Abstract
Gas chromatography was used to measure transepithelial transport of glycylsarcosine (Gly-Sar) by perfused lobster (Homarus americanus) intestine. Unidirectional and net fluxes of dipeptide across the tissue and luminal factors affecting their magnitude and direction were characterized by perfusing the lumen with the dipeptide and measuring its appearance in saline on the serosal side of the organ. Transmural transport of 10 mM Gly-Sar resulted in serosal accumulation of only the dipeptide; no appearance of corresponding monomeric amino acids glycine or sarcosine was observed. Carrier-mediated and diffusional transmural intestinal transport of Gly-Sar was estimated at 1–15 mM luminal concentrations and followed a curvilinear equation providing a K m = 0.44 ± 0.17 mM, a J max = 1.27 ± 0.12 nmol cm−2 min−1, and a diffusional coefficient = 0.026 ± 0.008 nmol cm−2 min−1 mM−1. Unidirectional mucosal to serosal and serosal to mucosal fluxes of 10 mM Gly-Sar provided a significant (p < 0.05) net absorptive flux toward the serosa of 3.54 ± 0.77 nmol cm−2 min−1, further supporting carrier-mediated dipeptide transport across the gut. Alkaline (pH 8.5) luminal pH more than doubled transmural Gly-Sar transport as compared to acidic (pH 5.5) luminal pH, while luminal amino acid-metal chelates (e.g., Leu-Zn-Leu), and high concentrations of amino acids alone significantly (p < 0.001) reduced intestinal Gly-Sar transfer by inhibiting carrier transport of the dipeptide. Proposed mechanisms accounting for intestinal dipeptide transport and luminal factors affecting this process are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Protein digestive activities of vertebrate and invertebrate gastrointestinal tracts result in a mixture of amino acids and short-chain peptides that are independently absorbed across the intestine to the blood by a variety of transport proteins (Matthews and Payne 1980; Daniel 2004; Bröer 2008). While a number of different amino acid transport proteins of the mammalian intestine and kidney have been identified, none of them appears to transport di- or tripeptides composed of the same monomer units (Bröer 2008). Instead, epithelial transport of small peptides occurs by members of the POT (Proton-coupled, Oligopeptide Transporter) family, including PEPT1 and PHT1, each of which have a wide spectrum of substrates (Herrera-Ruiz and Knipp 2003; Daniel 2004). Mammalian peptide transporters appear functionally conserved and have analogs throughout the vertebrates (Thamotharan et al. 1996a, b; Verri et al. 2000, 2010; Daniel et al. 2006; Rønnestad et al. 2007) and invertebrates (Thamotharan and Ahearn 1996).
In lobster (Homarus americanus), the hepatopancreas is the major organ of digestion and absorption, where digestive enzymes are produced and nutrients are absorbed (Wright and Ahearn 1997). Following hepatopancreatic digestive and absorptive action, chyme is passed to the tubular intestine, which is lined with a columnar epithelium, where further nutrient absorption occurs prior to defecation (Ahearn 1976; Ahearn and Maginniss 1977; Conrad and Ahearn 2005; Obi et al. 2011; Abdel-Malak and Ahearn 2014). The dipeptide, 3H-glycylsarcosine, was transported by lobster hepatopancreatic brush border membrane vesicles by a proton-dependent, carrier-mediated, transporter that responded to an imposed membrane potential and was sodium insensitive (Thamotharan and Ahearn 1996). Diethylpyrocarbonate and a variety of peptides significantly inhibited the vesicular transport of 14C-glycylsarcsine, suggesting that, in many respects, peptide transport in crustaceans appeared to follow the transport paradigm proposed for fish and mammals.
In recent years, an alternate transport pathway for amino acid movements across digestive organs in fish and crustaceans has been proposed that involves the chelation of two amino acids with a metallic ion such as zinc or copper (Conrad and Ahearn 2005; Glover and Wood 2008; Obi et al. 2011; Abdel-Malak and Ahearn 2014). The resulting bis-complexes (His-Zn-His; Leu-Cu-Leu) are transported by a putative peptide transport system in place of the normal substrate, as they structurally mimic dipeptides where two amino acids are linked by a peptide bond. As a result of bis-complex formation, net transport of zinc across lobster intestine toward the blood was doubled in the presence of luminal l-histidine and similar enhancing effects on net l-histidine transport were observed in the presence of luminal zinc (Conrad and Ahearn 2005, 2007). Results suggest that absorption of both amino acid and metal is synergistically affected by their luminal co-occurrence and as a result of employing a dipeptide transporter on the intestinal apical membrane.
The present investigation uses gas chromatography to extend the previous characterization of crustacean dipeptide intestinal absorption. The data suggest that glycylsarcosine is transported across lobster intestine as an intact molecule, without any measurable conversion to glycine and sarcosine, providing evidence for both apical and basolateral peptide transport systems. Absorption of the dipeptide is inhibited by amino acid chelates with zinc and is regulated by variations in luminal pH.
Materials and methods
Materials
Live male lobsters, H. americanus, were purchased from a local seller. They were maintained in a saltwater aquarium at 12 °C with a pH of 8.2. Lobsters were kept up to 4 days until used in experiments. They were dissected by cutting the ventral nerve cord, then opening the carapace and dorsal tail segments to expose the intestine which was removed and flushed with saline to remove waste.
Physiological saline was prepared to match the osmolarity and composition of crustacean hemolymph. The salt concentrations used in the buffer were as follows: 420 mM sodium chloride, 25 mM calcium chloride, 10 mM potassium chloride, 8.4 mM sodium sulfate, and 30 mM HEPES/tris for a final adjusted pH of 7.0 or 8.5. When the adjusted pH was 5.5, 30 mM MES/tris was used instead of HEPES/tris. In all preliminary experiments, the perfusion chamber buffer (serosal medium) was pH 7.0 and the perfusate buffer was pH 5.5, resulting in a proton gradient from lumen to bath. Adjustments to the physiological saline pH were as described in the “Results” section.
Intestinal perfusion technique
In vitro transmural mucosal to serosal transport of Gly-Sar was studied using a perfusion apparatus as described previously (Ahearn and Maginniss 1977). Isolated, intact intestines were flushed with buffered saline solution and mounted onto 18-gauge blunted stainless steel needles. Surgical thread was used to secure both ends to the needles. The perfusion chamber was filled with 35 mL of physiological saline, which served as the serosal medium for the duration of the experiment. The diameter and length of the intestine were measured and used in calculating its surface area with the equation SA = πld, where “SA” equals the calculated surface area in cm2, “l” equals the length and “d” equals the diameter of the intestine in cm. Experimental perfusate made with physiological saline was perfused through the intestine as the mucosal medium using a peristaltic pump (Instech Laboratories, Inc., Plymouth Meeting, PA, US) at a rate of 0.38 ml min−1 for up to 240 min. Various concentrations of l-leucine, zinc chloride, and glycylsarcosine (Sigma Chem. Co., St. Louis, MO) were added to the saline perfusate as needed for each experiment. All experiments were conducted at room temperature (23 °C) with the bath saline mixed by pipette every 15 min for periods of time up to 4 h. Previous electrophysiological and organic solute transport studies with arthropod gut for perfusions up to this length of time at room temperature did not result in significant effects due to apparent substrate depletion as might occur with similar mammal studies conducted at 37 °C (Ahearn 1980; Wyban et al. 1980; Chu 1986; Conrad and Ahearn 2005). Triplicate 200 µL samples were removed from the bath at set time points relative to the starting time, such that each experimental treatment was perfused for 60 min prior to samples being removed. For instance, samples would be taken 60 min after the start of the first treatment perfusion; the second treatment would begin and samples would be taken after 60 additional minutes, and so forth. Samples were placed in labeled microcentrifuge tubes, capped, and stored at 0 °C until analysis by gas chromatography (GC). An equal amount of physiological saline was added to the bath after removing samples to maintain a constant bath volume of 35 mL.
A similar procedure was followed when measuring the in vitro transmural serosal to mucosal transport of Gly-Sar. Experiments were conducted by adding Gly-Sar, zinc chloride, and/or l-leucine to the bath saline. Physiological saline was perfused through the intestine and various experimental treatments were added to the bath after each hour. Triplicate 200 µL samples were removed from the collected perfusate exiting the intestine after each experimental treatment. Since the perfusate effluent accumulated over the duration of the experiment, the total volume of the effluent was measured when samples were taken and used in calculations of Gly-Sar concentrations during sample analysis. Different animals were used to determine mucosal to serosal and serosal to mucosal unidirectional fluxes. To standardize experimental conditions, entire intestines removed from each animal used in both sets of experiments were only obtained from male lobsters in intermolt (Aiken 1973) which were kept unfed for up to 4 days in a seawater aquarium at 12 °C before sacrifice.
Gas chromatography
Once perfusion was complete, samples were prepared for GC analysis using the EZ:faast™ GC-FID Free (Physiological) Amino Acid Analysis Kit (Phenomenex Inc., Torrance, CA, US). Briefly, this kit allows the user to extract amino acids and di- and tripeptides from biological samples, including serum, plasma, and urine and rapidly derivatizes extracted amino acids to yield volatilized products for GC analysis. A standard curve was prepared for each experiment by diluting the experimental perfusate to 50, 200, and 500 µM in fresh saline prior to derivatization. Samples of the experimental perfusate (200 µL) and each of the three standards (glycylsarcosine, glycine, and sarcosine) were placed in labeled microcentrifuge tubes, capped, and stored at 0 °C until analysis. Derivatized treatment and control samples, as well as pure standards, were analyzed by an HP Agilent 6890 series GC-FID system with ChemStation software (Agilent Technologies, Inc., Santa Clara, CA, US). GC analyses were conducted with the column provided by the EZ-faast™ kit, following manufacturer recommendations for GC experimental parameters. GC-FID provides an analog signal, called a chromatogram, that displays ion abundance (pA) versus retention time (Fig. 1).
a Gas chromatogram of a 500 µM glycylsarcosine (Gly-Sar), glycine (Gly), and sarcosine (Sar) saline standard mixture, and b–d of three separate saline bath samples after 3 h of 10 mM glycylsarcosine perfusion through 3 lobster intestines. Glycylsarcosine was transported intact through the lobster intestines and was minimally broken down into glycine and sarcosine. The internal standard used to calculate concentrations in all samples was norvaline (NorV)
GC signals for individual analytes were identified through comparison with retention time for pure derivatized amino acid and dipeptide standards provided with the EZ-fast™ kit, and from Sigma Chem. Co., St. Louis, MO, and Fisher Scientific, Pittsburgh, PA (glycylsarcosine, glycine, sarcosine), as well as sample chromatograms that showed relative retention times for many amino acids (Phenomenex Inc., Torrance, CA, US). For experimental samples, areas were determined for GC signals at the appropriate retention time for individual analytes. These analyte areas were compared to the area of a norvaline internal standard of known concentration, and individual analytes were quantified by comparison of data for experimental samples with a standard curve of known analyte concentrations. A standard curve generated by the analysis of saline standards was included during every perfusion experiment.
The bath volume and intestine surface area were used in further calculations to quantify the amount of transport of each amino acid, measured in nmol cm−2. These concentrations were divided by the duration of treatment to calculate the rate of transport. These rates were displayed as histograms by plotting transport rate in nmol cm−2 min−1 versus experimental treatment. Each experiment was conducted three to five times using intestines from different animals and freshly prepared experimental treatments. All values shown are mean ± SEM. For some experiments, statistical differences between means were calculated using paired Student’s t tests with Bonferroni correction factor using Microsoft Excel and SPSS (IBM Corporation, Armonk, NY, USA). Family-wise significant difference was established at p < 0.05. With the correction factor, p (or α) was divided by the number of treatments (3) to reach a corrected p < 0.02 for significant difference. In other experiments, statistical differences between means were obtained using an ANOVA calculation and post hoc Tukey B and Ryan–Einot–Gabriel–Welsch Range tests.
Results
Chemical form of glycylsarcosine during transmural transport
To assess whether lobster intestine absorbed the dipeptide, glycylsarcosine, as an intact molecule or as its metabolites, glycine and sarcosine, 10 mM Gly-Sar was perfused through intestines for 3 h followed by sampling the serosal bath for substrates appearing from transmural transport. As shown in Fig. 1a, control GC analyses of Gly-Sar, glycine, and sarcosine added as standards to saline exhibit different GC retention times. It took approximately 4 min for the Gly-Sar standard to pass through the GC column, but only 2 min for both glycine and sarcosine when all three substrates were analyzed simultaneously. The three control compounds showed no overlap in their retention times, providing a clear means to distinguish between these three substances. Figure 1b–d clearly shows that in each replicate intestine, only Gly-Sar was found in the serosal bath following a 3 h perfusion, suggesting negligible conversion of the dipeptide to its monomer subunits during transfer across the gut.
Transmural transport of glycylsarcosine is carrier-mediated
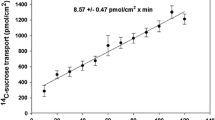
Transmural transport of Gly-Sar was measured over a range of Gly-Sar concentrations from 1 to 15 mM with luminal pH 5.5 and serosal bath pH 7.0. Transport rates were measured sequentially by perfusing a test gut with each dipeptide concentration for 1 h before changing the concentration to the next higher value, taking bath samples at the end of each perfusion interval for analysis. As the luminal concentration of Gly-Sar increased, the transport rate increased in a curvilinear manner consistent with the combination of Michaelis–Menten kinetics and diffusion (Fig. 2). Kinetic constants K m (apparent binding affinity), J max (maximal transport velocity), and D (diffusional permeability) were determined by curve-fitting with Sigma Plot 10.0 software and were 0.44 ± 0.17 mM, 1.27 ± 0.12 nmol cm−2 min−1, and 0.026 ± 0.008 nmol cm−2 min−1 mM−1, respectively.
Effect of increasing luminal glycylsarcosine concentration on the mucosal to serosal transmural transport of glycylsarcosine. Symbols are mean ± SEM of results from four lobster intestines (n = 4). The data were curve-fit with Sigma Plot 10.0 using the equation on the figure that includes both carrier-mediated and diffusion transport components. Values of the resulting kinetic constants are displayed in the figure with their standard errors
Transmural glycylsarcosine transport is inhibited by metal-amino acid chelates
Previous studies with lobster intestine have shown that transmural 3H-glycylsarcosine transport was inhibited by metal-amino acid chelates (His-Zn-His) and the suggestion was made that both chelates and dipeptide used a common carrier mechanism for transfer across the intestinal luminal membrane (Conrad and Ahearn 2005). To assess whether a similar response between a different chelate (Leu-Zn-Leu) and glycylsarcosine occurred in the same tissue and could be analyzed with gas chromatography, 1 mM glycylsarcosine was perfused through an intestine for 1 h, followed by incubation of the dipeptide with 25 µM zinc for a second hour, and finally exposed for the last hour to the dipeptide, metal, and 2 mM l-leucine (Fig. 3). As displayed in this figure, incubation of the dipeptide with zinc alone did not significantly (p > 0.05) affect the rate of glycylsarcosine transport across the tissue, but when both metal and l-leucine were present together a significant (p < 0.02) reduction of Gly-Sar transport from lumen to serosal bath occurred.
Effect of 25 µM Zn2+ and 2 mM l-leucine on 1 mM glycylsarcosine transport. Bars represent the average rate of amino acid transport in nmol cm−2 min−1 for three lobster intestines. Error bars represent ± SEM for the three replicates. Means with different letters denote significant difference between treatments (paired Student’s t test, Bonferroni correction, p < 0.02)
To ensure the metal-amino acid chelate combination was the effective inhibitory unit in the previous experiment, a second, similar experiment was conducted. In this experiment, the 1 h transmural transport of 10 mM Gly-Sar across the intestine was measured first, followed by a 1 h incubation of 10 mM Gly-Sar with 20 mM l-leucine, and finally 10 mM Gly-Sar was exposed to both 20 mM l-leucine and 50 µM zinc. As shown in Fig. 4, an ANOVA analysis, and post hoc Tukey B and Ryan–Einot–Gabriel–Welsch Range tests of the three groups indicated that as a whole the three treatments were significantly different from one another at p < 0.001. These results suggest that the amino acid alone was able to partially inhibit GlySar transport, but that when both the amino acid and metal were present together, as also shown in Fig. 3, the greatest reduction in dipeptide transport was observed. It, therefore, appears that while amino acids alone may be partial inhibitors of dipeptide transport across lobster intestine, zinc without amino acids, had no significant (p > 0.05) influence on GlySar transfer.
Effect of 20 mM l-leucine and 50 µM Zn2+ on 10 mM glycylsarcosine transport. Bars represent the average rate of amino acid transport in nmol cm−2 min−1 for five lobster intestines. Error bars represent ± SEM for the five replicates. Means with different letters denote significant difference between treatments (ANOVA with post hoc Tukey B and Ryan–Einot–Gabriet–Welsch Range tests, p < 0.001)
Net transmural glycylsarcosine transport is inhibited by luminal metal-amino acid chelates by reducing unidirectional mucosal to serosal dipeptide flux
Unidirectional mucosal to serosal (MS) and serosal to mucosal (SM) fluxes of 10 mM Gly-Sar across lobster intestine were independently measured in the presence and absence of cis concentrations of 20 mM l-leucine and 50 µM zinc to determine if a net flux (Net flux = MS–SM) of dipeptide occurred across the tissue and whether it was influenced by metal-amino acid chelates. As shown in Fig. 5, in the absence of either l-leucine or zinc, the MS flux of dipeptide was significantly (p < 0.05) greater than the SM flux by 3.54 ± 0.77 nmol cm−2 min−1, suggesting that under these conditions the lobster intestine displayed an absorptive transfer of this dipeptide from lumen to blood. Figure 5 also shows that the cis occurrence of 20 mM l-leucine plus 50 µM zinc significantly (p < 0.001) inhibited the MS dipeptide flux without affecting the SM flux. Because the presence of luminal 20 mM Leu alone partially reduced MS flux of the dipeptide, there was no significant (p > 0.05) net flux of GlySar across the tissue under these conditions. In addition, there were no significant differences (p > 0.05) between the SM fluxes in all three experimental treatments. These results support the presence of an intestinal dipeptide carrier system that can be inhibited by luminal Leu-Zn-Leu metal-amino acid chelates, thereby reducing net Gly-Sar transport across the tissue. No effect (p > 0.05) of the chelates was observed on SM dipeptide fluxes.
Net flux of glycylsarcosine was calculated from mucosal to serosal (M to S) transport rates and serosal to mucosal (S to M) transport rates under the same treatment conditions. Bars represent the average rate of amino acid transport in nmol cm−2 min−1 for lobster intestines. Error bars represent ± SEM. For M to S replicates (n = 5), the saline treatment was perfused through the intestine and measurements of the normal bath saline were taken. For S to M replicates (n = 3), the saline treatment was in the bath chamber and normal saline was perfused through the intestine. Measurements taken from the perfusate effluent were collected for the duration of the experiment. Means with different letters denote significant difference between treatments (ANOVA with post hoc Tukey B and Ryan–Einot–Gabriet–Welsch Range tests, p < 0.001). Asterisk represents significant difference between M to S and S to M transport rates with 10 mM glycylsarcosine (Student’s t test, p < 0.05)
Effects of luminal pH on transmural glycylsarcosine transport
In addition to the effect of metals and amino acids, the perfusate pH was examined for its effects on dipeptide transport since transmembrane proton concentrations are reported driving forces for peptide uptake into cells (Daniel 2004; Daniel and Kottra 2004). The effect of pH was tested by perfusing saline containing 10 mM glycylsarcosine through the lobster intestine at a range of pH from 5.5 to 8.5, with the bath saline maintained at pH 7.0. No difference (p > 0.05) was found between glycylsarcosine transport rates when the perfused saline was at pH 5.5, 6.5, or 7.5. However, at pH 8.5 the transport rate was significantly (p < 0.02) increased by almost a factor of three compared to the rates observed at the lower pH values (Fig. 6). These results depart considerably from published accounts of the effects of luminal proton concentration on transmembrane transport of dipeptides in vertebrates, and in addition, suggest that lobster intestine may have a more alkaline content that do hepatopancreatic tubules of the same animal known to be acidic and to transport glycylsarcosine from an acidic saline (Thamotharan and Ahearn 1996).
Effect of perfused saline pH on transport rate of 10 mM glycylsarcosine. Bath saline was maintained at pH 7.0 for all treatments. Data represent means from three lobster intestines and error bars represent ± SEM. The asterisk represents a significantly different transport rate between pH 8.5 and the other three pH values used (paired Student’s t test, Bonferroni correction, p < 0.02)
Discussion
Use of gas chromatography to characterize intestinal dipeptide transport
Many investigations examining the nature of transmural nutrient transport by intestinal epithelia employ the use of radioisotopes of the test molecule to measure unidirectional fluxes of solute from one side of the tissue to the other and determine the direction and magnitude of net flux as the difference between the rates in each direction. While this method is able to produce accurate estimates of radioactive fluxes across the tissue, the extent to which a specific intestine may metabolize the radiolabelled solute during transit through the epithelium may not be ascertained by strictly using the isotopic specific activity to quantitatively define molecular traffic. This potential problem may be particularly important in polymeric molecules where cytoplasmic enzymes may hydrolyze bonds between monomeric components of a test solute resulting in the cellular release of radiolabelled monomers rather than the original complex molecule. In the present investigation, gas chromatography was employed to examine the movements of the dipeptide, glycylsarcosine (Gly-Sar), across lobster intestine. Because this technique allows the chemical identification of all molecules crossing the intestine during experiments, the extent of molecular hydrolysis during transit can be assessed and used to characterize the processes involved in transmural movements of a complex substrate. Figure 1 shows that transmural transport of Gly-Sar across lobster intestine occurred without any metabolism of the original luminal substrate. Earlier studies of dipeptide transport suggested that small peptides entering cells might be enzymatically degraded into their amino acid components during the transit process (Rubino et al. 1971). Because no glycine or sarcosine was observed in the serosal bath during transmural fluxes of Gly-Sar in the present study, the conclusion can be reached that the tissue did not metabolize this dipeptide to any measurable extent during absorption. Furthermore, because only the dipeptide was measured in the serosal bath following the perfusion of intact Gly-Sar, it can also be concluded that carrier transport mechanisms for this dipeptide may occur on both luminal and basolateral membranes of lobster intestinal epithelial cells and work together to move the polymer through the tissue. More recent studies of dipeptide transport in other types of epithelia have also suggested that these types of substrates are not necessarily degraded by passage through cell layers (Dyer et al. 1990; Shepherd et al. 2002; Daniel and Kottra 2004). In addition, a number of studies of vertebrates have proposed dipeptide carrier systems on both epithelial poles often displaying significant functional differences (Thamotharan et al. 1996a, b; Herrera-Ruiz and Knipp 2003; Daniel 2004).
Carrier nature of Gly-Sar transport by lobster intestine
Figure 2 shows that transmural Gly-Sar transport across lobster intestine was rate-limited by at least one carrier system with a higher apparent binding affinity (lower K m, 0.44 ± 0.17 mM) than reported for vertebrates from fish to mammals (Thamotharan et al. 1996a, b; Herrera-Ruiz and Knipp 2003; Daniel 2004; Verri et al. 2000, 2010). Furthermore, 14C-glycylsarcosine transport by purified hepatopancreatic brush border membrane vesicles from H. americanus displayed an apparent binding affinity considerably lower (higher K m, 5.90 ± 0.13 mM) than that from the intestine of the same animal, but consistent with vertebrate values (Thamotharan and Ahearn 1996). The sequential increase in apparent binding affinity of the dipeptide transport system from proximal (anterior) to distal (posterior) aspects of the digestive system in the lobster is similar to what has been reported for 3H-d-glucose transport by fish gastrointestinal tract (Ahearn et al. 1992) and mammalian renal proximal tubule (Turner and Moran 1982a, b). In all three instances, the more proximal the transporter, the higher is the concentration of the transported substrate and the lower is the binding affinity of the respective carrier system, allowing maximal withdrawal of substrate during passage through this portion of the tubular system. In contrast, the relatively higher apparent binding affinities of the distal digestive and renal segments act as scavengers, efficiently removing most of the remaining substrate. Such combined sequential absorption processes allow the human renal proximal tubule to reabsorb essentially all d-glucose that passes glomerular filtration. Similar efficient absorption mechanisms may also occur in invertebrate, fish, and mammalian digestive systems due to the sequential coupling of low- and high-affinity carrier proteins.
Figure 2 also shows that in addition to carrier-mediated transcellular transport of Gly-Sar, a second pathway across the tissue was present and its rate appeared to be a linear function of luminal substrate concentration. This process was likely transfer of the dipeptide through the paracellular pathway between epithelial cells and appears to be diffusive in nature. At this time, however, a facilitated diffusion process, with a low apparent substrate binding affinity in the luminal membrane, cannot be ruled out.
Figure 5 clearly shows that there was greater unidirectional flux of Gly-Sar from a 10 mM solution from mucosa to serosa across the gut than occurred from serosa to mucosa, providing evidence for the net absorption of the dipeptide from luminal contents. Because no hydrolysis of the dipeptide to glycine and sarcosine or metabolism to other organic substrates took place (Fig. 1), these results imply that approximately three-fourths of the total unidirectional mucosal to serosal flux (3.54 ± 0.77 nmol cm−2 min−1) of unchanged Gly-Sar occurred through intestinal epithelial cells with the remainder likely transiting the paracellular pathway by diffusion. The data also suggest that unidirectional serosal to mucosal flux of the dipeptide probably only occurred by paracellular diffusional transport, since the rate of transport toward the lumen was unaffected by the simultaneous occurrence of 20 mM l-leucine and 50 µM zinc in the serosal bath. In contrast, unidirectional transport of Gly-Sar toward the serosal bath from the lumen was significantly (p < 0.001) reduced when either high concentrations of the amino acid alone (e.g., 20 mM l-leucine), or the simultaneous combination of the amino acid and metal, were present in the lumen. Significantly greater reduction in mucosal to serosal flux of GlySar was observed when both amino acid and metal were present together in the lumen (p < 0.001) than when only the amino acid was perfused through the lumen. These results suggest that mucosal to serosal flux of dipeptide occurred by a brush border carrier process that was more strongly inhibited by an amino acid dimer linked by zinc bonding (e.g., bis-complex), apparently mimicking a natural dipeptide bond, than by two dissociated monomeric amino acids. It is proposed that all three molecular configurations may share a common binding site.
Figures 3 and 4 provide additional evidence for the presence of a brush border peptide transporter that may be shared by dipeptides, high concentrations of amino acids, and by luminal l-leucine-zinc bis-complexes. The protocols used in these two experiments were complementary and show that the metal alone had no effect on Gly-Sar transport across the tissue. It was only when 20 mM l-leucine alone, or both l-leucine and zinc occurred simultaneously, that unidirectional mucosal to serosal flux of Gly-Sar was reduced as a result of the dipeptide, free amino acids, and bis-complexes potentially competing for transport by the same brush border carrier. Recent work with lobster intestine indicated that Gly-Sar was a mixed inhibitor of transmural mucosa to serosa 3H-l-histidine transport in the presence of Zn2+, the dipeptide affecting both the K m and J max of amino acid transport (Conrad and Ahearn 2005). In addition, this same study showed that luminal addition of 20 µM Zn2+ and 20 µM l-leucine together completely blocked 100 µM 3H-Gly-Sar transport across the gut (Conrad and Ahearn 2005), suggesting that the dipeptide transporter was unable to transfer Gly-Sar at all across the tissue when suitable amino acid bis-complexes co-occurred in the lumen. In contrast to the results of the present investigation showing a partial inhibition of GlySar transport in the presence of 20 mM luminal l-leucine alone, the previous study (Conrad and Ahearn 2005) used a much lower concentration of free l-leucine (20 µM) which did not significantly (p > 0.05) reduce 100 µM 3H-GlySar transport. These results might be explained by the presence of a low affinity shared transport site to free amino acids compared to that of either the dipeptide or bis-complex. While these previous experiments and those presented in this investigation clearly show inhibition between the dipeptide, free amino acids, and bis-complexes involving different amino acids and metals, additional kinetic studies indicating clear competitive interactions between the substrates are needed to strengthen the argument that a single carrier system is at work in all three situations.
The data in Figs. 1, 2, 3, 4, 5 argue that two carrier proteins, binding and transporting Gly-Sar across the tissue from mucosa to serosa, occur in lobster intestinal epithelia cells: a brush border protein that may bind dipeptides, free amino acids, and bis-complexes, and a basolateral facilitated diffusion process that transfers the dipeptide from the cytoplasm to the serosal bath without being influenced by the presence of free amino acids or amino acid-metal bis-complexes in the serosal compartment.
Regulation of Gly-Sar transport across lobster intestine by luminal pH
Dipeptide transport in fish and mammalian intestine occurs by the proton-dependent symporter PEPT1 (Thamotharan et al. 1996a, b; Verri et al. 2000; Herrera-Ruiz and Knipp 2003; Daniel 2004). The driving force for dipeptide accumulation by intestinal epithelial cells is an inwardly directed transapical proton gradient achieved by the co-occurrence on this membrane of a Na+/H+ antiporter (Thwaites et al. 2002; Kennedy et al. 2002). The acidic nature of dipeptide transport by epithelial cells of the digestive system has also been reported for 14C-glycylsarcosine uptake by purified brush border membrane vesicles of lobster hepatopancreas (Thamotharan and Ahearn 1996). This invertebrate dipeptide transporter had an apparent Gly-Sar binding affinity of 5.90 ± 0.12 mM, a high-affinity proton binding site (K H = 235 ± 25 nM; pK = 6.6), and an apparent transport stoichiometry of 1 H+: 1 Gly-Sar.
Figure 6 shows that transport of Gly-Sar across lobster intestine was increased by almost a factor of 3 when the luminal pH was increased from pH 5.5 to pH 8.5 which departed significantly from the effect of pH on dipeptide transport in vertebrate epithelia and in lobster hepatopancreas. The lobster hepatopancreas is known to have a slightly acidic lumen (Gibson and Barker 1979), and an apical, electrogenic, 2Na+/1H+ antiporter has been described in brush border membrane vesicles of this organ (Ahearn et al. 1994) which is able to provide the required symport substrate for dipeptide accumulation. The stimulation of intestinal Gly-Sar transport in lobsters by luminal alkalization suggests that the two absorptive organs in these invertebrates may have different pH values during absorptive activities. A similar enhancing effect of luminal alkaline pH on dipeptide transport was reported in the zebrafish (Danio rerio) (Verri et al. 2003), and these authors suggested that no Na+/H+ antiporter may be present in these fish to power dipeptide accumulation. At present, it is unclear whether lobster and zebrafish intestines share a similar alkaline-dependent dipeptide transport mechanism, and if so, what pH regulatory characteristics it displays.
References
Abdel-Malak R, Ahearn GA (2014) Regulation of transmural transport of amino acid/metal conjugates by dietary calcium in crustacean digestive tract. J Exp Zool Part A 321A:135–143
Ahearn GA (1976) Co-transport of glycine and sodium across the mucosal border of the midgut epithelium in the marine shrimp, Penaeus marginatus. J Physiol 258:499–520
Ahearn GA (1980) Intestinal electrophysiological and transmural ion transport in freshwater prawns. Am J Physiol 239:C1–C10
Ahearn GA, Maginniss LA (1977) Kinetics of glucose transport by the perfused mid-gut of the freshwater prawn, Macrobrachium rosenbegii. J Physiol 271:319–336
Ahearn GA, Behnke RD, Zonno V, Storelli C (1992) Kinetic heterogeneity of Na/d-glucose cotransport in absorptive organs of the teleost gastrointestinal tract. Am J Physiol 263:R1018–R1023
Ahearn GA, Zhuang Z, Duerr J, Pennington V (1994) Role of the invertebrate electrogenic 2Na+/1H+ antiporter in monovalent and divalent cation transport. J Exp Biol 196:319–336
Aiken DE (1973) Proecdysis, setal development, and molt prediction in the American lobster (Homarus americanus). J Fish Res Board Can 30:1334–1337
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286
Chu KH (1986) Glucose transport by the in vitro perfused midgut of the blue crab, Callinectes sapidus. J Exp Biol 123:325–344
Conrad EM, Ahearn GA (2005) 3H-l-Histidine and 65Zn2+ are cotransported by a dipeptide transport system in intestine of lobster, Homarus americanus. J Exp Biol 208:287–296
Conrad EM, Ahearn GA (2007) Transepithelial transport of zinc and l-histidine across perfused intestine of American lobster, Homarus americanus. J Comp Physiol 177:297–307
Daniel H (2004) Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66:361–384
Daniel H, Kottra G (2004) The proton oligopeptide contrasporter family SLC15 in physiology and pharmacology. Pflugers Arch 447(5):610–618
Daniel H, Spanier B, Kottra G, Weitz D (2006) From bacteria to man: Arachaic proton-dependent peptide transporters at work. Physiology 21:93–102
Dyer J, Beechey RB, Gorvel J-P, Smith RT, Wootoon R, Shirazi-Beechey SP (1990) Glycyl-l-proline transport in rabbit enterocyte basolateral membrane vesicles. Biochem J 269:565–571
Gibson R, Barker PL (1979) The decapod hepatopancreas. Oceanogr Mar Biol A Rev 17:285–346
Glover CN, Wood CM (2008) Absorption of copper and copper-histidine complexes across the apical surface of freshwater rainbow trout intestine. J Comp Physiol B 178:101–109
Herrera-Ruiz D, Knipp GT (2003) Current perspectives on established and putative mammalian oligopeptide transporters. J Pharmaceu Sci 92:691–714
Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT (2002) Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflugers Arch 445:139–146
Matthews DM, Payne JW (1980) Transmembrane transport of small peptides. In: Bronner F, Kleinzeller A (eds) Current topics in membranes and transport, vol 14. Academic Press, Newyork, pp 332–425
Obi I, Wells AL, Ortega P, Patel D, Farah L, Zanotto FP, Ahearn GA (2011) 3H-l-leucine transport by the promiscuous crustacean dipeptide-like co-transporter. J Exp Zool A 315(8):465–475
Rønnestad I, Gavaia PJ, Viegas CSB, Verri T, Romano A, Nilsen TO, Jordal A-EO, Kamisaka Y, Cancela ML (2007) Oligopeptide transporter PepT1 in Atlantic cod (Gadus morhua L.) cloning, tissue expression and comparative aspects. J Exp Biol 210:3883–3896
Rubino A, Field M, Shwachman H (1971) Intestinal transport of amino acid residues of dipeptides: Influx of the glycine residue of glycyl-l-proline across mucosal border. J Biol Chem 246(11):3542–3548
Shepherd EJ, Lister N, Affleck JA, Bronk JR, Kellet GL, Collier ID, Bailey PD, Boyd CAR (2002) Identification of a candidate membrane protein for the basolateral peptide transporter of rat small intestine. Biochem Bioph Res Co 296:918–922
Thamotharan M, Ahearn GA (1996) Dipeptide transport by crustacean hepatopancreatic brush-border membrane vesicles. J Exp Biol 199:635–641
Thamotharan M, Gomme J, Zonno V, Maffia M, Storelli C, Ahearn GA (1996a) Electrogenic, proton-coupled, intestinal dipeptide transport in herbivorous and carnivorous teleosts. Am J Physiol 270:R939–R947
Thamotharan M, Zonno V, Storelli C, Ahearn GA (1996b) Basolateral dipeptide transport by the intestine of the teleost Oreochromis mossambicus. Am J Physiol 270:R948–R954
Thwaites DT, Kennedy DJ, Raldua D, Anderson CM, Mendoza ME, Bladen CL, Simmons NL (2002) H+/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na+/H+ exchanger. Gastroenterology 122:1322–1333
Turner RJ, Moran A (1982a) Heterogeneity of sodium-dependent d-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol 242:F406–F414
Turner RJ, Moran A (1982b) Further studies of proximal tubular brush border membrane d-glucose transport heterogeneity. J Membrane Biol 70:37–45
Verri T, Maffia M, Danieli A, Herget M, Wenzel U, Daniel H, Storelli C (2000) Characterization of the H+/peptide cotransporter of eel intestinal brush-border membranes. J Exp Biol 203:2991–3001
Verri T, Kottra G, Romano A, Tiso N, Peric M, Maffia M, Boll M, Argenton F, Daniel H, Storelli C (2003) Molecular and functional characterization of the zebrafish (Danio rerio) PEPT1-type peptide transporter. FEBS Lett 549:115–122
Verri T, Romano A, Barca A, Kottr G, Daniel H, Storelli C (2010) Transport of di- and tripeptides in teleost fish intestine. Aquac Res 41:641–653
Wright SH, Ahearn GA (1997) Nutrient absorption in invertebrates. In: Handbook of physiology (Sect 13: Comparative Physiology), vol. II, Chap. 16, pp 1137–120. Oxford University Press, Oxford
Wyban JA, Ahearn GA, Maginniss LA (1980) Effects of organic solutes on transmural PD and Na transport in freshwater prawn intestine. Am J Physiol 239:C11–C17
Acknowledgments
This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2010-65206-20617 from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Peterson, M.L., Lane, A.L. & Ahearn, G.A. Analysis of glycylsarcosine transport by lobster intestine using gas chromatography. J Comp Physiol B 185, 37–45 (2015). https://doi.org/10.1007/s00360-014-0863-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-014-0863-7