Abstract
As temperatures continue to rise, adjustments to biological membranes will be key for maintenance of function. It is largely unknown to what extent Antarctic notothenioids possess the capacity to remodel their biological membranes in response to thermal change. In this study, physical and biochemical properties were examined in membranes prepared from gill epithelia (plasma membranes), cardiac ventricles (microsomes, mitochondria), and brains (synaptic membranes, myelin, mitochondria) from Notothenia coriiceps following acclimation to 5 °C (or held at ambient temperature, 0 °C) for a minimum of 6 weeks. Fluidity was measured between 0 and 30 °C in all membranes, and polar lipid compositions and cholesterol contents were analyzed in a subset of biological membranes from all tissues. Osmotic permeability was measured in gills at 0 and 4 °C. Gill plasma membranes, cardiac mitochondria, and cardiac microsomes displayed reduced fluidity following acclimation to 5 °C, indicating compensation for elevated temperature. In contrast, no fluidity changes with acclimation were observed in any of the membranes prepared from brain. In all membranes, adjustments to the relative abundances of major phospholipid classes, and to the extent of fatty acid unsaturation, were undetectable following thermal acclimation. However, alterations in cholesterol contents and acyl chain length, consistent with the changes in fluidity, were observed in membranes from gill and cardiac tissue. Water permeability was reduced with 5 °C acclimation in gills, indicating near-perfect homeostatic efficacy. Taken together, these results demonstrate a homeoviscous response in gill and cardiac membranes, and limited plasticity in membranes from the nervous system, in an Antarctic notothenioid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The extreme stenothermy of Antarctic notothenioids has been documented for more than 50 years (Somero and DeVries 1967), and these organisms are likely to be vulnerable to global climate change. Despite their stenothermy, several species of notothenioids can extend their thermal limits, as indicated by measurements of their critical thermal maxima (CTMAX) (i.e., upper thermal limit to acute change) following acclimation (i.e., adjustments following a 1- to 3-week period of exposure) to warmer temperatures (Bilyk and DeVries 2011). Adjustments to physiological systems likely will be critical to the survival of notothenioids at elevated temperatures, yet the mechanisms that govern thermal plasticity in these species have not been explored fully.
Homeoviscous adaptation (HVA), the preservation of fluidity among biological membranes in response to thermal variation (Sinensky 1974), will likely be critical to the survival of notothenioids in the future, as the physical state of the membrane depends highly on temperature (Hazel and Williams 1990). In the absence of compensatory changes, an increase in temperature will render biological membranes more fluid, resulting in an excess passive movement of solutes across the membrane (Lande et al. 1995; Choi et al. 2016; Kaddah et al. 2018). Furthermore, permeability to oxygen and water is highly sensitive to the physical properties of the membrane (Subczynski et al. 1989; Lande et al. 1995). For ectothermic organisms—whose body temperatures match that of their environment—HVA preserves membrane integrity upon shifts in temperature (Hazel 1995).
To date, no studies involving Antarctic notothenioids have examined directly how membrane fluidity is altered in response to thermal acclimation. Furthermore, the capacity for structural and/or compositional remodeling with thermal acclimation in membranes from cardiac and brain tissue—organs that are likely to be integral to thermal tolerance—from notothenioids is unknown. The membranes of Antarctic notothenioid fishes contain high proportions of polyunsaturated fatty acids (PUFAs), which enhance lipid movement at subzero temperatures (Logue et al. 2000). Long-term HVA (i.e., evolutionary divergence among species) has been demonstrated previously in notothenioids (Behan-Martin et al. 1993; Logue et al. 2000), but more acute adjustments to membrane fluidity have not yet been reported in these species.
Previous work in temperate fishes has shown that the proportion of saturated fatty acids increases with warm acclimation, decreasing membrane fluidity to offset the direct effects of elevated temperature (Roots 1968; Cossins 1977; Quinn 1981; Los et al. 2013). Further, longer fatty acyl chains contribute to reduced fluidity (van Meer et al. 2008). Studies in Antarctic fishes, however, suggest a limited thermal plasticity. Temperature-specific adjustments to fatty acid unsaturation have been reported in membranes from liver of an Antarctic notothenioid, Trematomus bernacchii, as well as in crude gill and white muscle tissues of this species (Malekar et al. 2018; Truzzi et al. 2018a, b). Other studies have demonstrated limited—or lack of—membrane restructuring in notothenioids (Gonzalez-Cabrera et al. 1995; Strobel et al. 2013; Malekar et al. 2018).
In the present study, we take the most comprehensive approach, to date, to investigate whether Notothenia coriiceps—a notothenioid whose CTMAX is extended from 16.2 to 17.4 °C following acclimation to 4 °C (Bilyk and DeVries 2011)—possesses the capacity for HVA with thermal acclimation. We have quantified biophysical and biochemical properties of biological membranes from gill epithelia (plasma membranes), cardiac ventricles (microsomes, mitochondria), and brains (synaptic membranes, myelin, mitochondria) following acclimation to 5 °C for a minimum of 6 weeks. Membranes from animals held under ambient temperature (0 °C) for the same duration were used to compare the effects of thermal acclimation. Fluidity was measured in all membrane types. Biochemical analyses (phospholipid class distribution, acyl chain chemistry, and cholesterol contents) were performed in select membranes. Additionally, osmotic permeability was measured in gills. Because previous studies in notothenioids indicate little or no evidence of lipid remodeling, we hypothesized that HVA would be absent in all membranes. Our results, however, demonstrate HVA (and accordant lipid remodeling) in membranes of the gill epithelia and cardiac ventricles, but no HVA in any of the biological membranes measured from the nervous system, in N. coriiceps.
Materials and methods
Animal collection and thermal acclimation
Adult N. coriiceps were collected in the Western Antarctic Peninsula region during the austral autumn of 2017 using otter trawls deployed from the ARSV Laurence M. Gould in Dallmann Bay (64°10′ S, 62°35′ W) and off the southwestern shore of Low Island (63°24′ S, 62°10′ W). Additional animals were also captured at these sites using baited pots. The animals were held in circulating seawater tanks on the vessel before being transferred to Palmer Station, Antarctica, where they were held in 4100 L flow-through (15–40 L/min) seawater tanks at ambient temperature (0 ± 1 °C) for at least 3 days prior to acclimation (Group A). Additionally, a second cohort of animals was caught using baited lines in Arthur Harbor during the same season (Group B). These animals were placed in buckets of seawater, transferred immediately to 4100 L flow-through seawater tanks on station, and allowed to recover for 3 days prior to acclimation period.
The animals were assigned randomly to warm (5 ± 1 °C) or control (0 ± 1 °C) acclimation groups. For the 5 °C group, temperature was increased at a rate of 1 °C per day using an immersion heater (Process Technology). Animals were held at 5 °C for a period of either 10 (Group A) or 6 (Group B) weeks. All animals were fed to satiation with ~ 10 g muscle fillets every other day.
Following the acclimation period, the animals were euthanized by a blunt blow to the head followed by severing the spinal cord. Hearts were excised and allowed to contract several times in ice-cold notothenioid Ringer’s solution (240 mM NaCl, 2.5 mM MgCl2, 5 mM KCl, 2.5 mM NaHCO3, 5 mM NaH2PO4, pH 8.0 at 4 °C). Next, brains were removed and bathed in ice-cold extraction buffer A (0.35 M sucrose, 5 mM EGTA, 10 mM HEPES, pH 7.8 at 1 °C). Gill arches were excised and assigned randomly to two groups: (1) flash frozen in liquid nitrogen for subsequent experiments or (2) bathed immediately in ice-cold notothenioid Ringer’s solution for osmotic permeability assays, which were performed on the day of collection. All samples were kept on ice during collection and tissue preparation. All animal experiments were approved by the Ohio University Animal Care and Use Committee (14-L-004).
Membrane preparations
Plasma membranes were prepared from gill epithelia as described (Robertson and Hazel 1995), with modifications for preparations from frozen tissue. Gills were thawed in beakers of ice-cold notothenioid Ringer’s solution for 20 min. The gills were then irrigated with fresh notothenioid Ringer’s solution, using a 10-mL syringe fitted with a 32-gauge needle, until the gills were clear of blood. Next, epithelial tissue was scraped gently from the filaments of each gill arch using a razor blade on an ice-cold glass tray. Tissues were pooled as needed; typically, filaments from two to three gill arches were combined per preparation. Tissues were pooled from the same individual when possible.
Next, pooled samples were homogenized in 15 mL extraction buffer A using a motor-driven Potter–Elvehjem grinder with six even strokes (5 s per stroke). The homogenate was filtered through cheesecloth and diluted to 25 ml with extraction buffer A. Next, 15 ml of 41% sucrose (weight/volume) was pipetted below the dilute homogenate. The sample was centrifuged at 23,000 g for 30 min at 4 °C in a Sorvall RC 6 + high-speed centrifuge with a Fiberlite F21-8 × 50y rotor, and a band formed at the homogenate–sucrose interface. The band was removed using a glass Pasteur pipette and diluted in 25 ml extraction buffer A.
The dilute suspension was centrifuged at 7000 g for 15 min, and a pellet formed at the bottom of the tube. The pellet was collected and resuspended in 1 ml extraction buffer A, which was then pipetted onto a self-generating gradient (18% Percoll, 0.25 M sucrose and 20 mM Tris, pH 7.4 at 4 °C). The gradient was centrifuged at 33,600 g for 25 min. A band formed towards the lower density portion. The band was collected, resuspended in 30 ml extraction buffer A, and centrifuged at 82,000 g for 2 h at 4 °C in a 50.2 Ti rotor, using either a Beckman Coulter L5-50 ultracentrifuge or a Beckman Coulter Optima L-80 XP ultracentrifuge. The plasma membrane pellet was reconstituted in four 50 µl aliquots of resuspension buffer A (20 mM Tris, pH 7.4 at 4 °C) and stored at − 70 °C.
Mitochondria were prepared from cardiac ventricles as described previously (Urschel and O’Brien 2009), with modifications (Mueller et al. 2011). Ventricles were diced on an ice-cold metal block, pooled, and homogenized in 8 volumes of extraction buffer B (0.1 M sucrose, 140 mM KCl, 10 mM EDTA, 10 mM MgCl2, 20 mM HEPES, pH 7.3 at 4 °C) using a 40 ml Tenbroeck ground glass homogenizer. Up to three hearts were pooled per preparation. The homogenate was centrifuged at 1400 g for 5 min at 4 °C in a Beckman–Avanti-JE with a JA-17 rotor. The supernatant was collected and centrifuged at 9000 g for 10 min at 4 °C. A pellet formed at the bottom of the tube. The pellet was collected and resuspended in 11 ml extraction buffer B and centrifuged at 1400 g for 5 min at 4 °C. The supernatant was collected and centrifuged at 11,000 g for 10 min at 4 °C. The mitochondrial pellet was collected and resuspended in ~ 0.5 ml resuspension buffer B (10 mM HEPES, pH 7.4 at 4 °C), flash frozen in liquid nitrogen, and stored at – 70 °C. During the mitochondrial preparation, the supernatant from the second centrifugation step was collected and centrifuged at 302,000 g for 90 min at 4 °C in a Beckman Coulter Optima XPN centrifuge with a 50.2 Ti rotor. A microsomal pellet formed at the bottom of the tube. The pellet was collected and resuspended in ~ 0.5 ml resuspension buffer B, flash frozen in liquid nitrogen, and stored at – 70 °C.
Synaptic membranes, myelin and mitochondria were fractionated from brain tissue as described previously (Dunkley et al. 2008), with modifications. Brains were diced, pooled, and homogenized in 6 volumes of extraction buffer A using a motor-driven Potter–Elvehjem grinder with eight even strokes (5 s per stroke). The samples were kept on ice throughout the preparations. Brains were pooled as needed; generally, two were pooled per preparation. The pooled homogenate was centrifuged at 4 °C for 8 min at 600 g in a Beckman Coulter Avanti J-E centrifuge with a JA-17 rotor.
The supernatant from the initial centrifugation was pipetted onto discontinuous Percoll gradients. Four gradients per preparation were prepared up to 4 h in advance. Percoll was filtered using Whatman Nuclepore track-etched membranes and made up to appropriate concentrations (3, 10, 15, and 23%) in gradient buffer (0.32 M sucrose, 1 mM EDTA, 0.25 mM DTT, 5 mM Tris, pH 7.4 at 25 °C). Four discrete 2 ml layers were generated in a 12 ml centrifuge tube. After the addition of 2 ml supernatant, the gradients were centrifuged at 13,000 g for 31 min at 4 °C in a Beckman Coulter Avanti J-E centrifuge with a JA-17 rotor. Five bands formed at the gradient interfaces and at the top and bottom of the gradient. The membrane fractions—myelin (band 2), synaptic membranes (band 4), and mitochondria (band 5)—were collected, resuspended in 20 ml extraction buffer A, and centrifuged at 302,000 g for 90 min at 4 °C in a Beckman Coulter Optima XPN centrifuge with a 50.2 Ti rotor. The membranes concentrated in a loose pellet above the hard Percoll pellet. The membrane pellets were collected, resuspended in 250 μl resuspension buffer A, separated into three aliquots, and frozen at – 70 °C.
Marker enzyme analyses
Enrichments of gill plasma membranes were determined by measuring the protein-specific activities of marker enzymes: sodium–potassium ATPase (NKA) for basolateral membranes (McCormick 1993), gamma-glutamyltransferase (GGT) for apical membranes (Silber et al. 1986), and succinate dehydrogenase (SDH) for mitochondria (Hollywood et al. 2010). Enrichments of brain membranes were determined by measuring the protein-specific activities of marker enzymes as described previously (Biederman et al. 2019a): acetylcholinesterase (AChE) for synaptic membranes (Ellman et al. 1961), cyclic nucleotide phosphodiesterase (CNPase) for myelin (Tsukada et al. 1980), and SDH for mitochondria (Hollywood et al. 2010). All marker assays were performed at ~ 20 °C. Marker enzymes were not quantified in cardiac mitochondria because the method used to isolate mitochondria has been performed extensively by our group (Mueller et al. 2011). Additionally, there was insufficient material to analyze marker enzymes from the cardiac microsomes.
Protein-specific activities (relative to those of crude homogenates) were calculated to determine enrichment factors for each membrane fraction, indicating the degree of membrane separation. Two controls—one in the absence of substrate and one in the absence of sample—were performed and subtracted from the measured activity for each marker assay. Total protein content was measured using a Sigma-Aldrich bicinchoninic acid assay kit.
Membrane fluidity assays
Membrane fluidity was quantified by fluorescence depolarization as described previously (Crockett and Hazel 1995). In brief, samples were added to a solution of 1,6-diphenyl-1,3,5-hexatriene (DPH) for a final phosphate-to-probe molar ratio of 500:1 and added to 2.5 ml resuspension buffer A with constant stirring in a quartz cuvette. Probe incorporation was conducted in a darkened room in a foil-covered amber glass vial to prevent quenching of the fluorescent signal. Change in polarization (excitation = 356 nm, emission = 430 nm) was measured between 0 and 30 °C using a Perkin-Elmer LS-50B spectrophotometer. Measurements were initiated at 0 °C, and assay temperature was increased at 2 °C intervals at a rate of ~ 0.3 °C min−1 using a circulating water bath containing 50% ethylene glycol. Polarization measurements were performed in triplicate at each temperature interval.
Membrane composition determination
Lipids were extracted as described previously (Bligh and Dyer 1959). First, 2.25 ml of deoxygenated methanol and chloroform (2:1 ratio) was added to 600 µl diluted sample and vortexed vigorously for 30 s. The mixture was centrifuged at 600 g for 10 min and distinct layers formed. The lower layer was collected using a glass Pasteur pipette. The extraction procedure was repeated three times for each sample. The samples were washed with deionized water and evaporated under dry nitrogen gas in 2-ml borosilicate glass vials with Teflon-lined caps. The extracts were sent to the Kansas Lipidomics Research Center for analysis, and a diacyl polar lipid profile dataset was generated by quadrupole mass spectrometry using an Applied Biosystems 4000 QTRAP mass spectrometer as described (Xiao et al. 2010). Relative abundances of the major phospholipid classes were compared between acclimation groups. The unsaturation index (UI) was calculated as described (Grim et al. 2010).
Cholesterol was quantified using a Cayman fluorometric assay kit and normalized to total phospholipid content, which was measured as hydrolyzed inorganic phosphate in membranes as described previously (Rouser et al. 1970). Diluted samples were hydrolyzed in covered glass culture tubes with full-strength perchloric acid at 180 °C until the samples clarified. The samples were cooled to room temperature before quantifying phosphate content by adding 40 µl sample (diluted as necessary to produce absorbance values within standard curve range) to 160 µl reaction medium (1.2 N H2SO4, 2% ascorbate, and 0.5% [NH4]2MoO4). The samples were incubated for 7 min at 60 °C, and absorbances were measured at 820 nm using a SpectraMax M2 microplate reader.
Osmotic permeability assays
Osmotic permeability was quantified in intact gill arches as described previously (Robertson and Hazel 1999), with modifications. After incubation in ice-cold notothenioid Ringer’s solution for 30 min, arches were washed with fresh notothenioid Ringer’s solution, blotted dry for 3 s using four layers of Kimwipe tissues, and weighed. The arches were transferred to beakers containing 100 ml ice-cold 20 mM CaCl2. Air stones were fixed to the bottom of the beakers to maintain consistent aeration. The beakers were held in either an ice bath (0 ± 0.5 °C) or on the countertop of a fixed-temperature cold room (4 ± 0.7 °C). Gill arch masses were measured in 10-min intervals using the blotting procedure described above. Arches were then incubated at 110 °C for 24 h to obtain dry weights. The osmotic gain (\(R\left( \% \right)\)) for each time point (t) was calculated using the following formula: \(R\left( \% \right) = \left( {W_{t} {-} W_{{\text{i}}} } \right)/\left( {W_{{\text{i}}} - W_{{\text{d}}} } \right) \times 100\), where \(W_{t}\) = the arch mass at time t, \(W_{{\text{i}}}\) = the initial arch mass, and \(W_{{\text{d}}}\) = the dry weight. The rate of osmotic gain was calculated over the linear range of data (i.e., before the rate of \(R\left( \% \right)\) began to stabilize).
Statistical analyses
Fluidities of gill plasma membranes, cardiac microsomes, synaptic membranes, myelin, and brain mitochondria were compared between acclimation groups by analysis of covariance (ANCOVA) using SPSS Statistics. Due to differences in slope between groups, the data for cardiac mitochondrial fluidity did not meet the requirements for ANCOVA and were analyzed by a mixed effects model in R Studio, with assay temperature designated as a random factor. For cardiac mitochondria and brain mitochondria, polar lipid compositions were compared between acclimation groups and tissue types by two-way analysis of variance (ANOVA). For gill plasma membranes, polar lipid compositions were compared between acclimation groups by two-tailed t test. Cholesterol contents and fatty acyl chain lengths were compared between acclimation groups by two-tailed-t test, as applicable.
Osmotic permeability measurements were calculated for each gill arch as described in the previous section. Rates of linear osmotic gain were compared between acclimation groups by ANCOVA. Protein-specific enzymatic activities were calculated in crude homogenates as described above and compared between acclimation groups by two-tailed t test. The effect of gill arch position (anterior-to-posterior) was assessed for all relevant assays (fluidity, biochemical composition, enzymatic assays) and was found to be insignificant.
For the gill and brain membranes, samples from the two acclimation cohorts (Groups A and B) were found to be statistically equivalent in all analyses (P > 0.50). For this reason, data from both groups were pooled to account for logistical issues during fieldwork. Data from cardiac membranes represent samples from the 6-week cohort (Group B) only.
Results
Membranes displayed enrichment and altered enzymatic activities upon acclimation
Gill plasma membranes were enriched 4.8- and 5.2-fold in the basolateral membrane marker NKA and the apical membrane marker GGT, respectively. The mitochondrial marker SDH was found to be a relatively minor component of the membrane fraction (data not shown).
In crude homogenate, gill NKA activity, normalized to protein content, increased 1.4-fold with 5 °C acclimation, compared with gills from animals held under ambient conditions (P < 0.05) (Fig. 1). In contrast, GGT activity expressed relative to protein was reduced 1.5-fold with 5 °C acclimation (P < 0.05) (Fig. 1). These trends in activity were consistent when normalized to wet tissue weight.
Synaptic membranes were enriched 4.2-fold in AChE, myelin was enriched 3.7-fold in CNPase, and brain mitochondria were enriched 4.2-fold in SDH. Contamination of the membrane types within the three fractions was relatively minor. Enzymatic activities for all markers in brain did not differ significantly between the acclimation groups (data not shown).
Evidence of HVA was observed in membranes from gill and heart but not brain
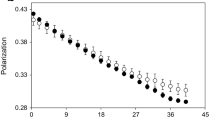
For gill plasma membranes, cardiac microsomes, and cardiac mitochondria, the 5 °C group displayed significantly greater polarization values, indicating reduced membrane fluidity compared to membranes from the 0 °C group (P < 0.0001) (Fig. 2a–c). Homeoviscous efficacy (HVE) (calculated as the ratio of polarization values for both acclimation groups at their respective physiological temperatures, expressed as a percent) was 100% for all membrane types measured in the gill and the heart. In contrast, polarization values did not differ significantly between acclimation groups for the three membranes from the brain, (Fig. 2d–f); no evidence of a homeoviscous response was observed. No significant discontinuities in membrane fluidity were observed, indicating the lack of a detectable phase transition over the temperature range measured.
Steady state polarization values (i.e., inverse of membrane fluidity) for the fluorescent probe DPH in a gill plasma membranes (N = 10 for 0 °C and N = 9 for 5 °C), b cardiac mitochondria (N = 5), c cardiac microsomes (N = 5), d synaptic membranes (N = 10), e myelin (N = 10), and f brain mitochondria (N = 10) from N. coriiceps held at 0 °C and 5 °C. Error bars represent means ± SEM
Lipid profiles differed between tissues and with thermal acclimation
In gill plasma membranes, cardiac mitochondria, and brain mitochondria the most abundant phospholipid classes were phosphatidylcholine (PC) (48–56 mol%), phosphatidylethanolamine (PE) (15–36 mol%), and plasmalogen PC (ePC) (6–12 mol%) (Table 1). In brain mitochondria, phosphatidylinositol (PI) and phosphatidylserine (PS), two phospholipids in lower abundance, increased by 1.3-fold and 1.6-fold, respectively, following 5 °C acclimation (P < 0.05). In cardiac mitochondria, the abundance of lyso-PC (LPC) was reduced by 1.4-fold following 5 °C acclimation (P < 0.01). No other significant differences in phospholipid class, nor changes in acyl chain unsaturation, were altered with temperature acclimation across the three membrane types.
The proportion of long-chain fatty acids (i.e., ≥ 20 carbon atoms per acyl chain) increased by 1.2-fold in cardiac mitochondria from the 5 °C-acclimated fish, compared with those maintained at ambient temperature (P < 0.01) (Table 2). Similarly, the proportion of long-chain fatty acids was increased by 1.1-fold in gill plasma membranes from the 5 °C-acclimated fish, compared with those from the fish maintained at ambient temperature (P < 0.05) (Table 2). In contrast, acyl chain lengths did not differ between acclimation groups in brain mitochondria.
In cardiac mitochondria from the 5 °C acclimation group, the relative proportion of hydrolyzed phospholipids (i.e., lyso-PC and lyso-PE, relative to PC and PE) was reduced by 1.3-fold (P < 0.01), while the abundance of the specific class lyso-PC (LPC) was reduced by 1.4-fold (P < 0.01) compared with the 0 °C group (Table 1). In gill plasma membranes and brain mitochondria, the extent of lipid hydrolysis did not differ between acclimation groups.
Cholesterol contents were measured in gill plasma membranes, cardiac microsomes, synaptic membranes, and myelin (i.e., membranes known to display cholesterol enrichment). In gill plasma membranes, cholesterol contents increased by 1.2-fold (P < 0.01) in the 5 °C group, compared to the 0 °C group (Table 3). Similarly, in cardiac microsomes, cholesterol contents increased by 1.6-fold in in membranes from the 5 °C acclimation group compared to those from the 0 °C group (P < 0.05) (Table 3). However, in both myelin and synaptic membranes, cholesterol contents did not differ between the 0 and 5 °C groups.
Osmotic permeability was reduced with acclimation
When compared at a common temperature, rates of osmotic gain were 1.5-fold lower in intact gills from the 5 °C group, compared with gills from animals held under ambient temperatures (P < 0.05), indicating greater permeability to water in the 0 °C group (Fig. 3).
Discussion
We explore in detail the question of whether Antarctic notothenioids have retained the capacity to mount a homeoviscous response to an elevated temperature of 5 °C, and given a compensatory response, what lipid constituents are responsible. Using a variety of biological membranes from three major organs, we report complete compensation of fluidity (i.e., HVA) in biological membranes from the gill and heart in response to thermal acclimation of N. coriiceps, yet an absence of modifications to membrane fluidity in any of the biological membranes we measured from the brain. Remodeling of cholesterol contents and fatty acyl chain length represent two mechanisms contributing to the homeoviscous response in the gill and heart. Given the lack of a homeoviscous modulation in the membranes from the brain, we suggest the likelihood that function within the nervous system, rather than cardiac performance or gill function, of Antarctic notothenioids may be most compromised in a warming world.
The cardiac and nervous systems have been identified as likely candidates limiting the thermal tolerance of ectothermic animals. Because cardiac arrhythmia occurs just prior to CTMAX, cardiac failure is a likely contributor to the loss of performance during acute warming (Ferreira et al. 2014; Joyce et al. 2018b). Furthermore, recent evidence suggests that cardiac failure with warming reflects changes in passive (i.e., resting ion leak) and active (i.e., inward charge movement) electrical properties of ventricular myocytes, which elevates the ventricle excitation threshold (Haverinen and Vornanen 2020).
Other reports suggest that brain function is compromised at elevated temperatures (Somero and DeVries 1967; Friedlander et al. 1976; Nilsson and Lefevre 2016; Jutfelt et al. 2019). Neuron firing rates increase with warming until a critical temperature is reached, beyond which firing becomes more random (Harper et al. 1990). While these studies indicate failures to the cardiac and nervous systems with warming, the present study provides a potential mechanism that may underlie changes to the functions of these systems. Further, our previous work suggests that differences in the fluidities of synaptic membranes and cardiac mitochondria may contribute to differences in thermal tolerance among notothenioids (Biederman et al. 2019a, b).
At the same time, the teleost gill is also likely to be affected by a shifting dynamic of both temperature and oxygen availability associated with climate change. Oxygen uptake incurs a significant energetic cost in passive ion and water movement across the gill epithelium (Gilmour and Perry 2018). Currently, for most Antarctic notothenioids, oxygen demand is relatively low while oxygen availability is relatively high (Davison et al. 1997). For notothenioids, optimization of gill function—particularly, maintenance of osmotic permeability—is likely to be critical in the allocation of an animal’s energy budget. Although relatively little work has focused attention on structural remodeling in the gills of Antarctic notothenioid fishes, previous work in T. bernacchii and T. newnesi demonstrate a decrease in serum osmolality following acclimation to 4 °C (Gonzalez-Cabrera et al. 1995), suggesting at least some degree of plasticity in gill function of notothenioids.
Capacity for membrane remodeling varies by species and tissues
At first glance, it would seem plausible that the varied thermal response of membrane remodeling among notothenioids stems from differences in thermal history. For example, notothenioids collected from higher latitudes (with relatively small swings in annual temperature) might display reduced thermal plasticity, compared to those from lower latitudes with larger (seasonal) fluctuations in temperature (Llano and Littlepage 1965; Clarke et al. 1984; Hunt et al. 2003). Consistent with this, samples collected from T. bernacchii in McMurdo Sound, a high latitude locale, (77°51′ S) do not appear to undergo lipid remodeling (Gonzalez-Cabrera et al. 1995; Malekar et al. 2018). However, in contrast with this hypothesis, a study of liver mitochondria from notothenioids collected from lower latitudes—King George Island (Notothenia rossii, 62°14′ S) and South Georgia Island (Lepidonotothen squamifrons, 53°24′ S)—also demonstrates a lack of adjustment in lipids from liver mitochondria (Strobel et al. 2013). Further, crude gill and muscle homogenates from T. bernacchii collected in another high latitude locale, Tethys Bay (74°42′ S) display rapid changes in lipid composition with acclimation (Truzzi et al. 2018a, b). Consequently, the capacity for thermal plasticity associated with lipid remodeling of notothenioids does not appear to be directly related to thermal habitat.
Neither a homeoviscous response, nor evidence of major compositional remodeling, were detected in the three distinct biological membranes from the brain. Our results here are in marked contrast with previous work demonstrating the capacity for remodeling in membranes from the nervous system in temperate species, which demonstrate a robust degree of HVA following thermal acclimation. For example, in Cassius auratus, changes to fatty acid unsaturation in brain lipids (Johnston and Roots 1964) and fatty acyl chain composition and plasmalogen contents in the optic nerves and optic tectum (Matheson et al. 1980) have been reported. Further, HVA has been reported in mitochondria (HVE = 43%), synaptic membranes (HVE = 35%), and myelin (HVE = 19%) of C. auratus (Cossins et al. 1977; Cossins 1977; Cossins and Prosser 1982). A recent study, however, has indicated that membranes from the brain might be less plastic under certain conditions than membranes from other tissues; membranes from C. auratus brain were incapable of remodeling after exposure to hypoxia, in contrast with membranes from gill, muscle, and liver (Farhat et al. 2019). We posit that the lack of HVA in synaptic membranes, myelin, and brain mitochondria observed in this study might reflect a similar difference in plasticity among tissues.
While our findings suggest limited thermal plasticity in the brain of this species, it is also possible that acclimation to 5 °C might be insufficient to prompt a homeoviscous response in the nervous system. We posit that—even with similar perturbations in fluidity—the brain and the heart require different threshold temperatures, only above which a homeoviscous response will occur. The physiological limits for acclimation in notothenioids are not fully understood; this topic warrants investigation in a future study.
In the brain, a longer time scale for achieving a homeoviscous response might be required, as evidence of a homeoviscous response exists—in comparisons to membranes of temperate and tropical animals—in synaptic membranes of notothenioids (Behan-Martin et al. 1993; Logue et al. 2000). Prior work in brain membranes suggests that thermal compensation may occur over an extended time course. For example, in a thermal acclimation study of synaptic membranes of C. auratus, HVA was observed within 20 days, but fatty acid remodeling occurred over a period of 50 days (Cossins et al. 1977). We posit that Antarctic notothenioids, which have adapted to an environment with a relatively narrow degree of thermal fluctuation, may require additional time to achieve HVA in brain tissue. This topic, as well as differences in functional temperature ranges among tissue types, may be explored in a future study.
Increases in membrane cholesterol and fatty acyl chain length are consistent with changes in fluidity in gill and cardiac membranes
Modulation of cholesterol content likely plays a significant role in the homeoviscous response we observed. Cholesterol stabilizes membranes by restraining movement of fatty acyl tails (Quinn 1981; Yeagle 1985) and typically imparts an ordering effect to resist increases in fluidity that occur with warming (Hazel 1995). Cholesterol’s role in HVA in gill and heart membranes in N. coriiceps is further supported by our observation that cholesterol was unchanged in myelin and synaptic membranes, neither of which exhibited any compensatory changes to membrane fluidity with thermal acclimation. A positive association between membrane cholesterol and acclimation temperature has been observed in several species of non-Antarctic ectotherms (Robertson and Hazel 1995; Hassett and Crockett 2009; Reynolds et al. 2014), although the trend is not always present (Crockett and Hazel 1995; Robertson and Hazel 1995). Malekar et al. (2018) did not detect changes in cholesterol contents of membranes from liver in T. bernacchii or Pagothenia borchgrevinki following thermal acclimation. Overall, these results may reflect species differences or variation in acclimation design (e.g., duration, temperature).
Like the modulation of cholesterol contents, changes in acyl chain length with warm acclimation were consistent with alterations in membrane fluidity, as extended acyl chains increase membrane order (Chintalapati et al. 2004). Alterations in chain length have been reported in membranes from temperate fishes following thermal acclimation (Hazel and Landrey 1988), consistent with the data reported herein.
Remodeling of phospholipid classes, fatty acid unsaturation and hydrolyzed phospholipids
The relative abundances of the two major phospholipid classes (PC and PE) were unchanged in select membranes analyzed (cardiac mitochondria, brain mitochondria, and gill plasma membranes) of all three tissues, yet changes in the proportions of three less abundant phospholipids (LPC, PI, and PS) were observed. These phospholipids represent a small portion (~ 1–5 mol%) of the biological membranes measured.
Although HVA was observed in gill and cardiac membranes, the extent of membrane unsaturation (i.e., UI) appeared unchanged in the tissues analyzed in this study. However, due to the analytical method employed in this work (which reported each phospholipid species by the sum of its acyl chain tails, rather than individually), we are unable to rule out the possibility that the distribution of unsaturation across individual acyl chains was altered with acclimation. Previous studies in notothenioids have demonstrated changes in the relative abundance of diacyl chains with warm acclimation (Malekar et al. 2018; Truzzi et al. 2018a, b). Thus, it is still possible that changes to fatty acyl chain unsaturation, not detected in this analysis, occurred. We point out, however, that changes in individual phospholipids likely would be reflected in total membrane unsaturation. Thus, our data suggest—albeit somewhat indirectly—that major restructuring of membrane unsaturation did not occur.
Hydrolysis of membrane phospholipids is associated with enhanced fluidity (Quinn 1981), and our observation of lower proportions of hydrolyzed phospholipids in cardiac mitochondria is consistent with HVA. Previous studies have indicated that lipid hydrolysis can indeed be modulated during times of environmental change. For example, enzymatic activity of phospholipase A2, which cleaves fatty acids from phospholipids, increases with cold acclimation in O. mykiss (Neas and Hazel 1985), and phospholipase A is enhanced at cold growth temperatures in Escherichia coli and is modulated by membrane fluidity (Audet et al. 1974; Michel and Stárka 1979). It is also possible that phospholipid hydrolysis contributes to preservation of membrane structure at subzero temperatures (i.e., ambient temperatures) for notothenioids, because phospholipid hydrolysis enhances cold tolerance in plants (Welti et al. 2002) and produces intermediates for membrane restructuring (Cowan 2006).
Reduced membrane fluidity accounts for decreased osmotic permeability and NKA activity
The reduced osmotic permeability in gills of the 5 °C group suggests thermal compensation, and consequently, preservation of at least one aspect of membrane function with warming. This decrease in osmotic permeability with 5 °C acclimation was matched by the higher contents of membrane cholesterol, which is thus likely to be responsible, at least in part, for the change in osmotic permeability. Cholesterol is well known to influence membrane permeability (Pfrieger 2003; McMullen et al. 2004). Additionally, changes to the distributions of other membrane components, such as aquaporins and/or claudins, may also modulate osmotic permeability (Fujiyoshi et al. 2002). The expression of these proteins in N. coriiceps with thermal acclimation warrants future study.
The activity of membrane-bound proteins is also likely to be affected by both physical and biochemical changes in the membrane lipid environment. For example, the relationship between environmental temperature and NKA activity is well documented in fishes from both polar and temperate habitats, with enhanced enzymatic activity with environmental warming most commonly associated with marine rather than freshwater fishes (Crockett and Londraville 2005). Consistent with the present data, enhanced NKA activity in gill has been reported following warm acclimation in the notothenioids T. bernacchii, T. newnesi, and Eleginops maclovinus (Gonzalez-Cabrera et al. 1995; Guynn et al. 2002; Oyarzún et al. 2018). Increased NKA activity with 5 °C acclimation may also reflect changes in the lipid environment; cholesterol, for example stabilizes the lipid environment in which the enzyme resides (Yeagle 1985; Cornelius et al. 2015). Alternatively, changes to NKA activity with thermal acclimation may reflect differences in gene expression (Urbina et al. 2013), post-translational modifications, such as phosphorylation (Feschenko and Sweadner 1995), and/or changes in the relative distribution of mitochondria-rich chloride cells, which are enriched in NKA (Evans et al. 2019). At this time, we cannot eliminate any of the possible mechanisms responsible for the increase in NKA activity with warm acclimation in N. coriiceps.
Perspectives
We demonstrate the presence of a homeoviscous response to thermal acclimation in gill and cardiac membranes, but an absence of a detectable response in membrane fluidity among the biological membranes from the brain in the Antarctic notothenioid N. coriiceps. Because preservation of membrane fluidity is likely to be critical for optimal physiological function, our results indicate that membrane lipid remodeling (e.g., cholesterol and acyl chain length) might provide continuity of some aspect(s) of gill and cardiac function at elevated temperatures. Consistent with these findings, work by our collaborators suggests evidence of thermal plasticity in cardiovascular function of N. coriiceps following acclimation to 5 °C (Joyce et al. 2018a).
Our observations suggest the possibility that disruption of membrane integrity in the brain may limit performance of notothenioids in a warmer world. Evidence of membrane compensation in the notothenioid brain—relative to those of temperate and tropical animals—is well documented (Behan-Martin et al. 1993; Logue et al. 2000). Yet it remains largely unknown whether biological membranes from the brains of notothenioid fishes can respond in a manner to keep pace with warming in the Southern Ocean.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Audet A, Nantel G, Proulx P (1974) Phospholipase A activity in growing Escherichia coli cells. Biochim Biophys Acta 348:334–343
Behan-Martin MK, Jones GR, Bowler K, Cossins AR (1993) A near perfect temperature adaptation of bilayer order in vertebrate brain membranes. Biochim Biophys Acta 1151:216–222
Biederman AM, Kuhn DE, O’Brien KM, Crockett EL (2019a) Physical, chemical, and functional properties of neuronal membranes vary between species of Antarctic notothenioids differing in thermal tolerance. J Comp Physiol B 189:213–222. https://doi.org/10.1007/s00360-019-01207-x
Biederman AM, Kuhn DE, O’Brien KM, Crockett EL (2019b) Mitochondrial membranes in cardiac muscle from Antarctic notothenioid fishes vary in phospholipid composition and membrane fluidity. Comp Biochem Physiol Part B Biochem Mol Biol 235:46–53
Bilyk KT, DeVries AL (2011) Heat tolerance and its plasticity in Antarctic fishes. Comp Biochem Physiol A 158:382–390. https://doi.org/10.1016/j.cbpa.2010.12.010
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Chintalapati S, Kiran MD, Shivaji S (2004) Role of membrane lipid fatty acids in cold adaptation. Cell Mol Biol 50:631–642. https://doi.org/10.1170/T553
Choi MSMK, Son S, Hong M et al (2016) Maintenance of membrane integrity and permeability depends on a patched-related protein in Caenorhabditis elegans. Genetics 202:1411–1420. https://doi.org/10.1534/genetics.115.179705
Clarke A, Doherty N, DeVries AL, Eastman JT (1984) Lipid content and composition of three species of Antarctic fish in relation to buoyancy. Polar Biol 3:77–83
Cornelius F, Habeck M, Kanai R et al (2015) General and specific lipid–protein interactions in Na, K-ATPase. Biochim Biophys Acta 1848:1729–1743. https://doi.org/10.1016/j.bbamem.2015.03.012
Cossins AR (1977) Adaptation of biological membranes to temperature. The effect of temperature acclimation of goldfish upon the viscosity of synaptosomal membranes. Biochim Biophys Acta 470:395–411
Cossins AR, Prosser CL (1982) Variable homeoviscous responses of different brain membranes of thermally-acclimated goldfish. Biochim Biophys Acta 687:303–309
Cossins AR, Friedlander MJ, Prosser CL (1977) Correlations between behavioral temperature adaptations of goldfish and viscosity and fatty-acid composition of their synaptic membranes. J Comp Physiol 120:109–121. https://doi.org/10.1007/BF00619309
Cowan AK (2006) Phospholipids as plant growth regulators. Plant Growth Regul 48:97–109. https://doi.org/10.1007/s10725-005-5481-7
Crockett EL, Hazel JR (1995) Cholesterol levels explain inverse compensation of membrane order in brush border but not homeoviscous adaptation in basolateral membranes from the intestinal epithelia of rainbow trout. J Exp Biol 198:1105–1113
Crockett EL, Londraville RL (2005) Temperature. In The physiology of fishes, 3rd edn. CRC Press, Boca Raton, pp 231–270
Davison W, Axelsson M, Nilsson S, Forster E (1997) Cardiovascular control in Antarctic notothenioid fishes. Comp Biochem Physiol Part A Physiol 118:1001–1008
Dunkley PR, Jarvie PE, Robinson PJ (2008) A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc 3:1718–1728. https://doi.org/10.1038/nprot.2008.171
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Evans DH, Piermarini PM, Choe KP (2019) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177. https://doi.org/10.1152/physrev.00050.2003
Farhat E, Turenne E, Choi K, Weber J (2019) Hypoxia-induced remodelling of goldfish membranes. Comp Biochem Physiol Part B 237:110326
Ferreira EO, Anttila K, Farrell AP (2014) Thermal optima and tolerance in the eurythermic goldfish (Carassius auratus): relationships between whole-animal aerobic capacity and maximum heart rate. Physiol Biochem Zool 5:599–611. https://doi.org/10.1086/677317
Feschenko M, Sweadner KJ (1995) Structural basis for species-specific differences in the phosphorylation of Na, K-ATPase by protein kinase C. J Biol Chem 270:14072–14077
Friedlander MJ, Kotchabhakdi N, Prosser CL (1976) Effects of cold and heat on behavior and cerebellar function in goldfish. J Comp Physiol A 112:19–45
Fujiyoshi Y, Mitsuoka K, De Groot BL et al (2002) Structure and function of water channels. Curr Opin Struct Biol 12:509–515. https://doi.org/10.1016/S0959-440X(02)00355-X
Gilmour KM, Perry SF (2018) Conflict and compromise: using reversible remodeling to manage competing physiological demands at the fish gill. Physiology 33:412–422. https://doi.org/10.1152/physiol.00031.2018
Gonzalez-Cabrera PJ, Dowd F, Pedibhotla VK et al (1995) Enhanced hypo-osmoregulation induced by warm-acclimation in Antarctic fish is mediated by increased gill and kidney Na+/K+-ATPase activities. J Exp Biol 198:2279–2291
Grim JM, Miles DRB, Crockett EL (2010) Temperature acclimation alters oxidative capacities and composition of membrane lipids without influencing activities of enzymatic antioxidants or susceptibility to lipid peroxidation in fish muscle. J Exp Biol 213:445–452. https://doi.org/10.1242/jeb.036939
Guynn S, Dowd F, Petzel D (2002) Characterization of gill Na/K-ATPase activity and ouabain binding in Antarctic and New Zealand nototheniid fishes. Comp Biochem Physiol A 131:363–374. https://doi.org/10.1016/S1095-6433(01)00488-3
Harper AA, Watt PW, Hancock NA, Macdonald AG (1990) Temperature acclimation effects on carp nerve: a comparison of nerve conduction, membrane fluidity and lipid composition. J Exp Biol 154:305–320
Hassett RP, Crockett EL (2009) Habitat temperature is an important determinant of cholesterol contents in copepods. J Exp Biol 212:71–77. https://doi.org/10.1242/jeb.020552
Haverinen J, Vornanen M (2020) Reduced ventricular excitability causes atrioventricular block and depression of heart rate in fish at critically high temperatures. J Exp Biol. https://doi.org/10.1242/jeb.225227
Hazel JR (1995) Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol 57:19–42
Hazel JR, Landrey SR (1988) Time course of thermal adaptation in plasma membranes of trout kidney. I. Headgroup composition. Am J Physiol 255:R622–R627. https://doi.org/10.1152/ajpregu.1988.255.4.R622
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–227. https://doi.org/10.1016/0163-7827(90)90002-3
Hollywood KA, Shadi IT, Goodacre R (2010) Monitoring the succinate dehydrogenase activity isolated from mitochondria by surface enhanced Raman scattering. J Phys Chem C 114:7308–7313. https://doi.org/10.1021/jp908950x
Hunt BM, Hoefling K, Cheng CHC (2003) Annual warming episodes in seawater temperatures in McMurdo Sound in relationship to endogenous ice in notothenioid fish. Antarct Sci 15:333–338. https://doi.org/10.1017/S0954102003001342
Johnston P, Roots BI (1964) Brain lipid fatty acids and temperature acclimation. Comp Biochem Physiol 11:303–309
Joyce W, Axelsson M, Egginton S et al (2018a) The effects of thermal acclimation on cardio-respiratory performance in an Antarctic fish (Notothenia coriiceps). Conserv Physiol 6:1–12. https://doi.org/10.1093/conphys/coy069
Joyce W, Egginton S, Farrell AP et al (2018b) Exploring nature’s natural knockouts: in vivo cardiorespiratory performance of Antarctic fishes during acute warming. J Exp Biol. https://doi.org/10.1242/jeb.183160
Jutfelt F, Roche DG, Clark TD et al (2019) Brain cooling marginally increases acute upper thermal tolerance in Atlantic cod. J Exp Biol 222:1–5. https://doi.org/10.1242/jeb.208249
Kaddah S, Khreich N, Kaddah F et al (2018) Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem Toxicol 113:40–48. https://doi.org/10.1016/j.fct.2018.01.017
Lande MB, Donovan JM, Zeidel ML (1995) The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J Gen Physiol 106:67–84. https://doi.org/10.1085/jgp.106.1.67
Llano GA, Littlepage GL (1965) Biology of the Antarctic Seas II. American Geophysical Union, Washington, DC
Logue JA, DeVries AL, Fodor E, Cossins AR (2000) Lipid compositional correlates of temperature-adaptive interspecific differences in membrane physical structure. J Exp Biol 203:2105–2115
Los DA, Mironov KS, Allakhverdiev SI (2013) Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth Res 116:489–509. https://doi.org/10.1007/s11120-013-9823-4
Malekar VC, Morton JD, Hider RN et al (2018) Effect of elevated temperature on membrane lipid saturation in Antarctic notothenioid fish. PeerJ Prepr. https://doi.org/10.7287/peerj.preprints.26472v1
Matheson DF, Oei R, Roots BI (1980) Changes in the fatty acyl composition of phospholipids in the optic tectum and optic nerve of temperature-acclimated goldfish. Physiol Biochem Zool 53:57–69
McCormick SD (1993) Methods for gill biopsy and measurement of Na+, K+-ATPase activity. Can J Fish Aquat Sci 50:656–658
McMullen TPW, Lewis RNAH, McElhaney RN (2004) Cholesterol–phospholipid interactions, the liquid-ordered phase and lipid rafts in model and biological membranes. Curr Opin, Colloid Interface Sci 8: 459
Michel GPF, Stárka J (1979) Phospholipase A activity with integrated phospholipid vesicles in intact cells of an envelope mutant of Escherichia coli. FEBS Lett 108:261–265. https://doi.org/10.1016/0014-5793(79)81224-7
Mueller IA, Grim JM, Beers JM et al (2011) Inter-relationship between mitochondrial function and susceptibility to oxidative stress in red- and white-blooded Antarctic notothenioid fishes. J Exp Biol 214:3732–3741. https://doi.org/10.1242/jeb.062042
Neas NP, Hazel JR (1985) Phospholipase A2 from liver microsomal membranes of thermally acclimated rainbow trout. J Exp Zool 233:51–60. https://doi.org/10.1002/jez.1402330108
Nilsson GE, Lefevre S (2016) Physiological challenges to fishes in a warmer and acidified future. Physiology 31:409–417. https://doi.org/10.1152/physiol.00055.2015
Oyarzún R, Muñoz JLP, Pontigo JP et al (2018) Effects of acclimation to high environmental temperatures on intermediary metabolism and osmoregulation in the sub-Antarctic notothenioid Eleginops maclovinus. Mar Biol 165:1–15. https://doi.org/10.1007/s00227-017-3277-8
Pfrieger FW (2003) Role of cholesterol in synapse formation and function. Biochim Biophys Acta 1610:271–280. https://doi.org/10.1016/S0005-2736(03)00024-5
Quinn PJ (1981) The fluidity of cell membranes and its regulation. Prog Biophys Mol Biol 38:1–104
Reynolds AM, Lee RE, Costanzo JP (2014) Membrane adaptation in phospholipids and cholesterol in the widely distributed, freeze-tolerant wood frog, Rana sylvatica. J Comp Physiol Part B. https://doi.org/10.1007/s00360-014-0805-4
Robertson JC, Hazel JR (1995) Cholesterol content of trout plasma membranes varies with acclimation temperature. Am J Physiol 269:R1113–R1119
Robertson JC, Hazel JR (1999) Influence of temperature and membrane lipid composition on the osmotic water permeability of teleost gills. Physiol Biochem Zool 72:623–632
Roots BI (1968) Phospholipids of goldfish Carassius auratus L. brain: the influence of environmental temperature. Comp Biochem Physiol 25:457–466
Rouser G, Fleischer S, Yamamoto A (1970) Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494–496. https://doi.org/10.1007/BF02531316
Silber PM, Gandolfi AJ, Brendel K (1986) Adaptation of a γ-glutamyl transpeptidase assay to microtiter plates. Anal Biochem 158:68–71. https://doi.org/10.1016/0003-2697(86)90590-7
Sinensky M (1974) Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci 71:522–525. https://doi.org/10.1073/pnas.71.2.522
Somero GN, DeVries AL (1967) Temperature tolerance of some Antarctic fishes. Science (80-) 156:257–258
Strobel A, Graeve M, Pörtner HO, Mark FC (2013) Mitochondrial acclimation capacities to ocean warming and acidification are limited in the Antarctic nototheniid fish, Notothenia rossii and Lepidonotothen squamifrons. PLoS ONE 8:1–11. https://doi.org/10.1371/journal.pone.0068865
Subczynski WK, Hyde JS, Kusumi A (1989) Oxygen permeability of phosphatidylcholine–cholesterol membranes. Proc Natl Acad Sci 86:4474–4478. https://doi.org/10.1073/pnas.86.12.4474
Truzzi C, Annibaldi AM, Scarponi G, Illuminati S (2018a) Gas chromatography–mass spectrometry analysis on effects of thermal shock on the fatty acid composition of the gills of the Antarctic teleost, Trematomus bernacchii. Environ Chem 15:424. https://doi.org/10.1071/en18130
Truzzi C, Illuminati S, Antonucci M et al (2018b) Heat shock influences the fatty acid composition of the muscle of the Antarctic fish Trematomus bernacchii. Mar Environ Res 139:122–128. https://doi.org/10.1016/j.marenvres.2018.03.017
Tsukada Y, Nagai K, Suda H (1980) Rapid micro method for 2′,3′-cyclic nucleotide 3′-phosphohydrolase assay using micro high performance liquid chromatography. J Neurochem 34:1019–1022
Urbina MA, Schulte PM, Bystriansky JS, Glover CN (2013) Differential expression of Na+, K+-ATPase α-1 isoforms during seawater acclimation in the amphidromous galaxiid fish Galaxias maculatus. J Comp Physiol B 183:345–357. https://doi.org/10.1007/s00360-012-0719-y
Urschel MR, O’Brien KM (2009) Mitochondrial function in Antarctic notothenioid fishes that differ in the expression of oxygen-binding proteins. Polar Biol 32:1323–1330. https://doi.org/10.1007/s00300-009-0629-y
van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124. https://doi.org/10.1038/nrm2330
Welti R, Li W, Li M et al (2002) Profiling membrane lipids in plant stress responses. J Biol Chem 277:31994–32002. https://doi.org/10.1074/jbc.M205375200
Xiao S, Gao W, Chen Q-F et al (2010) Overexpression of Arabidopsis acyl-CoA binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence. Plant Cell 22:1463–1482. https://doi.org/10.1105/tpc.110.075333
Yeagle PL (1985) Cholesterol and the cell membrane. Biochim Biophys Acta 822:267–287. https://doi.org/10.1016/0304-4157(85)90011-5
Acknowledgements
Field work for this study represented a collaborative effort between the authors, Dr. Michael Axelsson, Dr. Anthony Farrell, Dr. Stuart Egginton, Dr. William Joyce, Dr. Theresa Grove, Anna Rix, and Elizabeth Evans. We owe our thanks for logistic support to the staff at Palmer Station and the masters and crew of the ARSV Laurence M. Gould. The lipid analyses described in this work were performed at the Kansas Lipidomics Research Center Analytical Laboratory. Instrument acquisition and lipidomics method development was supported by National Science Foundation (EPS 0236913, MCB 1413036, DBI 0521587, DBI1228622), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20GM103418), and Kansas State University. We extend our thanks to Dr. Chris Griffin, Dr. Ahmed Faik and Tasleem Javaid for lending equipment and to Dr. John Robertson and Dr. Janet Duerr for technical and analytical guidance. Financial support for this research was provided by the US National Science Foundation [Grant no. PLR 1341602] and the Ohio University Student Enhancement Award Program.
Funding
Financial support for this research was provided by the US National Science Foundation [Grant no. PLR 1341602] and the Ohio University Student Enhancement Award Program.
Author information
Authors and Affiliations
Contributions
ELC, KOB and AMB: conceived and designed research; ELC, KOB, and AMB: performed experiments; AMB: analyzed data; AMB and ELC: interpreted results of experiments; AMB: prepared figures; AMB: drafted manuscript; ELC and KOB: edited and revised manuscript; ELC, KOB, and AMB: approved final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal experiments were approved by the Ohio University Animal Care and Use Committee (14-L-004).
Additional information
Communicated by Bernd Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biederman, A.M., O’Brien, K.M. & Crockett, E.L. Homeoviscous adaptation occurs with thermal acclimation in biological membranes from heart and gill, but not the brain, in the Antarctic fish Notothenia coriiceps. J Comp Physiol B 191, 289–300 (2021). https://doi.org/10.1007/s00360-020-01339-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01339-5