Abstract
Behavioral hearing thresholds and noise localization acuity were determined using a conditioned avoidance/suppression procedure for three Helmeted guineafowl (Numida meleagris). The guineafowl responded to frequencies as low as 2 Hz at 82.5 dB SPL, and as high as 8 kHz at 84.5 dB SPL. At a level of 60 dB SPL, their hearing range spanned 8.12 octaves (24.6 Hz–6.86 kHz). Like most birds, they do not hear sounds above 8 kHz. However, the guineafowl demonstrated good low-frequency hearing (frequencies below 32 Hz), showing thresholds that are more sensitive than both the peafowl and pigeon, both of which hear infrasound. It thus appears that infrasound perception may be more common than previously thought and may have implications for species that inhabit areas with wind energy facilities. The guineafowls’ minimum audible angle for a 100-ms broadband noise burst was 13.8 °, at the median for birds and near the mean for mammals. Unlike in mammals, the small sample of bird species and limited representation of lifestyles do not yet allow for meaningful interpretations of the selective pressures or mechanisms that underlie their abilities to locate sound sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The low-frequency sensitivity of mammals has been widely investigated revealing that mammals fall into two non-overlapping groups, one that hears low frequencies and one that does not. However, few mammals hear lower than humans (infrasound) (Heffner et al. 2022). Unlike mammals, surprisingly little is known about the low-frequency sensitivity of birds (Heffner et al. 2020). It has been more than 40 years since Kreithen and Quine’s astonishing report (1979) that pigeons hear as low as 2 Hz. Yet there had been little further investigation for the next 35 years of the ability of birds to hear below about 125 Hz—a gap of about six octaves. More recently, low-frequency hearing in birds has been pursued by determining the lowest frequencies detectable to a level of at least 60 dB SPL (Sound Pressure Level). Surprisingly both domestic chickens and peafowl have been shown to hear as well as or better than pigeons at low frequencies, whereas other birds (bullfinches, canaries, red-winged blackbirds, brown-headed cowbirds, and kea parrots) hear only as low as 80—350 Hz (Schwartzkopff 1949; Heinz et al. 1977; Okanoya and Dooling 1987; Heffner et al. 2013a, b; Hill et al. 2014; Schwing et al. 2016). Nevertheless, our knowledge of low-frequency hearing in birds remains limited and not representative of either phylogeny or lifestyle, leaving us with little understanding of either the adaptive advantages of low-frequency hearing, or its mechanisms (cf. Zeyl et al. 2020, 2023).
Because of the theoretical importance of low-frequency hearing for understanding the evolutionary adaptations and anatomical mechanisms underlying hearing in birds and the recent interest in the potential impact of low-frequency noise from wind turbines, we chose to examine the hearing of Helmeted guineafowl (Numida meleagris), a species in which midbrain neurons have been shown to respond to frequencies as low as 5 Hz (Theurich et al. 1984). We here report their behavioral audiogram between 2 Hz and 8 kHz. Furthermore, as there is also limited data on sound localization in birds, and because their localization cues are affected by the presence of an interaural canal (Bierman et al. 2014; Christensen-Dalsgaard 2011), which is not generally found in mammals (cf. Mason 2016), we took this opportunity to also determine the minimum audible angle for 100 ms broadband noise in guineafowl.
Method
Subjects
Helmeted guineafowl are in the order Galliformes along with chickens, pheasants, and quail. They belong to the earliest branching family, Numididae, within that order. They are large (2 kg) ground-dwelling omnivorous birds native to Africa, preferring open habitats of savannas and farmland. Their communication calls are not known to involve low frequencies (Maier et al. 1983).
Three Helmeted guineafowl (Numida meleagris), obtained from a local breeder, one male (labeled A) and two females (labeled B and C), were used in this study. They were group-housed in a room with free access to water. Game bird feed (Purina Game Bird crumbles) was used as a reward and the birds were weighed daily when on test to monitor their health and deprivation status. All birds were 6 months old and of mature weights (ranging from 1.68 to 1.95 kg) at the beginning of training.
Apparatus
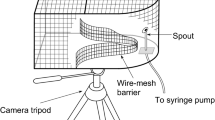
Training and testing were conducted in a double-walled sound chamber (Industrial Acoustic Co. 1204; 2.55 × 2.75 × 2.05 m), the walls and ceiling of which were lined with eggcrate foam and the floor carpeted to reduce sound reflections and floor vibrations. The birds were placed in a cage (50L × 30W × 42H cm) constructed of one-inch (2.54 cm) wire mesh, and mounted 92 cm above the floor on a tripod. The bottom of the cage was lined with two layers of thick carpeting (approx. 2.5 cm) to further prevent substrate-borne vibrations. A contact switch, consisting of a clear plastic disk (3 cm diameter, 1 mm thick) with an embedded red LED served as the response key. This key was mounted at the front of the cage, 37 cm above the cage floor. The lighted LED was momentarily switched off when the key was pecked, providing feedback to the bird. Access to the reward was provided using a solenoid-controlled food hopper that, when triggered, would come up at the bottom of the cage to allow the bird to feed for 2 s. As the entire feeder mechanism was placed below the cage floor, it did not interfere with the sound field.
Behavioral procedure
A conditioned suppression/avoidance procedure was used to determine both the bird’s audiogram and minimum audible angle (MAA) for broadband noise. In the audiogram task, the birds were trained to continuously peck the response key (when no sound was presented) to obtain access to food at 2-s trial intervals, and to stop pecking in the presence of a tone to avoid a mild electric shock (1.5 s duration, 0.3–0.8 mA), delivered via wire leads from the top of the cage to bead chains around the base of the bird’s wings.
Pecking rate generally varied between 3 and 5 times per 2-s trial (depending on the individual bird) when no sound was presented. A 25-W light bulb (placed 30 cm below and in front of the peck key), was turned on concurrently with the shock. Similarly, in the noise localization task, the birds were trained to continue pecking the key when the noise burst emanated from their right side, and to stop pecking when the noise was presented from a speaker to their left. Thus, in this procedure, the shock was avoidable, and the birds were rewarded for both hits and correct rejections, but not for misses and false alarms. The conditioned suppression/avoidance procedure has been used successfully to test birds as well as mammals (e.g., Heffner et al. 2013a, b, 2016, 2020; Hill et al. 2014).
Audiogram
Suppressing pecking when a tone was presented indicated that the bird had detected the sound and it was rewarded with access to food. If the bird continued pecking during a tone, a shock was delivered.
Pure tones were generated, pulsed, and gated on/off at zero crossing using Tucker-Davis Technologies (TDT) equipment and associated RPvds software. The output of the processor (TDT Real-Time Processor RP2) was then routed to an attenuator (TDT PA4), filtered (± 1/3 octave; Krohn-Hite 3550), amplified (Crown D75A), monitored on an oscilloscope (Tektronix TDS 210), and sent to the loudspeaker. Various loudspeakers, ranging from a 15-in (38.1 cm) subwoofer for frequencies 2–32 Hz, a 12-in (30.5 cm) woofer for frequencies 62.5–125 Hz, a 5.5-in woofer (14 cm) for frequencies 250 Hz–2 kHz, and a piezo tweeter for frequencies 2.8–8 kHz, were used to present the sounds. To allow at least 4 cycles of a tone during each stimulus, frequencies of 125 Hz and below were presented as single pulses (2 s on, 100 ms rise-decay), while frequencies from 250 to 750 Hz were presented using 2 pulses (900 ms on, 100 ms off, 50 ms rise-decay). Higher frequencies, from 1 to 8 kHz, were presented as 4 pulses (400 ms on, 100 ms off, 10 ms rise-decay).
All speakers were placed at least 1 m in front of the test cage, at the height of the response key. Unlike the placement of other speakers to directly face the bird (0° incidence), the subwoofer was turned 180° to prevent the bird from cueing to the movement of the speaker diaphragm. An added benefit to this arrangement was that intensity of the low-frequency signals (2–32 Hz) were boosted as much as 6 dB by standing waves, thus increasing the saliency of the signal at the beginning of the test.
The sound pressure level (SPL re 20 μN/m2) of the stimulus was measured using a 1-inch (2.54 cm) microphone (Bruel & Kjaer 4145, calibrated down to 2 Hz), measuring amplifier (Bruel & Kjaer 2610), and a spectrum analyzer (Zonic A&D 3525 FFT Analyzer) to verify the signal and check for overtones. This measuring system was calibrated with a pistonphone (Bruel & Kjaer 4220).
Sound measurements were taken by placing the microphone in the region occupied by a guineafowl’s head when it was pecking the response key and pointing it directly at the loudspeaker (0° incidence). Multiple readings were taken within the area of the bird’s head to further ensure that sound intensity was uniform within that space. No correction for orientation was required when measuring low frequencies presented from the subwoofer (2–32 Hz), as the microphone was omnidirectional at these frequencies.
A session consisted of a series of 2-s trials, each initiated by the first key peck, which also ensured that the guineafowl’s head was directly in front of the loudspeaker and the bird was attending to the task. The response of a bird was defined by whether or not it pecked during the 300-ms period immediately following the 2-s trial. If the bird suppressed pecking during this 300-ms period, a response was recorded. The response was classified as a hit if a tone was presented and as a false alarm if there was no tone. Pecking during the 300-ms period after a tone trial was scored as a miss, followed by a 1.5-s shock. The bird gained a 2-s access to food at the end of a trial if it had made a correct response, i.e., it pecked continuously during a silent trial (correct rejection) or it stopped pecking during a tone trial (hit).
Each trial had a 22% probability of containing a tone (for a discussion of trial sequences, see Heffner and Heffner 1995). Total number of trials varied between 40 and 70 tone trials (and approx. 160 –280 associated silent trials) per session, depending on the amount of food each bird had received in the previous session or during weekends on free feed. However, once trained, a threshold could be obtained for each bird in each session lasting 30–50 min.
Thresholds were determined by reducing the intensity of a tone in 5 dB steps until the bird could no longer detect it above chance level (p > 0.01, binomial distribution). At suprathreshold levels, fewer tone trials (usually four) per intensity were presented, while at intensities ranging from about 20 dB above to 10 dB below threshold, the number of trials per intensity was increased to eight. Hit and false alarm rates were determined for each block of tone and associated silent trials at each intensity. The hit rate was corrected for the false alarm rate to produce a performance measure according to the following formula: Corrected Hit Rate = Hit rate − (Hit rate × False alarm rate) (Heffner and Heffner 1995). This measure proportionally reduces the hit rate by the false alarm rate and varies from 0 (no hits) to 1 (100% hit rate with no false alarms).
Threshold was defined as the intensity at which the animal could detect a sound 50% of the time (corrected for false alarms), which was usually calculated by interpolation. Threshold testing for a particular frequency was considered complete when the thresholds obtained in at least three different sessions were stable (neither systematically increasing nor decreasing) and within 3 dB of each other. As such, the number of thresholds obtained at each frequency ranged from 4 to 7 for each bird. The best 3 thresholds were then averaged to represent the bird’s best hearing at that frequency.
Noise localization
For the sound-localization task, the bird was trained to continue pecking in the presence of noise bursts emanating from its right side, and to stop pecking when the sound came from its left, indicating that it had detected a shift in locus of the sound.
Brief broadband noise bursts (100 ms duration) were generated (Coulbourn S81-02) and equalized (Behringer FBQ 3102) to produce a relatively flat response (± 4 dB) from 250 Hz to 10 kHz. The signal was then randomly attenuated (up to 3.5 dB; Coulbourn S85-08), shaped (0 ms rise/decay; Coulbourn S84-04), amplified (Crown D75A), and routed to one of four pairs of matched speakers (Tang Band W3-319SF) in the sound chamber. The electrical signals to the speakers were continuously monitored on an oscilloscope (Tektronix TDS 210). The speakers were mounted symmetrically left and right of midline on a semi-circular perimeter bar (115 cm radius), and centered on the bird’s head at ear level. Angles of separation between speakers were fixed at 180, 120, 90, 60, 45, 30, 20, 15, 10, and 5 deg. The intensity of the noise bursts was set to 64 dB SPL, an easily detectable level for these birds. The intensity of the noise signal was measured and equated daily for each speaker pair. The procedure for measuring and calibrating the noise was the same as described for tones above.
Each 2-s trial consisted of a single noise burst (100 ms off, 100 ms on, 1.8 s off), presented from either a left or right speaker. To prevent acoustic interference between key-pecking and the short-duration noise burst, the 100-ms delay between the first key peck and noise onset was introduced for each trial. As in the audiogram procedure above, each trial had a 22% probability of the noise burst emanating from a speaker located to the left side of the bird. Each correct response (i.e., continue pecking when noise came from the right side, and stop pecking when the noise came from the left) was rewarded with a 2-s access to food at the end of the 2-s trial period. Continued pecking when the noise emanated from the left resulted in a 1.5-s shock at the end of the trial. False alarms, in which the bird stopped pecking when the noise emanated from its right side, were neither rewarded nor punished. In summary, only a single noise burst was presented every 2 s with a 22% probability of a noise burst emanating from the left. Following hits and correct rejections, an additional time elapsed while the bird ate its reward then returned to pecking.
Noise localization threshold was determined by successively reducing the angular separation between left and right speakers, in blocks of 8 left trials (and approx. 32 associated right trials), until the bird’s performance fell to chance (p > 0.01, binomial distribution). Testing was considered complete when scores at every angle stabilized and were no longer improving with practice. Asymptotic performance was calculated by averaging the three blocks of trials with the highest scores at each angle; these scores were taken from three different sessions. The means were then plotted as the performance curve for each bird. Threshold was defined as the angle at which mean performance equaled 50%, which was determined by interpolation.
Results
Audiogram
The guineafowl adapted relatively easily to the test cage and learned to peck the response key to receive rewards. After an initial 30-day demonstration that the birds would not peck reliably for sunflower treats (Brossia-Root et al. 2021), training based on food restriction proceeded quickly. All three birds learned to listen for sounds and all became reliable observers of very-low-intensity sounds within approximately 86 training sessions, after which they produced reliable thresholds. Complete audiogram testing and rechecking required 90 sessions.
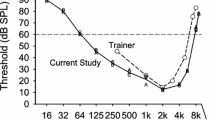
Mean thresholds for the three guineafowl are illustrated in Fig. 1. There is good agreement between the individual birds, with less than 3 dB difference between individuals at most frequencies. Beginning with an average threshold of 82.5 dB SPL at 2 Hz, hearing sensitivity gradually improves with increasing frequency to the best hearing at 2 kHz, with a threshold of 20 dB SPL. Above 2 kHz, there is gradual reduction in hearing sensitivity to 34 dB SPL at 5.6 kHz, followed by a steep loss in sensitivity to 85 dB SPL at 8 kHz, the highest frequency tested. The largest variation in thresholds between individual guineafowl (up to 5.5 dB) occurred at the birds’ upper limits of hearing at 7 and 8 kHz, where sensitivity also declines the fastest with increasing frequency. At a level of 60 dB SPL, the hearing of Helmeted guineafowl extends from 24.6 Hz to 6.86 kHz, a range of 8.12 octaves.
Psychophysical performance curves for frequencies near the low and high hearing limits, as well as the frequency of best hearing for guineafowl B are shown in Fig. 2. Note that the corresponding false alarm rates for the different intensities (also depicted in Fig. 2) remained low, indicating that the birds had become experienced observers by the time final thresholds were being determined.
The thresholds remained stable, as shown by a replication of guineafowl B’s threshold at 2 kHz (20.5 dB SPL) obtained 5 months after the initial audiogram had been completed; the replication did not differ significantly from the bird’s first mean threshold (19.7 dB SPL). Finally, inspection of the birds’ ears under anesthesia for possible obstructions revealed a clean meatus and intact eardrums. Although guineafowl are about 5 dB less sensitive than either pigeons or peafowl at their best frequency, their overall sensitivity is within the range reported for birds (e.g., Fay 1988).
Sound localization
Minimum audible angles were determined for one male and one female guineafowl (Fig. 3). There was excellent performance by both birds at angles of 30° and larger, and performance fell rapidly at smaller angles, with chance performance at both 5° and 10° separation indicating that the sounds were not otherwise discriminable except based on location. The 50% performance threshold was 13.8°.
Discussion
Hearing limits
The 6.86-kHz high-frequency hearing limit of the guineafowl reported here is consistent with observations that birds rarely hear above about 10 kHz. Unlike mammals, high-frequency hearing varies minimally among birds (cf. Gleich and Langemann 2011), possibly owing to their ability to localize sound without having to rely on the high-frequency interaural intensity (or spectral) differences that are increasingly important to mammals of decreasing size (cf. Heffner and Heffner 2016). Although barn owls are reported to hear higher frequencies (12 kHz) and use frequencies in that range for localization (Konishi 1973; Krumm et al. 2019), hearing above 10 kHz has only been reported in one other species of owl (cf. Dyson et al. 1998). As audiograms become available for additional avian species, we may gain insight into the factors that influence high frequency hearing in birds and how those factors compare to those that seem to drive high-frequency hearing in mammals.
Recent studies have also shown that species’ high-frequency hearing is affected by their vocalizations. Specifically, we now know that echolocating mammals hear slightly higher frequencies than predicted by their functional interaural distance (Heffner et al. 2013a, b). In this regard, audiograms of echolocating birds would be of interest. Unusually good high-frequency hearing has also been reported for the Ecuadorian Hillstar hummingbird, which showed behavioral and neural responses to field recordings of its vocalizations in the range of 11–15 kHz, suggesting that it hears above 10 kHz (Duque et al. 2020). It will be especially interesting to discover whether those high frequencies are also used in localization. Although its behavioral audiogram has yet to be determined, there is now strong evidence that the hearing of the Eqcadorian Hillstar hummingbird extends beyond the common 10-kHz limit for birds.
Comparative studies of hearing often link audiograms to the vocalizations of a species and its conspecifics. Specifically, some of the earliest bird audiograms (Dooling et al. 1971), and continuing to this day (Yeh 2023), show that the frequency of best hearing is often near or at the dominant frequency of their communication calls. The vocal repertoire of the Helmeted guineafowl contains frequencies ranging from 1.5 to 5.5 kHz (Maier 1982), which also encompass the region of best hearing in its behavioral audiogram. Thus, among birds as well as mammals (cf. Sales and Pye 1974), vocalizations tend to employ the frequencies to which an animal is most sensitive.
In contrast to high-frequency hearing and mid-range best sensitivity, low-frequency hearing varies considerably among birds, and it is becoming clear that good low-frequency hearing is not uncommon (Fig. 4). However, the sample of bird audiograms remains limited. Some investigators have attempted to achieve a larger sample by lowering the criterion for hearing limits to 30 dB, but that enlarged sample comes at a cost. There is, of course, a correlation between the 30 and 60-dB hearing limits (p < 0.001 at both low and high frequencies). However, the slope of the low-frequency portion of audiograms differs for species that hear low compared to those that do not, with the result that lowering the definition of low-frequency hearing from a 60-dB criterion to a 30-dB criterion reduces the low-frequency variation that is of interest. The current sample of 14 bird species whose low-frequency hearing limits at 60 dB are known includes only 10 families, with four of the fourteen species belonging to a single order, Galliformes. Among this small and unrepresentative sample, we do not yet see compelling correlates with low-frequency hearing of either phylogeny, lifestyle, or anatomy. For example, although plausible, body weight (based on the midrange for adults) remains a poor predictor of either low-frequency hearing (adjusted r2 = 20%) or hearing range (adjusted r2 = 7%).
Low-frequency hearing limits at 60 dB SPL, note log scale (Bullfinch, Schwartzkopff 1949; Red-winged blackbird and Brown-headed cowbird, Heinz et al. 1977; Pigeon, Kreithen and Quine 1979, Heffner et al. 2013a, b; Canary, Okanoya and Dooling 1987; Human, Jackson et al. 1999; Peafowl, Heffner et al. 2020; Orange-fronted conure, extrapolated from 52 dB threshold at 250 Hz, Wright et al. 2003; Budgerigar, Heffner et al. 2016; Domestic chicken, Hill et al. 2014; Kea parrot, Schwing et al. 2016; Great cormorant, extrapolated from 53 dB threshold at 500 Hz, Maxwell et al. 2017; Mallard duck, Hill 2017; Japanese quail, Strawn and Hill 2020)
At present, both the upper and lower frequency limits of hearing are likely to be more influenced by adventitious sounds—those made by animals as they interact with their environment (Joris 2022). The ability not only to detect adventitious sounds, but especially to use high frequencies to locate their sources seems to be clearly important for mammalian hearing (e.g., Heffner and Heffner 2016), but perhaps is less important for birds. The frequencies well above 10 kHz may not be important for birds because their sound-localization mechanism takes advantage of the enhanced binaural cues produced by their interconnected ears (e.g., Koppl 2019). The excellent low-frequency hearing in pigeons was initially proposed to have evolved as an aid in navigation and altitude control, but that function would not apply to either chickens, peafowl, or guineafowl. Perhaps a broader comparative sample of avian hearing will reveal environmental and geophysical sounds or unsuspected functions of very low frequencies that are important for hearing in some species (cf. Zeyl et al. 2020).
The fact that the ability to hear low frequencies may be relatively common among birds has implications for the current proliferation of wind farms where the turbines produce pulsing sound that begins at about 0.75 Hz and ranges up to 3 Hz, reaching levels of 100 dB (Stelling 2015). Such levels would be within the audible range of guinea fowl and other species, including Domestic chickens (Hill et al. 2014). It would be important to know if turbine noise affects flock communication, mating, or even perhaps egg production and rearing of young in animals that can hear the sound as well as feel the vibrations (Salt and Hullar 2010).
At this time, it seems inadvisable to speculate on which of the many potential factors—migratory or not, aerial or ground-dwelling, aquatic, diet (nectar/seed/vegetation/live or dead prey), nocturnal or diurnal, etc.—may have influenced the evolution of low-frequency hearing in birds. Yet the wide variation is intriguing and further data are likely to lead to better understanding of both the evolutionary pressures and the mechanisms underlying hearing in birds.
Sound localization
Long-duration sounds and continuously pulsing sounds allow scanning and tracking of a sound source rather than revealing the best possible neural discrimination of locus cues. In contrast, environmental sounds are often brief transients. For these reasons, thresholds for localizing very brief sounds are important for comparative analyses (Table 1). Only minimum audible angles for single brief sounds (200 ms or shorter) are listed in Table 1 with the exceptions of Great tits (300 ms duration) and Barn owls, Short-eared owls, Marsh hawks, Red-tailed hawks, and American kestrels (tested with repeated bursts of 200-ms noise). Most comparisons are based on performance measures to which a correction for false alarms or chance has been applied. Occasionally, when it has been particularly difficult for animals to learn the behavioral task used for testing, curve fitting and a d’ correction have been used to determine thresholds. However, although d’ corrects for response biases, it does not reveal the degree to which subjects are inattentive rather than under stimulus control and cannot correct for unobservant subjects (Green 1995). Such differences in how thresholds are calculated may have contributed to some of the variation observed so far for localization thresholds among birds, but we do not know by how much because traditional performance measures are not usually reported along with a d’ measure. (Among well practiced animals with steep psychophysical curves, d’ values at 0.50 performance thresholds tend to be slightly greater than 1.0, hence lowering reported thresholds only minimally. For example, the detection thresholds illustrated in Fig. 1 would be lower by no more than about 1 dB.) Localization thresholds determined using longer duration sounds or multiple presentations that permit some scanning are likely to produce smaller thresholds, but by an undetermined amount. Likewise, use of a d’ measure may result in a slightly lower threshold definition. Nevertheless, the sound-localization thresholds shown in Table 1 give us a current estimate of the variation among eleven species of birds despite what remains for now variation due to measurement error.
Minimum audible angles for brief noise bursts have been determined for only eleven species of birds (see Table 1). Although the measures are not comparable, they do illustrate that there is likely variation among the different species of birds. The 13.8-degree minimum audible angle for the Helmeted guineafowl lies at the median for the species tested so far; it is also very near the mean of 13.1 degrees for 41 mammals (Heffner et al. 2022). Despite the small sample of bird species, the range of thresholds, from 1.5 degrees to 93 degrees, is comparable to the broad range observed in mammals. With such a small sample and limited representation of avian orders and lifestyles, no pattern has yet emerged as to either the selective pressures or mechanisms that might underlie the wide variation in birds’ abilities to locate sound sources. It will be interesting to explore the degree to which the magnitude of locus cues, which does not seem to be a determining factor among mammals (e.g., Heffner et al. 2022), contributes to the variation in acuity among birds. Similarly, the role of vision and its interaction with hearing should be explored. Among mammals, the variation in sound localization acuity is highly correlated with the width of their field of best vision (r = 0.889, p < 0.0001), suggesting that that the function of the ears is to direct the eyes to the source of a sound (Heffner and Heffner 1992, 2016; Heffner et al. 2008). Such a relation should be among those explored in birds.
References
Bierman HS, Thorton JL, Jones HG, Koka K, Young BA, Brandt C, Christensen-Dalsgaard J, Carr CE, Tollin DJ (2014) Biophysics of directional hearing in the American alligator (Alligator mississippiensis). J Exp Bio 217:1094–1107
Brossia-Root L, Heffner RS, Heffner HE (2021) Comparison of 2 methods to train guinea fowl to perform a behavioral task. Abstr of Sci Present 2021 AALAS Nat Meet 60:669
Christensen-Dalsgaard J (2011) Vertebrate pressure-gradient receivers. Hear Res 273:37–45
Dooling RJ, Mulligan JA, Miller JD (1971) Auditory sensitivity and song spectrum of the common canary (Serinus canarius). J Acoust Soc Am 50:700–709
Duque FG, Rodriguez-Saltos CA, Ulma S, Nasir I, Monteros MF, Wilczynski W, Carruth LL (2020) High-frequency hearing in a hummingbird. Sci Adv 6:eabb9393
Dyson ML, Klump GM, Gauger B (1998) Absolute hearing thresholds and critical masking ratios in the barn owl: a comparison with other owls. J Comp Physiol A 182:695–702
Fay RR (1988) Hearing in Vertebrates: A Psychophysics Databook. Hill-Fay Associates, Winnetka, IL
Feinkohl A, Klump GM (2013) Azimuthal sound localization in the European starling (Sturnus vulgaris): II psychophysical results. J Comp Physiol A 199:127–138
Gleich O, Langemann U (2011) Auditory capabilities of birds in relation to the structural diversity of the basilar papilla. Hear Res 273:80–88
Green DM (1995) Maximum-likelihood procedures and the inattentive observer. J Acoust Soc Am 97:3749–3760
Heffner RS, Heffner HE (1992) Visual factors in sound localization in mammals. J Comp Neurol 317:219–232
Heffner HE, Heffner RS (1995) Conditioned avoidance. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC (eds) Methods in comparative psychoacoustics. Birkhäuser, Basel, pp 73–87
Heffner HE, Heffner RS (2016) The evolution of mammalian sound localization. Acoustics Today 12:20–35
Heffner HE, Koay G, Heffner RS (2008) Sound localization acuity and its relation to vision in large and small fruit-eating bats: II Non-echolocating species, Eidolon helvum and Cynopterus brachyotis. Hear Res 241:80–86
Heffner HE, Koay G, Hill EM, Heffner RS (2013a) Conditioned suppression/avoidance as a method for testing hearing in birds: the domestic pigeon (Columba livia). Behav Res Meth 45:383–392
Heffner RS, Koay G, Heffner HE (2013b) Hearing in American leaf-nosed bats. IV: the common vampire bat. Desmodus Rotundus Hear Res 296:42–50
Heffner HE, Koay G, Heffner RS (2016) Budgerigars (Melopsittacus undulatus) do not hear infrasound: the audiogram from 8 Hz to 10 kHz. J Comp Physiol A 202:853–857
Heffner RS, Cumming JF, Koay G, Heffner HE (2020) Hearing in Indian peafowl (Pavo cristatus): sensitivity to infrasound. J Comp Physiol A 206:899–906
Heffner RS, Koay G, Heffner HE, Mason MJ (2022) Hearing in African pygmy hedgehogs (Atelerix albiventris): audiogram, sound localization, and ear anatomy. J Comp Physiol A 208:653–670
Heinz RD, Sinnott JM, Sachs MB (1977) Auditory sensitivity of the redwing blackbird (Agelaius phoeniceus) and brown-headed cowbird (Molothrus ater). J Comp Physiol Psychol 91:1365–1376
Hill EM (2017) Audiogram of the mallard duck (Anas platyrhynchos) from 16 Hz to 9 kHz. J Comp Physiol A 203:929–934
Hill EM, Koay G, Heffner RS, Heffner HE (2014) Audiogram of the chicken (Gallus gallus domesticus) from 2 Hz to 9 kHz. J Comp Physiol A 200:863–870
Jackson LL, Heffner RS, Heffner HE (1999) Free-field audiogram of the Japanese macaque (Macaca fuscata). J Acoust Soc Am 106:3017–3023
Joris PX (2022) In praise of adventitious sounds. Hear Res 425:108592. https://doi.org/10.1016/j.heares.2022.108592
Klump GM, Windt W, Curio E (1986) The great tit’s (Parus major) auditory resolution in azimuth. J Comp Physiol A 158:383–390
Konishi M (1973) How the owl tracks its prey. Am Sci 61:414–424
Koppl C (2019) Internally coupled middle ears enhance the range of interaural time differences heard by the chicken. J Exptl Biol 222:1–11. https://doi.org/10.1242/jeb.199232
Kreithen ML, Quine DB (1979) Infrasound detection by the homing pigeon: a behavioral audiogram. J Comp Physiol 129:1–4
Krumm B, Klump GM, Köppl C, Langemann U (2019) The barn owls’ minimum audible angle. PLoS ONE 14(8):e0220652. https://doi.org/10.1371/journal.pone.0220652
Krumm B, Klump GM, Koppl C, Beutelmann R, Langemann U (2022) Chickens have excellent sound localization ability. J Exptl Biol. 225:243601
Maier V (1982) Acoustic communication in the guinea fowl (Numida meleagris): Structure and use of vocalizations, and the principles of message coding. Z Tierpsychol 69:29–83
Maier V, Rasa OAE, Scheich H (1983) Call-system similarity in a ground-living social bird and a mammal in the bush habitat. Behav Ecol Sociobiol 12:5–9
Maldarelli G, Firzlaff U, Luksch H (2022) Azimuthal sound localization in the chicken. PLoS ONE 17:e0277190
Mason MJ (2016) Internally coupled ears in living mammals. Biol Cybernetics 110:345–358
Maxwell A, Hansen KA, Ortiz ST, Larsen ON, Siebert U, Wahlberg M (2017) In-air hearing of the great cormorant (Phalacrocorax carbo). Biol Open 6:496–502
Okanoya K, Dooling RJ (1987) Hearing in passerine and psittacine birds: a comparative study of masked and absolute auditory thresholds. J Comp Psychol 101:7–15
Park TJ, Dooling RJ (1991) Sound localization in small birds: absolute localization in azimuth. J Comp Psychol 105:125–133
Rice WR (1982) Acoustical location of prey by the marsh hawk: adaptation to concealed prey. Auk 99:403–413
Sales G, Pye D (1974) Ultrasonic Communication by Animals. Chapman and Hall, London
Salt AN, Hullar TE (2010) Responses of the ear to low-frequency sounds, infrasound and wind turbines. Hear Res 268:12–21
Schwartzkopff J (1949) Über Sitz und Leistung von Gehör und Vibra- tionssinn bei Vögeln. Z Vergl Physiol 31:527–608
Schwing R, Nelson XJ, Parsons S (2016) Audiogram of the kea parrot, Nestor notabilis. J Acoust Soc Am 140:3739–3744
Stelling K (2015) Infrasound: low frequency noise and industrial wind turbines. Report prepared for the Multi-municipal wind turbine working group. Chesley, England, pp 1–46
Strawn SN, Hill EM (2020) Japanese quail (Coturnix japonica) audiogram from 16 Hz to 8 kHz. J Comp Physiol 206:665–670
Theurich M, Langner G, Scheich H (1984) Infrasound responses in the midbrain of the guineafowl. Neurosci Let 49:81–86
Wright TF, Dortopassi KA, Bradbury JW, Dooling RJ (2003) Hearing and vocalizations in the orange-fronted conure (Aratinga canicularis). J Comp Psychol 117:87–95
Yeh Y-T, Rivera M, Woolley SMN (2023) Auditory sensitivity and vocal acoustics in five species of estrildid songbirds. Animal Behav 195:107–116
Zeyl JN, denOuden O, Koppl C, Assink J, Christensen-Dalsgaard J, Patrick SC, Clusella-Trullas S (2020) Infrasonic hearing in birds: a review of audiometry and hypothesized structure-function relationships. Biol Rev. https://doi.org/10.1111/brv12596
Zeyl JN, Snelling EP, Joo R, Clusella-Trullas S (2023) Scaling of ear morphology across 127 bird species and its implications for hearing performance. Hear Res 428:108679
Author information
Authors and Affiliations
Contributions
H.H., G.K., and R.H. contributed similarly to the conception of this study, its execution, analysis of the data, and the writing and approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Andrea Megela Simmons.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heffner, H.E., Koay, G. & Heffner, R.S. Hearing in helmeted guineafowl (Numida meleagris): audiogram from 2 Hz to 10 kHz and localization acuity for brief noise bursts. J Comp Physiol A 210, 65–73 (2024). https://doi.org/10.1007/s00359-023-01645-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-023-01645-7