Abstract
The channels commonly responsible for maintaining cell resting membrane potentials are referred to as K2P (two-P-domain K+ subunit) channels. These K+ ion channels generally remain open but can be modulated by their local environment. These channels are classified based on pharmacology, pH sensitivity, mechanical stretch, and ionic permeability. Little is known about the physiological nature of these K2P channels in invertebrates. Acidic conditions depolarize neurons and muscle fibers, which may be caused by K2P channels given that one subtype can be blocked by acidic conditions. Doxapram is used clinically as a respiratory aid known to block acid-sensitive K2P channels; thus, the effects of doxapram on the muscle fibers and synaptic transmission in larval Drosophila and crawfish were monitored. A dose-dependent response was observed via depolarization of the larval Drosophila muscle and an increase in evoked synaptic transmission, but doxapram blocked the production of action potentials in the crawfish motor neuron and had a minor effect on the resting membrane potential of the crawfish muscle. This indicates that the nerve and muscle tissues in larval Drosophila and crawfish likely express different K2P channel subtypes. Since these organisms serve as physiological models for neurobiology and physiology, it would be of interest to further investigate what types of K2P channel are expressed in these tissues. (212 words)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the initial discovery of “K+ ion leak channels,” comprising a family of K+ pores known as K2P (two-P-domain K+ subunit) channels (Lesage et al. 1996a, b; Goldstein 2011; Goldstein et al. 1996; Plant and Goldstein 2015), many subtypes have been identified and characterized. For years, these channels have been recognized as being responsible for maintaining resting membrane potential, as well as for the change in membrane potential observed in relation to external K+ concentration, [K+]o (Nernst 1888, 1889; Goldman 1943; Hodgkin and Katz 1949; Hodgkin and Huxley 1952; Hodgkin et al. 1952; for review see Hille 1992); however, the specific channel protein makeup had not yet been identified. Now described and illustrated with schematics (Kamuene et al. 2021), K2P channels are dimers of subunits. After the initial discovery of the channels’ genetic sequence through genome screening, they have been identified in both yeast (Saccharomyces cerevisiae) and a nematode (Caenorhabditis elegans) (Ketchum et al. 1995; Yang and Jan 2008; Plant and Goldstein 2015), though the genes for these channels occur in plants, animals, yeast, and fungi. Upon discovery of these channels in the genome of Drosophila melanogaster, these K2P channels are referred to as K2PØ, KCNKØ, or dORK channels (Lesage et al. 1996b). It is now known that the human genome contains 15 human genes designated “KCNK” for the K2P channels (Lesage and Barhanin 2011; Yang and Jan 2008; Kamuene et al. 2021). However, even with extensive screening of various organisms and the tissues they contain, there are still large gaps in the current understanding of K2P channel genetic diversity, especially since many organisms have not yet been screened for the genes that might code for these channels. 11 KCNK0 genes code for two K2P channels in Drosophila (Adams et al. 2000; Littleton and Ganetzky 2000), while two members of the K2P family KCNK have thus far been identified in crustacean species C. borealis (CbKCNK1, CbKCNK1) and H. americanus (HaKCNK1, HaKCNK2) (Northcutt et al. 2016). However, there are certainly more to be discovered in various genomes of crustaceans and insects (i.e., arthropods).
Screening of K2P channel expression has been directed towards various pathological conditions or diseases in humans (Lee et al. 2021; Wiedmann et al. 2021). Cancerous tissues and diseases, for example, have been shown to accompany increased expression within some subtypes, or decreased with others; however, it is not yet known whether these altered expressions represent a cause of the pathological conditions or a consequence of them (Lee et al. 2021; Wiedmann et al. 2021). In Drosophila screening, it was shown that dORK1 is expressed in the heart tissue (Buckingham et al. 2005; Lalevée et al. 2006), but recent experimentation has indicated that overexpression of dORK1 in mesodermal tissue (i.e. Drosophila cardiac and skeletal muscle) results in larval death at the second instar stage, demonstrating lethality upon altered expression of this K2P channel (Elliott et al. 2023). Selective overexpression of ORK1 channels in the pupal heart has also been observed to leave the heart tube in a diastolic state, and selective knockdown of ORK1 expression with RNAi in the heart resulted in an increased pupal heart rate, implying that the cardiac cells were depolarized (Lalevée et al. 2006). To confirm this general finding, selective overexpression of dORK1 was carried out in the larval Drosophila heart tube and was observed to result in a depressed heart rate (Elliott et al. 2023).
K2P channels have been identified by how they respond to pH changes (both extra- and intracellularly), mechanical deformation, pharmacological agents, and various metals. More recently, the K2P channel has been described using gene sequence comparisons and protein identification. Protons bind to the histidine residue present in the K2P channel’s protein structure to influence its conformation (Rajan et al. 2000; Lopes et al. 2001; O’Connell et al. 2002). Several extensive reviews have been published on K2P channel subtypes, as well as what can activate and/or block their function (Goldstein et al. 1998; lan and Goldstein 2001; Kim 2005; Enyedi and Czirják 2010; Enyedi and Czirják 2010; Mathie et al. 2010; Noël et al. 2011; Lesage and Barhanin 2011; Kuang et al. 2015; Feliciangeli et al. 2015; Plant and Goldstein 2015; Kamuene et al. 2021).
While many subtypes of K2P channels exist, this investigation has, for many reasons, focused on those that showed sensitivity to low external pH, as well as those that have exhibited transient responses to the Gram-negative bacterial endotoxin lipopolysaccharides (LPS). First, the larval Drosophila body wall muscle and crawfish skeletal muscle both demonstrate pH sensitivity, which suggests that low pH inhibits a subtype of K2P channel present in the muscle. Second, exposure to known K2P-channel-blocker doxapram (trade names: Stimulex or Respiram) results in a depolarization of Drosophila muscle similar to that observed under acidic conditions (Vacassenno et al. 2023a, b). Doxapram initially enhances evoked synaptic responses but, in time, depresses transmission at the crawfish neuromuscular junction (NMJ) (Brock and Cooper 2023). Interestingly, doxapram only slightly depolarizes crawfish skeletal muscle but is speculated to depolarize the motor neuron, producing enhanced transmission until continued depolarization results in inactivation of voltage-gated Na+ channels and, subsequently, depression of evoked nerve stimulation (Brock and Cooper 2023). Since quantal responses are still observed even when evoked transmission is depressed, the postsynaptic glutamate receptors do not seem to be blocked, indicating that doxapram’s effects are presynaptic. It also appears that the rapid hyperpolarization of the larval Drosophila muscle induced by LPS from Serratia marcescens activates a doxapram-sensitive K2P channel (Cooper et al. 2019; Cooper and Krall 2022; Vacassenno et al. 2023a, b), and LPS exposure tends to slow the doxapram-induced depression of evoked transmission at the crawfish NMJ (Brock and Cooper 2023).
One way to better understand the diversity present in K2P channels is through physiological investigation into various organisms, addressing how they are affected by pharmacological agents and alterations to their environment to, in turn, shed light on how these changes affect channel function. No known pharmacological profiling has been conducted for potential K2P channels in crustaceans, and only limited profiling (that through alterations in pH) has been preformed in Drosophila; certain animal models (Drosophila, crawfish, and crab) have been investigated with doxapram (McCubbin et al. 2020; Ison et al. 2022; Cooper and Krall 2022; Vacassenno et al. 2023a, b). Doxapram is used clinically to increase respiration during therapeutic hypothermia and COVID-19 (Sanders et al. 2020; Baxter 1976; Kim 2005; Fathi et al. 2020; Cotten et al. 2006) with some side effects. It has been established that doxapram inhibits the K2P channel subtype called TASK (Cotten et al. 2006; Kim 2005). The acid-sensitivity of this channel, as well as its presence in carotid bodies, allows doxapram to depolarize the membrane potential and drive respiration (Martin 1973; Yost 2006; Flint et al. 2021; Karklus et al. 2021).
Invertebrate models, such as the Drosophila and crawfish models used here, have long been recognized as instruments for studying various physiological processes, from investigating the effects of K+ on membrane potential, to genetically blocking or emphasizing protein expression, to measuring synaptic transmission and the properties of neural electrical activity. These investigations focus on these models to address the variation in response to doxapram reported between the two preparations. Additionally, more descriptions of doxapram’s effects on animal models at the physiological level allow for a better understanding of its actions in general.
Materials and methods
Animals
Drosophila melanogaster Canton S (CS) flies were used in physiological assays. This strain has been isogenic in the laboratory for several years after originally being obtained from the Bloomington Drosophila Stock Center (BDSC). Early third-instar Drosophila CS larvae were used (50–70 h) post-hatching. The CS larvae were maintained at room temperature, ~ 21 °C, in vials partially filled with a cornmeal-agar-dextrose-yeast medium. Overexpression of the ORK1 receptor in larval body wall muscles (m6 and m7) was achieved by crossing homozygous males of P{w[+ mW.hs] = GawB}BG487 (BDSC stock # 51,634) with female virgins of UAS-ORK1 (BDSC stock # 6586; y1 w*; P{w[+ mC] = UAS-Ork1.Delta-C}2), a transgene that lacks the protein’s Ork1 C terminal regulatory domain (Nitabach et al. 2002). Progeny carrying one copy each of GAL4 driver and UAS-ORK1, referred to as body muscle M6-M7 > ORK1, were used for physiological analyses. BG487-Gal4 expression pattern occurs as an anteroposterior gradient in larval body wall muscles 6/7. This allows BG487 to drive UAS–ORK1 specifically in muscles 6 and 7. (Budnik et al. 1996; Sulkowski et al. 2014). UAS-ORK1 alone were used for control comparisons. The line used for expression in motor neurons was D42-Gal4 (w[*]; P{w[+ mW.hs] = GawB}D42 BDSC stock number: 8816, under UAS control. Female virgins of UAS-ORK1 were crossed with males of D42-Gal4. The over expressers are referred to as D42 > ORK1. These Drosophila strains were obtained from the Bloomington Drosophila Stock Center (BDSC).
Red Swamp Crawfish (Procambarus clarkii) were obtained from a distribution center in Atlanta, GA, USA and delivered to a local supermarket in Lexington, KY, USA, whence they were purchased for use in this investigation. When necessary, some were ordered directly from Kyle LeBlanc Crawfish Farms, 302 Saint Peter St., Raceland, LA USA, 70,394. Throughout the study, mid-sized crawfish of 6–10 cm in body length and 12.5–25 g in body weight were used. Each animal was housed in individual standardized plastic containers with dry fish food (exchanged weekly) and aerated water (20–21 °C).
Neuromuscular junction
Drosophila
The early third-instar larval body wall muscle m6 was used to monitor transmembrane potentials with sharp intracellular electrodes (30 to 40 megaOhm resistance) filled with 3 M K-acetate. An Axonclamp 2B (Molecular Devices, Sunnyvale, CA, USA) amplifier and 1 X LU head stage was used. The EJPs and spontaneous mEJPs were collected and analyzed with LabChart 7.0 (ADInstruments, USA) as previously detailed (Cooper et al. 2019). Three experimental paradigms were used to investigate the effects of doxapram at the larval Drosophila NMJ (Fig. 1).
The paradigms used for investigating the effects of doxapram on synaptic transmission and membrane potential of body wall muscle in larval Drosophila. The three paradigms were: (a) monitor the resting membrane potential of muscle fibers and occurrence of spontaneous quantal events before, during, and after exposure to doxapram; (b) monitor the amplitude of the evoked compound EJPs before, during, and after exposure to doxapram while stimulating the segmental nerve at ½ Hz.; (c) monitor the resting membrane potential and amplitude of EJPs before, during, and after exposure to doxapram when the glutamate receptors are desensitized by glutamate
Crawfish: The details of the dissection and electrophysiological recording processes at the opener neuromuscular junction for crawfish walking legs have been described in video format (Cooper and Cooper 2009). Short-term facilitation was induced by providing a train of 25 or 40 stimuli at 40–60 Hz, respectively. The excitatory nerve was stimulated in the meropodite region of the leg with a suction electrode in an isolated motor nerve. Intracellular excitatory junction potential (EJP) recordings were performed by standard procedures (Crider and Cooper 2000). Analysis of responses used the amplitudes of the EJPs from the short-term facilitation pulse train. The amplitudes of the 25th EJPs were measured from the proceeding trough to the peak response. Evoked action potentials were recorded within the excitatory motor neuron that innervates the opener muscle in crayfish with 3 M-KCl-filled microelectrodes. The nerve was stimulated at 1 Hz while the amplitude of the action potential was monitored, both prior to and during doxapram exposure. The technique by which intracellular action potentials were recorded in this preparation is described in detail (He et al. 1999).
Also monitored were the extracellular compound action potentials (CAPs) of the abdominal segments, taken from the second root of the ventral nerve cord innervating the dorsal extensor muscle and muscle receptor organ (MRO); these allowed verification of doxapram’s effect on electrical conduction along nerves. The second root was dissected out of the second or third abdominal segment before being transected at one end near the ganglion and at the other near the nerve as it innervates the dorsal DEL1, DEL2, DEM, SEM, SEL and MRO tissues (Sohn et al. 2000). The nerve was stimulated with a suction electrode on the proximal nerve root and recorded with a suction electrode on the distal end. The nerves were tightly fitted into the opening of the suction electrode using petroleum jelly, allowing for low voltage stimulation and maximal electrical signal recording. The voltage was increased until the CAP’s maximum amplitude was obtained. The nerve was then stimulated with ½ Hz frequency, and the bath exchanged from saline, to one containing doxapram, and through three washes of fresh saline to remove the doxapram (10 mM). The doxapram solution needed to remain on the preparation only for about five minutes before the CAP was no longer discernable. The procedure used to make the suction electrodes is described in video format (Baierlein et al. 2011).
Chemicals
The crawfish saline used was a modified Van Harreveld’s solution (in mM: 205 NaCl, 5.3 KCl, 13.5 CaCl2·2H2O, 2.45 MgCl2·6H2O, and 5 HEPES adjusted to pH 7.4), and so was fly saline haemolymph-like 3(HL3) (de Castro et al. 2014; Stewart et al. 1994): (in mmol/L) 70 NaCl, 5 KCl, 20 MgCl 2, 10 NaHCO 3, 1 CaCl 2, 5 trehalose, 115 sucrose, 25 N, N-bis(2-hydroxyethyl)-2-aminoethane sulfonic acid (BES) and pH at 7.1. Doxapram was dissolved directly in saline to be used. Vigorous vortexing is required to dissolve it as best as possible. All chemicals listed above were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Statistical analysis
Analysis was performed using SigmaStat software. P of ≤ 0.05 was considered statistically significant. Paired t-tests and Sign tests were used for statistical analysis. All averaged data is expressed as a mean (± SEM). A post analysis on effect size was determined to be less than 1. Effect size was determined by the null hypothesis of no change (i.e., 0) minus the mean percent change with the product being divided by the standard deviation of the values for the percent changed obtained. A post-hoc power calculation was not used as that would mean using a mean of sample estimates and not population estimates; however, values were already determined for significance (Zhang et al. 2019) while conducting the experiments, such that pre-determining the number of organisms required was unnecessary.
Results
The larval Drosophila NMJ
The effect of doxapram on the membrane potential was examined without stimulation of the motor nerve. Exposure to doxapram at 10 mM generally resulted in the muscle producing small twitches, as observed in Fig. 2. The membrane potential depolarized in proportion to concentrations of doxapram exposure (Table 1, N = 6, p < 0.05 paired t-test). The membrane potentials were not voltage clamped, allowing their natural values to be observed; this also allowed investigation into the effect of increased K2p channel expression in skeletal muscle. A representative response to exposure of doxapram at 10 mM is shown in Fig. 2.
The effect of doxapram on the resting membrane potential and on the frequency of spontaneous quantal events at the larval Drosophila (CS) neuromuscular junction. In the example shown, the recording is lost while changing the media temporarily until the bathing media is replaced (blue highlighted areas). The muscle tends to twitch upon exposure to 10 mM doxapram, as shown with asterisk(*)
Doxapram’s effect on EJP amplitude and resting membrane potential was investigated using stimulation of a segmental nerve at a frequency of ½ Hz. Surprisingly, as doxapram exposure depolarized the muscle’s resting membrane potential, the EJP amplitude was observed to increase, despite the ionotropic glutamate receptors exhibiting a decrease in driving gradients towards the reversal potential. A representative response is shown in Fig. 3a for the effect of doxapram at 1 mM. Even though the membrane depolarized substantially, the membrane potential was able to recover upon removal of the doxapram. Enlarged views of a few EJPs before, during, and after exposure to doxapram are shown in Fig. 3b, c, and d. Representative responses to doxapram at 2.5 mM, 5 mM, and 10 mM on separate preparations are shown in Figs. 4 and 5, and 6 respectively. The overall effects for various concentrations of doxapram on EJP amplitude and membrane potential are listed in Table 1. Each concentration resulted in depolarization of the muscle membrane potential and an increase in EJP amplitude (N = 6(+), p < 0.05 paired t-test). Each concentration was examined in at least six different preparations, though some concentrations necessitated more.
The effect on membrane potential and amplitude of the evoked EJPs for muscle of larval Drosophila. (a) Overview of a minute’s exposure to 1 mM doxapram, followed by a rinse to remove doxapram from the bath at the larval Drosophila (CS) neuromuscular junction. The blue highlighted area indicates the point at which the preparation was flushed thrice with fresh saline. The arrows indicated as (b), (c), and (d) are regions of the trace enlarged below. Note the amplitude of the EJP is increased during the doxapram exposure. A slight increase in the frequency of spontaneous quantal events was observed under doxapram exposure, as indicated in trace (c), as compared to the trace in (b)
The effect on membrane potential and amplitude of the evoked EJPs during a minute’s exposure to 5 mM doxapram, followed by a rinse to remove doxapram from the bath at the larval Drosophila (CS) neuromuscular junction. The blue highlighted area indicates the point at which the preparation was flushed thrice with fresh saline. Note the amplitude of the EJP is increased during the doxapram exposure. There is an increase in the frequency of spontaneous quantal events with doxapram exposure
The effect on membrane potential and amplitude of the evoked EJPs during a minute’s exposure to 5 mM doxapram, followed by a rinse to remove doxapram from the bath at the larval Drosophila (CS) neuromuscular junction. The blue highlighted area indicates the point at which the preparation was flushed thrice with fresh saline. Note the amplitude of the EJP is increased during the doxapram exposure
The effect on membrane potential and amplitude of the evoked EJPs during a minute’s exposure to 10 mM doxapram, followed by a rinse to remove doxapram from the bath at the larval Drosophila (CS) neuromuscular junction. The blue highlighted area indicates the point at which the preparation was flushed thrice with fresh saline. Note the amplitude of the EJP is increased during the doxapram exposure, as well as the series of downward deflections in the trace that indicate twitching of the muscle fiber. The evoked stimulation was ceased for a short period after the saline flush so as to relax the muscle fiber
To investigate whether doxapram still depolarized the muscle fibers after the desensitization of a large fraction of the glutamate receptors and the depolarization of the muscle, preparations were exposed to glutamate (1 mM) and then a combination of glutamate (1 mM) and doxapram (10 mM). The glutamate exposure depressed the EJP amplitudes, and the subsequent exposure to glutamate-doxapram solution maintained the membrane potential depolarization and amplitude of the EJP depression, as shown in a representative response (Fig. 7). There was a significant depression of the EJP amplitudes upon exposure to glutamate (P < 0.05; N = 7; Sign Test). The membrane potentials initially depolarized as the added glutamate blocked receptors; though this was followed by some desensitization to glutamate, these receptors remained blocked against the glutamate released by nerve stimulation. The membrane potentials observed in each preparation after one minute of glutamate exposure were used to determine percentages of depolarization (-28.01 ± 6.95; Mean ± SEM; N = 7) from saline, after which the percent change from the initial saline to the subsequent depolarization induced by glutamate-doxapram exposure was determined (-41.44 ± 4.1, Mean ± SEM; N = 7). A significant depolarization was observed under both conditions (P < 0.05; paired T-test; N = 7).
The effect of doxapram on the resting membrane potential following desensitization of the glutamate receptors by exposure to 1 mM glutamate at the larval Drosophila (CS) neuromuscular junction. (a) Overview of the membrane potential during exposure to glutamate (1 mM) and then glutamate (1 mM) combined with doxapram (10 mM), followed by a flush with fresh saline. The blue highlighted areas indicate bath exchanges to and from saline. Arrows b and c depict the regions of the enlarged traces shown below. (b) EJPs are present while exposed to glutamate (1 mM) as well as for (c) glutamate (1 mM) combined with doxapram (10 mM)
The overexpression of K2P channels in the motor neuron (D42 > ORK1) was predicted to result in a more pronounced effect on the EJP amplitude with exposure to doxapram than for the UAS-ORK1 alone (no GAL4 driver) or for the wild type CS strain. As illustrated in Table 1, the overall effects were similar, both in the degree of muscle depolarization and EJP increase upon exposure to doxapram for CS, UAS-ORK1 and D42 > ORK1. A representative response for a larval of D42 > ORK1 before, during, and after exposure to doxapram (5 mM) is shown in Fig. 8.
The effect on membrane potential and evoked EJP amplitude at the neuromuscular junctions of larval Drosophila with overexpressed K2p channels in the motor neurons (D42 > ORK1) given a minute’s exposure to 5 mM doxapram, followed by a rinse to remove doxapram from the bath. The blue highlighted areas indicate periods of saline exchange. Note the increase in EJP amplitude during doxapram exposure
The strain exhibiting ORK1 overexpression in muscles m6 & m7 showed a larger enhancement of evoked EJPs under doxapram exposure even though the muscle fibers had a range of resting membrane potentials similar to that observed in the UAS-ORK1 line. In some cases, the muscle fibers even produced action potentials in the muscle fiber (Fig. 9; Table 1). The evoked EJPs showed a higher amplitude variability, even with full recruiting of both Ib & Is motor neurons. This may also account for a decrease in larval survival. A majority of the instars die by the late 3rd instar stage. The dead larvae carcasses suggest that the larvae survive well through 1st and 2nd instar stages but start to die off in the later 3rd instar stage. There are few pupae which form and eclose, but some F1 adults did appear from the cross as M6-M7 > ORK1. Graphical representation of the mean in the percentage changes for each paradigm is shown in Fig. 10.
The crawfish NMJ
The effect of doxapram on synaptic transmission at the NMJ of a crawfish’s walking leg opener muscle was quite different than that at the larval Drosophila NMJ. In both, doxapram exposure (10 mM) resulted in an increase in the EJP amplitude and a decrease within a few minutes of exposure. As shown in a representative response in Fig. 11, the EJPs would progressively fail within a stimulus train until evoked synaptic transmission was completely blocked. This same trend was observed in 8 of 9 preparations (p < 0.05, Sign test). Even with the failure of evoked EJPs, spontaneous quantal events could be observed between stimulus trains. Thus, doxapram was not blocking the postsynaptic glutamate receptors.
In preparations wherein only spontaneously occurring events were recorded (i.e. without nerve stimulation) before and during doxapram exposure (5 mM), the frequency of spontaneous quantal events and changes in resting membrane potential were measured. Changes in resting membrane potential were negligible in some cases but showed a few millivolts’ change in others, so it appears measuring membrane potential without evoking EJPs for this 5 mM exposure did not have a significant effect. Also, the frequency of spontaneous quantal events decreased in 4 of 6 preparations and increased in 2 of 6. There was an average percent change of a depolarization of the membrane potential of 3.1 (SEM ± 1.4; N = 6) and an average percent decrease in the number of quantal events over a minute of 19.6 (SEM ± 41; N = 6). There was not a consistent effect for all 6 preparations upon 5 mM doxapram exposure.
The effect on membrane potential and evoked EJP amplitude at the neuromuscular junctions of larval Drosophila with overexpressed K2p channels in the m6 & m7 muscle fibers (M6-M7 > ORK1) given a minute’s exposure to 5 mM doxapram, followed by a rinse to remove doxapram from the bath. (a) The intracellular recordings were made in segment 2 of m6. The blue highlighted areas indicate saline exchange periods. Note that the amplitude of the EJP is increased during doxapram exposure. (b) The evoked EJPs were variable and produced action potentials within the muscle fiber during doxapram exposure
In another series of experiments, evoked EJPs were examined at the same time as the resting membrane potential. The muscle fibers depolarized when exposed to doxapram (5 mM) in 4 of 6 preparations, though 1 of 6 preparations saw no change (Table 2). Exposure to 5 mM doxapram showed a depression in EJP amplitude in most preparations (4 of 6), but two showed an increase. At 10 mM exposure, 7 of 7 preparations saw decreased EJP amplitudes. When a preparation demonstrated a silencing of EJP responses, it was rapidly flushed with fresh saline in order to recover the EJPs. Generally, the EJP amplitude did not recover after being flushed with fresh saline. It is likely that, at 10 mM, doxapram had a greater effect on the nerve terminal than the muscle fibers, resulting in a failure to excite the nerve for electrical signal conduction. With the wide variation in detection of spontaneous quantal events in saline and during exposure to doxapram, no significant trends were observed for either the 5 or 10 mM exposure to doxapram (Table 2).
The mean percent changes in the number of quantal events, membrane potential, and the EJP amplitudes with exposure to various concentrations of doxapram at larval NMJs of the following strains: CS (Canton S) strain, D42 > ORK1 (overexpressors of K2p channel subtype ORK1 in the motor neurons), M6-M7 > ORK1 (overexpressors of ORK1 in muscles m6/m7), and UAS-ORK1 (a parental strain for the overexpressors to serve as genetic control). Spontaneous quantal events in saline and during exposure to doxapram increased in the overall percentage. All conditions presented a decrease in the percent change for the resting membrane potential and a percent increase in the EJP amplitudes
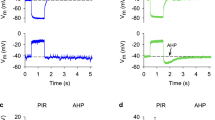
Intracellular recordings of excitatory motor neuron axons were performed to examine whether doxapram altered presynaptic function. In all three preparations, doxapram at 10 mM blocked the production of an action potential in the axon (Fig. 12). Flushing the bath with fresh saline did not regain an action potential. To examine doxapram’s effects further, extracellular recordings were taken of compound action potentials (CAPs) at the the second root of the ventral nerve cord, innervating the dorsal extensor muscle and muscle receptor organ (MRO). The CAP before, during, and after doxapram exposure (10 mM) is illustrated in Fig. 13. Note that the presence of doxapram accompanies a stimulus artifact preceding the extracellular potential and the disappearance of the CAP. In all three preparations, extracellular CAPs were depressed after about three minutes of exposure and were regained after removal of the doxapram (Fig. 13). Thus, six nerve preparations exposed to doxapram saw a loss of action potential conduction in the axon (P < 0.05, Sign test; N = 6).
The facilitated excitatory junction potentials (EJPs) in the opener muscle of the crawfish walking leg, both before and during exposure to 10 mM doxapram during 40 Hz stimulation of the motor neuron. (a) Responses in saline showed a progressive short-term facilitation in EJP amplitude. (b) After 1 min of exposure to doxapram, the nerve’s ability to conduct action potentials was failing; thus, the EJPs are also failing later in the stimulus train. Note that the EJPs exhibit a more rapid degree of facilitation and larger amplitude EJPs than prior to doxapram exposure (i.e. in saline alone). (c) After 3 min of doxapram exposure, the EJPs fail at an earlier point in the stimulation train. Note also that the initial EJPs (i.e. at the start of the stimulus train) are not facilitating as quickly as before
Intracellular recordings of action potentials in an opener motor neuron while exposed to 10 mM doxapram, before, during, and after doxapram exposure (10 mM). Note the stimulus artifact precedes the action potential. Doxapram resulted in a depolarization of the axon and a failure to evoke an action potential
Extracellular recordings of the compound action potential at the second root of the ventral nerve cord, innervating the dorsal extensor muscle and muscle receptor organ (MRO). (a1) An image of the second nerve root. (a2) The stimulating and recording arrangement for evoking compound action potentials (CAPs) along the nerve root. (b) The compound action potential before (1-saline), during (2-Doxapram), and after (3-Saline washout) doxapram exposure (10 mM). Note the stimulus artifact preceding the extracellular potential and the disappearance of the CAP in the presence of doxapram. The region wherein a CAP was obtained is highlighted in blue shading
Discussion
This study was inspired by previous investigations into doxapram’s interactions with the direct effects of lipopolysaccharides (LPS) at the crawfish and Drosophila neuromuscular junctions, since unexpected responses were observed. The mechanism of action of doxapram in mammals acts upon a subtype of acid-sensitive K2P channels, and since low pH also depolarizes the muscle in both crawfish and Drosophila, it was postulated that doxapram would also depolarize the tissues. Low pH and exposure to doxapram both appear to block K2P channels, causing a depolarization of the neurons driving respiratory neural circuitry and enhanced respiration in humans in clinical settings (Martin 1973; Baxter 1976; Kim 2005; Cotten et al. 2006; Yost 2006; Sanders et al. 2020; Fathi et al. 2020; Flint et al. 2021; Karklus et al. 2021). Undoubtedly, other tissues are affected by doxapram and can be discovered through systematic application of the compound. Doxapram’s mechanisms of action more broadly can be investigated using model preparations like the crawfish or the larval Drosophila. Additionally, selectively altered expression of K2p channels in motor neurons or muscles can allow determination of whether doxapram acts on specific subtypes.
The assumed actions of doxapram at the Drosophila and crawfish NMJs, as assembled from the results of investigations both past and present, are modeled here. Doxapram’s effects on the fly NMJ (i.e. muscle depolarization, possible motor neuron depolarization, etc.) were predictable. However, the fact that doxapram exposure brought with it continued EJP amplification was not fully expected, since depolarization could have led to a much wider EJP, spontaneous firing of the motor neuron, and/or the muscle existing in a state of continual contraction. The muscle’s depolarization does not appear to be caused by persistent activation of quisqualate-subtype glutamate receptors. The depolarization of the glutamate receptors upon exposure to glutamate and then, subsequently, the glutamate-doxapram cocktail still results in further depolarization. This implies that doxapram is not activating a current through ionotropic glutamate receptors. It is interesting that EJPs broaden upon desensitization of the glutamate receptors (Fig. 7B, C), which is likely due to the rapid depolarization of one type of glutamate receptor at the Drosophila NMJ but not others (Tour et al. 2000; Heckmann and Dudel 1997).
Overexpression of ORK1 in the motor neurons (D42 > ORK1) did not show any significant differences in evoked EJPs compared to the parental line (UAS-ORK1) or wild type (CS) flies, either when bathed in saline or when exposed to doxapram (5 mM). However, it was not expected that ORK1 overexpression in muscle fibers (M6-M7 > ORK1) would have a similar resting membrane potential as UAS-ORK1, CS, and D42 > ORK1, as it was assumed that a more negative RMP would have been present to be closer to the K+ equilibrium potential. Since LPS can induce a RMP change from − 60 mV to -80 mV, it was assumed that LPS might transiently activate or recruit more K2p channels (Cooper and Krall 2022; Vacassenno et al. 2023a, b). This would be feasible, as experiments with the muscles of both adult Drosophila and moths estimated the K+ equilibrium potential at over − 90 mV (Ikeda et al. 1976; Salkoff and Wyman 1983). It is possible that the muscle maintained a normal membrane potential using ionic pumps and exchangers, but, in doing so, had some retrograde influence on the motor neuron. The large EJP amplitude variation within a saline-bathed preparation of the M6-M7 > ORK1 strain before and during doxapram exposure may be due to changes in synaptic properties within both the motor neuron and muscle. It would be of interest to know whether the muscle motor nerve terminal has normal morphological and physiological properties. Future studies could address the quantal content over nerve terminal regions on m6 & m7 muscles, as well as examining the innervation pattern development and maintenance, as conducted in earlier studies (Li et al. 2002; Harrison and Cooper 2023).
Given that the motor nerve stays viable to evoked stimuli and produces larger EJPs during doxapram exposure, it is inferred that doxapram has a presynaptic action. A generalized model for the larval Drosophila NMJ is presented in Fig. 13.
Schematic model to explain the potential mechanisms of action for doxapram at the larval Drosophila neuromuscular junction. Doxapram blocks K2P channels on both the muscle and the presynaptic nerve terminal, resulting in depolarization of both, which, in turn, results in the opening of more evoked voltage-gated Ca2+ channels, more evoked vesicular fusion events, and a larger amplitude of excitatory junction potential. This even happens while a fraction of the postsynaptic glutamate receptors are desensitized with exogenous glutamate
The proposed action of doxapram at the crawfish NMJ is primarily a presynaptic action, since an acute increase in EJP amplitude was observed just before complete depression. This decrease is caused by the axon’s inability to conduct the evoked action potential. When a preparation was flushed well with fresh saline, the evoked action potential and EJP reappeared. The acute increases of EJP amplitude and frequency of spontaneous quantal events observed in some preparations were likely due to the depolarization of the presynaptic motor nerve terminal. However, any increase in quantal events was short lived. Even while the motor nerve terminal was depolarized during doxapram exposure, the spontaneous quantal events tended to dissipate over time, indicating that doxapram may also depress Ca2+ entry within the presynaptic terminal. Whatever specific mechanisms of action reduced the spontaneous quantal events, the removal of doxapram via flushing reversed the action. The mechanism by which the production and conduction of the action potential were blocked could be that doxapram blocks voltage-gated Na+ channels; alternatively, the blockage might be caused by depolarization of the neuron, keeping the Na+ channels in an inactivated state while the axon remains depolarized.
A generalized model for the crawfish NMJ is presented in Fig. 14. Prior to recording at the motor neuron axon, it was postulated that doxapram might depolarize the inhibitory axon, causing a release of GABA and a blockage of excitatory EJPs. However, given that inhibitory junction potentials (i.e., IJPs) were not observed upon addition of doxapram and that intracellular recordings in the excitatory axon showed a failure of the action potential and CAPs in the segmental abdominal roots, the evoked EJPs may be absent due to an inability to induce action potentials in the axons. One might also assume that the evoked action potential in the inhibitory motor neuron would be blocked by doxapram, but that has not yet been directly examined. The crawfish opener NMJs are a good model for investigation into synaptic transmission as both excitatory and inhibitory inputs exist on the muscle, and as the inhibitory nerve produces presynaptic inhibition on the excitatory nerve terminal (Dudel 1963, 1983; Cooper and Cooper 2009), much like vertebrate preparations’ neural circuitry within the CNS (see Fig. 15).
Schematic model to explain the potential mechanisms of action for doxapram at the crawfish neuromuscular junction. Doxapram has little if any effect on membrane potential of the muscle fiber and does not seem to alter the frequency of spontaneous quantal events. Thus, doxapram may bind to a type of K2P channels that does not contribute to the resting membrane potential in the axon or muscle fiber. It was initially assumed that doxapram depolarized the inhibitor motor neuron, thus blocking the excitatory junction potentials, but intracellular recordings within the axon of motor neurons indicated that doxapram depresses evoked transmission by blocking the motor neuron’s electrical excitability without altering its resting membrane potential. This may occur thanks to doxapram blocking voltage gated Na+ channels in the motor axons
It is surprising that doxapram’s actions were different for the crawfish NMJ as compared to the Drosophila NMJ; larval preparations saw, given exposure to 10 mM doxapram, a prolonged period of time in which EJPs remained larger, a high rate of spontaneous quantal events, and substantial depolarization of the muscle. Since one cannot record intracellularly in the NMJ motor axons of the larvae as one can for the crawfish, it is not known how doxapram affects the shape of the action potential for motor axons in larval Drosophila. As stated for the model, it is likely that doxapram depolarizes the motor neuron, thus allowing for a greater influx of Ca2+ for the evoked responses. This would suggest that the voltage-gated Na+ channels in the motor axons are not inactivated in larval Drosophila as might be the case in crawfish preparations.
The NMJs of crawfish and larval Drosophila are similar in many ways, given that both are arthropods and both produce graded postsynaptic responses. In both NMJs, the postsynaptic receptors are ionotropic glutamate channels and pharmacologically defined as a quisqualate subtype (Titlow and Cooper 2018). However, the resting membrane potentials of the muscles are affected differently by doxapram, and so is evoked synaptic transmission. Given that doxapram blocks both human TASK-1 and TASK-3 acid-sensitive K2p channels in humans (Cunningham et al. 2020) and that the crawfish and larval Drosophila muscles will depolarize given lowered pH, it is likely that the K2P subtypes are different as well as that the density of subtypes. Since doxapram (10 mM) depolarizes the muscle in Drosophila but does not bring the membrane potential to zero, it is implied that other K2P channel subtypes may be responsible for maintaining the depolarized state of the membrane potential. It has not yet been established how many of the 11 known KCNKØ genes are expressed in various Drosophila tissues (Adams et al. 2000; Littleton and Ganetzky 2000); also, the genes for K2P channel expression in crustacean muscles have yet to be identified.
The initial reasoning behind examination of doxapram’s effect on these two model preparations was to determine if it would block the responses induced by lipopolysaccharides (LPS) by Serratia marcescens (Cooper and Krall 2022; Vacassenno et al. 2023a, b). Exposure to this specific type of LPS (i.e. from Serratia marcescens) results in hyperpolarization of the resting membrane potential in both larval Drosophila and crawfish, though the former occurs to a greater extent. Additionally, LPS blocks the glutamatergic receptors on the larval Drosophila muscle (Cooper and Krall 2022), thus inhibiting spontaneous quantal synaptic transmission. However, LPS promotes synaptic transmission at the crawfish NMJ by increasing EJP amplitude. Doxapram blocks the effect of LPS in both preparations, but it likely does not block the direct effect of LPS in the crawfish preparations, since doxapram’s mechanisms are different from LPS’s in that organism. However, it does appear that doxapram is targeting the mechanisms by which LPS acts in the larval Drosophila, as it can block LPS-induced hyperpolarization and reduces blockage of the postsynaptic glutamate receptors. The action of LPS on the larval muscle may transiently activate K2P channels on the muscle, but LPS action on the glutamate receptor has a different effect. Doxapram was not expected to dampen LPS-induced blockage of the glutamate receptors at the Drosophila NMJ without exhibiting the same response at crawfish NMJs.
This investigation is important because it provides insight into potential blockers of K2P channels through the use of two different invertebrate model preparations known to be good representations of synaptic transmission and physiology. It appears the K2P channel potentially responsible for acidic sensitivity on the crayfish muscle is not affected by doxapram but is in the muscle of larval Drosophila. Interestingly, doxapram appears to act differently on the motor neurons of Drosophila and crayfish despite so many similarities in basic neuronal function of these two arthropod species. The findings in this study emphasize the need to better understand the expression profiles of the K2P subtypes in various tissues, their roles in cellular physiology, and their pharmacological profiling.
Data availability
Data is provided within the manuscript and available upon request.
References
Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE et al (2000) The genome sequence of Drosophila melanogaster. Science 287(5461):2185–2195. https://doi.org/10.1126/science.287.5461.2185
Baierlein B, Thurow AL, Atwood HL, Cooper RL (2011) Membrane potentials, synaptic responses, neuronal circuitry, neuromodulation and muscle histology using the crayfish: student laboratory exercises. J Vis Exp 47:e2322. https://doi.org/10.3791/2322
Baxter AD (1976) Side effects of doxapram infusion. Eur J Intensive Care Med 2(2):87–88. https://doi.org/10.1007/BF01886121
Brock KE, Cooper RL (2023) The effects of doxapram blocking the response of Gram-negative bacterial toxin (LPS) at glutamatergic synapses. BIOLOGY 12(8):1046. https://doi.org/10.3390/biology12081046
Buckingham SD, Kidd JF, Law RJ, Franks CJ, Sattelle DB (2005) Structure and function of two-pore-domain K + channels: contributions from genetic model organisms. Trends Pharmacol Sci 26:361–367. https://doi.org/10.1016/j.tips.2005.05.003
Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M (1996) Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron 17(4):627–640. https://doi.org/10.1016/s0896-6273(00)80196-8
Cooper AS, Cooper RL (2009) Historical view and physiology demonstration at the nmj of the crayfish opener muscle. J Vis Exp 33:e1595. https://doi.org/10.3791/1595
Cooper RL, Krall RM (2022) Hyperpolarization induced by lipopolysaccharides but not by chloroform is inhibited by doxapram, an inhibitor of two-p-domain k+ channel (K2P). Int J Mol Sci 23:15787. https://doi.org/10.3390/ijms232415787
Cooper RL, McNabb M, Nadolski J (2019) The effects of a bacterial endotoxin LPS on synaptic transmission at the neuromuscular junction. Heliyon 5:e01430. https://doi.org/10.1016/j.heliyon.2019.e01430
Cotten JF, Keshavaprasad B, Laster MJ, Eger EI 2, Yost CS (2006) The ventilatory stimulant doxapram inhibits TASK tandem pore (K2P) potassium channel function but does not affect minimum alveolar anesthetic concentration. Anesth Analg 102(3):779–785. https://doi.org/10.1213/01.ane.0000194289.34345.63
Crider ME, Cooper RL (2000) Differential facilitation of high- and low output nerve terminals from a single motor neuron. J Appl Physiol 88:987–996. https://doi.org/10.1152/jappl.2000.88.3.987
Cunningham KP, MacIntyre DE, Mathie A, Veale EL (2020) Effects of the ventilatory stimulant, doxapram on human TASK-3 (KCNK9, K2P9.1) channels and TASK-1 (KCNK3, K2P3.1) channels. Acta Physiol (Oxf) 228(2):e13361. https://doi.org/10.1111/apha.13361
de Castro C, Titlow J, Majeed ZR, Cooper RL (2014) Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J Comp Physiol A 200(1):83–92. https://doi.org/10.1007/s00359-013-0864-0
Elliott ER, Taul AC, Abul-Khoudoud MO, Hensley N, Cooper RL (2023) Effect of doxapram, bacterial endotoxin and pH on heart rate: Larval Drosophila model. Appl Biosci 2:406–420. https://doi.org/10.3390/applbiosci2030026
Enyedi P, Czirják G (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90:559–605. https://doi.org/10.1152/physrev.00029.2009
Fathi M, Massoudi N, Nooraee N, Beheshti Monfared R (2020) The effects of doxapram on time to tracheal extubation and early recovery in young morbidly obese patients scheduled for bariatric surgery: a randomised controlled trial. Eur J Anaesthesiol 37(6):457–465. https://doi.org/10.1097/EJA.0000000000001144
Feliciangeli S, Chatelain FC, Bichet D, Lesage F (2015) The family of K2P channels: salient structural and functional properties. J Physiol 593(12):2587–2603. https://doi.org/10.1113/jphysiol.2014.287268
Flint RB, Simons SHP, Andriessen P, Liem KD, Degraeuwe PLJ, Reiss IKM et al (2021) The bioavailability and maturing clearance of doxapram in preterm infants. Pediatr Res 89(5):1268–1277. https://doi.org/10.1038/s41390-020-1037-9
Goldman DE (1943) Potential, impedance, and rectification in membranes. J Gen Physiol 27:37–60. https://doi.org/10.1085/jgp.27.1.37
Goldstein SA (2011) K2P potassium channels, mysterious and paradoxically exciting. Sci Signal 4(184):pe35. https://doi.org/10.1126/scisignal.2002225
Goldstein SA, Price LA, Rosenthal DN, Pausch MH (1996) ORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster by expression in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 93(23):13256–13261. https://doi.org/10.1073/pnas.93.23.13256. Erratum in: Proc Natl Acad Sci U S A 1999;96(1):318
Goldstein SA, Wang KW, Ilan N, Pausch MH (1998) Sequence and function of the two P domain potassium channels: implications of an emerging superfamily. J Mol Med (Berl) 76:13–20. https://doi.org/10.1007/s001090050186
Harrison DA, Cooper RL (2023) Characterization of development, behavior and neuromuscular physiology in the phorid fly, Megaselia scalaris. Comp Biochem Physiol A 136(2):427–439. https://doi.org/10.1016/s1095-6433(03)00200-9
He P, Southard RC, Whiteheart SW, Cooper RL (1999) Role of α-SNAP in promoting efficient neurotransmission at the crayfish neuromuscular junction. J Neurophysiol 82:3406–3416. https://doi.org/10.1152/jn.1999.82.6.3406
Heckmann M, Dudel J (1997) Desensitization and resensitization kinetics of glutamate receptor channels from Drosophila larval muscle. Biophys J 72(5):2160–2169. https://doi.org/10.1016/S0006-3495(97)78859-3
Hille B (1992) Ionic channels of excitable membranes, 2nd edn. Sinauer Assoc, Sunderland, Mass, USA
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Lond) 117:500–544. https://doi.org/10.1007/BF02459568
Hodgkin AL, Katz B (1949) The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol 108:37–77. https://doi.org/10.1113/jphysiol.1949.sp004310
Hodgkin AL, Huxley AF, Katz B (1952) Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol (Lond) 116:424–448. https://doi.org/10.1113/jphysiol.1952.sp004716
Ikeda K, Ozawa S, Hagiwara S (1976) Synaptic transmission reversibly conditioned by single-gene mutation in Drosophila melanogaster. Nature 259:489–491. https://doi.org/10.1038/259489a0
ISBN 9780199847839
Ison BJ, Abul-Khoudoud MO, Ahmed SM, Alhamdani AW, Ashley C, Bidros PC et al (2022) The effect of Doxapram on proprioceptive neurons: invertebrate model. NeuroSci 3:566–588. https://doi.org/10.3390/neurosci3040041
Kamuene JM, Xu Y, Plant LD (2021) The pharmacology of two-pore domain potassium channels. Handb Exp Pharmacol 267:417–443. https://doi.org/10.1007/164_2021_462
Karklus AA, Sladky KK, Johnson SM (2021) Respiratory and antinociceptive effects of dexmedetomidine and doxapram in ball pythons (Python regius). Am J Vet Res 82(1):11–21. https://doi.org/10.2460/ajvr.82.1.11
Ketchum KA, Joiner WJ, Sellers AJ, Kaczmarek LK, Goldstein SA (1995) A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature 376(6542):690–695. https://doi.org/10.1038/376690a0
Kim D (2005) Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des 11(21):2717–2736. https://doi.org/10.2174/1381612054546824
Kuang Q, Purhonen P, Hebert H (2015) Structure of potassium channels. Cell Mol Life Sci 72(19):3677–3693. https://doi.org/10.1007/s00018-015-1948-5
Lalevée N, Monier B, Sénatore S, Perrin L, Sémériva M (2006) Control of cardiac rhythm by ORK1, a Drosophila two-pore domain potassium channel. Curr Biol 16(15):1502–1508. https://doi.org/10.1016/j.cub.2006.05.064
lan N, Goldstein SA (2001) KCNKO: single, cloned potassium leak channels are multi-ion pores. Biophys J 80(1):241–253. https://doi.org/10.1016/S0006-3495(01)76010-9
Lee LM, Müntefering T, Budde T, Meuth SG, Ruck T (2021) Pathophysiological role of K2P channels in human diseases. Cell Physiol Biochem 55(S3):65–86. https://doi.org/10.33594/000000338
Lesage F, Barhanin J (2011) Molecular physiology of pH-sensitive background K(2P) channels. Physiol 26(6):424–437. https://doi.org/10.1152/physiol.00029.2011
Lesage F, Reyes R, Fink M, Duprat F, Guillemare E, Lazdunski M (1996a) Dimerization of TWIK-1 K + channel subunits via a disulfide bridge. EMBO J 15(23):6400–6407 PMID: 8978667
Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J (1996b) A pH-sensitive yeast outward rectifier K + channel with two pore domains and novel gating properties. J Biol Chem 271(8):4183–4187. https://doi.org/10.1074/jbc.271.8.4183
Li H, Peng X, Cooper RL (2002) Development of Drosophila larval neuromuscular junctions: maintaining synaptic strength. Neurosci 115(2):505–513. https://doi.org/10.1016/s0306-4522(02)00380-9
Littleton JT, Ganetzky B (2000) Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron 26(1):35–43. https://doi.org/10.1016/s0896-6273(00)81135-6
Lopes CM, Zilberberg N, Goldstein SA (2001) Block of Kcnk3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. J Biol Chem 276(27):24449–24452. https://doi.org/10.1074/jbc.C100184200
Martin JL (1973) Clinical evaluation of doxapram hydrochloride, a respiratory stimulant. J Okla State Med Assoc 66(12):481–487 PMID: 4762565
Mathie A, Al-Moubarak E, Veale EL (2010) Gating of two pore domain potassium channels. J Physiol 588(17):3149–3156. https://doi.org/10.1113/jphysiol.2010.192344
McCubbin S, Jeoung A, Waterbury C, Cooper RL (2020) Pharmacological profiling of stretch activated channels in proprioceptive neuron. Comp Biochem Physiol C 233:108765. https://doi.org/10.1016/j.cbpc.2020.108765
Nernst WH (1888) Zur Kinetik Der in Lösung Befindlichen Körper: Theorie Der Diffusion. Z Phys Chem 3:613–637
Nernst WH (1889) Die elektromotorische Wirksamkeit Der Ionen. Z Phys Chem 4:129–181
Nitabach MN, Blau J, Holmes TC (2002) Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell 109(4):485–495. https://doi.org/10.1016/s0092-8674(02)00737-7
Noël J, Sandoz G, Lesage F (2011) Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin) 5(5):402–409. https://doi.org/10.4161/chan.5.5.16469
Northcutt AJ, Lett KM, Garcia VB, Diester CM, Lane BJ, Marder E, Schulz DJ (2016) Deep sequencing of transcriptomes from the nervous systems of two decapod crustaceans to characterize genes important for neural circuit function and, modulation. BMC Genomics 17(1):868. https://doi.org/10.1186/s12864-016-3215-z
O’Connell AD, Morton MJ, Hunter M (2002) Two-pore domain K + channels-molecular sensors. Biochim Biophys Acta 1566(1–2):152–161. https://doi.org/10.1016/s0005-2736(02)00597-7
Plant LD, Goldstein SAN. Two-Pore Domain Potassium Channels, Handbook of Ion Channels. Edition 1st Edition, CRC, Press (2015) ISBN 9780429193965 Zheng, J., & Trudeau, M.C. (Eds.)
Rajan S, Wischmeyer E, Xin Liu G, Preisig-Müller R, Daut J, Karschin A, Derst C (2000) TASK-3, a novel tandem pore domain acid-sensitive K + channel. An extracellular histiding as pH sensor. J Biol Chem 275(22):16650–16657. https://doi.org/10.1074/jbc.M000030200
Salkoff LB, Wyman RJ (1983) Ion currents in Drosophila flight muscles. J Physiol 337:687–709. https://doi.org/10.1113/jphysiol.1983.sp014649
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID-19). Rev JAMA 323(18):1824–1836. https://doi.org/10.1001/jama.2020.6019
Sohn J, Mykles DL, Cooper RL (2000) Characterization of muscles associated with the articular membrane in the dorsal surface of the crayfish abdomen. J Exp Zool 287(5):353–377 PMID: 10980494
Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF (1994) Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A 175:179–191. https://doi.org/10.1007/BF00215114
Sulkowski M, Kim YJ, Serpe M (2014) Postsynaptic glutamate receptors regulate local BMP signaling at the Drosophila neuromuscular junction. Develop 141(2):436–447. https://doi.org/10.1242/dev.097758
Titlow JS, Cooper RL (2018) Glutamatergic synthesis, recycling, and receptor pharmacology at Drosophila and Crustacean Neuromuscular junctions. In: Parrot S, Denoroy L (eds) Biochemical approaches for glutamatergic neurotransmission. Neuromethods, vol 130. Humana, New York, NY, pp 263–291. https://doi.org/10.1007/978-1-4939-7228-9_9
Tour O, Parnas H, Parnas I (2000) On the mechanism of desensitization in quisqualate-type glutamate channels. J Neurophysiol 84(1):1–10. https://doi.org/10.1152/jn.2000.84.1.1
Vacassenno RM, Haddad CN, Cooper RL (2023a) The effects on resting membrane potential and synaptic transmission by Doxapram (blocker of K2P channels) at the Drosophila neuromuscular junction. Comp Biochem Physiol C 263:109497. https://doi.org/10.1016/j.cbpc.2022.109497
Vacassenno RM, Haddad CN, Cooper RL (2023b) Bacterial lipopolysaccharide hyperpolarizes the membrane potential and is antagonized by the K2p channel blocker doxapram. Comp Biochem Physiol C 266:109571. https://doi.org/10.1016/j.cbpc.2023.109571
Wiedmann F, Frey N, Schmidt C (2021) Two-Pore-Domain potassium (K2P-) channels: cardiac expression patterns and disease-specific remodelling processes. Cells 10(11):2914. https://doi.org/10.3390/cells10112914
Yang S-B, Jan LY (2008) Thrilling moment of an inhibitory channel. Neuron 58:823–824. https://doi.org/10.1016/j.neuron.2008.06.003
Yost CS (2006) A new look at the respiratory stimulant doxapram. CNS Drug Rev 12(3–4):236–249. https://doi.org/10.1111/j.1527-3458.2006.00236.x
Zhang Y, Hedo R, Rivera A, Rull R, Richardson S, Tu XM (2019) Post hoc power analysis: is it an informative and meaningful analysis? Gen Psychiatr 32(4):e100069. https://doi.org/10.1136/gpsych-2019-100069
Acknowledgements
We thank Stacey A. Slone with Predictive Analytics and Data Science (PADS) Hub at the University of Kentucky for statistical services.
Funding
College of Arts and Sciences Summer Research Fellowship and a Gertrude Flora Ribble Scholarship from the Department of Biology at the University of Kentucky (E.R.E.). Beckman Scholarship (K.E.B.). Chellgren Endowed Funding (R.L.C.). Alumni of the research group (RLC).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written in parts by all authors and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Uwe Homberg.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elliott, E.R., Brock, K.E., Vacassenno, R.M. et al. The effects of doxapram and its potential interactions with K2P channels in experimental model preparations. J Comp Physiol A (2024). https://doi.org/10.1007/s00359-024-01705-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00359-024-01705-6