Abstract
Plants, algae, and photosynthetic bacteria experience frequent changes in environment. The ability to survive depends on their capacity to acclimate to such changes. In particular, fluctuations in temperature affect the fluidity of cytoplasmic and thylakoid membranes. The molecular mechanisms responsible for the perception of changes in membrane fluidity have not been fully characterized. However, the understanding of the functions of the individual genes for fatty acid desaturases in cyanobacteria and plants led to the directed mutagenesis of such genes that altered the membrane fluidity of cytoplasmic and thylakoid membranes. Characterization of the photosynthetic properties of the transformed cyanobacteria and higher plants revealed that lipid unsaturation is essential for protection of the photosynthetic machinery against environmental stresses, such as strong light, salt stress, and high and low temperatures. The unsaturation of fatty acids enhances the repair of the damaged photosystem II complex under stress conditions. In this review, we summarize the knowledge on the mechanisms that regulate membrane fluidity, on putative sensors that perceive changes in membrane fluidity, on genes that are involved in acclimation to new sets of environmental conditions, and on the influence of membrane properties on photosynthetic functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The extent of molecular disorder and molecular motion within a lipid bilayer is referred to as the fluidity of the membrane. Temperature stress causes alterations in the fluidity of the membranes in living cells. Cold causes a decrease in the membrane fluidity (membrane rigidification), which can be compensated by desaturation of membrane lipids by fatty acid desaturases (FADs). Alternatively, heat causes fluidization of the membranes. This is compensated by the synthesis of the membrane-stabilizing proteins and by the replacement of unsaturated fatty acids (UFAs) in membrane lipids by the de novo synthesized saturated FAs. Living organisms are usually capable of synthesizing the saturated FAs (Fig. 1) that are further desaturated by the specific enzymes, the FADs (Los and Murata 1998). Certain exceptions though exist, such as Escherichia coli, which produces monounsaturates during FA synthesis and elongation (Feng and Cronan 2009). Introduction of double bonds between carbon atoms of the FA chains of membrane lipids alter their conformation and affect physical properties of the membranes (Los and Murata 2004). These changes in membrane properties are perceived by cells via sensory proteins embedded in their membranes (Murata and Los 1997; Los and Zinchenko 2009; Cybulski and de Mendoza 2011). These sensors transfer the signals about the changes in the membranes from the environment (shifts in temperature) to networks of signal-transduction pathways, with the resulting regulation of gene expression (Los and Murata 2004).
The example of saturated and unsaturated fatty acids with 18 carbon atoms, which may be esterified to glycerol backbone to form the membrane diacylglycerolipids. The appearance of double bonds in the FA chains is strictly regulated. Carbon atoms are circled, ©; hydrogen atoms are shown by small dark circles. The position of double bonds may be counted from carboxyl-terminus of the fatty acid chain (Δ), or form the methyl-terminus of the chain (ω). Introduction of double bonds appears sequentially: first double bond is formed at position Δ9, second double bond, at position Δ12(ω), third, at position Δ15(ω3). See text for further details
Modulation in FA unsaturation is not the only mechanism, which determines the changes in membrane fluidity. Environmentally induced dynamic changes of lipid composition, lipid/protein ratio, and the presence of plant sterols in plant membranes are also important factors controlling membrane dynamics (Hartmann 1998; Espenshade and Hughes 2007; Mullineaux and Kirchhoff 2009).
In some bacteria, cis–trans isomerization of UFAs plays an important role in the regulation of membrane fluidity (Okuyama et al. 1991; Heipieper et al. 2003). The conversion of cis-UFAs to trans-UFAs apparently occurs with considerable efficiency during the adaptation of membrane fluidity to changes in the cellular environment.
Physical (temperature), chemical (membrane fluidizers, e.g., some drugs and alcohols), and genetic modification (knock-outs of genes for FADs) of the membrane fluidity often have similar effects on the expression of genes that are involved in the acclimation of cells to changing temperature (Los and Murata 2004; Horváth et al. 2012). For a long time, studies of the influence of membrane fluidity on gene expression have been limited to a small number of known genes that are responsible for the maintenance of the physical properties of membrane lipids. The availability of DNA microarrays that cover the entire genomes of various organisms made it possible to analyze the genome-wide transcription of genes associated with temperature acclimation (Los et al. 2008). It became possible to identify new, earlier unknown, genes, expression of which is affected by changes in membrane fluidity. Since membrane fluidity can directly regulate the activity of membrane-bound proteins, such as various translocators, ion channels, receptor-associated protein kinases (Ruelland and Zachowski 2010; Digel 2011), and sensor proteins (McClung and Davis 2010; Cybulski and de Mendoza 2011), it became the intriguing challenge—to solve a puzzle of temperature and fluidity sensing at the gene level. Many things still remain mysterious. At the same time, a considerable progress has been made in this field. Here, we will summarize recent findings and point to the unsolved questions.
Fatty acids of the membrane lipids
Living organisms, which few exceptions (Feng and Cronan 2009) synthesize the saturated FAs (Fig. 1) that are further desaturated by the FADs (Los and Murata 1998). The cell and photosynthetic membrane lipids are primarily glycerolipids formed by diglycerides, or diacylglycerols (DAGs), consisting of two FA chains covalently bonded to a molecule of tri-carbon alcohol glycerol through ester linkages. Glycerol has three backbone positions for esterification, thus, stereochemical numbering (sn) is usually applied to characterize the structure of membrane lipids. The numbering starts from the sn-1 carbon at the top and sn-3 carbon at the bottom of the molecule projection. The positions sn-1 and sn-2 may be occupied by saturated or unsaturated FAs with 14–22 carbon length (C14–C22). The sn-3 position is occupied by phosphate group, neutral, or charged molecules (Fig. 2). If all three sn-positions are occupied by fatty acyl chains, triglycerides (fats in animals and oils in plants) are formed.
Lipids form double layer of the cellular membrane. a One of possible configurations of a phosphatidylglycerol molecule: oleic acid (C18:1Δ9cis) is esterified to the sn-1 position of the glycerol backbone, palmitic acid (C16:0) is esterified to the sn-2 position of the glycerol backbone, phosphatidyl group is bound to sn-3 position of glycerol backbone. Fatty acids are bound to glycerol by their carboxy-termini (Δ-position). The double bond in the oleic acid is at cis-Δ9-position. A phosphate group represents charged hydrophilic “head” of a lipid, whereas fatty acids represent hydrophobic part of a lipid. b Schematic molecular model of a lipid molecule represented in (a). Such molecules form a membrane lipid bilayer (c) in aqueous solutions. The definitions and symbols are the same as in Fig. 1
The major lipids in the chloroplast membranes are monogalactosyl diacylglycerol (MGDG), digalactosyl diacylglycerol (DGDG), sulfoquinovosyl diacylglycerol (SQDG), phosphatidylglycerol (PG), phosphatidylcholine (PC). Monoglucosyl diacylglycerol (MGlDG) and phosphatidylinositol (PI) are minor components (Shimojima et al. 2009). MGDG and DGDG amount, respectively, to 50 and 25 % of chloroplast or cyanobacterial glycerolipids.
It should be noted that unsaturated double bonds theoretically may be formed in cis- or trans-positions, which refer to the configuration of a double carbon–carbon bond. These positions determine different spatial structure of the fatty acyl chain (see monounsaturated elaidic and oleic acids in Fig. 1). In nature, trans fatty acids are generated by direct enzymatic isomerization of the respective cis configuration of the double bond without a shift of its position (Heipieper et al. 2003). Trans-FAs are abundantly formed during the processing of cis-UFAs in food production, such as oil hydrogenation, refining, frying (Martin et al. 2007). With rare exceptions (Gao et al. 2009), FADs produce only cis-isomers of UFAs; therefore, we will further focus only on cis-UFAs. The indentified FADs of Arabidopsis are listed in Table 1.
Among photosynthesizing cells, cyanobacteria possess acyl-lipid FADs that are integrated into both cytoplasmic and thylakoid membranes (Mustardy et al. 1996). These enzymes generate double bonds in the fatty acids esterified to glycerol backbone. Plants possess two types of FADS. 1) soluble acyl–acyl-carrier-protein-FADs (acyl-ACP-desaturases) that introduce first double bonds at position Δ9 of the fatty acyl chains bound to ACP; 2) membrane-located acyl-lipid desaturases that are similar to cyanobacterial FADs (Table 1). Some of plant FADs are targeted to plastids, and other—to endoplasmic reticulum (Dyer and Mullen 2001; McCartney et al. 2004). In plants, acyl-ACP desaturases with the Δ4 or Δ6 positions are known (Guy et al. 2007 and references therein) that participate in oil biosynthesis.
Yeast and animals possess acyl-coenzyme A FADs (acyl-CoA desaturases), which are also located in membranes and introduce double bonds to the fatty acids bound to CoA. These enzymes have little amino acid sequence homology to acyl-lipid FADs of cyanobacteria and plants, however, their hydrophobic profiles look very similar (Wada et al. 1990).
Galactolipids (MGDG and DGDG) of photosynthetic plant cells usually contain a high proportion of polyunsaturated FAs, up to 95 % of which can be trienoic alpha-linolenic acid (Fig. 1). The most abundant molecular species of mono- and digalactosyldiacylglycerols have 18:3 at both sn-l and sn-2 positions of the glycerol backbone. Pea (Pisum sativum), which have 18:3 as almost the only FA in the MGDGs, have been termed “18:3 plants.” Other species, including the “model” plant Arabidopsis thaliana, contain appreciable amounts of hexadecatrienoic acid (16:3) in the MGDG, and they are termed “16:3 plants.” This acid is located exclusively at the sn-2 position of the glycerol backbone. Saturated palmitic acid (16:0) appears only in DGDGs, usually in small amounts. In non-photosynthetic tissues, such as tubers, roots or seeds, C18 FAs are usually more saturated.
On the basis of these structures, galactolipids are classified into two groups. The first has mainly C18 FAs at the sn-l position of the glycerol backbone, and only C16 FAs at the sn-2 position. Such lipids are termed as lipids of “prokaryotic” structure, as it is characteristic of cyanobacteria. The second class has C16 or C18 FAs at the sn-l position, but only C18 FAs in the sn-2 position. These lipids are of “eukaryotic” structure, as they are present in most glycerolipids, such as the phospholipids, of all eukaryotic cells. The exception is PG, which is synthesized in chloroplasts via the prokaryotic pathway (Li-Beisson et al. 2013).
The “counting” mechanism of FADs, by which they determine the exact place (regiospecificity) of desaturation, was debated for a long time. First double bond is formed at position Δ9 (after 9th carbon atom counting from the carboxyl-terminus of the chain). In case of C18 FAs, the Δ9-position and ω9-position (counting from the methyl-terminus of the acyl chain) coincide. Second double bond is formed at position Δ12(ω6). Third double bond is formed at position Δ15(ω3). It was unclear, whether Δ9-FAD counts from the methyl (ω) or carboxyl (Δ) end of the fatty acid. Similar question was applied also to Δ12(ω6) and Δ15(ω3)-FADs. The available experimental data on desaturation in cyanobacterial cells suggest that the Δ9 desaturase counts the carbon number from the carboxy terminus, whereas the so-called Δ15 desaturase is, in fact, the ω3-desaturase, which counts the carbon number from the methyl-terminus (Higashi and Murata 1993). Although a significant progress has been made in understanding the molecular basis of regiospecific desaturation by soluble acyl–acyl-carrier-protein desaturases (Cahoon et al. 1997; Guy et al. 2011), the counting order of the acyl-lipid membrane-bound Δ12-desaturase is still under question. It is also important to note that the ω3-desaturase of the cyanobacterium Synechocystis cannot introduce double bonds into Δ9 monounsaturated fatty acids, and it requires Δ9,12 di-unsaturated substrate for its activity (Mironov et al. 2012b).
Temperature-dependent changes in membrane fluidity
The effects of changes in temperature on membrane fluidity have been demonstrated by DPH (1,6-diphenyl-1,3,5-hexatriene) fluorescence polarization in fish (Cossins 1977), bacteria (Sinensky 1974), and cyanobacteria (Horváth et al. 1998). DPH is incorporated into membranes in parallel to the acyl chains of membrane lipids. Its fluorescence is weakly depolarized when it interacts stably with rigidified membranes, and it increases in the fluidized membranes. These studies demonstrated low temperatures cause a decrease in membrane fluidity (Fig. 3). Those changes have been confirmed by Fourier transform infrared (FTIR) spectroscopy which monitors the disorder of the acyl chains of lipids and the interactions between lipids and membrane proteins in terms of the frequency of the symmetric CH2 stretching mode. Low and high frequencies of the CH2 stretching mode correspond to the rigidified or fluid states of membrane lipids (Szalontai et al. 2000). The frequency of the CH2 stretching mode in cytoplasmic membranes and thylakoid membranes isolated from the cyanobacterium Synechocystis sp. PCC 6803 (Synechocystis) decreases with a decrease in temperature (Szalontai et al. 2000; Inaba et al. 2003).
Schematic representation of changes in membrane structure and in the behavior of lipid bilayers at low and high temperatures. Low temperatures cause an increase in lipid order and rigidification of membranes, whereas high temperatures cause a decrease in lipid order and fluidization of membranes. (Los and Murata 2004)
Heat shock causes the fluidization of membranes (Fig. 3), which at the extremes can lead to disintegration of the lipid bilayer (Vigh et al. 1998; Horváth et al. 2012). Aliphatic alcohols also cause the fluidization of membranes and they are often used in the experiments to mimic heat stress (Horváth et al. 1998, 2008, 2012).
Membrane fluidity and fatty acid unsaturation
The dependence of membrane fluidity on the extent of unsaturation of FAs in membrane lipids is a well-characterized phenomenon, which has been demonstrated in animals (Thewke et al. 2003), fish (Tiku et al. 1996), fungi (Maresca and Kobayashi 1993), yeasts (Bossie and Martin 1989; Rodríguez-Vargas et al. 2007), plants (Lyons and Raison 1970; Nishida and Murata 1996), and bacteria (Sinensky 1974; Cybulski et al. 2002), including cyanobacteria (Los and Murata 2004). Cyanobacteria are particularly suitable for studies of such phenomena (Glatz et al. 1998) because the number of unsaturated bonds in their FAs can be altered by targeted mutagenesis of the genes that encode the FADs (Tasaka et al. 1996; Mironov et al. 2012a). Two strains of cyanobacteria, namely, Synechocystis and Synechococcus sp. PCC 7942 (Synechococcus), have been used to study the effects of the unsaturation of FAs on membrane fluidity. Synechocystis is characterized by the presence of four genes, designated desA, desB, desC, and desD, for FADs and by its ability to synthesize FAs with four double bonds. Thus, its membrane lipids contain high levels of UFAs (Fig. 4).
When the desA and desD genes were inactivated in Synechocystis, a dramatic decrease in membrane fluidity (Szalontai et al. 2000; Inaba et al. 2003; Mironov et al. 2012a) was detectable in the resultant desA −/desD − strain, which lost the ability to survive at low temperatures.
Cells of Synechococcus naturally possess only one desaturase and they synthesize only monounsaturated 16:1Δ9 or 18:1Δ9 FAs (Fig. 4). These cells transformed with the desA gene (Δ12 desaturase) of Synechocystis produced considerable amounts of di-unsaturated 18:2Δ9,12 FAs (Wada et al. 1990). They were characterized by a considerable increase in membrane fluidity (Sarcina et al. 2003), which was linked to the newly gained and important feature—the ability to survive at low temperatures.
Desaturation of polar glycerolipids in cyanobacterial cells. a Desaturation in wild-type cells of Synechocystis (WT) is catalyzed by four acyl-lipid desaturases (Δ6, Δ9, Δ12, ω3) with formation of C18:4 fatty acid. Site-directed mutagenesis of the desA and desD genes (shown by crosses) prevents the synthesis of polyunsaturated fatty acids and only monounsaturated fatty acids are produced in the desA −/des
D − mutant cells (b). The molecular species of lipids in Synechocystis are mainly C18 with minor levels of C16 at the sn-1 position and C16:0 at the sn-2 position. Desaturation occurs exclusively at the sn-1 position. c Desaturation in wild-type cells of Synechococcus (WT) is catalyzed only by the acyl-lipid Δ9-desaturase and only monounsaturated fatty acids are produced. Transformation of this strain with the desA gene for the Δ12-desaturase of Synechocystis (desA +) leads to enhanced formation of di-unsaturated fatty acids (d). In Synechococcus, most of the molecular species are C16 with minor levels of C18 at the sn-1 position and C16:0 at the sn-2 position. Desaturation occurs at the sn-1 and sn-2 positions. n = 0, 1, 2, 3, 4 corresponds to the number of double bonds in the fatty acyl chains
Thus, an increase in desaturation of FAs fluidizes the membranes, which allows cyanobacterial and plant cells to tolerate chilling and to survive at low temperatures (Tasaka et al. 1996; Ishizaki-Nishizawa et al. 1996; Orlova et al. 2003; Mironov et al. 2012a). Similar effects of engineered membrane fluidization on growth and survival have been recently observed in yeast cells transformed with plant gene for the Δ12 desaturase (Rodríguez-Vargas et al. 2007).
Feedback of desaturation on membrane fluidity
Three of the four genes for desaturases in Synechocystis (desA, desB, and desD) are cold-inducible (Los et al. 1997). The enhanced synthesis de novo of these three FADs under cold stress and the subsequent introduction of additional double bonds into the fatty acyl chains of membrane lipids are involved in the maintenance of membrane fluidity in the liquid-crystalline phase and prevent the membranes from undergoing phase transition to the lethal gel phase (Hazel 1995). The cold-induced expression of desaturase genes to compensate for a decrease in membrane fluidity, is a widespread phenomenon that can be observed in almost all taxa of poikilothermic organisms, from bacteria to plants, fish, and animals (Macartney et al. 1994; Los and Murata 2004; Aguilar and de Mendoza 2006).
Synthesis of UFAs as a compensatory response to a decrease in membrane fluidity at low temperatures was demonstrated first in E. coli, and the phenomenon was designated “homeoviscous acclimation” (Sinensky 1974). Later on, the existence of a feedback loop between membrane rigidification and the compensatory expression of genes for FADs was demonstrated by the chemical hydrogenation of UFAs in the plasma-membrane lipids of Synechocystis (Vigh et al. 1993), or by the use of genetically engineered mutants with decreased unsaturation of membrane lipids (Tasaka et al. 1996; Mironov et al. 2012a, b). Palladium-catalyzed saturation of a small portion of plasma-membrane lipids at the optimal growth temperature caused rapid induction in transcription of the desA gene for the Δ12 desaturase, which is responsible for the synthesis of dienoic FAs. This enzyme compensated the chemically induced rigidification of membrane lipids and returned them to their optimal fluid state (Vigh et al. 1993).
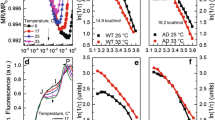
The desB gene for the ω3 desaturase is responsible for the generation of the trienoic FAs. Genetically engineered rigidification of the membranes (the desA −/desD − cells of Synechocystis) resulted in the induction of the desB at 32 °C, whereas its induction in wild-type cells was evident only at 26 °C (Fig. 5). Thus, the existence of the feedback link between the fluidity of biological membranes and the expression of genes responsible for the maintenance of the optimal physical state of membranes during a change of the ambient temperature has been confirmed using two independent approaches (Vigh et al. 1993; Mironov et al. 2012a).
Analysis of the desB gene transcription in the Synechocystis wild-type (a) and the desA −/desD − mutant (b) cells (northern blotting). Cells were grown at 36 °C and incubated at different temperatures (from 34 to 24 °C) for 20 min. Then, RNA was isolated from cells for northern blotting with the desB and 16S rRNA gene fragments as probes. c Data for the desB expression are graphically presented as a dependence of the level of expression (in % from the maximal level) on the temperature of the incubation of wild-type (open symbols) and the desA −/desD − (dark symbols) cells
It is important that the cold-induced enhancement of the expression of the genes for desaturases depends on the extent of the shift in temperature but not on the absolute temperature (Mironov et al. 2012a). Similar conclusions have been made from the studies of non-photosynthesizing bacteria (Porta et al. 2010a, b). In plants, however, experimental evidence suggests the existence of two types of the thermometers: one type may detect absolute temperature, and the other—a rate of change in temperature (Knight and Knight 2012).
Membrane rigidification and cold sensing
Cold-inducible genes have been grouped into several categories, as follows: (1) genes for FADs that are responsible for adjustments in membrane fluidity; (2) genes for RNA-binding proteins—RNA chaperones and RNA helicases (rbpA, crhR) that destabilize the secondary structures of mRNAs, thereby overcoming inhibition of the initiation of translation at low temperatures; (3) genes for ribosomal proteins and translation factors (rps, rpl, fus) an excess of which is necessary for acclimation of the translational machinery to cold; (4) genes for caseinolytic proteases that participate in the renewal of photosystem II; (5) genes for subunits of RNA polymerase (rpoA, sigD); several stress-activated genes (cytM, hli-family) (Los et al. 2008). In total, cold strongly induces more than 50 genes in Synechocystis (Suzuki et al. 2001; Inaba et al. 2003).
The cold sensor Hik33 (histidine kinase 33) regulates the expression of about a half of cold-inducible genes (Suzuki et al. 2000, 2001). The expression of these Hik33-regulated genes depends on the fluidity of cell membranes, and it is regulated by light, though it does not require the activity of the photosynthetic apparatus (Mironov et al. 2012b). The expression of another group of cold-induced genes, which is not controlled by Hik33, does not depend on the membrane fluidity or light. Thus, membrane fluidity determines the temperature dependence of the expression of cold-induced genes that are under control of the Hik33, which might be the sensor of changes in the membrane fluidity.
It might be that Hik33 perceives cold-induced membrane rigidification as the primary signal of cold stress. The transmembrane sensor protein Hik33 contains several conserved domains, namely, a type-P linker, a leucine zipper and a PAS domain (Fig. 6). The type-P linker might be responsible for intermolecular dimerization of the protein under cold stress, when rigidification of the membranes takes place. The PAS domain senses oxidative stress, which might accompany cold stress shortly after a drop in temperature (Los and Murata 2004).
Cold sensors. a The histidine kinase Hik33 of Synechocystis consists of two transmembrane domains, type-P linker, leucine zipper (LZ), and PAS domain (Murata and Los 2006). b The histidine kinase DesK of Bacillus subtilis includes five transmembrane segments. The rigidification of the membrane promotes autophosporylation and transfer the phosphate group to the response regulator DesR, which activates expression of the des gene coding for a Δ5-desaturase. c A truncated chimera of DesK represents the “minimum sensor” (DesK min), in which the N-terminal domain of TM1 is fused to the C-terminal domain of TM5. This engineered sensor allows near-normal control of membrane-mediated DesK signaling (Cybulski and de Mendoza 2011)
The molecular mechanism of this sensing is still unclear. It was suggested that lateral diffusion of the membrane-spanning domains of the Hik33 may lead to a drift of its subunits, to dimerization and to autophosphorylation (Los and Murata 2004; Los and Zinchenko 2009). Dimerization of the PAS domain of Hik33 in vivo was recently confirmed by bacterial two-hybrid assay (Shimura et al. 2012).
As a member of the two-component regulatory system, Hik33 should operate via its cognate response regulator (Rre) to transduce a signal of membrane rigidification and to induce the responsive genes. Recently, such transducer, Rre26, has been identified (Kappell and van Waasbergen 2007).
A typical prokaryotic two-component system consists of a Hik and a Rre. The Hik perceives changes in the environment via its sensor domain and then a histidine residue within the conserved histidine kinase domain is autophosphorylated, with ATP as the donor of the phosphate group (Fig. 6). The phophate group is transferred from the Hik to an aspartate residue in the conserved receiver domain of the cognate Rre. Upon phosphorylation, the Rre changes its conformation, and this change allows the Rre to bind to the promoter regions of genes that are located further downstream in the signal-transduction pathway. The Hiks in Synechocystis appear to be involved in the perception and transduction of environmental signals due to low temperature and high temperature stress, hyperosmolarity and high NaCl concentrations, phosphate and manganese deprivation, excess nickel ions, etc. (for reviews, see Los et al. 2008, 2010).
Recent findings on the temperature sensor histidine kinase DesK of Gram-positive Bacillus subtilis provide another clue for understanding the molecular mechanisms of sensing the changes in membrane properties (Cybulski et al. 2010; Cybulski and de Mendoza 2011). The DesK sensor kinase includes five TM-spanning segments that sense an increase in the order of membrane lipids promoting a kinase dominant state of DesK, which undergoes autophosphorylation and transfer of the phosphate group to the response regulator DesR (Aguilar et al. 2001; Cybulski et al. 2002). Phospho-DesR activates expression of the des gene (Albanesi et al. 2004, 2009; Najle et al. 2009) for the Δ5-desaturase, the only FAD in B. subtilis that is involved in the maintenance of the membrane fluidity (Mansilla et al. 2004; Mansilla and de Mendoza 2005).
A truncated DesK, in which the N-terminal domain of TM1 is fused to the C-terminal domain of TM5, still allows near-normal control of membrane-mediated DesK signaling state (Fig. 7). The temperature-dependent signaling of the engineered sensor protein with just one transmembrane domain implies that lateral movements of the transmembrane domains are not so important for sensing the membrane physical state. It is now thought that the most important parameters of sensing is the match between hydrophobic thickness of the membrane and the hydrophobic thickness of transmembrane segments of integral membrane protein. Any mismatch between the thicknesses of the lipid bilayer and the protein may lead to modification in the structure of the protein (Cybulski et al. 2010), which may trigger the low temperature response (Cybulski and de Mendoza 2011).
The defects in polyunsaturated fatty acids in the desA −/desD − mutant cells of Synechocystis accelerated the NaCl-induced inactivation of PS II and reduced the light-dependent recovery of PS II activity. a Wild-type and desA −/desD − cells were incubated for 12 h in darkness in the presence of 0.5 M NaCl. Then, cells were exposed to light at 50 μmol photons m−2 s−1 for 20 h. b Wild-type and desA −/desD − cells were incubated with 0.5 M NaCl in light at 50 μmol photons m−2 s−1 for 12 h. Then, cells were collected by centrifugation, resuspended in fresh medium with no added NaCl, and incubated in light at 50 μmol photons m−2 s−1 for 20 h. Activity of PS II was monitored by means of the light-dependent evolution of oxygen in the presence of 1.0 mM 1,4-benzoquinone. Open circles: wild-type cells, dark circles: desA −/desD − cells (Allakhverdiev et al. 1999)
The DesK of Bacillus does not have PAS domain or type-P linker. It should be noted that the DesK was unable to substitute the inactivated cold sensor Hik33 in Synechocystis. On the other hand, the Hik33-homolog from Synechococcus sp. PCC 7942 could successfully complement the knock-out of Hik33 in Synechocystis (L. Kiseleva, I. Suzuki, D.A. Los, N. Murata—the unpublished observations). This may reflect the differences in membrane properties of cyanobacteria and Bacillus. Thus, the additional experiments on truncation of transmembrane domains of cyanobacterial Hik33 (Shimura et al. 2012) are necessary to confirm the importance of membrane thickness for fluidity and temperature sensing.
Engineered rigidification of membranes in Synechocystis
As mentioned above, a reduction in the extent of unsaturation of FAs was achieved by targeted mutagenesis of the FADs in the cyanobacterium Synechocystis (Fig. 4). The effects of the unsaturation of FAs on the thermotropic behavior of thylakoid membranes from wild-type and mutant cells was examined first by differential scanning calorimetry, DPH fluorescence polarization, FTIR and Raman spectrometry (Tasaka et al. 1996; Szalontai et al. 2000; Mironov et al. 2012a, b). The replacement of PUFAs in membrane lipids by a monounsaturated FAs rigidified cytoplasmic and thylakoid membranes and raised the temperature for the phase transition of the lipids in thylakoids.
The effects of the rigidification of membranes on the growth of cells were examined at various temperatures (Tasaka et al. 1996; Mironov et al. 2012a). Maximum growth of wild-type cells was observed at ~30–35 °C and the cells were able to grow even at 20 °C. By contrast, desA −/desD − mutant cells could not grow at 20 °C. These results indicated that replacement of PUFAs by a monounsaturated species had a significant effect on the proliferation of cells at low temperature.
Since membrane-bound protein complexes, such as PSI, PSII, the cytochrome b 6 /f complex and ATP synthase, are associated directly with lipids in thylakoid membranes, it is reasonable to predict that their activities are affected by the properties of membrane lipids. In this context, the effects of membrane rigidification on the electron transport (from water to ferricyanide), on the uptake of protons, and on the synthesis of ATP have been examined (Dilley et al. 2001). These activities in wild-type cells were reduced with a decrease in temperature but were retained at certain low levels at temperatures close to freezing. By contrast, desA − /desD − mutant cells lost the ability to synthesize ATP but not the ability to transport electrons and take up protons at ~5 °C. These findings suggest that a decrease in the membrane fluidity might block the ability of thylakoid membranes to couple a bioenergetically competent proton-motive force to the synthesis of ATP at low temperatures.
Membrane rigidification stimulates photoinhibition at low temperatures
The inactivation of photosystem II (PS II) by strong light is a phenomenon known as photoinhibition, which is due to an imbalance between the rate of photodamage to PS II and the rate of repair of the damaged PS II. Photodamage is a result of the direct inactivation of the oxygen-evolving complex and the photochemical reaction center by light (Ohnishi et al. 2005; Hakala et al. 2005). In addition, strong light induces the production of reactive oxygen species, which inhibit the repair of photodamaged PS II by suppressing, primarily, the synthesis of proteins de novo (Murata et al. 2007; Takahashi and Murata 2008; Nishiyama et al. 2011). The effects of environmental stress on damage to and repair of PS II can be examined separately and it appears that environmental stresses, such as low temperature, act primarily by inhibiting the repair of PS II (Nishiyama et al. 2011).
A comparison of the turnover of the Dl protein, which is an important component of the photochemical reaction center of PS II, in wild-type and desA − /desD − cells of Synechocystis revealed that post-translational carboxy-terminal processing of the precursor to the D1 protein was extremely sensitive to low temperature and was also dependent on the fluidity of thylakoid membranes (Kanervo et al. 1995, 1997). A decrease in temperature from 33 to 18 °C caused a failure to recover from photodamage in desA −/desD − mutant cells due to their inability to process the newly synthesized precursor to the D1 protein (Kanervo et al. 1997). Thus, membrane fluidity helps to mitigate photoinhibition by counteracting the inhibitory effects of low temperature on the processing of the precursor to D1 (Takahashi and Murata 2008; Nishiyama et al. 2011).
Membrane rigidification raises sensitivity to salt stress
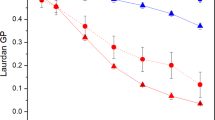
The ability of the photosynthetic machinery to tolerate salt stress has been examined in detail in wild-type and desA −/desD − mutant cells of Synechocystis. The disappearance of the oxygen-evolving activity of PS II under salt stress due to 0.5 M NaCl, both in darkness and in light, was much more rapid in desA − /desD − cells with rigid membranes than in wild-type cells with fluid membranes (Fig. 4, Allakhverdiev et al. 1999). Moreover, the photosynthetic activity of wild-type cells recovered more rapidly than that of the mutant cells after removal of NaCl from the growth medium (Fig. 7). Recovery in the presence of NaCl depended on light and ATP was required for the synthesis de novo of the proteins necessary for reactivation of the oxygen-evolving machinery (Allakhverdiev et al. 1999).
The recovery of the oxygen-evolving activity under salt stress depends on the activity of the Na+/H+ antiporter system, which, in its turn, is regulated by the membrane fluidity. Incubation of wild-type and desA −/desD − cells in the presence of 1.0 M NaCl in darkness resulted in total inactivation of the Na+/H+ antiporter system. Upon exposure to low light, the activity of the Na+/H+ antiporter system in wild-type cells returned to 30 % of the original level while, in desA − /desD − cells, it returned to only to 5 % of the original level. The NaCl-induced inactivation of the oxygen-evolving complex in thylakoid membranes isolated from desA −/desD − cells also occurred much more rapidly than in membranes from wild-type cells (Allakhverdiev et al. 1999). In general, desA − /desD − cells were characterized by enhanced sensitivity to NaCl and a reduced ability to recover from salt stress, as compared to wild-type cells.
This may be explained by the following: (a) the membrane fluidity might act directly to protect the oxygen-evolving machinery against salt-induced inactivation; (b) the activity of the Na+/H+ antiporter system might be regulated by membrane fluidity; (c) the recovery of Na+/H+ antiporter activity is more efficient in unsaturated (fluid) membranes (Allakhverdiev et al. 1999; Allakhverdiev and Murata 2008).
Resent results obtained with the mutants of Arabidopsis defective in Δ12 fatty acid desaturase FAD2 confirmed the importance of unsaturation for the maintenance of the Na+/H+ antiporter activity and for tolerance to salt stress (Zhang et al. 2012).
Engineered fluidization of membranes in Synechococcus
The increase in unsaturation of FAs by genetic engineering was achieved by transformation of Synechococcus cells with the gene for the Δ12 desaturase of Synechocystis (Wada et al. 1990). Synechococcus synthesizes only monounsaturated FAs, mainly 16:1Δ9 and 18:1Δ9. It cannot grow and propagate at low temperatures. The desA +-transformed Synechococcus cells were able to introduce a double bond at the Δ12 position of the FA that was esterified to the sn-1 position of the glycerol moiety (Fig. 4). The resulting change in the FA composition was recognized as an increase in the level of di-unsaturated FAs at the expense of monounsaturated FA (Wada et al. 1990): the transformed cells contained almost equal amounts of monounsaturated and di-unsaturated FAs.
Membrane fluidization mitigates photoinhibition at low temperatures
As mentioned above, photoinhibition in vivo reflects the effects of photodamage to PS II and subsequent repair (Murata et al. 2007; Nishiyama et al. 2011). The damage corresponds to the light-induced inactivation of PS II due to a damage to the D1 protein, while repair consists of a series of steps, namely, degradation of the D1 protein in the photodamaged PS II, synthesis of the precursor to the D1 protein de novo, incorporation of the precursor into the PS II complex and, finally, processing of the precursor to yield the mature D1 protein.
The desA +-transformed Synechococcus cells, glycerolipids of which included di-unsaturated FAs, were more resistant to photoinhibition at low temperatures than were wild-type cells, which contained monounsaturated FAs exclusively (Gombos et al. 1997). Studies of the light-induced inactivation and recovery of the activity of the PS II complex revealed that the recovery process was markedly accelerated in di-unsaturated membranes. The synthesis of the D1 protein de novo at low temperature was much faster in the desA +-transformed cells than in the wild-type cells. Thus, increased unsaturation of membrane lipids enhanced the ability of cells to withstand strong light by accelerating the de novo synthesis and turnover of the D1 protein (Gombos et al. 1997).
Three isoforms of the D1 protein are encoded by three psbA genes in Synechococcus. The psbAI gene encodes D1 form I (D1:1). The psbAII and psbAIII genes are identical and they encode the transiently expressed D1 form II (D1:2). Transfer of cultures of Synechococcus cells from 32 to 25 °C under light at 100 μmol photons m−2 s−1 resulted in replacement of D1:1, the prevailing form at the higher temperature, by D1:2 in both wild-type cells and the desA +-transformed cells, with no loss of PS II activity (Sippola et al. 1998). A further downward shift in temperature to 18 °C caused a dramatic decrease in PS II activity in wild-type cells, while the desA +-transformed cells were affected to a much smaller extent. It appeared that wild-type cells were incapable of accumulating D1:2 to compensate for the loss of D1:1, with resultant disassembly of PS II at the low temperatures (Sippola et al. 1998). These results suggested a crucial role of the UFAs and membrane fluidity in promotion of the exchange of the prevailing forms of the D1 protein and, thus, in the maintenance of the activity of PS II at low temperature (Takahashi and Murata 2008; Nishiyama et al. 2011).
Fluidization of membranes enhances tolerance to salt stress
In Synechococcus, the photoinactivation of PS II under salt stress was more prominent in wild-type cells than in desA +-transformed cells (Allakhverdiev et al. 2001). A similar effect was also observed when the activity of PS I was examined: the reduction of P700+ in the PS I complex was more resistant to the damaging action of a high concentration of NaCl in desA +-transformed cells than in wild-type cells.
The NaCl-induced inactivation of PS I and PS II, as well as that of the Na+/H+ antiporter system, consists of a rapid phase and a slow phase (Allakhverdiev et al. 2000; Allakhverdiev and Murata 2008). The rapid phase is reversible and is induced by the osmotic effect of NaCl, which reduces the amount of water in the cytosol via the efflux of water through water channels. The slow phase is irreversible and is induced by ionic effects that are due to the influx of Na+ ions through K+/Na+ channels.
Unsaturation of FAs in membrane lipids protects PS I and PS II against both rapid phase and slow phase of NaCl-induced inactivation (Allakhverdiev et al. 2001). Experiments with isolated thylakoid membranes demonstrated that the NaCl-induced inactivation occurred more slowly in membranes isolated from desA +-transformed cells than in those from wild-type cells.
Recovery of the activities of PS I and PS II was alleviated in desA +-transformed cells than in wild-type cells. The available evidence suggests that the unsaturation of FAs has two effects: (a) it mitigates the NaCl-induced damage to PS I and PS II both in vivo and in vitro; (b) it enhances the repair of damaged PS I and PS II in vivo. These phenomena might be due to the activities of the Na+/H+ antiporter system and H+-ATPase(s), as well as to water channels and K+(Na+) channels, which might be influenced by the fluidity of membranes. Thus, there is the direct evidence that membrane fluidity plays important role in the maintenance of the photosynthetic machinery under salt stress.
Modulation of lipid unsaturation in higher plants
Genetics of fatty acid unsaturation
Large numbers of cold-inducible genes have been identified in plants (Seki et al. 2001; Fowler and Thomashow 2002; Benedict et al. 2006; Zeller et al. 2009; Matsui et al. 2010). These genes could be classified into several groups, such as genes for transcription factors, signal transducers, transporters, enzymes involved in the synthesis of cell walls and enzymes involved in the response to oxidative stress, FADs (Maruyama et al. 2009). The level of unsaturation of membrane FAs in higher plants correlates with growth temperature. Thus, desaturation plays the key role in the regulation of membrane fluidity and the acclimation of plants to low temperatures (Orvar et al. 2000; Vaultier et al. 2006; Knight and Knight 2012). Although the induction of the expression of genes for desaturases and the experiments with fluidizers and rigidifiers of membrane lipids (Sangwan et al. 2002) suggest that membrane rigidification might also participate in cold signaling, the distinct knowledge about cold sensors in plants is still missing (for updates on the topic, please, see Thomashow 2010; Knight and Knight 2012). Future efforts will be required to identify the sensor proteins that act as thermosensors in plants and linking the perception of temperature to response.
In parallel with the development of techniques for the genetic engineering of various aspects of plant metabolism, including the unsaturation of FAs, extensive efforts have been made to isolate and characterize mutant plants with defects in the unsaturation of FAs by a classical genetic approach (Wallis and Browse 2002). The Arabidopsis mutant with reduced activity of the Δ12 desaturases accumulated high levels of 16:1 and 18:1 in lipids. This mutant was characterized by abnormal chloroplast ultrastructure, the protein and chlorophyll contents of thylakoid membranes, electron- transport activity, and the thermal stability of photosynthetic membranes (Hugly et al. 1989). These observations pointed to the central role for di-unsaturated FAs in lipids in determining chloroplast structure and maintaining normal photosynthetic functions. These studies demonstrated that the unsaturation of FAs has a direct effect on the thermal stability of photosynthetic membranes.
In unicellular prokaryotic Synechocystis (Tasaka et al. 1996) and in eukaryotic protozoan Acanthamoeba castellanii (Harwood 2007), the Δ12-desaturase also plays the crucial role in cold acclimation and survival at low temperatures.
The complete absence of trienoic FAs in thylakoid lipids of Arabidopsis due to triple mutation of genes for ω3 desaturases (fad3-2/fad7-2/fad8) stipulated hypersensitivity of such plants to photoinhibition (Vijayan and Browse 2002). The rate of photodamage to PS II was similar in wild-type and triple-mutant plants. However, the recovery of photodamaged PS II was much slower in triple-mutants. These results support the proposed importance of PUFAs in the recovery of PS II activity at low temperatures (Moon et al. 1995). Similar conclusions have been drawn from the lessons learned from the studies of cyanobacteria (Tasaka et al. 1996; Allakhverdiev et al. 1999, 2000).
It should be mentioned that cold-induced desaturation in cyanobacteria occurs mainly due to transcriptional induction and due to the increase in desaturase mRNA stability (Los and Murata 2004). In plants, as well as in fish (Tiku et al. 1996) desaturases, in addition to transcriptional level, are regulated at the post-transcriptional and post-translational levels including phosphorylation and modulation of protein stability (Matsuda et al. 2005; Tang et al. 2005).
The effect of changes in membrane fluidity on the oxygen-evolving capability of isolated thylakoids was recently studied. Membranes were rigidified by incorporation of the plant sterol stigmasterol. Such rigidification was accompanied by a reduction of the activity of PSII and by even more pronounced inhibition of PSI-mediated electron transport (Popova et al. 2007; Velitchkova et al. 2009).
For the assembly and function of photosynthetic machinery, the translocation of proteins through the thylakoid membranes is very important. This transport across membranes occurs via one of three distinct pathways (Gutensohn et al. 2006). A study of three mutant lines of Arabidopsis with depressed unsaturation, namely, fad6, fad5, and fad3/fad7/fad8, has provided useful information relevant to the characterization of these three translocation pathways (Ma and Browse 2006). The mutations in desaturases reduced, by up to 50 %, the rate of transport of the 18-kDa extrinsic protein of PS II via the Tat pathway, which depends on a pH gradient across membranes. By contrast, the mutations substantially increased the transport of the 33-kDa protein of PS II via the Sec pathway. This increased capacity for protein import might compensate partially for the reduced capacity for thylakoid transport via the Tat pathway. It means that the transport of proteins might depend on the properties of the chloroplast membranes. Impaired transport of proteins in the mutants might contribute to their low-temperature-sensitive phenotype (Ma and Browse 2006).
Engineered fatty acid unsaturation in plants
Another approach to modulate the unsaturation of FAs involves transformation of tobacco plants with the desC gene for Δ9 desaturase from Synechococcus (Ishizaki-Nishizawa et al. 1996). The expression of this acyl-lipid desaturase increased the relative level of monounsaturated FAs in the PG of chloroplasts in tobacco leaves. The excess of monounsaturated FAs served as substrates for further desaturation. As a result, such transformation of tobacco plants dramatically increased levels of PUFAs and decreased levels of saturated FAs from 44 to 24 %. These changes dramatically enhanced the ability of the transgenic tobacco plants to tolerate low temperatures.
The tobacco plants have also been transformed with the desC gene from the thermophilic cyanobacterium Synechococcus vulcanus (Orlova et al. 2003). The lipid content and extent of unsaturation of FAs were significantly higher in leaves of transgenic plants if compared to parental plants. The chilling tolerance of the desC + plants also increased, as estimated by measurements of the leakage of electrolytes from tissues upon exposure to low temperatures (Fig. 8a). Seeds of the transgenic plants exhibited greater chilling tolerance than the control seeds. Strong light at low temperature caused leaf bleaching in wild-type plants, but not in the desC + transformants (Fig. 8b, c), indicating that unsaturation of lipids protects photosynthetic machinery under strong light at low temperatures.
Tests for cold sensitivity of wild-type and desC +-transgenic tobacco. a Wild-type tobacco plants (white bars) and the desC + tobacco transformants (shaded bars) have been tested for chilling sensitivity by measuring the electric conductivity of water extracts of plant tissues. Plants have been exposed at 2 or 0 °C for 24 h; at −5 °C for 30 min (no ice nucleation) or for 60 min (with ice nucleation). The index of injury represented leakage of electrolytes from the damaged tissues as percent of the leakage from the tissues completely destroyed by boiling (Orlova et al. 2003). Higher index of injury reflects higher cold sensitivity of plants. Lower panel: tobacco plants of wild-type (b) and desC + transformant (c) were exposed to high light intensity (HL—1,000 μmol photons m−2 s−1) at 4 °C for 3 days
Overexpression in tomato plants of the fad7 gene for ω3 fatty acid desaturase from tomato increased the level of α-linolenic acid (18:3) in the glycerolipids of thylakoid membranes (Liu et al. 2008). Upon exposure to chilling stress at 4 °C under light at 100 μmol photons m−2 s−1, the fad7 +-transgenic plants maintained greater oxygen-evolving activity and greater photochemical efficiency than wild-type plants.
Together with the mutants with defects at certain steps in desaturation pathway, these transgenic plants should serve as useful models for direct assessment of the roles of FA unsaturation in photosynthesis and related physiological activities in higher plants.
Changes in fatty acids unsaturation by acyltransferases
Glycerol-3-phosphate acyltransferase (GPAT), encoded by the ATS1 gene (Nishida et al. 1993) and localized in plastids, catalyzes the transfer of the acyl group of acyl-ACP to the sn-1 position of glycerol 3-phosphate (Nishida and Murata 1996). In chilling-resistant plants, such as spinach (Spinacea oleracea) and Arabidopsis, this enzyme uses 18:1-ACP specifically as its substrate. By contrast, in chilling-sensitive squash (Cucurbita moschata), the enzyme utilizes both 18:1-ACP and 16:0-ACP (Frentzen et al. 1987; Nishida et al. 1987).
The extent of unsaturation of FAs was altered in transgenic tobacco (Nicotiana tabacum) that expressed GPAT from plants that differed in terms of chilling sensitivity (Murata et al. 1992). In tobacco that expressed GPAT derived from squash, the fluidity of thylakoid membranes decreased in response to the increase in the proportion of saturated plus trans-monounsaturated molecular species of PG from 24 to 65 % (Szalontai et al. 2003). These plants displayed more pronounced sensitivity to photoinhibition at low temperatures and lower rates of PSII recovery than non-transformed plants. These results suggested that the unsaturation of FAs of PG in thylakoid membranes might stabilize the photosynthetic machinery against photoinhibition at low temperature by accelerating the repair of PS II (Moon et al. 1995).
Chilling stress also damaged inflorescences: the abscission of flower buds and inflorescence meristems occurred more frequently in transgenic plants than wild-type plants. Thus, a decrease in the proportion of cis-unsaturated PG provoked the sensitivity of reproductive organs to chilling (Sakamoto et al. 2003).
Sui et al. (2007a) overexpressed cDNA for GPAT of tomato in tomato plants under the control of the strong 35S promoter of cauliflower mosaic virus (CaMV) and observed an increase in relative level of cis-unsaturated PG. This enhanced the tolerance of plants to cold stress and accelerated the recovery of PS II after photodamage during cold-induced photoinhibition. When the GPAT was repressed in tomato by the antisense method, an increase in the relative level of saturated FAs in PG from leaves was observed. A decrease in unsaturation reduced male fertility, increased the size of tapetal cells, and mitigated photoinhibition of PS II under mild-heat conditions (Sui et al. 2007b).
In rice leaves (Oryza sativa L.), the level of UFAs in PG have been genetically raised by introduction of cDNA for GPAT from spinach (Yokoi et al. 1998; Ariizumi et al. 2002). At 25 °C, transformed and non-transformed plants revealed similar activities of photosynthesis. However, at chilling temperature (14 °C), the photosynthetic activity of transgenic plants was higher than that of wild-type plants. Moreover, growth at chilling temperature of transgenic plants was greater than that of wild-type plants (Ariizumi et al. 2002). These results demonstrate the strict dependence of photosynthetic reactions on the lipid environment.
Recently, the leaves of transgenic tobacco plants with decreased levels of FA unsaturation in PG (Murata et al. 1992) have been investigated in terms of function and stability of the PSI reaction center P700 (Ivanov et al. 2012). The PSI photochemistry of wild-type plants was only slightly affected by high light treatments, whereas transgenic lines exhibited much higher susceptibility to photoinhibition of PSI. Thus, FA unsaturation and membrane fluidity play important roles in the function of both PSII and PSI in plant cells.
Freezing-induced lipid remodeling in chloroplast membranes
Moderate cold or chilling stress, which does not imply intracellular ice nucleation, causes metabolic rearrangements that result in activation of multiple pathways leading to acclimation: increases in intracellular solutes, the accumulation of cryoprotective metabolites and proteins, adjustments of membrane fluidity, etc. (Uemura et al. 1995; Nishida and Murata 1996). This process is known as cold acclimation. However, recent studies of plants exposed to freezing temperatures indicate a distinct mechanism required for freezing tolerance. Freezing destroys cellular and photosynthetic membranes due to ice formation, dehydration, the formation of non-bilayer lipid structures, their transition to gel phase. Thus, stabilization of membranes within the dehydrated plant cells is a key factor that determines the survival of plants under freezing.
Thylakoid membranes of plants and cyanobacteria contain high amounts of galactolipids (~50 % of MGDG and ~25 % of DGDG of total chloroplast glycerolipids) that are indispensable for the efficiency of photosynthetic light reactions. The stability of the thylakoid membrane bilayer is determined by the ratio of MGDG to DGDG (Shimojima and Ohta 2011). Under freezing, a certain proportion of MGDG is converted into DGDG, tri- or tetra-galactolipids by the galactolipid:galactolipid galactosyltransferase (GGGT) enzyme (Moellering and Benning 2011). The reaction of such transgalactosylation is accomplished by the transfer of the galactosyl moiety from one MGDG or DGDG to another. The GGGT-encoding gene was recently identified to be associated with the SENSITIVE TO FREEZING 2 (SFR2) phenotype of Arabidopsis (Moellering et al. 2010). It was proposed that a decrease in MGDG content accompanied by the proportional increase in oligogalactolipids leads to the remodeling of the thylakoids that prevents fissions and fusions of chloroplast membranes provoked by freezing-induced dehydration (Moellering and Benning 2011).
This hypothesis, for the involvement of GGGT in freezing tolerance, needs to be further tested experimentally. So far, the location of GGGT associated with the SFR phenotype has been restricted to the plastid envelope membranes. If the scenario of lipid remodeling is extrapolated to the whole cell and organism, one should expect the existence of lipid-transferase-like enzyme(s) associated with cytoplasmic and/or mitochondrial or nuclear membranes.
Membrane fluidization and heat sensing
Heat-induced gene expression has been studied in Synechocysis on a genome-wide level (Inaba et al. 2003; Suzuki et al. 2005). 59 genes were induced more than threefold in Synechocystis under heat stress (Suzuki et al. 2005). Mostly the genes were characterized by the transient mode of induction: they were highly induced by heat stress within 10–20 min, but 1 h after the onset of stress their transcription levels significantly declined. These genes encode proteins that belong mostly to the chaperone class (hspA, groESL, groEL2, dnaJ, htpG, dnaK2). Heat stress also induced the expression of htrA and clpB1genes for proteases, sigB for RNA polymerase sigma factor and hik34 for histidine kinase 34, as well as sodB for superoxide dismutase and hliC for high light-inducible protein C. The expression of most genes involved in energy and lipid metabolism, pigment biosynthesis and photosynthesis decreased under heat stress conditions (Suzuki et al. 2005).
Proteomic studies of heat shock response in Synechocystis revealed over 100 spots, corresponding to 65 different proteins alter heat shock (Slabas et al. 2006). Changes occured not only in the classical heat shock proteins but also in the protein biosynthetic machinery, amino acid biosynthetic enzymes, components of the light and dark acts of photosynthesis and energy metabolism. In particular, following heat shock treatment, there was a twofold increase in the level of the manganese-stabilizing protein, PsbO. Phycobilisome proteins decrease in abundance upon heat shock, and RUBISCO large subunit RbcL decreased over twofold (Slabas et al. 2006).
The majority of the heat-induced genes and proteins, the so-called HSPs, are not specific for heat stress, but they are also induced by osmotic, salt, as well as by oxidative stress, strong visible light and UV-B light (Los et al. 2008, 2010). Thus, these proteins should be rather considered as general stress proteins, GSPs, but not HSPs. In Synechocystis, the genes that are specifically induced by heat are limited to a short list of open reading frames with unknown function: sll0441, sll0688, sll1106, sll1884, slr0852, slr0095, and slr1597.
Heat stress causes membrane fluidization (Horváth et al. 2008). If we consider that the cold sensor is activated by rigidification of the plasma-membrane (Los and Murata 2004; Cybulski and de Mendoza 2011), one would suggest that a putative heat sensor in the membrane might be activated by its fluidization (Vigh et al. 1998; Mittler et al. 2012). However, no membrane-located candidate for a heat sensor has been identified in photosynthetic cells to date.
In S. cerevisiae, the engineered rigidification of the cytoplasmic membrane activated transcription of the hsp90 gene (Carratu et al. 1996). This demonstrates the dependence of the expression of HSPs on membrane fluidity. The supporting evidence was obtained in studies of the responses of Synechocystis cells to heat shock and to chemical membrane fluidizers. At normal growth temperatures, the membrane fluidizer benzyl alcohol activated transcription of the hspA gene as efficiently as heat stress (Horváth et al. 1998; Török et al. 2001). However, engineered rigidification of membranes in Synechocystis did not affect the heat-induced transcription of genes (Inaba et al. 2003). It should be taken into account that the membrane fluidity in the engineered strain with defective genes for the FADs may be compensated by some unknown mechanism, which differs from FA unsaturation (Mironov et al. 2012b). Thus, the consensus on heat sensing via membrane fluidization, at least, in cyanobacteria, still require additional studies.
The identified heat sensor of Synechocystis, the histidine kinase Hik34, is a soluble protein that negatively controls transcription of several GSP genes (Suzuki et al. 2005). Overexpressed and purified recombinant Hik34 was autophosphorylated in vitro at physiological temperatures, but not at elevated temperatures, such as 44 °C. Unlike wild-type cells, the knock-out mutant cells deficient in Hik34 survived incubation at 48 °C for 3 h. The Hik34-deficient mutant displayed the enhanced levels of transcripts of a number of GSP genes, including htpG, groESL1, and groEL2. Alternatively, overexpression of the Hik34 repressed transcription of these GSP genes (hspA, htpG, dnaK2, groESL1, and groEL2).
Two transcription factors, Sll1670 (HrcA) and Sll1130, negatively control transcription of GSP genes in Synechocystis (Nakamoto et al. 2003; Krishna et al. 2013). HrcA represses nearly the same set of genes (groESL operon and the groEL2 gene), which are under control of Hik34 (Kojima and Nakamoto 2007). Sll1130 represses htpG, hspA, isiA, isiB, frpC, rre5, and several hypothetical genes. Thus, the Hik34 and two transcription factors control the full set of GSP genes in Synechocystis. It is still unknown whether Hik34 and transcription factors HrcA and/or Sll1617 interact with each other in vivo.
Conclusions and perspectives
It has been postulated that a change in membrane fluidity might be the primary signal in the perception of cold stress that triggers the acclimatory responses in cells. Now, we have the example of how the sensory transmembrane histidine kinase may perceive the changes in the physical state of the biological membranes (Cybulski and de Mendoza 2011). The whole-genome and proteome studies in combination with the targeted mutagenesis and modifications of certain signal-transduction and biochemical pathways allow identification of stress sensors, transducers, individual genes, and groups of genes that are induced specifically and non-specifically under certain stress conditions.
The sensor proteins, such as DesK of B. subtilis or Hik33 of Synechocystis, may recognize the rigidification of membranes due to decrease in ambient temperature. Although, other mechanisms of cold signaling, which differ from classical two-component regulatory systems, may operate in cyanobacterial cells (Prakash et al. 2009), we still do not know the whole picture of this network. We do not know whether membrane-associated heat sensors exist in photosynthetic cells, and how such sensors are controlled by the membrane fluidity. Nothing is known about cold and heat sensors in higher plants. It is essential that efforts now be made to identify these molecules and the complete pathways of temperature perception and transduction in photosynthetic organisms.
Temperature sensors of plant cells remain to be identified. According to our knowledge on “cell thermometers,” the successful candidates might be found among protein kinases, ion (Ca2+?) channels, small RNAs, proteins that regulate the structure of chromatin (McClung and Davis 2010; Knight and Knight 2012).
Abbreviations
- ACP:
-
Acyl carrier protein
- DPH:
-
1,6-Diphenyl-1,3,5-hexatriene
- FA:
-
Fatty acid
- FAD:
-
Fatty acid desaturase
- FTIR spectroscopy:
-
Fourier transform infrared spectroscopy
- HSP:
-
Heat shock protein
- PS I:
-
Photosystem I
- PS II:
-
Photosystem II
- PUFA:
-
Polyunsaturated fatty acid
- UFA:
-
Unsaturated fatty acid
- TM:
-
Transmembrane
References
Aguilar PS, de Mendoza D (2006) Control of fatty acid desaturation: a mechanism conserved from bacteria to humans. Mol Microbiol 62:1507–1514. doi:10.1111/j.1365-2958.2006.05484.x
Aguilar PS, Hernandez-Arriaga AM, Cybulski LE, Erazo AC, de Mendoza D (2001) Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J 20:1681–1691. doi:10.1093/emboj/20.7.1681
Albanesi D, Mansilla MC, de Mendoza D (2004) The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator. J Bacteriol 186:2655–2663. doi:10.1128/JB.186.9.2655-2663.2004
Albanesi D, Martín M, Trajtenberg F, Mansilla MC, Haouz A, Alzari PM, de Mendoza D, Buschiazzo A (2009) Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc Natl Acad Sci USA 106:16185–16190. doi:10.1073/pnas.0906699106
Allakhverdiev SI, Murata N (2008) Salt stress inhibits photosystems II and I in cyanobacteria. Photosynth Res 98:529–539. doi:10.1007/s11120-008-9334-x
Allakhverdiev SI, Nishiyama Y, Suzuki I, Tasaka Y, Murata N (1999) Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress. Proc Natl Acad Sci USA 96:5862–5867. doi:10.1073/pnas.96.10.5862
Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N (2000) Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol 123:1047–1056. doi:10.1104/pp.123.3.1047
Allakhverdiev SI, Kinoshita M, Inaba M, Suzuki I, Murata N (2001) Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol 125:1842–1853. doi:10.1104/pp.125.4.1842
Ariizumi T, Kishitani S, Inatsugi R, Nishida I, Murata N, Toriyama K (2002) An increase in unsaturation of fatty acids in phosphatidylglycerol from leaves improves the rates of photosynthesis and growth at low temperatures in transgenic rice seedlings. Plant Cell Physiol 43:751–758. doi:10.1093/pcp/pcf087
Arondel V, Lemieux B, Hwang I, Gibson S, Goodman HM, Somerville CR (1992) Map-based cloning of a gene controlling omega-3 fatty acid desaturation in Arabidopsis. Science 258:1353–1355. doi:10.1126/science.1455229
Benedict C, Geisler M, Trygg J, Huner N, Hurry V (2006) Consensus by democracy. Using meta-analyses of microarray and genomic data to model the cold acclimation signaling pathway in Arabidopsis. Plant Physiol 141:1219–1232. doi:10.1104/pp.106.083527
Bossie MA, Martin CE (1989) Nutritional regulation of yeast Δ-9 fatty acid desaturase activity. J Bacteriol 171:6409–6413. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC210528/
Cahoon EB, Lindqvist Y, Schneider G, Shanklin J (1997) Redesign of soluble fatty acid desaturases from plants for altered substrate specificity and double bond position. Proc Natl Acad Sci USA 94:4872–4877. doi:10.1073/pnas.94.10.4872
Carratu L, Franceschelli S, Pardini CL, Kobayashi GS, Horváth I, Vigh L, Maresca B (1996) Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc Natl Acad Sci USA 93:3870–3875. doi:10.1073/pnas.93.9.3870
Cossins AR (1977) Adaptation of biological membranes to temperature. The effect of temperature acclimation of goldfish upon the viscosity of synaptosomal membranes. Biochim Biophys Acta 470:395–411. doi:10.1016/0005-2736(77)90131-6
Cybulski LE, de Mendoza D (2011) Playing with transmembrane signals. Commun Integr Biol. 4:69–71. doi:10.4161/cib.4.1.13778
Cybulski LE, Mansilla MC, Aguilar PS, de Mendoza D (2002) Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase. Mol Microbiol 45:1379–1388. doi:10.1046/j.1365-2958.2002.03103.x
Cybulski LE, Martín M, Mansilla MC, Fernández A, de Mendoza D (2010) Membrane thickness cue for cold sensing in a bacterium. Curr Biol 20:1539–1544. doi:10.1016/j.cub.2010.06.074
Digel I (2011) Primary thermosensory events in cells. Adv Exp Med Biol 704:451–468. doi:10.1007/978-94-007-0265-3_25
Dilley RA, Nishiyama Y, Gombos Z, Murata N (2001) Bioenergetic responses of Synechocystis 6803 fatty acid desaturase mutants at low temperatures. J Bioenerg Biomembr 33:135–141. doi:10.1023/A:1010752531909
Dyer JM, Mullen RT (2001) Immunocytological localization of two plant fatty acid desaturases in the endoplasmic reticulum. FEBS Lett 494:44–47. doi:10.1016/S0014-5793(01)02315-8
Espenshade PJ, Hughes AL (2007) Regulation of sterol synthesis in eukaryotes. Annu Rev Genet 41:401–427. doi:10.1146/annurev.genet.41.110306.130315
Falcone DL, Gibson S, Lemieux B, Somerville CR (1994) Identification of a gene that complements an Arabidopsis mutant deficient in chloroplast ω6 desaturase activity. Plant Physiol 106:1453–1459. doi:10.1104/pp.106.4.1453
Feng Y, Cronan JE (2009) Escherichia coli unsaturated fatty acid synthesis. Complex transcription of the fabA gene and in vivo identification of the essential reaction catalyzed by FabB. J Biol Chem 284:29526–29535. doi:10.1074/jbc.M109.023440
Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold-response pathway. Plant Cell 14:1675–1690. doi:10.1105tpc.003483
Frentzen M, Nishida I, Murata N (1987) Properties of the plastidial acyl-(acyl-carrier protein): glycerol-3-phosphate acyltransferase from the chilling-sensitive plant squash (Cucurbita moschata). Plant Cell Physiol 28:1195–1201. http://pcp.oxfordjournals.org/content/28/7/1195.abstract
Gao J, Ajjawi I, Manoli A, Sawin A, Xu C, Froehlich JE, Last RL, Benning C (2009) FATTY ACID DESATURASE4 of Arabidopsis encodes a protein distinct from characterized fatty acid desaturases. Plant J 60:832–839. doi:10.1111/j.1365-313X.2009.04001.x
Gibson S, Arondel V, Iba K, Somerville C (1994) Cloning of a temperature-regulated gene encoding a chloroplast ω-3 desaturase from Arabidopsis thaliana. Plant Physiol 106:1615–1621. doi:10.1104/pp.106.4.1615
Glatz A, Vass I, Los DA, Vigh L (1998) The Synechocystis model of stress: from molecular chaperones to membranes. Plant Physiol Biochem 37:1–12. doi:10.1016/S0981-9428(99)80061-8
Gombos Z, Kanervo E, Tsvetkova N, Sakamoto T, Aro EM, Murata N (1997) Genetic enhancement of the ability to tolerate photoinhibition by introduction of unsaturated bonds into membrane glycerolipids. Plant Physiol 115:551–559. doi:10.1104/pp.115.2.551
Gutensohn M, Fan E, Frielingsdorf S, Hanner P, Hou B, Hust B, Klösgen RB (2006) Toc, Tic, Tat et al.: structure and function of protein transport machineries in chloroplasts. J Plant Physiol 163:333–347. doi:10.1016/j.jplph.2005.11.009
Guy JE, Whittle E, Kumaran D, Lindqvist Y, Shanklin J (2007) The crystal structure of the ivy Δ4–16:0-ACP desaturase reveals structural details of the oxidized active site and potential determinants of regioselectivity. J Biol Chem 282:19863–19871. doi:10.1074/jbc.M702520200
Guy JE, Whittle E, Moche M, Lengqvist J, Lindqvist Y, Shanklin J (2011) Remote control of regioselectivity in acyl–acyl carrier protein-desaturases. Proc Natl Acad Sci USA 108:16594–16599. doi:10.1073/pnas.1110221108
Hakala M, Tuominen I, Keränen M, Tyystjärvi T, Tyystjärvi E (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of photosystem II. Biochim Biophys Acta 1706:68–80. doi:10.1016/j.bbabio.2004.09.001
Hartmann MA (1998) Plant sterols and membrane environment. Trends Plant Sci 3:170–175. doi:10.1016/S1360-1385(98)01233-3
Harwood JL (2007) Temperature stress: reacting and adapting: lessons from poikilotherms. Ann NY Acad Sci 1113:52–57. doi:10.1196/annals.1391.025
Hazel JR (1995) Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol 57:19–42. doi:10.1146/annurev.ph.57.030195.000315
Heilmann I, Mekhedov S, King B, Browse J, Shanklin J (2004) Identification of the Arabidopsis palmitoyl-monogalactosyldiacylglycerol Δ7-desaturase gene FAD5, and effects of plastidial retargeting of Arabidopsis desaturases on the fad5 mutant phenotype. Plant Physiol 136:4237–4245. doi:10.1104/pp.104.052951
Heipieper HJ, Meinhardt F, Segura A (2003) The cis-trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: biochemistry, molecular biology and physiological function of a unique stress-adaptive mechanism. FEMS Microbiol Lett 229:1–7. doi:10.1016/S0378-1097(03)00792-4
Higashi S, Murata N (1993) Desaturases and acyltransferases in lipid synthesis in Synechocystis PCC 6803. Plant Physiol 102:1275–1278. doi:10.1104/pp.102.4.1275
Horváth I, Glatz A, Varvasovszki V, Török Z, Pali T, Balogh G, Kovacs E, Nadasdi L, Benko S, Joo F, Vigh L (1998) Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci USA 95:3513–3518. doi:10.1073/pnas.95.7.3513
Horváth I, Multhoff G, Sonnleitner A, Vígh L (2008) Membrane-associated stress proteins: more than simply chaperones. Biochim Biophys Acta 1778:1653–1664. doi:10.1016/j.bbamem.2008.02.012
Horváth I, Glatz A, Nakamoto H, Mishkind ML, Munnik T, Saidi Y, Goloubinoff P, Harwood JL, Vigh L (2012) Heat shock response in photosynthetic organisms: membrane and lipid connections. Prog Lipid Res 51:208–220. doi:10.1016/j.plipres.2012.02.002
Hugly S, Kunst L, Browse J, Somerville C (1989) Enhanced thermal tolerance of photosynthesis and altered chloroplast ultrastructure in a mutant of Arabidopsis deficient in lipid desaturation. Plant Physiol 90:1134–1142. doi:10.1104/pp.90.3.1134
Iba K, Gibson S, Nishiuchi T, Fuse T, Nishimura M, Arondel V, Hugly S, Somerville C (1993) A gene encoding a chloroplast ω-3 fatty acid desaturase complements alterations in fatty acid desaturation and chloroplast copy number of the fad7 mutant of Arabidopsis thaliana. J Biol Chem 268: 24099–24105. http://www.jbc.org/content/268/32/24099.full.pdf+html
Inaba M, Suzuki I, Szalontai B, Kanesaki Y, Los DA, Hayashi H, Murata N (2003) Gene-engineered rigidification of membrane lipids enhances the cold inducibility of gene expression in Synechocystis. J Biol Chem 278:12191–12198. doi:10.1074/jbc.M212204200
Ishizaki-Nishizawa O, Fujii T, Azuma M, Sekiguchi K, Murata N, Ohtani T, Toguri T (1996) Low-temperature resistance of higher plants is significantly enhanced by a nonspecific cyanobacterial desaturase. Nature Biotechnol 14:1003–1006. doi:10.1038/nbt0896-1003
Ivanov AG, Allakhverdiev SI, Huner NPA, Murata N (2012) Genetic decrease in fatty acid unsaturation of phosphatidylglycerol increased photoinhibition of photosystem I at low temperature in tobacco leaves. Biochim Biophys Acta 1817:1374–1379. doi:10.1016/j.bbabio.2012.03.010
Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF (2001) A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA 98:9448–9453. doi: 10.1073/pnas.151258398
Kachroo A, Shanklin J, Whittle E, Lapchyk L, Hildebrand D, Kachroo P (2007) The Arabidopsis stearoyl-acyl carrier protein-desaturase family and the contribution of leaf isoforms to oleic acid synthesis. Plant Mol Biol 63:257–271. doi:10.1007/s11103-006-9086-y
Kanervo E, Aro EM, Murata N (1995) Low unsaturation level of thylakoid membrane lipids limits turnover of the D1 protein of photosystem II at high irradiance. FEBS Lett 364:239–242. doi:10.1016/0014-5793(95)00404-W
Kanervo E, Tasaka Y, Murata N, Aro EM (1997) Membrane lipid unsaturation modulates processing of the photosystem II reaction-center protein D1 at low temperatures. Plant Physiol 114:841–849. doi:10.1104/pp.114.3.841
Kappell AD, van Waasbergen LG (2007) The response regulator RpaB binds the high light regulatory 1 sequence upstream of the high-light-inducible hliB gene from the cyanobacterium Synechocystis PCC 6803. Arch Microbiol 187:337–342
Knight MR, Knight H (2012) Low temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol 195:737–751. doi:10.1111/j.1469-8137.2012.04239.x
Kojima K, Nakamoto H (2007) A novel light- and heat-responsive regulation of the groE transcription in the absence of HrcA or CIRCE in cyanobacteria. FEBS Lett 581:1871–1880. doi:10.1016/j.febslet.2007.03.084
Krishna PS, Rani BR, Mohan MK, Suzuki I, Shivaji S, Prakash JS (2013) A novel transcriptional regulator, Sll1130, negatively regulates heat-responsive genes in Synechocystis sp. PCC6803. Biochem J 449:751–760. doi:10.1042/BJ20120928
Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, Franke RB, Graham IA, Katayama K, Kelly AA, Larson T, Markham JE, Miquel M, Molina I, Nishida I, Rowland O, Samuels L, Schmid KM, Wada H, Welti R, Xu C, Zallot R, Ohlrogge J (2013) Acyl-lipid metabolism. Arabidopsis Book 11:e0161. doi:10.1199/tab.0133
Liu X-Y, Li B, Yang J-H, Sui N, Yang X-M, Meng Q-W (2008) Overexpression of tomato chloroplast omega-3 fatty acid desaturase gene alleviates the photoinhibition of photosystems 2 and 1 under chilling stress. Photosynthetica 46:185–192. doi:10.1007/s11099-008-0030-z
Los DA, Murata N (1998) Structure and expression of fatty acid desaturases. Biochim Biophys Acta 1394:3–15. doi:10.1016/S0005-2760(98)00091-5
Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochim Biophys Acta 1666:142–157. doi:10.1016/j.bbamem.2004.08.002
Los DA, Zinchenko VV (2009) Regulatory role of membrane fluidity in gene expression. In: Wada H, Murata N (eds) Lipids in photosynthesis. Essential and regulatory functions. Springer Science, Dordrecht, pp 329–348. doi:10.1007/978-90-481-2863-1_15
Los DA, Ray MK, Murata N (1997) Differences in the control of the temperature-dependent expression of four genes for desaturases in Synechocystis sp. PCC 6803. Mol Microbiol 25:1167–1175. doi:10.1046/j.1365-2958.1997.5641912.x
Los DA, Suzuki I, Zinchenko VV, Murata N (2008) Stress responses in Synechocystis: regulated genes and regulatory systems. In: Herrero A, Flores E (eds) The cyanobacteria: molecular biology, genomics and evolution. Caister Academic Press, Norfolk, pp 117–157
Los DA, Zorina A, Sinetova M, Kryazhov S, Mironov K, Zinchenko VV (2010) Stress sensors and signal transducers in cyanobacteria. Sensors 10:2386–2415. doi:10.3390/s100302386
Lyons JM, Raison JK (1970) Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol 45:386–389. doi:10.1104/pp.45.4.386
Ma X, Browse J (2006) Altered rates of protein transport in Arabidopsis mutants deficient in chloroplast membrane unsaturation. Phytochemistry 67:1629–1636. doi:10.1016/j.phytochem.2006.04.008
Macartney AI, Maresca B, Cossins AR (1994) Acyl–CoA desaturases and the adaptive regulation of membrane lipid composition. In: Cossins AR (ed) Temperature adaptation of biological membranes. Portland, London, pp 129–139
Mansilla MC, de Mendoza D (2005) The Bacillus subtilis desaturase: a model to understand phospholipid modification and temperature sensing. Arch Microbiol 183:229–235. doi:10.1007/s00203-005-0759-8
Mansilla MC, Cybulsky LE, Albanesi D, de Mendoza D (2004) Control of membrane fluidity by molecular thermosensors. J Bacteriol 186:6681–6688. doi:10.1128/JB.186.20.6681-6688.2004
Maresca B, Kobayashi G (1993) Changes in membrane fluidity modulate heat shock gene expression and produced attenuated strains in the dimorphic fungus Histoplasma capsulatum. Arch Med Res 24:247–249
Martin CA, Milinsk MA, Visentainer JV, Matsishita M, De-Souza NE (2007) Trans fatty acid-forming processes in foods: a review. Ann Brazil Acad Sci 79:343–350. doi:10.1590/S0001-37652007000200015
Maruyama K, Takeda M, Kidokoro S, Yamada K, Sakuma Y, Urano K, Fujita M, Yoshiwara K, Matsukura S, Morishita Y, Sasaki R, Suzuki H, Saito K, Shibata D, Shinozaki K, Yamaguchi-Shinozaki K (2009) Metabolic pathways involved in cold acclimation identified by integrated analysis of metabolites and transcripts regulated by DREB1A and DREB2A. Plant Physiol 150:1972–1980. doi:10.1104/pp.109.135327
Matsuda O, Sakamoto H, Hashimoto T, Iba K (2005) A temperature-sensitive mechanism that regulates post-translational stability of a plastidial ω-3 fatty acid desaturase (FAD8) in Arabidopsis leaf tissues. J Biol Chem 280:3597–3604. doi:10.1074/jbc.M407226200
Matsui A, Ishida J, Morosawa T, Okamoto M, Kim JM, Kurihara Y, Kawashima M, Tanaka M, To TK, Nakaminami K, Kaminuma E, Endo TA, Mochizuki Y, Kawaguchi S, Kobayashi N, Shinozaki K, Toyoda T, Seki M (2010) Arabidopsis tiling array analysis to identify the stress-responsive genes. Methods Mol Biol 639:141–155. doi:10.1007/978-1-60761-702-0_8
McCartney AW, Dyer JM, Dhanoa PK, Kim PK, Andrews DW, McNew JA, Mullen RT (2004) Membrane-bound fatty acid desaturases are inserted co-translationally into the ER and contain different ER retrieval motifs at their carboxy termini. Plant J 37:156–173. doi:10.1111/j.1365-313X.2004.01949.x
McClung CR, Davis SJ (2010) Ambient thermometers in plants: from physiological outputs towards mechanisms of thermal sensing. Curr Biol 20:R1086–R1092. doi:10.1016/j.cub.2010.10.035
Michaelson LV, Zäuner S, Markham JE, Haslam RP, Desikan R, Mugford S, Albrecht S, Warnecke D, Sperling P, Heinz E, Napier JA (2009) Functional characterization of a higher plant sphingolipid Δ4-desaturase: defining the role of sphingosine and sphingosine-1-phosphate in Arabidopsis. Plant Physiol 149:487–498. doi:10.1104/pp.108.129411
Mironov KS, Maksimov EG, Maksimov GV, Los DA (2012a) Feedback between fluidity of membranes and transcription of the desB gene for the ω3-desaturase in the cyanobacterium Synechocystis. Mol Biol 46:134–141. doi:10.1134/S002689331201013X. PMID: 22642112
Mironov KS, Sidorov RA, Trofimova MS, Bedbenov VS, Tsydendambaev VS, Allakhverdiev SI, Los DA (2012b) Light-dependent cold-induced fatty acid unsaturation, changes in membrane fluidity, and alterations in gene expression in Synechocystis. Biochim Biophys Acta 1817:1352–1359. doi:10.1016/j.bbabio.2011.12.011
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37:118–125. doi:10.1016/j.tibs.2011.11.007
Moellering ER, Benning C (2011) Galactoglycerolipid metabolism under stress: a time for remodeling. Trends Plant Sci 16:98–107. doi:10.1016/j.tplants.2010.11.004
Moellering ER, Muthan B, Benning C (2010) Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330:226–228. doi:10.1126/science.1191803
Moon BY, Higashi S, Gombos Z, Murata N (1995) Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc Natl Acad Sci USA 92:6219–6223. doi:10.1073/pnas.92.14.6219
Mullineaux CW, Kirchhoff H (2009) Role of lipids in the dynamics of thylakoid membranes. In: Wada H, Murata N (eds) Lipids in photosynthesis essential and regulatory function. Springer Science, Dordrecht, pp 283–294. doi:10.1007/978-90-481-2863-1_13
Murata N, Los DA (1997) Membrane fluidity and temperature perception. Plant Physiol 115:875–879. doi:10.1104/pp.115.3.875
Murata N, Los DA (2006) Histidine kinase Hik33 is an important participant in cold signal transduction in cyanobacteria. Physiol Plant 126:17–27. doi:10.1111/j.1399-3054.2005.00608.x
Murata N, Ishizaki-Nishizawa O, Higashi S, Hayashi H, Tasaka Y, Nishida I (1992) Genetically engineered alteration in the chilling sensitivity of plants. Nature 356:710–713. doi:10.1038/356710a0 357: 607 (correction)
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421. doi:10.1016/j.bbabio.2006.11.019
Mustardy L, Los DA, Gombos Z, Murata N (1996) Immunocytochemical localization of acyl-lipid desaturases in cyanobacterial cells: evidence that both thylakoid membranes and cytoplasmic membranes are sites of lipid desaturation. Proc Natl Acad Sci USA 93:10524–10527. doi:10.1073/pnas.93.19.10524
Najle SR, Inda ME, de Mendoza D, Cybulski LE (2009) Oligomerization of Bacillus subtilis DesR is required for fine tuning regulation of membrane fluidity. Biochim Biophys Acta 1790:1238–1243. doi:10.1016/j.bbagen.2009.07.002
Nakamoto H, Suzuki M, Kojima K (2003) Targeted inactivation of the hrcA repressor gene in cyanobacteria. FEBS Lett 549:57–62. doi:10.1016/S0014-5793(03),00768-3
Nishida I, Murata N (1996) Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol 47:541–568. doi:10.1146/annurev.arplant.47.1.541
Nishida I, Frentzen M, Ishizaki O, Murata N (1987) Purification of isomeric forms of acyl-[acyl-carrier-protein]:glycerol-3-phosphate acyltransferase from greening squash cotyledons. Plant Cell Physiol 28:1071–1079
Nishida I, Tasaka Y, Shiraishi H, Murata N (1993) The gene and the RNA for the precursor to the plastid-located glycerol-3-phosphate acyltransferase of Arabidopsis thaliana. Plant Mol Biol 21:267–277. doi:10.1007/BF00019943
Nishiyama Y, Allakhverdiev SI, Murata N (2011) Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant 142:35–46. doi:10.1111/j.1399-3054.2011.01457.x
Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) The two-step mechanism of photodamage to photosystem II: step one occurs at the oxygen-evolving complex and step two occurs at the photochemical reaction center. Biochemistry 44:8494–8499. doi:10.1021/bi047518q
Okuley J, Lightner J, Feldmann K, Yadav N, Lark E, Browse J (1994) Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 6:147–158. doi:10.1105/tpc.6.1.147
Okuyama H, Okajima N, Sasaki S, Higashi S, Murata N (1991) The cis/trans isomerization of the double bond of a fatty acid as a strategy for adaptation to changes in ambient temperature in the psychrophilic bacterium, Vibrio sp. strain ABE-1. Biochim Biophys Acta 1084:3–20. doi:10.1016/0005-2760(91)90049-N PMID: 2054374
Orlova IV, Serebriiskaya TS, Popov V, Merkulova N, Nosov AM, Trunova TI, Tsydendambaev VD, Los DA (2003) Transformation of tobacco with a gene for the thermophilic acyl-lipid desaturase enhances the chilling tolerance of plants. Plant Cell Physiol 44:447–450. doi:10.1093/pcp/pcg047
Orvar BL, Sangwan V, Omann F, Dhindsa RS (2000) Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J 23:785–794. doi:10.1046/j.1365-313x.2000.00845.x
Popova AV, Velitchkova M, Zanev Y (2007) Effect of membrane fluidity on photosynthetic oxygen production reactions. Z Naturforsch C 62:253–260
Porta A, Eletto A, Török Z, Franceschelli S, Glatz A, Vígh L, Maresca B (2010a) Changes in membrane fluid state and heat shock response cause attenuation of virulence. J Bacteriol 192:1999–2005. doi:10.1128/JB.00990-09
Porta A, Török Z, Horvath I, Franceschelli S, Vígh L, Maresca B (2010b) Genetic modification of the Salmonella membrane physical state alters the pattern of heat shock response. J Bacteriol 192:1988–1998. doi:10.1128/JB.00988-09
Prakash JSS, Sinetova M, Kupriyanova E, Zorina A, Suzuki I, Murata N, Los DA (2009) DNA supercoiling regulates the stress-inducible expression of genes in the cyanobacterium. Mol BioSyst 5:1904–1912. doi:10.1039/B903022k
Rodríguez-Vargas S, Sánchez-García A, Martínez-Rivas JM, Prieto JA, Randez-Gil F (2007) Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl Environ Microbiol 73:110–116. doi:10.1128/AEM.01360-06
Ruelland E, Zachowski A (2010) How plants sense temperature? Environ Exp Bot 69:225–232. doi:10.1016/j.envexpbot.2010.05.011
Ryan PR, Liu Q, Sperling P, Dong B, Franke S, Delhaize E (2007) A higher plant Δ8 sphingolipid desaturase with a preference for (Z)-isomer formation confers aluminum tolerance to yeast and plants. Plant Physiol 144:1968–1977. doi:10.1104/pp.107.100446
Sakamoto A, Sulpice R, Hou CX, Kinoshita M, Higashi S, Kanaseki T, Nonaka H, Moon BY, Murata N (2003) Genetic modification of fatty acid unsaturation of phosphatidylglycerol in chloroplasts alters the sensitivity of tobacco plants to cold stress. Plant Cell Environ 27:99–105. doi:10.1046/j.0016-8025.2003.01131.x
Sangwan V, Orvar BL, Beyerly J, Hirt H, Dhindsa RS (2002) Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J 31:629–638. doi:10.1046/j.1365-313X.2002.01384.x
Sarcina M, Murata N, Tobin MJ, Mullineaux CW (2003) Lipid diffusion in the thylakoid membranes of the cyanobacterium Synechococcus sp.: Effect of fatty acid desaturation. FEBS Lett 553:295–298. doi:10.1016/S0014-5793(03)01031-7
Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K (2001) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 13:61–72. doi:10.1105/tpc.13.1.61
Shimojima M, Ohta H (2011) Critical regulation of galactolipid synthesis controls membrane differentiation and remodeling in distinct plant organs and following environmental changes. Prog Lipid Res 50:258–266. doi:10.1016/j.plipres.2011.03.001
Shimojima M, Ohta H, Nakamura Y (2009) Biosynthesis and function of chloroplast lipids. In: Wada H, Murata N (eds) Lipids in photosynthesis. Essential and regulatory functions. Springer Science, Dordrecht, pp 35–55. doi:10.1007/978-90-481-2863-1_3
Shimura Y, Shiraiwa Y, Suzuki I (2012) Characterization of the subdomains in the N-terminal region of histidine kinase Hik33 in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 53:1255–1266. doi:10.1093/pcp/pcs068
Sinensky M (1974) Homeoviscous adaptation: a homeostatic process that regulates viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA 71:522–525. doi:10.1073/pnas.71.2.522
Sippola K, Kanervo E, Murata N, Aro EM (1998) A genetically engineered increase in fatty acid unsaturation in Synechococcus sp. PCC 7942 allows exchange of D1 protein forms and sustenance of photosystem II activity at low temperature. Eur J Biochem 251:641–648. doi:10.1046/j.1432-1327.1998.2510641.x
Slabas AR, Suzuki I, Murata N, Simon WJ, Hall JJ (2006) Proteomic analysis of the heat shock response in Synechocystis PCC6803 and a thermally tolerant knockout strain lacking the histidine kinase 34 gene. Proteomics 6:845–864. doi:10.1002/pmic.200500196
Smith MA, Dauk M, Ramadan H, Yang H, Seamons LE, Haslam RP, Beaudoin F, Ramirez-Erosa I, Forseille L (2013) Involvement of Arabidopsis ACYL-COENZYME A DESATURASE-LIKE2 (At2g31360) in the biosynthesis of the very-long-chain monounsaturated fatty acid components of membrane lipids. Plant Physiol 16:81–96. doi:10.1104/pp.112.202325
Sui N, Li M, Zhao SJ, Li F, Liang H, Meng QW (2007a) Overexpression of glycerol-3-phosphate acyltransferase gene improves chilling tolerance in tomato. Planta 226:1097–1108. doi:10.1007/s00425-007-0554-7
Sui N, Li M, Shu DF, Zhao SJ, Meng QW (2007b) Antisense-mediated depletion of tomato chloroplast glycerol-3-phosphate acyltransferase affects male fertility and increases thermal tolerance. Physiol Plant 130:301–314. doi:10.1111/j.1399-3054.2007.00907.x
Suzuki I, Los DA, Kanesaki Y, Mikami K, Murata N (2000) The pathway for perception and transduction of low-temperature signals in Synechocystis. EMBO J 19:1327–1334. doi:10.1093/emboj/19.6.1327
Suzuki I, Kanesaki Y, Mikami K, Kanehisa M, Murata N (2001) Cold-regulated genes under control of the cold sensor Hik33 in Synechocystis. Mol Microbiol 40:235–244. doi:10.1046/j.1365-2958.2001.02379.x
Suzuki I, Kanesaki Y, Hayashi H, Hall JJ, Simon WJ, Slabas AR, Murata N (2005) The histidine kinase Hik34 is involved in thermotolerance by regulating the expression of heat shock genes in Synechocystis. Plant Physiol 138:1409–1421. doi:10.1104/pp.104.059097
Szalontai B, Nishiyama Y, Gombos Z, Murata N (2000) Membrane dynamics as seen by Fourier transform infrared spectroscopy in a cyanobacterium, Synechocystis PCC 6803: the effects of lipid unsaturation and the protein-to-lipid ratio. Biochim Biophys Acta 1509:409–419. doi:10.1016/S0005-2736(00)00323-0
Szalontai B, Kota Z, Nonaka H, Murata N (2003) Structural consequences of genetically engineered saturation of the fatty acids of phosphatidylglycerol in tobacco thylakoid membranes. An FTIR study. Biochemistry 42:4292–4299. doi:10.1021/bi026894c
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13:178–182. doi:10.1016/j.tplants.2008.01.005
Tang G-Q, Novitzky WP, Griffin HC, Huber SC, Dewey RE (2005) Oleate desaturase enzymes of soybean: evidence of regulation through differential stability and phosphorylation. Plant J 44:433–446. doi:10.1111/j.1365-313X.2005.02535.x
Tasaka Y, Gombos Z, Nishiyama Y, Mohanty P, Ohba T, Ohki K, Murata N (1996) Targeted mutagenesis of acyl-lipid desaturases in Synechocystis: evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. EMBO J 15:6416–6425 PMCID: PMC452467
Thewke D, Kramer M, Sinensky MS (2003) Transcriptional homeostatic control of membrane lipid composition. Biochem Biophys Res Commun 273:1–4. doi:10.1006/bbrc.2000.2826
Thomashow MF (2010) Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol 154:571–577. doi:10.1104/pp.110.161794
Tiku PE, Gracey AY, Macartney AI, Beynon RJ, Cossins AR (1996) Cold-induced expression of Δ9-desaturase in carp by transcriptional and posttranslational mechanisms. Science 271:815–818. doi:10.1126/science.271.5250.815
Török Z, Goloubinoff P, Horváth I, Tsvetkova NM, Glatz A, Balogh G, Varvasovszki V, Los DA, Vierling E, Crowe JH, Vigh L (2001) Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc Natl Acad Sci USA 98:3098–3103. doi:10.1073/pnas.051619498
Uemura M, Joseph RA, Steponkus PL (1995) Cold acclimation of Arabidopsis thaliana: effect on plasma membrane lipid composition and freeze-induced lesions. Plant Physiol 109:15–30. doi:10.1104/pp.109.1.15
Vaultier MN, Cantrel C, Vergnolle C, Justin AM, Demandre C, Benhassaine-Kesri G, Ciçek D, Zachowski A, Ruelland E (2006) Desaturase mutants reveal that membrane rigidification acts as a cold perception mechanism upstream of the diacylglycerol kinase pathway in Arabidopsis cells. FEBS Lett 580:4218–4223. doi:10.1016/j.febslet.2006.06.083
Velitchkova M, Lazarova D, Popova A (2009) Response of isolated thylakoid membranes with altered fluidity to short term heat stress. Physiol Mol Biol Plants 15:43–52. doi: 10.1007/s12298-009-0004-z. http://springerlink.bibliotecabuap.elogim.com/article/10.1007%2Fs12298-009-0004-z
Vigh L, Los DA, Horváth I, Murata N (1993) The primary signal in the biological perception of temperature: Pd-catalyzed hydrogenation of membrane lipids stimulated the expression of the desA gene in Synechocystis PCC6803. Proc Natl Acad Sci USA 90:9090–9094. doi:10.1073/pnas.90.19.9090
Vigh L, Maresca B, Harwood JL (1998) Does the membrane’s physical state control the expression of heat shock and other genes? Trends Biochem Sci 23:369–374. doi:10.1016/S0968-0004(98)01279-1
Vijayan P, Browse J (2002) Photoinhibition in mutants of Arabidopsis deficient in thylakoid unsaturation. Plant Physiol 129:876–885. doi:10.1104/pp.004341
Wada H, Gombos Z, Murata N (1990) Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature 347:200–203. doi:10.1038/347200a0
Wallis JG, Browse J (2002) Mutants of Arabidopsis reveal many roles for membrane lipids. Prog Lipid Res 41:254–278. doi:10.1016/S0163-7827(01)00027-3
Yokoi S, Higashi S, Kishitani S, Murata N, Toriyama K (1998) Introduction of the cDNA for Arabidopsis glycerol-3-phosphate acyltransferase (GPAT) confers unsaturation of fatty acids and chilling tolerance of photosynthesis on rice. Mol Breed 4:269–275. doi:10.1023/A:1009671231614
Zeller G, Henz SR, Widmer CK, Sachsenberg T, Rätsch G, Weigel D, Laubinger S (2009) Stress-induced changes in the Arabidopsis thaliana transcriptome analyzed using whole-genome tiling arrays. Plant J. 58:1068–1082. doi:10.1111/j.1365-313X.2009.03835.x
Zhang J, Liu H, Sun J, Li B, Zhu Q, Chen S, Zhang H (2012) Arabidopsis fatty acid desaturase FAD2 is required for salt tolerance during seed germination and early seedling growth. PLoS One 7:e30355. doi:10.1371/journal.pone.0030355
Acknowledgments
This work was supported, in part, by Grants from the Russian Foundation for Basic Research (Nos. 11-04-01389, 11-04-92101, 12-04-00473, 12-04-31496) and by Grants from the “Molecular and Cell Biology Program” of the Russian Academy of Sciences to DAL and SIA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Los, D.A., Mironov, K.S. & Allakhverdiev, S.I. Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth Res 116, 489–509 (2013). https://doi.org/10.1007/s11120-013-9823-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-013-9823-4