRt and 1.9 ± 1.6 days before conductance trials before being released.
Snow bunting distribution and location of summer and winter study sites (A). Note that snow buntings are associated with snow cover in winter and may not be observed in areas of their southern wintering range where snow is absent during warm winters. Temperatures presented are averages for the periods of captures. Snow bunting annual cycle (B). Colored months (darker) represent months where our data were collected on breeding and wintering sites, while lighter-colored months represent months where snow buntings are present at the site. Information presented is based on field observations, Macdonald et al. (2012), McKinnon et al. (2016), Montgomerie and Lyon (2020) and eBird (2023)
We also captured snow buntings (87 adults and 102 juveniles) during the winter months (January to April) from 2016 to 2018 in open fields near Rimouski, Canada (48°20′49″N, 68°43′32″W), using walk-in traps baited with cracked corn. Although they had completed their postjuvenal molt before their autumn migration, buntings in their first winter of life were categorized by plumage as wintering juveniles (Fig. 1). They were easily distinguished from adults using the coloration patterns of their flight feathers and wing covers (Pyle 1997; Love et al. 2012). We weighed and measured the birds as noted above and then transported them to the avian facilities of the Université du Québec à Rimouski for RMRt and conductance measurements (RMRt, n = 99; conductance, n = 51) and ultrasonography. Additionally, we measured the summit metabolic rate (Msum), an indicator of shivering cold endurance (Swanson 2001; Swanson and Liknes 2006, see Le Pogam et al. 2020, 2021a) on 75 birds. The birds were kept in indoor cages (117 cm × 39 cm × 31 cm) for an average of 2.4 ± 1.7 days before both RMRt and Msum and for 1.6 ± 0.9 days before conductance with ad libitum access to mixed seeds and water between metabolic trials and until being released.
It should be noted that the wintering range of snow buntings breeding at Alert is unknown as no birds banded at Alert have been recovered in winter yet. However, while the population wintering in eastern Québec is known to breed in western Greenland (Macdonald et al. 2012), recent evidence based on the genetic signature of blood cells also suggests that birds from Alert are likely mixing with buntings breeding in the low Arctic (East-Bay, Southampton Island) and wintering in the Canadian prairies and, to a lesser degree, Ontario (Macdonald et al. 2012, 2016; Patel 2022). Wintering environmental conditions for these birds are comparable to that experienced by birds wintering in Eastern Québec.
Fat score and flight muscle size
We estimated fat reserves and flight muscle size in all buntings using visual scores. Fat score ranged from 0, (no visible fat), to 6, (tracheal pit is full and bulging with fat; Canadian Snow Bunting Network 2012; Gosler 1996). The scores for flight muscles were based on muscle shape and ranged from 0 (muscle depressed and the sternum is sharp) to 3 (muscles rounded above the sternum; Bairlein 1995; Busse 2000).
We also estimated the size of flight muscles non-invasively by measuring muscle thickness with ultrasonography (Royer-Boutin et al. 2015; Le Pogam et al. 2020, 2021a). Flight muscle thickness and keel height were measured on all adults and 12 juveniles in summer and on 37 adults and 48 juveniles in winter using a LOGIQe ultrasound scanner fitted with a linear probe (12 MHz, GE Healthcare, Wauwatosa, WI, USA, see Le Pogam et al. 2020). Muscle thickness was obtained from a cross-sectional image by measuring, at 45° relative to the keel, the length between the muscle surface and the base of the bone. We included keel height as a covariate in statistical analyzes to control for variation in muscle thickness generated by the positioning of the probe (Le Pogam et al. 2020). Each measurement was repeated three to four times and average values were used in the analyses.
Metabolic performance
Physiological maintenance costs are measured as basal metabolic rate (BMR) in birds (Swanson et al. 2017). However, by definition BMR refers to animals not involved in generating new tissues (e.g. growth). Although our measurements respected the experimental standards for BMR (resting phase of the day, thermoneutrality, postabsorptive state), our juvenile birds were in the last stages of growth. We, therefore, use the term resting metabolic rate at thermoneutrality (RMRt) for both adults and juveniles in this study to avoid confusion. We performed summer measurements of RMRt and conductance at Alert using the instruments and setup described by Le Pogam et al. (2021b) and O’Connor et al. (2021). We used the instruments and setup described by Le Pogam et al. (2020, 2021a) for winter measurements of RMRt, conductance and Msum at UQAR. As these methods have been described in detail previously, they are presented briefly below.
RMRt and conductance
At Alert, we placed buntings in 1.5L stainless steel metabolic chambers and pushed dry CO2-free air (scrubbed with Silica gel, Soda lime and Drierite) at a constant rate of 650 ml·min−1, controlled by mass flow controllers (Omega FMA5418A, previously calibrated with a Bubble-O-Meter), for both RMRt and conductance measurements. The excurrent chamber airstream was scrubbed of water vapor and CO2 before entering one of the two FoxBox oxygen analyzers, each able to monitor two chambers in alternance using a MUX multiplexer (Sable Systems, Las Vegas, NV, USA). During RMRt trials, the airstreams sent to the analyzers alternated between a baseline channel (scrubbed ambient air) for 10 min and chamber channels. We measured up to 4 birds at a time (2 chambers per analyzer) for 40 min between baselines or for 60 min when analyzers were each monitoring a single chamber. We sampled the fractional oxygen concentration (\(\dot{\mathrm{V}}{\mathrm{O}}_{2})\) in the excurrent airstreams every 20 s. On average, buntings were fasted for 4.76 ± 2.86 h prior to measurements. We measured RMRt overnight, lasting on average 9.4 ± 3.7 h. We measured conductance during the day (mean fasting time of 1.94 ± 0.58 h), on a maximum of 4 birds in parallel. On average, conductance measurements lasted 3.0 ± 0.05 h. We used the same protocol as for RMRt except that baseline air was measured for 10 min before and after chamber measurements, which lasted 160 min. During conductance measurements \(\dot{\mathrm{V}}{\mathrm{O}}_{2}\) was sampled at a rate of 5 s. We measured chamber temperatures every 5 s with copper-constantan thermocouples (TC-2000 thermocouple reader, Sable Systems, Las Vegas, NV, USA). We maintained chamber temperature during RMRt trials at a thermoneutral temperature of 25 ºC (Scholander et al. 1950). For conductance, adults were exposed to − 20.6 ± 1.0 ºC and juveniles were exposed to − 8.4 ± 1.3 ºC, both below the lower critical temperature of 10 ºC for this species (Scholander et al. 1950).
At UQAR, we placed the birds in the same 1.5L metabolic chambers for measurements, which were also supplied with dry, CO2-free air. Flow rates, controlled by mass flow controllers (Sierra Instruments, SideTrak®, Monterey, CA, USA), were 650 and 700 ml.min−1 for RMRt and conductance, respectively. This system can measure air coming from 4 chambers in parallel, using a 4 channel Servomex gas purity analyzer (model 4100, Boston, MA, USA). We, therefore, used an alternating sequence of 10 min baseline and 40 min chamber for RMRt (at 25 ºC), which lasted 12.9 ± 2.3 h overnight (after 6.66 ± 2.54 h fasting). For conductance, chamber measurements lasted 100 min in total and baseline air was measured for 10 min three times during a sequence i.e., the first 10 min, after 40 min of chamber measurement and after a further 60 min of chamber measurement. Birds were fasted for 1.75 ± 0.66 h before conductance and metabolic chambers were maintained at − 21.4 ± 6.8 ºC during trials.
Summit metabolic rate
We used the same metabolic chambers for Msum. During a trial, birds were first exposed to scrubbed air for 10 min at a flow rate of 1200 ml.min−1, after which chamber inflow was replaced by a Helox gas mixture (21% oxygen, 79% helium) to increase heat loss (Rosenmann and Morrison 1974, mass flow controllers were calibrated for Helox using a Bubble-O-Meter). After 5 min at − 18 ℃, birds O2 consumption was measured and we subsequently dropped chamber temperature by 3 ℃ every 20 min (Swanson et al. 1996) until the birds showed signs of hypothermia (indicated by a decline in O2 consumption for several minutes) or reached the end of a 185 min automated program (see Le Pogam et al. 2020). Summit metabolic rate measurements lasted for 1.92 ± 0.67 h and birds were not fasted prior to measurements.
We weighed snow buntings before and after all metabolic measurements and average body mass was used in analyses. We also measured body temperature (Tb) for conductance and Msum, just before entering and immediately after exiting metabolic chambers, by inserting a type-T thermocouple approximately 10 mm into the cloaca (Omega model HH-25KC, NIST-traceable, Omega, Montréal, QC, Canada). We conducted respirometry calculations, for both summer and winter, with ExpeData V.1.8.4 (Sable Systems, Las Vegas, NV, USA), using the lowest averaged 10 min of \(\dot{\mathrm{V}}{\mathrm{O}}_{2}\) for conductance and RMRt and the highest averaged 10 min of \(\dot{\mathrm{V}}{\mathrm{O}}_{2}\) for Msum. Oxygen consumption was calculated using Eq. 10.1 from (Lighton 2019) and then converted to watts using a thermal equivalent of 19.8 kJ·L−1O2 (representing an assumed respiratory quotient of 0.71) (Gessaman and Nagy 1988). Conductance was calculated as
where MR = metabolic rate, Tb = average body temperature and Ta = average ambient temperature in the chamber during the lowest 10 min \(\dot{\mathrm{V}}{\mathrm{O}}_{2}\).
Statistical analysis
We used permutation tests for linear models (LMP) using the lmPerm package (Wheeler and Torchiano 2016) to assess morphological differences between adults and juveniles at Alert and between adults and first winter birds captured on their wintering grounds in Rimouski. Permutation tests were used because the distribution of residuals for some morphometric variables deviated from normality. Permutation tests for linear models compared traits (body mass, length of the head plus beak, tarsus, wing, tail as well as fat and muscle scores) between age classes (adults and juveniles). For structural size variables, models also included the fixed factor “observer” to control for potential measuring bias among observers. As winter data were collected over three winters, we also added “year” as a fixed factor in models comparing wintering birds. We then performed post-hoc pairwise comparisons between years using permutation t-tests when the year effect was significant. For variables with a normal distribution of residuals, the results obtained from the LMP were very similar to those obtained with linear models and led to the same conclusions. We, therefore, used LMP throughout for consistency. We used ANCOVA models, with body mass as a covariate, to assess whether RMRt, conductance and Msum differed between age classes within seasons.
All analyses were conducted in the R 4.1.1 (R Core Team 2021) environment, and we confirmed the normality of residuals visually for analyses on metabolic traits. For permutation tests, the exchangeability postulate was met as our morphometric measurements could belong to any age class under the null hypothesis (i.e., no difference between adult and juvenile traits). This assumption can be made with observational data such as ours (Anderson 2001). Data are presented as mean ± s.e.m.
Results
Comparing juveniles and adults in summer—Arctic
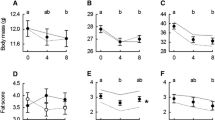
Our data revealed that, at the time of capture, juveniles had a body mass similar to adults but had yet to complete feather growth and the development of some structural traits (Fig. 3). Specifically, the length of the head plus beak in juveniles was 97.5 ± 0.8% of that measured in adults (Nperm = 5000, P = 0.02), and the same pattern was apparent for wing length (93.0 ± 1.3%, Nperm = 5000; P < 0.0001) and tail length (95.6 ± 2.0%, Nperm = 1500; P = 0.06). We found no evidence that body mass and tarsus length differed among age class (Nperm = 681; P = 0.1 and Nperm = 247; P = 0.3 respectively).
We observed no significant difference in fat score between juveniles and adults (Nperm = 51; P = 0.8), although muscle scores were higher, on average, in juvenile birds (adults = 1.6 ± 0.5; juveniles = 1.9 ± 0.3; Nperm = 2875; P = 0.03). However, this difference did not transpose into muscle thickness as measured by ultrasonography (Nperm = 154; P = 0.4).
Resting metabolic rate at thermoneutrality and conductance increased with body mass in both juvenile and adult snow buntings (mass effect: RMRt: P < 0.0001, conductance: P = 0.01) (Fig. 4). For a given body mass, RMRt was 11.7 ± 2.3% higher in juvenile birds compared to adults (F1,46 = 11.59; P = 0.001) (Fig. 4a). Young buntings also lost more heat to the environment for their mass than adults, with conductance being 11.3 ± 3.5% higher in juveniles than in adults (F1, 32 = 4.60; P = 0.04) (Fig. 4b).
Comparing first winter birds and adult buntings in winter—Québec
We found a significant difference in body mass between age classes in winter. First winter buntings were, on average, 2.8 ± 0.8% lighter than adults (Nperm = 5000; P = 0.001) (Fig. 3). We also observed a significant year effect on body mass (Nperm = 5000; P = 0.01) with birds being heavier in 2018 (Nperm = 999; P = 0.024 for comparisons with both 2016 and 2017). There were slight differences in structural size between age classes. Indeed, the length of the head plus beak in first winter birds was 99.1 ± 0.2% of that measured in adults (Nperm = 4160; P = 0.02) (Fig. 3). Our results further showed that wings and tail feathers were significantly shorter during the first winter of life compared to adults (no difference in tarsus length P = 0.24, Nperm = 325). Wing and tail lengths were respectively 98.2 ± 0.3% (Nperm = 5000; P < 0.0001) and 98.1 ± 0.6% (Nperm = 5000; P = 0.007) of adult size (Fig. 3).
There was no significant difference in fat reserves carried by adults and wintering juveniles (Nperm = 306; P = 0.25) and both had similar flight muscles sizes (Score: Nperm = 87, P = 0.54, thickness: Nperm = 99, P = 0.50). However, muscle scores did differ among years (Nperm = 5000; P < 0.0001, pectoral thickness P = NS) with higher muscle scores observed in 2018 relative to the two previous years (Nperm = 999; P = 0.003 for comparison with both 2016 and 2017).
Considering the significant influence of body mass on RMRt (F 1,96 = 33.19; P < 0.0001) and Msum (F 1,72 = 18.22; P < 0.0001), winter metabolic performance did not differ between first winter snow buntings and adults (age effect P > 0.10 in both RMRt and Msum). In contrast, winter conductance varied independently from body mass (mass effect P = 0.3) with adults losing 12.3 ± 3.4% more heat on average than juveniles (F1,48 = 7.61; P = 0.008) (Fig. 5).
Discussion
In this study, we examined whether juvenile snow buntings in their last stages of growth experience elevated maintenance costs relative to adults and compared body heat loss between juveniles and adults. Our data from the Arctic show that recently fledged juveniles had not completed structural growth at the time of capture based on differences in wing and head plus beak length. Juveniles also experienced a 12% higher resting metabolic rate relative to adults, which is consistent with juveniles investing more energy in growth following fledging (Rintamäki et al. 1983). We further observed that recently fledged juvenile buntings in the Arctic had a rate of heat loss of 14% higher than that of adults, which suggests that, despite being a cold specialized species, buntings display similar trends to passerines breeding at lower latitudes regarding plumage insulation (e.g. Andreasson et al. 2020). Comparing snow bunting age classes in winter revealed similar metabolic performance in adults and first-winter birds. However, heat loss was 12% higher in adults, a pattern opposite to that observed on the breeding ground.
Juveniles and adults in summer—Arctic
While juvenile snow buntings had body mass and fat stores comparable to adults, they fledged before fully completing their feather and structural body growth. Snow buntings are known to fledge before reaching adult size (see Fig. 2) and continue to grow their remiges and rectrices even after starting their postjuvenal body molt (Maher 1964; Hussell 1972). This pattern is consistent with other passerines where nestlings typically leave the nest before finalizing growth (Cheng and Martin 2012; Portelli 2016; Remeš et al. 2020), likely to reduce nest predation risk (Naef-Daenzer and Grüebler 2016; Remeš and Matysioková 2016). In fact, despite snow buntings nesting inside enclosed structures, such as rock cavities (Montgomerie and Lyon 2020), Hussell (1972) reported heavy nest predation by arctic foxes (Alopex lagopus) and weasels (Mustela erminea) on Devon Island. These two predators are also present in Alert.
We found that juvenile snow buntings had larger flight muscles than adults based on visual scores, although this did not translate into differences in muscle thickness. This is likely due to scores evaluating the 3-dimensional shape of muscles, whereas ultrasonography measures muscle thickness cross-sectionally, which may underestimate muscle volume differences (Swanson and Merkord 2013; Vézina et al. 2021; see also Royer-Boutin et al. 2015). Larger flight muscles in juvenile buntings could be a response to higher wing loading as they must fly with similar mass but with shorter wings than adults. Lind and Jakobsson (2001) also showed that molting tree sparrows (Passer montanus) increased their pectoral muscle size to body mass ratio with an increase in wing loading.
We found 11% higher conductance in juveniles compared to adult buntings at Alert, a finding compatible with observations by Andreasson et al. (2020) in blue tits and by Rintamäki et al. (1983) in black grouse. Higher conductance in juveniles likely reflects feather structural differences and lower feather mass relative to adults, as reported by Butler et al. (2008) for 10 species of warblers. Additionally, Butler et al. (2008) found a positive correlation between feather quality (based on barb count and feather mass) and duration of the nestling period among these species, suggesting that some may trade off feather insulation for early fledging. Callan et al. (2019) observed similar cost–benefit trade-offs between feather quality (based on barb density) and nestling duration among 123 temperate and tropical species. With juvenile snow buntings fledging at 13 days on average (Maher 1964), the benefit of fledging early (e.g., predator avoidance) may outweigh the cost of lower insulation and the accompanying thermoregulatory disadvantages during unpredictable cold days. Furthermore, the insulative property of feathers grown in the nest also depends on the timing of their renewal (i.e., feather lifespan) (Kiat and Sapir 2018). As juvenile buntings go through a complete molt of body feathers before their fall migration (Hussell 1972), low insulation would thus only be an ephemeral constraint as they could improve insulation before departure. Thus, the fitness costs of producing ephemeral body feathers with poor insulative properties is likely outweighed by the fitness benefits of a shorter development time (Callan et al. 2019), which is crucial in Arctic species experiencing short favorable conditions for breeding. Furthermore, juvenile buntings do fledge with a dark grey plumage which may partly compensate for heat loss by maximising heat gain from solar radiation, often 24 h per day in the high Arctic. Additionally, juvenile buntings could compensate for higher heat loss in summer by increasing shivering heat production capacity (Swanson 2010; Mckechnie and Swanson 2010; Le Pogam et al. 2020, 2021a), and this could be associated with larger flight muscles as observed here (O’Connor 1995; Dubois et al. 2016; Le Pogam et al. 2020).
Resting metabolic rate was 12% higher in juvenile buntings than in adults of comparable mass. This is likely not a thermal acclimation response (cf. Vézina et al. 2009b) as we found no correlation between conductance and RMRt in birds measured at Alert (data not shown). Given that juveniles still had to complete feather development and part of structural growth at the time of capture (Fig. 2), and that growth rate is known to correlate with BMR in other passerines (Ton and Martin 2016; see also Vézina et al. 2009a), the higher RMRt in juvenile buntings could reflect energy invested in the last stages of growth after fledging, including internal organ development and tissue maturation. For example, post-fledging development of organs has been reported in altricial domestic pigeons (Columba livia) (Liang et al. 2018) as well as in precocial greater snow geese (Chen caerulescens atlantica) (Lesage and Gauthier 1997). Vézina et al. (2009a) further showed that some organs, such as the heart, kidney, pancreas, pectoral muscles and intestines, are still developing a few days before fledging in European starlings (Sturnus vulgaris). Higher metabolic rates have also been reported in nestlings house martin (Delichon urbicum) compared to adults (Prinzinger and Siedle 1988) and in fledged black grouse (Rintamäki et al. 1983).
Juveniles and adults in winter—Québec
Comparing adult and juveniles on the wintering grounds, we found differences in morphometric measurements. Juveniles had shorter head plus beak, wing and tails than adults. This corroborates findings by Meltofte (1983) and Smith (1994) who also reported shorter wings in juvenile snow buntings during the first winter of life compared to adults in Greenland and Scotland respectively (but see Banks et al. 1989; Rae and Marquiss 1989). In their first winter, juveniles were also on average 3% lighter than adults. As juveniles snow buntings are dominant over adults on the wintering ground, they have priority access to food and may store less fat reserves (Smith and Metcalfe 1994, 1997; Laplante 2018). This difference was not reflected in our fat score results however, which may be due to the relatively small difference between age classes. We further observed significantly heavier birds and higher muscles scores in 2018 compared to 2016 and 2017. Interestingly, 2018 was the year with the coldest winter, (lowest mean temperature = − 10.8 ± 5.7 ºC) compared to − 8.6 ± 5.3 ºC in 2016 and − 8.3 ± 6.1 ºC in 2017. Laplante et al. (2019) showed that snow buntings increase their body mass and fat reserves in colder and snowier conditions. Thus, the year effect on body mass and muscle size observed here might result from colder temperatures requiring improved thermogenic capacity and larger fat reserves (Swanson and Olmstead 1999).
Surprisingly, juvenile buntings on their winter grounds lost 12% less body heat than adults. This suggests that first-winter juveniles become better insulated than adults following their postjuvenal molt on their breeding grounds. This differs from observations in blue tits where first-winter birds typically suffer greater heat loss than adults during the cold season (Andreasson et al. 2020). However, blue tits typically experience milder temperatures in winter than buntings. The reason for the switch in conductance patterns between juvenile and adults during breeding and wintering is not immediately obvious and could potentially be linked to population differences. However, it may also result from the timing of breeding and molt on the Arctic breeding ground. Indeed, adult snow buntings are known to begin molting while still provisioning nestlings (Hussell 1972; Green and Summers 1975; Montgomerie and Lyon 2020). In fact, post-breeding molt is very rapid (28 to 35 days Green and Summers 1975; Hussell 1972) and involves the loss of several flight feathers at once, which can lead to flightlessness (Parmelee 1968; Green and Summers 1975; Ryzhanovsky 2015; Montgomerie and Lyon 2020). As nestling provisioning and molt can be energetically demanding (Hussell 1972; Drent and Daan 1980; Walsberg 1983; Murphy and King 1992; Lindström et al. 1993), it is likely that adult snow buntings face energy and time constraints restricting the production of high-quality feathers. Furthermore, while both juveniles and adults molt into their winter plumage before departing the breeding grounds, adults have to carry out a complete post-breeding molt while post-juvenal molt is only partial, with body feathers and some secondary coverts being replaced (Hussell 1972; Pyle 1997; Ryzhanovsky 2015; Montgomerie and Lyon 2020). Time and energy constraints on molt have been observed before and can lead to the production of feathers with poorer insulative properties (Dawson et al. 2000; Hall and Fransson 2000; Koskenpato et al. 2016). For example, Nilsson and Svensson (1996) reported a trade-off between reproduction and molt in late-breeding blue tits that were found to spend more energy in thermoregulation the following winter. The negative effect of increased feather growth rate on feather structure was also observed in both juveniles (Butler et al. 2008) and adult passerines (Dawson et al. 2000; De La Hera et al. 2009). Therefore, no matter the location of their breeding ground, juvenile snow buntings appear less restricted than adults in producing their first winter plumage and this could explain the better insulation observed in first year wintering individuals compared to adults. Nevertheless, the greater heat loss in adults does not appear to be offset by a higher thermogenic capacity (Msum) or basal heat production (RMRt). This suggests that experienced wintering adults could rely on other means, such as behavioural thermoregulation, to reduce the impact of heat loss on their daily energy budget.
Data availability
Data are not available for this article.
References
Anderson MJ (2001) Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58:626–639. https://doi.org/10.1139/f01-004
Andreasson F, Nord A, Nilsson J-Å (2020) Age differences in night-time metabolic rate and body temperature in a small passerine. J Comp Physiol B. https://doi.org/10.1007/s00360-020-01266-5
Astheimer LB, Buttemer WA, Wingfield JC (1995) Seasonal and acute changes in adrenocortical responsiveness in an Arctic-breeding bird. Horm Behav 29:442–457. https://doi.org/10.1006/hbeh.1995.1276
Bairlein F (1995) Manual of feild methods. European Science Foundation, Germany
Banks KW, Clark H, Mackay IRK et al (1989) Biometrics and pre-migratory fattening in the snow bunting Plectrophenax nivalis. Ringing Migr 10:141–158. https://doi.org/10.1080/03078698.1989.9673953
Brooks WS (1968) Comparative adaptations of the Alaskan redpolls to the Arctic environment. The Wilson Bulletin 80:253–280
Busse P (2000) Bird station manual. SE European Bird Migration Network, University of Gdansk
Butler LK, Rohwer S, Speidel MG (2008) Quantifying structural variation in contour feathers to address functional variation and life history trade-offs. J Avian Biol 39:629–639. https://doi.org/10.1111/j.1600-048X.2008.04432.x
Callan LM, La Sorte FA, Martin TE, Rohwer VG (2019) Higher nest predation favors rapid fledging at the cost of plumage quality in nestling birds. Am Nat 193:717–724. https://doi.org/10.1086/702856
Cheng Y-R, Martin TE (2012) Nest predation risk and growth strategies of passerine species: grow fast or develop traits to escape risk? Am Nat 180:285–295. https://doi.org/10.1086/667214
Dawson A, Hinsley SA, Ferns PN et al (2000) Rate of moult affects feather quality: a mechanism linking current reproductive effort to future survival. The Royal Society 267:2093–2098
De La Hera I, Pérez-Tris J, Tellería JL (2009) Migratory behaviour affects the trade-off between feather growth rate and feather quality in a passerine bird: Bird migration and moult trade-offs. Biol J Linn Soc 97:98–105. https://doi.org/10.1111/j.1095-8312.2008.01189.x
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 68:225–252. https://doi.org/10.5253/arde.v68.p225
Dubois K, Hallot F, Vézina F (2016) Basal and maximal metabolic rates differ in their response to rapid temperature change among avian species. J Comp Physiol B 186:919–935. https://doi.org/10.1007/s00360-016-1001-5
eBird (2023) eBird: an online database of bird distribution and abundance [web application]. eBird, Cornell Lab of Ornithology, Ithaca, New York. http://www.ebird.org. Accessed 16 Mar 2019
Gessaman JA, Nagy KA (1988) Energy metabolism: errors in gas-exchange conversion factors. Physiol Zool 61:507–513. https://doi.org/10.1086/physzool.61.6.30156159
Gosler AG (1996) Environmental and social determinants of winter fat storage in the great tit Parus major. J Anim Ecol 65:1. https://doi.org/10.2307/5695
Green GH, Summers RW (1975) Snow bunting moult in Northeast Greenland. Bird Study 22:9–17. https://doi.org/10.1080/00063657509476435
Hahn TP, Wingfteld JC, Mullen R, Deviche PJ (1995) Endocrine bases of spatial and temporal opportunism in Arctic-breeding birds. Am Zool 35:259–273. https://doi.org/10.1093/icb/35.3.259
Hall KSS, Fransson T (2000) Lesser Whitethroats under time-constraint moult more rapidly and grow shorter wing feathers. J Avian Biol 31:583–587. https://doi.org/10.1034/j.1600-048X.2000.310419.x
Hussell DJT (1972) Factors affecting clutch size in Arctic Passerines. Ecol Monogr 42:317–364. https://doi.org/10.2307/1942213
Kiat Y, Sapir N (2018) Life-history trade-offs result in evolutionary optimization of feather quality. Biol J Linn Soc 125:613–624. https://doi.org/10.1093/biolinnean/bly135
Koskenpato K, Ahola K, Karstinen T, Karell P (2016) Is the denser contour feather structure in pale grey than in pheomelanic brown tawny owls Strix aluco an adaptation to cold environments? J Avian Biol 47:1–6. https://doi.org/10.1111/jav.00746
Laplante M-P (2018) Facteurs environnementaux et sociaux influençant la gestion des réserves énergétiques chez le plectrophane des neiges (Plectrophenax nivalis) en hiver. MSc thesis, Université du Québec à Rimouski
Laplante M-P, McKinnon EA, Love OP, Vézina F (2019) Flexible response to short-term weather in a cold-adapted songbird. J Avian Biol 50:1–10. https://doi.org/10.1111/jav.01766
Le Pogam A, Love OP, Régimbald L et al (2020) Wintering snow buntings elevate cold hardiness to extreme levels but show no changes in maintenance costs. Physiol Biochem Zool 93:417–433. https://doi.org/10.1086/711370
Le Pogam A, O’Connor RS, Love OP et al (2021a) Coping with the worst of both worlds: phenotypic adjustments for cold acclimatization benefit northward migration and arrival in the cold in an Arctic breeding songbird. Funct Ecol 35:1240–1254. https://doi.org/10.1111/1365-2435.13793
Le Pogam A, O’connor RS, Love OP et al (2021b) Snow buntings maintain winter-level cold endurance while migrating to the High Arctic. Front Ecol Evol 9:1–9. https://doi.org/10.3389/fevo.2021.724876
Lesage L, Gauthier G (1997) Growth and organ development in greater snow goose goslings. Auk 114:229–241. https://doi.org/10.2307/4089164
Liang X, Yu J, Wang H, Zhang Z (2018) Post-hatching growth of the pectoralis muscle in pigeon and its functional implications: growth of pigeon pectoralis muscle. Anat Rec 301:1564–1569. https://doi.org/10.1002/ar.23850
Lighton JRB (2019) Measuring metabolic rates: a manual for scientists. 2nd edn. Oxford University Press
Lind J, Jakobsson S (2001) Body building and concurrent mass loss: flight adaptations in tree sparrows. Proc R Soc Lond B 268:1915–1919. https://doi.org/10.1098/rspb.2001.1740
Lindström Å, Visser GH, Daan S (1993) The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol Zool 66:490–510
Love OP, Macdonald C, Mckinnon E (2012) Canadian Snow Bunting Banding Network Protocol. https://doi.org/10.6084/m9.figshare.1588581.v1
Macdonald CA, Fraser KC, Gilchrist HG et al (2012) Strong migratory connectivity in a declining arctic passerine. Animal Migration 1:23–30. https://doi.org/10.2478/ami-2012-0003
Macdonald CA, McKinnon EA, Gilchrist HG, Love OP (2016) Cold tolerance, and not earlier arrival on breeding grounds, explains why males winter further north in an Arctic-breeding songbird. J Avian Biol 47:7–15. https://doi.org/10.1111/jav.00689
Maher WJ (1964) Growth rate and development of endothermy in the snow bunting (Plectrophenax nivalis) and Lapland longspur (Calcarius lapponicus) at Barrow, Alaska. Ecology 45:520–528. https://doi.org/10.2307/1936105
Martin TE (1995) Avian life history evolution in relation to nest sites, nest predation, and food. Ecol Monogr 65:101–127. https://doi.org/10.2307/2937160
Mckechnie AE, Swanson DL (2010) Sources and significance of variation in basal, summit and maximal metabolic rates in birds. Curr Zool 56:741–758. https://doi.org/10.1093/czoolo/56.6.741
McKinnon EA, Macdonald CM, Gilchrist HG, Love OP (2016) Spring and fall migration phenology of an Arctic-breeding passerine. J Ornithol 157:681–693. https://doi.org/10.1007/s10336-016-1333-7
McNab BK (1980) On estimating thermal conductance in endotherms. Physiol Zool 53:145–156. https://doi.org/10.1086/physzool.53.2.30152577
Meltofte H (1983) Arrival and pre-nesting period of the snow bunting Plectrophenax nivalis in East Greenland. Polar Res 1:185–198. https://doi.org/10.3402/polar.v1i2.6983
Montgomerie R, Lyon B (2020) Snow bunting (Plectrophenax nivalis), version 1.0. Birds NAm. https://doi.org/10.2173/bna.198
Murphy ME, King JR (1992) Energy and nutrient use during moult by white-crowned sparrows Zonotrichia leucophrys gambelii. Ornis Scand 23:304. https://doi.org/10.2307/3676654
Naef-Daenzer B, Grüebler MU (2016) Post-fledging survival of altricial birds: ecological determinants and adaptation. J Field Ornithol 87:227–250. https://doi.org/10.1111/jofo.12157
Nilsson J-Å, Svensson E (1996) The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proc R Soc Lond B 263:711–714. https://doi.org/10.1098/rspb.1996.0106
Nord A, Folkow LP (2018) Seasonal variation in the thermal responses to changing environmental temperature in the world’s northernmost land bird. J Exp Biol 221:1–10. https://doi.org/10.1242/jeb.171124
O’Connor TP (1995) Metabolic characteristics and body composition in house finches: effects of seasonal acclimatization. J Comp Physiol B 165:298–305. https://doi.org/10.1007/BF00367313
O’Connor RS, Le Pogam A, Young KG et al (2021) Limited heat tolerance in an Arctic passerine: thermoregulatory implications for cold-specialized birds in a rapidly warming world. Ecol Evol. https://doi.org/10.1002/ece3.7141
Parmelee DF (1968) Life histories of North American cardinals, buntings, sparrows, and allies: snow buntings. In: Austin OL (ed) Life histories of North American cardinals, buntings, sparrows, and allies. U.S. Government Printing Office, New York, pp 1652–1675
Patel KK (2022) Using genetic approaches to study local adaptation and reproductive success in snow buntings (Plectrophenax nivalis). MSc thesis, University of Windsor
Portelli DJ (2016) Nestling and post-fledging growth and development in an Australian passerine: Hall’s Babbler Pomatostomus halli. Corella 40:81–90
Prinzinger R, Siedle K (1988) Ontogeny of metabolism, thermoregulation and torpor in the house martin Delichon u. urbica (L.) and its ecological significance. Oecologia 76:307–312. https://doi.org/10.1007/BF00379969
Pyle P (1997) Identification guide to North American birds, part I : Columbidae to Ploceidae. Slate Creek Press, Californie
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rae R, Marquiss M (1989) Ageing and sexing of snow buntings wintering on the Aberdeenshire coast, their biometrics and sex ratio. Ringing Migr 10:133–140. https://doi.org/10.1080/03078698.1989.9673952
Remeš V, Matysioková B (2016) Survival to independence in relation to pre-fledging development and latitude in songbirds across the globe. J Avian Biol 47:610–618. https://doi.org/10.1111/jav.00841
Remeš V, Matysioková B, Vrána J (2020) Adaptation and constraint shape the evolution of growth patterns in passerine birds across the globe. Front Zool 17:1–13. https://doi.org/10.1186/s12983-020-00377-7
Rintamäki H, Saarela S, Marjakangas A, Hissa R (1983) Summer and winter temperature regulation in the black grouse Lyrurus tetrix. Physiol Zool 56:152–159. https://doi.org/10.1086/physzool.56.2.30156048
Robinson RA, Baillie SR, Crick HQP (2007) Weather-dependent survival: implications of climate change for passerine population processes: Weather-dependent passerine survival. Ibis 149:357–364. https://doi.org/10.1111/j.1474-919X.2006.00648.x
Rosenmann M, Morrison P (1974) Maximum oxygen consumption and heat loss facilitation in small homeotherms by He-O2. Am J Physiol 226:490–495. https://doi.org/10.1152/ajplegacy.1974.226.3.490
Royer-Boutin P, Cortés PA, Milbergue M et al (2015) Estimation of muscle mass by ultrasonography differs between observers and life states of models in small birds. Physiol Biochem Zool 88:336–344. https://doi.org/10.1086/680016
Russell EM (2000) Avian life histories: is extended parental care the southern secret? Emu 100:377–399. https://doi.org/10.1071/MU0005S
Ryzhanovsky VN (2015) Comparative ecology of horned lark Eremophila alpestris flava Gm. and snow bunting Plectrophenax nivalis L. in subarctic and arctic zones. Contemp Probl Ecol 8:309–316. https://doi.org/10.1134/S1995425515030117
Scholander PF, Hock R, Walters V et al (1950) Heat regulation in some arctic and tropical mammals and birds. Biol Bull 99:237–258. https://doi.org/10.2307/1538741
Serreze MC, Barry RG (2014) The Arctic climate system. Cambridge University Press
Smith RD, Metcalfe NB (1994) Age, sex and prior site experience have independent effects on the foraging success of wintering snow buntings. Behaviour 129:99–111. https://doi.org/10.1163/156853994X00370
Smith RD, Metcalfe NB (1997) Why does dominance decline with age in wintering snow buntings? Anim Behav 53:313–322. https://doi.org/10.1006/anbe.1996.0403
Stettenheim P (1972) The integument of birds. In: Avian biology: vol II. Academic Press, New York, pp 1–63
Stienen EWM, Brenninkmeijer A (2002) Variation in growth in Sandwich Tern chicks Sterna sandvicensis and the consequences for pre- and post-fledging mortality. Ibis 2002:567–576
Swanson D (2001) Are summit metabolism and thermogenic endurance correlated in winter-acclimatized passerine birds? J Comp Physiol 171:475–481. https://doi.org/10.1007/s003600100197
Swanson DL (2010) Seasonal metabolic variation in birds: functional and mechanistic correlates. In: Thompson CF (ed) Current ornithology volume 17. Springer New York, New York, pp 75–129
Swanson DL, Liknes ET (2006) A comparative analysis of thermogenic capacity and cold tolerance in small birds. J Exp Biol 209:466–474. https://doi.org/10.1242/jeb.02024
Swanson DL, Merkord C (2013) Seasonal phenotypic flexibility of flight muscle size in small birds: a comparison of ultrasonography and tissue mass measurements. J Ornithol 154:119–127. https://doi.org/10.1007/s10336-012-0877-4
Swanson DL, Olmstead KL (1999) Evidence for a proximate influence of winter temperature on metabolism in passerine birds. Physiol Biochem Zool 72:566–575. https://doi.org/10.1086/316696
Swanson DL, Drymalski MW, Brown JR (1996) Sliding vs static cold exposure and the measurement of summit metabolism in birds. J Therm Biol 21:221–226. https://doi.org/10.1016/0306-4565(96)00005-8
Swanson DL, McKechnie AE, Vézina F (2017) How low can you go? An adaptive energetic framework for interpreting basal metabolic rate variation in endotherms. J Comp Physiol B 187:1039–1056. https://doi.org/10.1007/s00360-017-1096-3
Ton R, Martin TE (2016) Metabolism correlates with variation in post-natal growth rate among songbirds at three latitudes. Funct Ecol 30:743–748. https://doi.org/10.1111/1365-2435.12548
Vézina F, Gustowska A, Jalvingh KM et al (2009a) Hormonal correlates and thermoregulatory consequences of molting on metabolic rate in a northerly wintering shorebird. Physiol Biochem Zool 82:129–142. https://doi.org/10.1086/596512
Vézina F, Love OP, Lessard M, Williams TD (2009b) Shifts in metabolic demands in growing altricial nestlings illustrate context-specific relationships between basal metabolic rate and body composition. Physiol Biochem Zool 82:248–257. https://doi.org/10.1086/597548
Vézina F, O’Connor RS, Le Pogam A et al (2021) Snow buntings preparing for migration increase muscle fiber size and myonuclear domain in parallel with a major gain in fat mass. J Avian Biol 52:1–10. https://doi.org/10.1111/jav.02668
Walsberg GE (1983) Avian ecological energetics. In: Farner DS, King JR, Parkes KC (eds) Avian biology. Academic Press, New York, pp 161–200
Walsberg GE (1988) Heat flow through avian plumages: the relative importance of conduction, convection, and radiation. J Therm Biol 13:89–92. https://doi.org/10.1016/0306-4565(88)90018-6
Weathers WW, Sullivan KA (1989) Juvenile foraging proficiency, parental effort, and avian reproductive success. Ecol Monogr 59:223–246. https://doi.org/10.2307/1942600
Wheeler B, Torchiano M (2016) lmPerm: permutation tests for linear models. Version 2.1.0., https://CRAN.R-project.org/package=lmPerm
Wolf BO, Walsberg GE (2000) The role of the plumage in heat transfer processes of birds. Amer Zool 40:575–584
Wunderle JM (1991) Age-specific foraging proficiency in birds. Curr Ornithol 8:273–324
Acknowledgements
We thank François Fournier from Environment and Climate Change Canada for his help with logistical support in the initial Alert phase of this project, Francis Robitaille for his assistance in data collection at Alert along with members of the 2019 Hare Force team. We are also grateful to Gabrielle Roy, Claire Bottini, Yann Bouchez, Emily Cornelius Ruhs, Polan-Devi Darboven, Alexandre Paiement, Kim Régimbald Bélanger, Charles Richard, Laurence Rondeau, and Josianne Ruest for their help in catching birds in winter as well as Gratien Bélanger and Sylvie Foucault for granting access to their land for snow bunting captures. We are grateful to Alain Caron for statistical advice. We also thank Chris McRae and Nathan Koutroulides for their help with logistics and the personnel from CFS Alert for their support during fieldwork. This work benefited from a generous donation from the Kenneth M. Molson Foundation. It was also supported by NSERC Discovery grants and Northern Supplement to FV, Canada Foundation for Innovation (CFI) awards to FV as well as logistical support and funding from the Department of National Defence to FV and DB.
Author information
Authors and Affiliations
Contributions
RD and FV conceived the ideas and designed the study. ALP, KY and FV collected the data. RD analyzed the data. RD, AL, FV, RO, led the writing of the manuscript. All authors contributed to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Ethical approval
All bird handling at Rimouski and at Alert was approved by the Animal Care Committee of the Université du Québec à Rimouski (Rimouski: CPA-54-13-130 and CPA-71- 17-195 and Alert: CPA-61-15-163 and CPA-68-17-186) and was conducted under banding (10889) and scientific (SC-48, NUN- SCI-15-05) permits from Environment and Climate Change Canada, and under scientific permits from the Department of Environment of Nunavut (WL 2015-055, 2018- 010, 2019-002).
Additional information
Communicated by P. Withers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demers, R., O’Connor, R.S., Le Pogam, A. et al. Born in the cold: contrasted thermal exchanges and maintenance costs in juvenile and adult snow buntings on their breeding and wintering grounds. J Comp Physiol B 193, 557–568 (2023). https://doi.org/10.1007/s00360-023-01502-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-023-01502-8