Abstract
The role of the marine elasmobranch gastrointestinal tract in nitrogen-recycling and osmotic homeostasis has become increasingly apparent, with the gut microbial community likely playing a significant role converting urea, an important osmolyte in elasmobranchs, into ammonia. The Pacific spiny dogfish can experience and tolerate reduced environmental salinities, yet how this environmental challenge may affect the microbiome, and consequently nitrogen transport across the gut, is as of yet unknown. In the present study, excised gut sac preparations were made from dogfish acclimated to the following: full-strength seawater (C), low salinity for 7 days (LS), and after acute transfer of LS-acclimated fish to full-strength SW for 6 h (AT). Significantly reduced microbial derived urease activity was observed in the mucosal saline of gut sac preparations from the LS (by 81%) and AT (by 89%) treatments relative to the C treatment. Microbial derived cellulase activity from mucosal saline samples tended to follow similar patterns. To further ensure an effective decrease in the spiral valve microbial population, an antibiotic cocktail was applied to the mucosal saline used for in vitro measurements of ion, water, and nitrogen flux in these gut sac preparations. This caused a further 57–61% decrease in the mucosal saline urease activity of the C and LS treatments. Overall, we observed relatively little flux across the stomach for all measured parameters aside from water movement, which switched from a net efflux in control fish to a net influx in acutely transferred fish, indicative of drinking. While no significant differences were observed in terms of nitrogen flux (urea or ammonia), we tended to see the accumulation of ammonia in the spiral valve lumen and a switch from efflux to influx of urea in control versus acutely transferred fish. The increased ammonia production likely occurs as a result of heightened metabolism in a challenging environment, while the retention and acquisition of urea is suggestive of nitrogen scavenging under nitrogen-limiting conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine elasmobranchs are hypo-ionic osmoconformers with Na+ and Cl− ions contributing 50–60% of total osmolality (250–300 mM), while the remaining osmotic gap is primarily filled by urea (~ 350 mM) comprising 30–40% of total osmolality (Smith 1936). Although urea is often an ‘incompatible’ and toxic solute owing to its destabilization of proteins, trimethylamine oxide (TMAO) mitigates these effects and contributes another 5–10% to plasma osmolality (Yancey et al. 1982; Yancey 2015). In fact, where other organisms would experience toxic effects, some marine elasmobranchs require a minimum threshold of urea for normal bodily functions (e.g. 200 mM for a heartbeat, Mines 1912; Simpson and Ogden 1932). A subset of marine elasmobranchs (~ 20%) can tolerate dilution of their surrounding environment (Martin 2005) and, as osmoconformers these elasmobranchs will primarily offload urea and minimize ion loss to maintain intracellular ion homeostasis (Smith 1936; Goldstein and Forster 1971; Payan et al. 1973). Conversely, in hypersaline environments a rapid acquisition or synthesis of urea is required following an increase in ions, in order to rectify plasma osmolality (Hazon and Henderson 1985; Armour et al. 1993; Anderson et al. 2002, 2005).
Coordinated actions of the classic osmoregulatory organs in elasmobranchs (i.e. gills, kidney, and rectal gland) are required to combat these environmental challenges and to maintain osmotic and nitrogen homeostasis in these ureosmotic organisms (Burger and Hess 1960; Burger 1965; Boylan 1967; Goldstein and Forster 1971; Payan et al. 1973, Wood et al. 1995; reviewed in Hazon et al. 2003). The role of the intestine, however, has often been overlooked since osmoconformers were thought not to drink (Smith 1936). Yet, fasting marine elasmobranchs do drink (0.1–0.16 mL/kg/h; Webb and Wood 2000; De Boeck et al. 2001; Anderson et al. 2002) and drinking rates have been shown to increase during acclimation to increased environmental salinity (Anderson et al. 2002). In addition to the ion and water regulation that accompanies drinking, the role of the intestine in nitrogen acquisition has become increasingly apparent in recent years. The gastrointestinal tract (GIT) of fasting elasmobranchs absorbs urea in all regions apart from the spiral valve (i.e. intestine), where a net secretion is observed (Liew et al. 2013). Postprandially, however, there is a significant resorption of urea from the spiral valve (Liew et al. 2013), a component of which is thought to occur via active transport using phloretin-sensitive, sodium-coupled urea transporters (UT; Anderson et al. 2015). Nitrogen can also be acquired in the form of ammonia and may well be favoured over urea given its relatively higher metabolic potential (Wood et al. 2019). Indeed, in the little skate (Raja erinacea) GIT, ammonia transport proteins (Rhbg) are expressed at orders of magnitude above that of UT (Anderson et al. 2012), emphasizing the relative importance of ammonia compared to urea as a nitrogenous compound to a marine elasmobranch.
The carnivorous diet of elasmobranchs supplies ammonia as the result of protein catabolism (Bucking 2017), but additional ammonia could be derived from ureolytic bacteria within the GIT (Wood et al. 2019). The vertebrate microbiome is composed of Protista, Fungi, Archaea, and Bacteria, which together enhance nutrient acquisition and disease resistance (reviewed in Egerton et al. 2018). Although few studies have examined elasmobranch microbiomes (Grimes et al. 1985; Givens et al. 2015), early studies demonstrated that antibiotic application eliminated ammonia production in the gut of R. erinacea (Goldstein and Dewitt-Harley 1972). More recently, GIT microbial urease (responsible for converting urea into ammonia) has been identified and shown to increase with feeding in the spiny dogfish (Wood et al. 2019). The nitrogen dependence of this community provides reasoning for the observed urea loss during fasting in these nitrogen-limited animals (Liew et al. 2013; Wood et al. 2019) and while evidence for a nitrogen-recycling role of the elasmobranch microbiome continues to grow, whether this community is affected by changing environments is currently unknown.
Environmental salinity modifies the microbial gut community structure within anadromous fishes. Microbes best suited to the new environment will ultimately dominate (Yoshimizu and Kimura 1976; Schmidt et al. 2015; Llewellyn et al. 2016). Given the requisite changes in osmotic homeostasis during a low-salinity (LS) challenge, we hypothesized that a 1-week acclimation to LS would alter the microbial community of the spiny dogfish, leading to changes in ion and nitrogen transport across the GIT. We further examined recovery post-transfer by examining transport rates after a 6-h acute transfer (AT) from LS (70% seawater; 21 ppt) back to control seawater (C; 100% seawater; 32 ppt). The 6–h timeframe of AT was chosen based upon previous reports where drinking rate is elevated and plasma osmolality is significantly increased from that of elasmobranchs held at low salinity, while urea balance is not yet compensated for (Anderson et al. 2002). As these animals need to rectify urea loss, we predicted that ion and nitrogen transport rates would be elevated in this condition and the importance of the microbiome magnified. The contribution of the microbiome was assessed by measuring the activities of enzymes specific to non-vertebrate organisms (e.g. urease and cellulase (Yokoe and Yasumasu 1964; Mobley and Hausinger 1989) in response to altered salinity and antibiotic treatment. Finally, the impacts of both the environment and antibiotic application on GIT nitrogen, ion, and water transport were assessed using in vitro gut sac preparations.
Methods
Experimental animals and materials
Male Pacific spiny dogfish (Squalus acanthias suckleyi, 2.33 ± 0.14 kg) were collected from Barkley Sound, British Columbia, Canada using baited hook and line. Collection permits were obtained from the Department of Fisheries and Oceans Canada (XR99 2019) and all experimental procedures were approved by the Bamfield Marine Sciences Animal Care Committee (RS-19-03). Upon capture, animals were transported to the Bamfield Marine Sciences Centre and housed in a ~ 150,000 L tank with continuously flowing seawater (12–14 °C, 31–33 ppt salinity), where they were fed a 2% body mass ration of hake (Merluccius merluccius) every other day. Following an overnight fast, animals (groups of 6) were transferred to one of three 5000 L tanks with continuously flowing seawater for a 1-week period of fasting: control (C; 3–33 ppt salinity), low salinity (LS; 20–21 ppt salinity), or acute transfer (AT; 20–21 ppt salinity). After the 1-week period the AT animals were transferred to control seawater (31–33 ppt) for 6 h. All animals were then terminally anaesthetized with tricaine methanesulfonate (0.2 g L−1) and caudal blood samples were obtained (see below), prior to dissection. All chemicals and reagents were purchased from Millipore-Sigma unless otherwise noted.
Blood sampling and gut sac preparation
Caudal blood (2 mL) was withdrawn using a heparinized 24G needle and syringe. Samples were immediately centrifuged (12, 000 × g, 2 min) and the plasma was aliquoted and frozen at − 80 °C for later analysis (see analytical techniques). The dogfish was then dissected along the ventral surface and the stomach and spiral valve were removed and prepared as previously described (Liew et al. 2013) with some modification. Briefly, the stomach (pyloric and cardiac as one) and the spiral valve were extracted and gently flushed. The solution used throughout these experiments matched the experimental condition of the animals (Anderson et al. 2010), in that C and AT fish used standard dogfish Ringer (in mM: NaCl 257; Na2SO4 7; NaHCO3 6; Na2HPO4 0.1; KCl 4; MgSO4 3; CaCl2 2; glucose 5; TMAO 80; urea 350; pH 7.4), whereas a LS Ringer (in mM: NaCl 180; Na2SO4 7; NaHCO3 6; Na2HPO4 0.1; KCl 4; MgSO4 3; CaCl2 2; glucose 5; TMAO 57; urea 250; pH 7.4) was utilized for those animals exposed to LS. Mucosal and serosal solutions were of identical composition and the gut sacs were aerated using a pre-equilibrated precision gas mixture of 99.7% O2 and 0.3% CO2 for the flux periods. An initial 1-h flux was conducted after which time the stomach sacs and spiral valve sacs were drained and 3 mL mucosal samples were obtained. The removed sample volume was replaced with either Ringer (stomach) or an antibiotic cocktail dissolved in Ringer (spiral valve; 0.12 mg/kg animal Amphomycin, 150 mg/kg animal Oxytetracycline, 125 kU/ animal Penicillin G – purchased from Drugs.com) and a second 1.5 h flux period was run. The flux time was extended to permit action of the antibiotics while still maintaining a functioning preparation. Samples were collected from the mucosal solution at the start and end of each flux period. Differences were not apparent between the first and second flux periods in the stomach and so both time periods were combined for analysis, resulting in 12 possible measurements (see below). Aliquots were stored at – 80 °C for later analysis of ammonia and urea concentration, osmolality and ion composition. Samples of the mucosal saline collected before and after antibiotic application were stored at 4 °C and analysed within 3 days for urease and cellulase activity measurements (see below). Once mucosal samples were obtained, the sacs were dissected bilaterally and spread on graphing paper, where ImageJ Software (National Institute of Health) was utilised to determine surface area in cm2.
Analytical techniques and flux rate calculations
Osmolality was determined using a Vapro Osmometer 5600 (Wescor Inc, Logan, UT, USA). Urea was quantified via the colourimetric methods of Rahmatullah and Boyde (1980), while ammonia concentration was determined using a Thermo Scientific Orion ammonia gas sensing ISE electrode (Thermo Scientific, Waltham, MA, USA). To determine ion concentrations, samples were digested for 72 h at 50 °C in 70% nitric acid, prior to measurement on a 5110 ICP-OES (Agilent Technologies INC, Santa Clara, CA, USA). The flux rate (J) for each of these parameters was calculated using the following equation:
where Vi and Vf are the initial and final volumes, Ci and Cf are the initial and final calculated concentrations, T is the duration of the flux (h), and A is the surface area of the gut sac (cm2). Urease activity was calculated in mucosal intestinal samples collected before and after the addition of the antibiotic cocktail using a commercial kit as per the manufacturer’s instructions (MAK120; Millipore Sigma). Cellulase activity was measured using the DNS method of Miller (1959). Briefly, intestinal fluid samples were incubated with or without carboxymethyl cellulose, vortexed and incubated for 1 h at 37 °C. Next, 3,5-dinitrosalicylic acid (DNS) was added at a 3:1 ratio to the sample volume and incubated at 96 °C for 10 min. A volume of 200 µL was plated and read at A574 on a Spectramax 190 (Molecular Devices, Sunnyvale, CA, USA). For each of urease and celluase, activities were standardized to the total mucosal volume of the sac.
Statistical analyses
Data are expressed as means ± standard error of the mean (SEM). Statistical analyses and graphing were conducted using GraphPad Prism 6 (La Jolla, CA, USA) or SigmaPlot 11 (Systat software Inc., San Jose, CA, USA). Normality was assessed using the Shapiro–Wilk test, while homogeneity of variance was tested using the Brown-Forsythe test. Where possible, data were transformed to conform to these assumptions. Data were then assessed using an ANOVA on ranks (Kruskal–Wallis), a one-way ANOVA, or a two-way repeated measures ANOVA, where appropriate. Differences were identified using a Holm-Sidak, or Tukey posthoc analysis with significance set at p = 0.05 (see figure legends for details).
Results
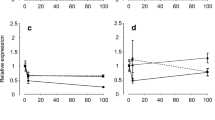
Urease activity in the mucosal saline was significantly reduced by ~ 61% following gut sac antibiotic treatment in control animals (p = 0.04). Following 1 week of LS, mucosal saline urease activity was reduced by ~ 81% (p = 0.048) and the addition of the antibiotic cocktail to the gut sac further reduced urease activity by ~ 57% (p = 0.004). There was no effect of gut sac antibiotic application within the AT group (p = 0.40). The environmental treatment had a significant impact on urease activity in the mucosal saline (p = 0.03; Fig. 1a) with AT animals having a 89% reduction in urease activity when compared to controls following pre-antibiotic treatment (p = 0.042), while LS animals had significantly reduced mucosal saline urease activity compared to controls following gut sac antibiotic applications (p = 0.032). No interactive effect between environment and antibiotic application was found regarding mucosal saline urease activity (p = 0.21). Cellulase activity was effectively undetectable following gut sac antibiotic treatment and so only data pre-AB treatment were analysed with a one-way ANOVA on ranks. While trends followed a similar pattern to urease activity, there was no significant effect of environment on mucosal saline cellulase activity (p = 0.09; Fig. 1b).
Urease (a) or cellulase (b) activity in the mucosal saline of spiral valve preparations pre-antibiotic (AB; black bars) treatment or post-AB treatment (white bars) from animals held in control (C; 100% SW 1 wk), low salinity (LS; 70% SW 1 wk), or acute transfer (AT; 70% SW 1 wk > 100% SW 6 h) conditions. Data are presented as means ± SEM of 4–5 preparations. B.D.L indicates measurements were below detectable limits. Asterisk (*) indicates significant effect of AB application (p < 0.05), while different letters denote differences between environmental salinities in the pre-AB or post-AB condition as detected using a 2-way repeated measures ANOVA with a Holm–Sidak analysis
Dogfish plasma osmolality was significantly reduced after 1 week at LS (760 ± 46 mOsm) when compared to control animals housed at full-strength seawater (945 ± 20 mOsm). Following 6 h of acute transfer to 100% SW, plasma osmolality was not significantly different from either control or LS groups (796 ± 35 mOsm, p = 0.05; Fig. 2a). Plasma sodium was significantly reduced in LS, (190 ± 26 mM, p = 0.04) however, AT animals had started to increase plasma sodium levels (274 ± 16 mM, p = 0.99) to values that were not significantly different from control (270 ± 16 mM; Fig. 2b). Similarly to plasma osmolality, plasma urea showed a significant reduction from C values (340 ± 16 mM; p = 0.081) in LS exposed fish (urea = 280 ± 13 mM), which was not sufficiently compensated for in AT animals (291 ± 15 mM; Fig. 2c). No significant changes in plasma magnesium, calcium, or potassium were observed across treatments however; plasma potassium followed similar trends to that of sodium (Table 1). Levels of plasma ammonia were not altered across treatments (ave = 0.93 ± 0.08 mM, p = 0.57; Table 1).
Effect of environmental salinity on plasma: osmolality (a), sodium (b), or urea (c) in Pacific spiny dogfish. C: control 100% SW (1 wk); LS: low salinity 70% SW (1 wk); AT: acute transfer 70% SW (1wk) to 100% SW (6 h). Datasets are presented as means + SEM of 4–6 animals. Different letters denote significant differences as detected by a one-way ANOVA with a Tukey’s posthoc test (p < 0.05)
Water flux rates in the stomach were significantly impacted when comparing C to AT dogfish (p = 0.035); however, no significant difference was noted after a one-week acclimation to LS when compared to control animals (p = 0.25; Fig. 3a). A net gain in the weight of the stomach compared to the body mass was evident in the AT fish only (~ 39%; p = 0.019; Fig. 3b). Spiral valve flux rates were not altered by antibiotic treatment (p = 0.52) or environmental salinity (p = 0.21) and organ mass was likewise unchanged (p = 0.25; data not shown).
Water flux in the stomach (a) and percent organ mass of the stomach relative to whole body mass (b) of dogfish held at control (C; 100% SW), low salinity (LS; 70% SW), or acutely transferred (AT) from 70% SW to 100% SW for 6 h. Positive values denote an increase in mucosal water content, whereas negative values denote a decrease in mucosal water content. Datasets are presented as means + SEM of 6 preparations (12 data points in the instance where stomach flux 1 and 2 are combined) and analysed using a Kruskal–Wallis (a), or a one-way ANOVA (b) with Tukey multiple comparison tests to determine where differences lie (p < 0.05)
The rates of stomach ion flux for all measured ions (i.e. Na+, K+, Ca2+, Mg2+) were not significantly altered as a result of environmental salinity (p = 0.22–0.86; Table 2). Within the spiral valve, potassium was the only ion significantly impacted by environment (p = 0.008). No effects of antibiotic application (p = 0.067) or interactive effects (p = 0.522) were found regarding potassium flux (Fig. 4a). Spiral valve flux rates for the other measured ions were not significant regarding environment (p = 0.15–0.69), antibiotic application (p = 0.22–0.44), or interactive effects (p = 0.16–0.99; Fig. 4b–d).
Potassium (K+; a), sodium (Na+; b), magnesium (Mg2+; c) and calcium (Ca2+; d) flux in the spiral valve of dogfish held at control (C; 100% SW), low salinity (LS; 70% SW), or acutely transferred (AT) from 70% SW to 100% SW for 6 h. Samples were measured pre-antibiotic (AB; black bars) and post-AB (white bars). Positive values denote mucosal accumulation, whereas negative values denote mucosal disappearance. Datasets are presented as means + SEM of 5–6 preparations and analysed using a two-way repeated measures ANOVA with a Holm–Sidak post hoc test to determine where differences lie. Different letters denote significant difference (p < 0.05)
Mucosal NH3 flux (JNH3) was consistent between flux periods in the stomach, so both periods were combined for analysis. The lowering of environmental salinity did not alter mucosal ammonia appearance (p = 0.71; Fig. 5a). Spiral valve JNH3 was not significantly affected by antibiotic treatment (p = 0.45) or by environmental salinity (p = 0.17; Fig. 5b), and no interactive effect was found (p = 0.44). Urea flux (Jurea) in the stomach was unaffected by environmental condition (p = 0.69; Fig. 5c). Similarly, the spiral valve preparations were unaffected by antibiotic treatment (p = 0.08) and by environmental salinity (p = 0.18), with no observed interaction (p = 0.45; Fig. 5d) despite a change in directionality from mucosal accumulation in C to mucosal disappearance in AT.
Ammonia flux rate (JNH3) from dogfish stomach (a) and spiral valve preparations (b) or urea flux rate (Jurea) from dogfish stomach (c) and spiral valve (d) following 1-week exposure at control (C; 100% SW), low salinity (LS; 70% SW), or acute transfer (AT; 70% SW 1 wk > 100 % SW 6 h) conditions. Spiral valve samples were measured pre-antibiotic (AB; black bars) and post-AB treatment (white bars). Positive values denote mucosal accumulation, whereas negative values denote mucosal disappearance. Data are presented as means ± SEM of 4–6 preparations (12 data points in the instance where stomach flux 1 and 2 are combined). Different letters denote statistical significance as detected using a Kruskal–Wallis with a Tukey’s posthoc analysis (a), one-way ANOVA (c) or a two-way repeated measures ANOVA (b, d; p < 0.05)
Discussion
Nitrogen recycling within the marine elasmobranch gut may involve ureolytic bacteria (Goldstein and Dewitt Harley 1972; Anderson et al. 2010; Wood et al. 2019). Although the microbial make-up of the spiny dogfish is unknown, we detected urease activity in the fluids collected from the spiral valves of control animals, consistent with previous reports from this species (Wood et al. 2019). As expected, the addition of an antibiotic cocktail significantly reduced both urease and cellulase activities (Fig. 1). Overall, cellulase activity was undetected in all but one sample after administration, signifying that cellulase-containing microbes were effectively eradicated. Of note, significant decreases in urease activity were observed with changing salinity and as a result, antibiotic treatment in the AT animals was deemed insignificant. Similarly, a downward trend in levels of cellulase activity was observed with LS and AT animals. These changes in microbial enzyme activity may be indicative of a shift in microbial community assembly, as has been observed in anadromous fishes (Schmidt et al. 2015; Dehler et al. 2017). Following changes in environmental salinity, the microbial species best suited to compete in the new environment will dominate (Schmidt et al. 2015) and a recolonization of the gut can occur after 3 weeks with new microbial sources potentially derived from the diet and/or water column (Zarkasi et al. 2016; Dehler et al. 2017). Ninety percent of the ocean’s biomass is predicted to be of microbial origin (International Council for the Exploration of the Sea [ICES] 2011) and, therefore, the increased drinking rate upon return to full-strength seawater may have a dual effect of aiding in osmoregulatory processes and the recolonization of the gut in marine elasmobranchs. Furthermore, estuarine environments are rich in organic matter, which has the potential to increase the species richness of the microbiome following recolonization. The limited duration of recovery (6 h) we examined herein does not provide any insight on how recolonization after a low salinity excursion will occur or over what timeframe. However, we do know that microbial proliferation occurs following a feeding event (Wood et al. 2019). Had feeding occurred alongside the low-salinity exposure, the influx of osmolytes accompanying feeding could lead to the retention of microbes that would otherwise be lost in low salinity. These retained microbes could then proliferate to maintain ureolytic processes and mitigate nitrogen losses in environments of reduced salinity. From an ecological standpoint, estuaries may provide more feeding opportunities alongside spatial refuge from predators (Branstetter 1990), which may offer a reason for these nitrogen-limited animals to enter into an environment where nitrogen loss ensues. Whether nitrogen losses can be lessened with feeding in these osmotically challenged animals remains to be determined.
Associated with exposure to low salinity, we observed an expected ~ 20% decrease in plasma osmolality. After a 1-week acclimation period to LS, plasma sodium values were at ~ 30% of control values, whereas plasma urea was only reduced by ~ 17%. Measured plasma urea content was similar (albeit slightly higher) in our study compared to previous studies which report ~ 250 mM in similar external environments (Payan et al. 1973; Pillans and Franklin 2004; Guffey and Goss 2014). It is probable that the 2% ration of food 24 h prior to the 1-week low-salinity exposure provided an additional nitrogen load, which enabled the maintenance of urea content above 280 mM. This may also explain why we observed no ill effects regarding animal health, whereas Guffey and Goss (2014) report a near 40% mortality rate of animals held under the same conditions. The ~ 30% loss of sodium in LS is near equivalent to the 33% reported previously (Guffey and Goss 2014), while plasma potassium, magnesium and calcium concentrations were maintained across all treatments. We do observe a significant recuperation of sodium in those animals acutely transferred back to control seawater, whereas plasma urea is not significantly increased after 6 h. Preferential gain of ions over urea has been noted in conditions where small-spotted catsharks (Scyliorhinus canicula) are nitrogen-limited and thus the osmotic deficit must be met by means other than urea synthesis (Armour et al. 1993). Drinking contributes to elasmobranch ion gain during the transition to hyperosmotic environments (Anderson et al. 2002), and indeed, we observe a significant influx of water in AT animals alongside a net increase in stomach mass, supporting this notion. The direction of water flux also shifts towards influx in the spiral valve of AT animals. The water loss we observe in control conditions can be attributed to the hyperosmotic Ringer (~ 990 mOsm) relative to the plasma osmolality (~ 940 mOSm), wherein thermodynamic gradients favour water loss and ion gain. Despite changing water movement, the net flux of ions is consistent across treatments in the stomach, as has been reported in the little skate (Anderson et al. 2010). Spiral valve ion movement was essentially abolished in LS treatment groups, likely owing to the elimination of the respective ion gradients between the tissue and mucosal fluid (in mM: Ringer Na+ 190, K+ 3; plasma Na+ 193, K+ 4, Fig. 2b). However, under control conditions we observed sodium uptake alongside magnesium and calcium exclusion, as has previously been observed in LS challenged L. erinacea (Anderson et al. 2010) and post-prandial S. acanthias (Wood et al. 2007). The degree of exclusion appears slightly diminished under altered salinity conditions, perhaps owing to more disparate gradients and a need to retain/regain ions. The mucosal accumulation of these divalent cations is also seemingly diminished after antibiotic treatment. However, both cations are known to chelate with tetracycline (Arayne et al. 2005; Guz-Regner and Bugla-Ploskońska 2007), which would manifest as a reduced concentration within the gut sacs. Interestingly, under all conditions we observe potassium efflux that is significantly diminished in LS conditions. Again we believe this is the result of artificial gradients established between the Ringer (K+ = 4 mM) and the plasma of these animals (C ~ 5.4 mM, AT ~ 4.5), leading to a net efflux from the tissue.
To our knowledge, no published report exists detailing plasma ammonia levels in elasmobranchs held at reduced salinity. However, it is known that plasma ammonia content is maintained following a feeding event, with excess ammonia presumably directed towards protein or urea synthesis (Kajimura et al. 2006). Given the trend towards reduced plasma ammonia concentration at LS, it is possible the dogfish are diverting plasma ammonia towards maintaining urea at a concentration required for bodily functions (min. 200 mM; Simpson and Ogden 1932). Negligible net ammonia flux has been observed in the stomach of fasting animals (Wood et al. 2019) and likewise ammonia flux is consistent in stomachs of all treatment groups in this study. In keeping with previous reports of an apparent ammonia efflux in the spiral valve, we observe a mucosal accumulation of ammonia in control animals. The continued accumulation despite the loss of urease-containing microbes in altered salinity or post-antibiotic treatment may be the result of heightened metabolism for osmoregulation. Osmoregulation constitutes 10–15% of standard metabolic rate in elasmobranchs (Kirschner 1993) and this percentage can only be expected to increase under times of osmotic stress, leading to elevated production of ammonia as a by-product. In fact bat rays (Myliobatis californica) and leopard sharks (Triakis semifasciata) show increased organismal metabolic rate as salinity decreases (Meloni et al. 2002; Dowd et al. 2010). Likewise, antibiotic application has been shown to increase zebrafish (Danio rerio) metabolic rate (Zhou et al. 2018), which again could yield these increases in ammonia production. This contradicts a report in the plainfin midshipman (Porichthys notatus) wherein antibiotic application reduced mucosal ammonia appearance (Bucking et al. 2013). While this is to be expected in animals that utilise ureolytic bacteria for nitrogen metabolism like the dogfish, these teleosts were not simultaneously challenged with a low-salinity environment, perhaps prompting such a difference.
Urea flux within the stomach varied widely between animals, yet net values hovered around zero. A slight mucosal accumulation of urea was observed in the spiral valve of control fish, consistent with prior observations (Liew et al. 2013). Antibiotic application tended to limit the amount of urea secretion, albeit not significantly, in control animals. The reduced microbial presence may limit the nitrogen requirement within the gut lumen and simultaneously restrict the amount of urea that can be converted to ammonia. Therefore, the animal may benefit from urea retention under these conditions, as the nitrogen-recycling machinery has become compromised. Under altered salinity environments, there is a tendency for urea acquisition suggestive of nitrogen scavenging under periods of extreme nitrogen limitation (i.e. LS) or when osmotic/nitrogen balance must be recovered (i.e. AT). The degree of urea uptake is exacerbated in AT animals that have been treated with antibiotics. Given the complete elimination of both urease and cellulase activity under this combination, alongside the highest rates of mucosal ammonia accumulation, these animals are likely more severely nitrogen compromised. As energetics must be maintained for osmoregulatory purposes, the acquisition of nitrogen is required and results in heightened rates of urea uptake from the mucosal solution. In an environmentally relevant set-up, the GI tract would not be filled with saline of a similar composition to that of the animal tissues. Yet, as mentioned above, there is potential for a meal to contribute substantially toward enhanced nitrogen scavenging which remains to be studied. In this scenario where plasma and mucosal compositions are nearly matched, we still observe trends of nitrogen scavenging under extreme periods of nitrogen limitation, with a role for the microbiome evident.
Overall, we note that environmental salinity has a profound impact on the microbial community as measured via urease and cellulase activity in the mucosal saline. We continue to observe mucosal accumulation of ammonia in the spiral valve even in conditions where the microbiome is diminished (i.e. LS, AT or antibiotic treated), which we attribute to a heightened metabolism as the result of the metabolization of the antibiotics and the energy required for osmoregulatory purposes. Although our data are not significant regarding nitrogen flux, we do tend to see nitrogen scavenging. This scavenging appears elevated under severely compromised conditions (i.e. AT with antibiotics). Whether antibiotic treatment leads to changes in whole-animal nitrogen metabolism remains to be seen, but given the trends herein it is likely that nitrogenous stores will need to be mobilized to compensate for nitrogen loss during estuarine excursions.
Availability of data
All data is available upon request.
Change history
13 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00360-022-01455-4
References
Anderson WG, Dasiewicz PJ, Liban S, Ryan C, Taylor JR, Grosell M, Weihrauch D (2010) Gastro-intestinal handling of water and solutes in three species of elasmobranch fish, the white-spotted bamboo shark, Chiloscyllium plagiosum, little skate, Leucoraja erinacea and the clear nose skate Raja eglanteria. Comp Biochem Phys A 155:493–502
Anderson WG, Good JP, Pillans RD, Hazon N, Franklin CE (2005) Hepatic urea biosynthesis in the euryhaline elasmobranch Carcharhinus leucas. J Exp Zool A 303A:917–921
Anderson WG, McCabe C, Brandt C, Wood CM (2015) Examining urea flux across the intestine of the spiny dogfish, Squalus acanthias. Comp Biochem Physiol A 181:71–78
Anderson WG, Nawata CM, Piercey-Normore WCM, MD, Weihrauch D, (2012) Body fluid osmolytes and urea and ammonia flux in the colon of two chondrichthyan fishes, the ratfish, Hydrolagus colliei, and spiny dogfish, Squalus acanthias. Comp Biochem Phys A 161:27–35
Anderson WG, Takei Y, Hazon N (2002) Osmotic and volaemic effects on drinking rate in elasmobranch fish. J Exp Biol 205:1115–1122
Arayne MS, Sultana N, Hussain F (2005) Interactions between ciprofloxacin and antacids - Dissolution and adsorption studies. Drug Metab Drug Interact 21:117–129
Armour KJ, O’Toole LB, Hazon N (1993) The effect of dietary protein restriction on the secretory dynamics of 1a-hydroxycorticosterone and urea in the dogfish, Scyliorhinus canicula: a possible role for 1a-hydroxycorticosterone in sodium retention. J Endocrinol 138:275–282
Boylan JW (1967) Gill permeability in Squalus acanthias. In: Gilbert P, Mathewson RF, Rall DP (eds) Sharks, Skates and Rays. Johns Hopkins University Press, Baltimore, pp 197–206
Branstetter S (1990) Early life-history implications of selected carcharhinoid and lamnoid sharks of the Northwest Atlantic. NOAA Tech Rep NMFS 90:17–28
Bucking C (2017) A broader look at ammonia production, excretion, and transport in fish: a review of impacts of feeding and the environment. J Comp Physiol B 187:1–18
Bucking C, LeMoine CMR, Craig PM, Walsh PJ (2013) Nitrogen metabolism of the intestine during digestion in a teleost fish, the plainfin midshipman (Porichthys notatus). J Exp Biol 216:2821–2832
Burger JW (1965) Roles of the rectal gland and the kidneys in salt and water excretion in the Spiny Dogfish. Physiol Zool 38:191–196
Burger JW, Hess WN (1960) Function of the rectal gland in the Spiny Dogfish. Science 131:670–671
De Boeck G, Grosell M, Wood C (2001) Sensitivity of the spiny dogfish (Squalus acanthias) to waterborne silver exposure. Aquat Toxicol 54:261–275
Dehler CE, Secombes CJ, Martin SAM (2017) Seawater transfer alters the intestinal microbiota profiles of Atlantic salmon (Salmo salar L.). Sci Rep 7:13877
Dowd WW, Harris BN, Cech JJ Jr, Kueltz D (2010) Proteomic and physiological responses of leopard sharks (Triakis semifasciata) to salinity change. J Exp Biol 213:210–224
Egerton S, Culloty S, Whooley J, Stanton C, Ross RP (2018) The gut microbiota of marine fish. Front Microbiol 9:873
Givens C, Ransom B, Bano N, Hollibaugh J (2015) Comparison of the gut microbiomes of 12 bony fish and 3 shark species. Mar Ecol Prog Ser 518:209–223
Goldstein L, Dewitt-Harley S (1972) The role of intestinal bacteria in urea metabolism by the skate, Raja erinacea. Bull Mt Desert Isl Biol Lab 12:37–39
Goldstein L, Forster RP (1971) Osmoregulation and urea metabolism in the little skate Raja erinacea. Am J Physiol 220:742–746
Grimes DJ, Brayton P, Colwell RR, Gruber SH (1985) Vibrios as autochthonous flora of neritic sharks. Syst Appl Microbiol 6:221–226
Guffey SC, Goss GG (2014) Time course of the acute response of the North Pacific spiny dogfish shark (Squalus suckleyi) to low salinity. Comp Biochem Phys A 171:9–15
Guz-Regner K, Bugla-Ploskońska G (2007) The immunomodulatory and anti-inflammatory properties of different antimicrobial agents. Postepy Hig Med Dosw 61:828–837
Hazon N, Henderson IW (1985) Factors affecting the secretory dynamics of 1-α hydroxycorticosterone in the dogfish, Scyliorhinus canicula. Gen Comp Endocrinol 59:50–55
Hazon N, Wells A, Pillans RD, Good JP, Anderson WG, Franklin CE (2003) Urea based osmoregulation and endocrine control in elasmobranch fish with special reference to euryhalinity. Comp Biochem Physiol B 136:685–700
Kajimura M, Walsh PJ, Mommsen TP, Wood CM (2006) The dogfish shark (Squalus acanthias) increases both hepatic and extrahepatic ornithine urea cycle enzyme activities for nitrogen conservation after feeding. Physiol Biochem Zool 79:602–613
International Council for the Exploration of the Sea [ICES] (2011) Report of the working group on biodiversity science (WGBIODIV). ICES, Copenhagen, p 94
Kirschner LB (1993) The energetics of osmotic regulation in ureotelic and hypoosmotic fishes. J Exp Zool 267:19–26
Liew HJ, De Boeck G, Wood CM (2013) An in vitro study of urea, water, ion and CO2/HCO3- transport in the gastrointestinal tract of the dogfish shark (Squalus acanthias): the influence of feeding. J Exp Biol 216:2063–2072
Llewellyn MS, McGinnity P, Dionne M, Letourneau J, Thonier F, Carvalho GR, Creer S, Derome N (2016) The biogeography of the atlantic salmon (Salmo salar) gut microbiome. ISME J 10:1280–1284
Martin RA (2005) Conservation of freshwater and euryhaline elasmobranchs: a review. J Mar Biol Ass 85:1049–1073
Meloni CJ, Cech JJJ, Katzman SM (2002) Effect of brackish salinities on oxygen consumption of bat rays (Myliobatis californica). Copeia 462–465
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Mines GR (1912) On the relations to electrolytes of the hearts of different species of animals. J Physiol 43:467–506
Mobley HL, Hausinger RP (1989) Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev 53:85–108
Payan P, Goldstein L, Forster R (1973) Gills and kidneys in ureosmotic regulation in euryhaline skates. Am J Physiol 224:367–372
Pillans RD, Franklin CE (2004) Plasma osmolyte concentrations and rectal gland mass of bull sharks Carcharhinus leucas, captured along a salinity gradient. Comp Biochem Physiol A 138:363–371
Rahmatullah M, Boyde TRC (1980) Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin Chim Acta 107:3–9
Schmidt VT, Smith KF, Melvin DW, Amaral-Zettler LA (2015) Community assembly of a euryhaline fish microbiome during salinity acclimation. Molec Ecol 24:2537–2550
Simpson WW, Ogden E (1932) The physiological significance of urea I - The elasmobranch heart. J Exp Biol 9:1–5
Smith HW (1936) The retention and physiological role of urea in the Elasmobranchii. Biol Rev 11:49–82
Webb NA, Wood CM (2000) Bioaccumulation and distribution of silver in four marine teleosts and two marine elasmobranchs: influence of exposure duration, concentration, and salinity. Aquat Toxicol 49:111–129
Wood CM, Kajimura M, Bucking C, Walsh PJ (2007) Osmoregulation, ionoregulation and acid-base regulation by the gastrointestinal tract after feeding in the elasmobranch (Squalus acanthias). J Exp Biol 210:1335–1349
Wood CM, Liew HJ, De Boeck G, Hoogenboom JL, Anderson WG (2019) Nitrogen handling in the elasmobranch gut: a role for microbial urease. J Exp Biol 222: jeb194787
Wood CM, Pärt P, Wright PA (1995) Ammonia and urea metabolism in relation to gill function and acid-base balance in a marine elasmobranch, the Spiny dogfish (Squalus acanthias). J Exp Biol 198:1545–1558
Yancey PH (2015) Organic osmolytes in elasmobranchs. In: Farrell AP, Brauner CJ (eds) Shadwick R E. Internal processes. Academic Press, Physiology of elasmobranch fishes, pp 222–264
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
Yokoe Y, Yasumasu I (1964) The distribution of cellulase in invertebrates. Comp Biochem Physiol 13:323–338
Yoshimizu M, Kimura T (1976) Study on the intestinal microflora of salmonids. Fish Pathol 10:243–259
Zarkasi KZ, Taylor RS, Abell GCJ, Tamplin ML, Glencross BD, Bowman JP (2016) Atlantic Salmon (Salmo salar :L) gastrointestinal microbial community dynamics in relation to digesta properties and diet. Microb Ecol 71:589–603
Zhou L, Limbu SM, Shen M, Zhai W, Qiao F, He A, Du Z-Y, Zhang M (2018) Environmental concentrations of antibiotics impair zebrafish gut health. Environ Pollut 235:245–254
Acknowledgements
The authors wish to acknowledge Dr. Eric Clelland and Tao Eastham and the staff at the Bamfield Marine Sciences Centre for their assistance in this study, as well as Alexandra Schoen and Jessica MacPherson for assistance in dogfish collection and maintenance. Additional thanks to Mark Miller-Williams and Wayne Blonski for providing access to the ICP-OES. Funding was provided by the University of Manitoba Faculty of Science Fieldwork support program and a Natural Science and Engineering Research Council of Canada (NSERC) Discovery grant #05348–15 to WGA and #29314–01 to CB.
Funding
Funding was provided by the University of Manitoba Faculty of Science Fieldwork support program and NSERC Discovery grant #05348–15 to WGA and #29314–01 to CB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing/conflicts of interest.
Ethics approval
All experiments were approved by the Bamfield Marine Sciences Animal Care Committee (RS-19-03). Animals were collected with approval from the Department of Fisheries and Oceans Canada (XR99 2019).
Additional information
Communicated by H.V. Carey.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weinrauch, A.M., Folkerts, E.J., Blewett, T.A. et al. Impacts of low salinity exposure and antibiotic application on gut transport activity in the Pacific spiny dogfish, Squalus acanthias suckleyi. J Comp Physiol B 190, 535–545 (2020). https://doi.org/10.1007/s00360-020-01291-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01291-4