Abstract
The tarsal attachment devices of the southern green stink bug Nezara viridula, a cosmopolitan pest of different crops, encompass a pair of claws, distal pretarsal smooth pulvilli, and a proximal hairy pad on the ventral basitarsus. To evaluate the role of these attachment devices in generating attachment, behavioural experiments testing locomotion of insects with ablated pulvilli, shaved hairs and cut-off claws were performed. Using traction force experiments, insect attachment performance was evaluated on artificial substrates characterised by different roughness and on two substrates with different surface energies in the air and under water. To examine the contact area of attachment devices during resting, pulling and inverted walking, intact insects and those without claws were video-recorded using a high-speed camera. The present data reveal a great involvement of pulvilli in insect attachment on all the tested surfaces, while the hairy pad seems to have a role in producing friction forces only on smooth surfaces and on surfaces with intermediate roughness. The hairy pad was revealed to be important in adhesion to hydrophobic substrates under water, a function that could be relevant for N. viridula insects in consideration that many plant leaves tend to have hydrophobic surfaces and may be often covered by water film.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects are well adapted to walk and adhere to different kinds of surfaces due to their leg attachment devices encompassing pads and claws developed to adhere to smooth and interlock with rough surfaces, respectively. Two kinds of pads can be recognised: hairy pads covered by relatively long deformable setae typical of flies, beetles and earwigs and smooth pads, such as arolia and euplantulae, typical of cockroaches, bees, grasshoppers and some bugs. Because of the flexibility of the material of attachment pads, both systems are able to adapt and maximize the contact area with a wide range of substrate profiles (see reviews in Gorb 2001, 2005, 2008; Federle 2006). The contact between insect attachment devices and the substrate is mediated by a thin film of liquid secretion which helps to increase the pad’s contact area, especially on rough surfaces and is responsible for generating capillary forces on various surfaces (see review by Dirks and Federle 2011).
Hairy and smooth adhesive pads have evolved repeatedly in different taxa (Beutel and Gorb 2001, 2006), and it is still not well understood, whether the performance of the two alternative designs is different. Bullock et al. (2008) compared smooth attachment pads of the stick insect Carausius morosus and the hairy ones of the leaf beetle Gastrophysa viridula and concluded that adhesion and friction forces per unit pad area were very similar in both systems. Bußhardt et al. (2012) have compared adhesive and frictional forces in two stick insects, Cuniculina impigra having smooth euplantulae and C. morosus bearing small nubs on their euplantulae. They concluded that smooth pads are specialized for rather smooth substrates, whereas nubby pads are better adapted to generate stronger forces on a broader range of surfaces.
Some insects, such as for example pentatomid bugs, bear an interesting combination of smooth and hairy attachment devices, which raises the question about relative contribution of different structures to the overall attachment. The southern green stink bug, Nezara viridula L. (Heteroptera: Pentatomidae) is a serious cosmopolitan pest of different crops in most areas of the world (Todd 1989; Panizzi et al. 2000). A recent study (Rebora et al. 2018) investigated in detail the tarsal attachment devices of this species encompassing claws and both smooth and hairy pads. Each pretarsus of N. viridula bears a pair of smooth flexible pulvilli, sac-like structures formed by complex cuticular layers that vary in their structure and resilin content. The dorsal side of each pulvillus consists of sclerotised chitinous material, while the ventral cuticle consists mainly of resilin and shows a very thin epicuticle and a thick exocuticle. The setae of the hairy adhesive pad are pointed and socketed exhibiting a pronounced longitudinal and transverse gradient in the resilin content (Rebora et al. 2018). Both attachment devices release fluid on the substrate and leave traces of such fluid at each step (Rebora et al. 2018). The attachment ability of males and females of this insect on different host plant species, on hydrophilic and hydrophobic artificial surfaces, on adaxial and abaxial leaf surfaces of the model plant species V. faba and on artificial substrates with different roughness (Salerno et al. 2017) has been characterized. In this last paper, it has been hypothesized that different types of pads (smooth pulvilli and hairy pads) of the tarsi of N. viridula, have a division of tasks, as demonstrated in other insects. Indeed, previous studies on Hymenoptera (Endlein and Federle 2015), Blattodea (Roth and Willis 1952; Clemente and Federle 2008) and Phasmatodea (Labonte and Federle 2013; Labonte et al. 2014) showed a division of labour between the two pad types with distal pads characterized by strong adhesion and a main involvement in pulling forces while proximal pads serving mainly friction generation, mainly responsible for pushing forces during locomotion. Some of these species, such as cockroaches and mantophasmids, evenly keep their arolia conspicuously out off the surface, when no adhesive force is required. So it is primarily used during vertical and inverted climbing and in other situations, when adhesion is required (Clemente and Federle 2008; Eberhard et al. 2009). Certain labor division was also studied for leg pads and the abdominal pad in larval Coleoptera (Zurek et al. 2015) and for the distal and proximal attachment pad of adult beetles (Bullock and Federle 2009).
The aim of the present investigation is to test the hypothesis that the different types of pads (smooth pulvilli and hairy pads) of the tarsi of N. viridula have a division of tasks as shown in the above reported insects. To evaluate the role of the different attachment devices of N. viridula we used behavioural experiments, using a traction force experiments set up, testing insects with ablated pulvilli, hairs and claws on flat surfaces. To examine the contact area of pulvilli and hairy pad with the substrate during resting, pulling and inverted climbing, intact insects and insects with ablated claws were filmed using a high-speed camera.
The attachment ability of the ablated insects was evaluated on artificial (epoxy resin) substrates characterised by different roughness. In consideration that, as previously observed, N. viridula can walk also on wet surfaces, we measured the friction force produced by intact and ablated insects on two substrates with different surface energies (glass and plastic Petri dish) in air and in water. In this regard, taking into account that some beetles may use air bubbles trapped between their adhesive setae to walk under water (Hosoda and Gorb 2012), we tested the effectiveness of the different attachment structures for generating underwater attachment force.
Materials and methods
Insects
Nezara viridula L. (Heteroptera: Pentatomidae) bugs were collected in the field in June 2017 close to Ponte Felcino (Perugia, Umbria region, Italy) and reared in a controlled condition chamber (14 h photophase, temperature of 25 ± 1 °C; RH of 70 ± 10%), inside clear plastic food containers (300 mm x 195 mm x 125 mm) with 5 cm diameter mesh-covered holes. All stages were fed with seeds, fruits and vegetative parts of their preferred food plants. In particular, sunflower seeds (Helianthus annus L.) and French beans (Phaseolus vulgaris L.) were used to feed insects. In consideration that the behavioural investigations on the attachment ability of males and females of N. viridula on hydrophilic and hydrophobic artificial surfaces and on the adaxial and abaxial leaf surfaces of the model plant species Vicia faba (Salerno et al. 2017) did not reveal any difference between the sexes, the present investigation has been performed only on adult virgin females.
Contact area of pulvilli and hairy pad
To examine the contact area of pulvilli and hairy pad involved during insect resting and pulling on a smooth surface (plastic Petri dish), N. viridula adults were analysed with reflection contrast microscopy (RCM) using an inverted bright-field microscope ZEISS Axio Observer.A1 (Carl Zeiss Microscopy GmbH) in combination with a high-speed camera Photron FASTCAM SA1.1 (VKT Video Kommunikation GmbH—Technisches Fernsehen, Pfullingen, Germany) at 500 fps with a 10x lens (Carl Zeiss MicroImaging GmbH), as described earlier (Ploem 1975; Federle et al. 2002; Heepe et al. 2014). To examine the contact area of pulvilli and hairy pad involved during insect inverted climbing on the same surface, N. viridula adults were analysed with reflection contrast microscopy (RCM) using a light microscope (Zeiss Axioplan) in combination with the same high-speed camera. Considering the results obtained in traction force experiments using insects with ablated claws, and in order to evaluate the possible effect of claw ablation on the contact area of pulvilli during pulling, some behavioural observations have been carried using ablated insects.
The laboratory temperature and relative humidity during measurements were 23 ± 2 °C and 50%, respectively. Insects were anaesthetized with carbon dioxide (for 60 s) and were made incapable of flying by carefully gluing their forewings together with a small droplet of melted wax. In each insect, one end of a 5 cm long human hair was fixed with a droplet of molten wax on the pronotum, while the other end was glued to the wall of a plastic Petri dish (9 cm diameter), where the insect was allowed to walk. The maximum contact area during insect resting (forelegs, n = 12, midlegs, n = 7, hindlegs, n = 9), pulling (forelegs, n = 6, midlegs, n = 10, hindlegs, n = 14) and inverted climbing (forelegs, n = 7, midlegs, n = 3, hindlegs, n = 6) was evaluated by processing the video with the open source image processing program ImageJ (Schneider et al. 2012).
Ablations
To test the role of different attachment structures in N. viridula, ablation experiments were carried out. The females were anaesthetized with carbon dioxide (for 120 s) and immobilized with Patafix (UHU Bostik, Milano, Italy) under the stereomicroscope. Pulvilli and claws were carefully cut off with microscissors, hairs of the hairy pads were carefully cut off with a scalpel-blade. Insects used as control were handled in exactly the same way as the cut groups, but without ablations. Ten insects for each treatment (no pulvilli, no hairs, no claws, intact) were prepared. The insects were left to recover for 24 h before carrying out the experiments to avoid any negative effect due to the manipulations and to the possible bleeding after the ablations.
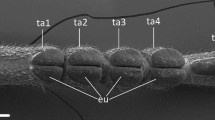
To check the quality of the ablations (see Fig. 1), some leg specimens from individuals used in the experiments were observed in a cryo-scanning electron microscope (cryo-SEM) Hitachi S-4800 (Hitachi High-Technologies Corp., Tokyo, Japan) equipped with a Gatan ALTO 2500 cryo-preparation system (Gatan Inc., Abingdon, UK). For details of sample preparation and mounting for cryo-SEM, see Gorb and Gorb (2009). Specimens were sputter-coated in frozen conditions with gold–palladium and examined at 3 kV acceleration voltage and temperature of − 120 °C.
Force measurements
The experiments were performed using a traction force experiment set up. Prior to the force measurements, adult females of N. viridula were weighted using micro-balances (Mettler Toledo AG 204 Delta Range, Greifensee, Switzerland). Experimental insects were anaesthetized with carbon dioxide (for 60 s) and were made incapable of flying by carefully gluing their forewings together with a small droplet of melted wax. In each insect, one end of a 15–20 cm long human hair was fixed with a droplet of melted wax on the pronotum. Before starting the experiments, insects were left to recover for 30 min.
All the experiments were performed during the daytime at 23 ± 2 °C temperature and 50 ± 5% relative humidity.
Two sets of experiments were performed: one to evaluate the role of different attachment devices of N. viridula in its attachment ability on artificial (epoxy resin) substrates with different roughness (1st set of experiments) and one to evaluate its attachment ability on two substrates with different surface energies (glass and Petri dish) immersed in water and the role of the different attachment devices in underwater adhesion (2nd set of experiments).
The traction force experiments set up consisted of a force sensor FORT-10 (10 g capacity; World Precision Instruments Inc., Sarasota, FL, USA) connected to a force transducer MP 100 (Biopac Systems Ltd, Goleta, CA, USA) (Gorb et al. 2010). Data were recorded using AcqKnowledge 3.7.0 software (Biopac Systems Ltd, Goleta, CA, USA). The insect was attached to the force sensor by means of the hair glued to its pronotum and was allowed to move on the substrate, in order to be tested in a direction perpendicular to the force sensor. To try to standardize the tests as much as possible, the force generated by the insect walking horizontally on the test substrates was measured until the insect stopped pulling. Force–time curves were used to estimate the maximal friction force produced by insects.
In the first set of experiments, each insect walked on seven surfaces (A–G) with different roughness, presented in a random order. In total, 42 females (10 females for intact, 10 females for no pulvilli, 11 females for no hairs and 11 females for no claws condition) were tested on all the surfaces. The maximal friction force produced by insects on different test surfaces was normalized by the body weight, to obtain the safety factor.
In the second set of experiments, each insect walked (1) on glass in air, (2) on the bottom of a plastic Petri dish (14 cm diameter) in air, (3) on glass immersed in water (depth = 4 mm) and (4) on a Petri dish (14 cm diameter) filled with water (depth = 4 mm). The water was changed after every two tested insects. In total 42 females (10 females for intact, 10 females for no pulvilli, 11 females for no hairs and 11 females for no claws condition) were tested on all the surfaces. Force–time curves were used to estimate the maximal friction force produced by insects on different test surfaces.
Substrate preparation and characterization
Artificial (epoxy resin) substrates with different roughness have been prepared to minimize the possible influence of chemical properties of different substrates on experimental data. Epoxy resin casts of a clean glass surface and polishing papers with defined asperity sizes (0.3, 1, 3, 9, 12 and 260 µm) were made using a two-step molding method (Gorb 2007) in order to get artificial substrates with different roughness parameters (A–G).
The roughness of A–F substrates has been characterized (as reported in Salerno et al. 2017) using the white light interferometer NewView 6000 (Zygo Middlefield, CT, USA) with the objectives x5 and x50 (N.A. 0.4, window size 1400·µm x 1050·µm and 140·µm × 105·µm, respectively). The optical surface scanner microscope (VR-3000 Series, Keyence, Osaka, Japan) was used for the surface with highest roughness (G). Five individual measurements (n = 5) were performed for each substrate. Roughness data for above surfaces are given in Table 1.
The wettability of surfaces used in experiments (glass, plastic Petri dish) was characterized by determining the contact angles of water (aqua Millipore, droplet size = 1 µl, sessile drop method) using a high-speed optical contact angle measuring instrument OCAH 200 (Dataphysics Instruments GmbH, Filderstadt, Germany). Ten measurements (n = 10) were performed for each substrate. Obtained values of contact angles of water are given in Table 2.
Statistical analysis
Maximal friction force obtained in traction force experiments was normalized by the body weight to obtain the safety factor. Safety factor data obtained from insects in different conditions were compared independently but considering that the same insect walked on the different surfaces. Data were analysed with two-way repeated measures ANOVA (Statistica 6.0, Statsoft Inc. 2001) considering the ablation type and the surface type as main factors. F-tests were used to assess the significance of the effects and their interactions. For significant factors, the Fisher LSD test was used as post hoc test (Statistica 6.0, Statsoft Inc., 2001). The effect of ablation on safety factor was expressed as percentage on the mean safety factor recorded with intact insects on each surface. One-way ANOVA was used to compare the percentages obtained on different surfaces for each kind of ablation and to compare the contact area of hairy pads and pulvilli during resting, pulling and inverted climbing. For significant factors, unequal Tukey’s HSD post hoc test for multiple comparisons between means was used. Contact areas of pulvilli in intact insects and in insects with ablated claws during pulling for each leg, as well as the water contact angles of glass and plastic Petri dish were compared using the Student’s t test for independent samples. Before the analysis, all the data were subjected to Box–Cox transformations, in order to reduce data heteroscedasticity (Sokal and Rohlf 1998). All the statistical tests were performed to verify the rejection of a null hypothesis with a p < 0.05. For p > 0.05 our tests were not able to verify acceptance of the null hypothesis even if along the text, for the sake of brevity, we reported the results as not significantly different.
Results
Contact area of pulvilli and hairy pad
In the comparison among resting, pulling and inverted climbing, the maximum total contact area (contributed by both pulvilli and hairy pad) was not statistically different for midlegs (F = 0.42; df = 2, 17; P = 0.66), while for forelegs (F = 3.04; df = 2, 22; P = 0.07) and hindlegs (F = 2.64; df = 2, 26; P = 0.09) was almost significant with an apparently higher total contact area during resting. In forelegs (F = 0.32; df = 2, 22; P = 0.73), midlegs (F = 1.68; df = 2, 17; P = 0.22) and hindlegs (F = 2.62; df = 2, 26; P = 0.09), the area of pulvilli appears to be not statistically different among the three conditions, while the contact area of the hairy pad was higher during resting, if compared both to inverted climbing and pulling in forelegs (F = 15.02; df = 2, 22; P < 0.0001) and in hindleg (F = 7.06; df = 2, 22; P = 0.0043), but was not different in midlegs (F = 2.35; df = 2, 16; P = 0.13) (Fig. 2).
Contact area of pulvilli and hairy pad of N. viridula adults. Maximum total contact area (pulvilli + hairy pad) (arithmetic mean ± s.e.m.) during resting (forelegs, n = 12, midlegs, n = 7, hindlegs, n = 9), pulling (forelegs, n = 6, midlegs, n = 10, hindlegs, n = 14) and inverted climbing (forelegs, n = 7, midlegs, n = 3, hindlegs, n = 6) on a smooth surface (plastic Petri dish). The comparisons were performed among resting, pulling and inverted climbing conditions; ns = not significant for the total contact area; columns with different capital letters (for hairy pad contact area) and columns with different low case letters (for pulvilli contact area) are significantly different at P < 0.05, one-way ANOVA, Tukey HSD post hoc test
In insects with ablated claws (4862 ± 1082 µm2), the maximum contact area contributed by pulvilli during pulling was lower than in intact insects (12,727 ± 2076 µm2) (t = 3.64; df=13; P = 0.003) in forelegs, while in midlegs (ablated: 10,148 ± 1443, intact: 13,571 ± 890) and in hindlegs (ablated: 17,539 ± 916, intact: 13,352 ± 1271), the maximum contact area of pulvilli was not statistically different (midleg: t = 0.99; df = 12; P = 0.34; hindleg: t = 1.71; df = 16; P = 0.11) between intact insects and insects with ablated claws.
Role of different attachment devices on substrates with different roughness
In the 1st set of experiments, aiming to evaluate the role of different attachment devices of N. viridula on its attachment ability on artificial (epoxy resin) substrates with different roughness, the safety factor varied significantly depending on the kind of ablation (pulvilli, hairs or claws), as well as on the surface roughness (see Table 1). The interaction between ablation and different surfaces was also statistically significant (Fig. 3 and table inset). The safety factors recorded with the intact insects were similar to those observed in a previous investigation (Salerno et al. 2017), with higher values on smooth surface (A) and on the surface with the highest asperity size (G) and minimum values on those with Ra = 0.54 µm (C) and Ra = 2.74 µm (F). The safety factor of N. viridula with ablated pulvilli was statistically lower in comparison with that of intact insects on all the tested surfaces (Fig. 3 and table inset). The safety factor of N. viridula with ablated hairs was statistically lower in comparison with that of intact insects on surfaces A and D, while there was no difference between intact and ablated insects on all the other surfaces (Fig. 3). The safety factor of N. viridula with ablated claws was statistically lower in comparison with that of intact insects only on surfaces A, B, C, D, E and G, but not different on surface F (Fig. 3). The mean percentage of the safety factor for insects with ablated pulvilli on mean safety factor recorded with intact insects was 22.93 ± 7.31 and was statistically different on different surfaces (F = 6.62; df = 6, 63; P < 0.0001). In particular, the percentage was higher on the surface with the highest asperity size (G) in comparison with surfaces A, B, C, D and E, thus, revealing a lower effect of pulvilli ablation on this surface. Moreover, the percentage was higher on the surface F in comparison with surfaces A, and D (Fig. 4). No difference was recorded in the percentage on surfaces A, B, C, D and E (Fig. 4). In insects with ablated hairs, the mean percentage was 70.94 ± 11.18, while in insects with ablated claws, it was 60.40 ± 10.77. The effect of the ablation of hairs (F = 0.18; df = 6, 70; P = 0.98) and claws (F = 0.36; df = 6, 70; P = 0.90) was not statistically different on different surfaces (Fig. 4).
Role of N. viridula attachment devices on substrates with different roughness. Safety factor (friction force divided by the insect weight) obtained with intact (n = 10) and ablated (no pulvilli, n = 10; no hairs, n = 11; no claws, n = 11) females on artificial epoxy resin surfaces with increasing roughness (A–G, see Table 1). Boxplots show the interquartile range and the median, whiskers indicate 1.5 × interquartile range and Х shows the arithmetic mean. Boxplots with asterisk (*) are significantly different from the white boxplot (intact insects) at P < 0.05, Fisher LSD post hoc test. Table inset shows the statistical parameters of two-way repeated measures ANOVA. Insets show height maps and 3D oblique maps of rough test surfaces obtained with white light interferometer (A–F) and with an optical surface scanner microscope (G) (for details, see Table 1)
Effect of ablation on the safety factor expressed as percentage on the mean safety factor recorded with intact (n = 10) insects on each surface with increasing roughness (A–G, see Table 1). Boxplots show the interquartile range and the median, whiskers indicate 1.5× interquartile range and Х shows the arithmetic mean. For ablated pulvilli (n = 10), boxplots with different letters are significantly different at P < 0.05, one-way ANOVA, Tukey HSD post hoc test. For ablated hairs (n = 11) and claws (n = 11), there was no significant difference among surfaces
Attachment ability on underwater substrates and role of the attachment devices
In the 2nd set of experiments, aiming to evaluate: (1) the attachment ability of N. viridula on two smooth substrates with different (water contact angle: t = 16.70, df = 18, P < 0.0001) surface energies (glass, more hydrophilic; plastic Petri dish, more hydrophobic) in air and on surfaces immersed in water and (2) the role of different attachment devices in underwater adhesion, the safety factor varied significantly depending on the kind of ablation (pulvilli, hairs or claws), as well as on the type tested surfaces (see Table 2). The interaction between the ablation type and the surface type was statistically significant for experiments on glass (Fig. 5a and table inset), and on plastic Petri dish (Fig. 5b and table inset). For intact and all ablated insects, the safety factor recorded on glass in air, was statistically different from that on the glass immersed in water. In insects with ablated pulvilli, the safety factor was lower than that recorded in intact insects both on glass in air and on glass under water. In insects with ablated hairs and in those with ablated claws, the safety factor was not statistically different from that recorded in intact insects both on glass in air and on glass under water (Fig. 5a).
Role of N. viridula attachment devices on two substrates with different surface energies in air and under water. Safety factor (friction force divided by the insect weight) obtained with intact (n = 10) and ablated (no pulvilli, n = 10; no hairs, n = 11; no claws, n = 11) females on glass (A) and plastic Petri dish (B) (see Table 2) in air and under water. Boxplots show the interquartile range and the median, whiskers indicate 1.5× interquartile range and Х shows the arithmetic mean. For each surface, boxplots with the same colour in air and under water are compared P < 0.05. Boxplots with asterisk (*) are significantly different from the white boxplot (intact insects) at P < 0.05, Fisher LSD post hoc test. Table inset shows the statistical parameters of two-way repeated measures ANOVA
For insects with ablated hairs and insects with ablated claws, the safety factor recorded on plastic Petri dish in air was statistically higher as compared to the underwater treatment, while for intact insects and for insects with ablated pulvilli there was no significant difference between safety factors obtained on plastic Petri dish in air and under water. The safety factor on plastic Petri dish was lower in insects with ablated pulvilli than that recorded in intact insects in both air and under water. In insects with ablated hairs, the safety factor was lower than that recorded in intact insects only on underwater plastic Petri dish, but not in air. The safety factor on plastic Petri dish was not statistically different in insects with ablated claws from that recorded in intact insects in both air and under water (Fig. 5b).
Discussion
Contact area of pulvilli and hairy pad
Footprints from resting individuals on a smooth surface show that N. viridula creates contact to the substrate with the ventral surface of: (1) the distal portions of pulvilli, (2) the setae of the hairy pad, (3) the two paraempodia representing mechanosensory setae, and (4) the tip of the claws (Rebora et al. 2018). The data of the present paper analyse the maximum contact areas of pulvilli and hairy pad involved in contact with the substrate during N. viridula’s resting, pulling and inverted climbing on a smooth surface (Fig. 2). They reveal that, when standing upright (resting), the insect touches the ground with both pads. The involvement of proximal and distal pads seems to change when insect is hanging upside down (inverted climbing), a situation in which the insect uses mainly pulvilli, while the hairy pad of the basitarsus is marginally involved. The involvement of the hairy pad is lower in comparison with resting also during insect pulling, while the role of pulvilli seems to be constant. These observations suggest the primary involvement of pulvilli in adhesion in comparison with hairy pad and this is in agreement with the data reported on other insects showing distal pads characterized by strong adhesion and a main involvement in pulling forces (Roth and Willis 1952; Clemente and Federle 2008; Labonte and Federle 2013; Labonte et al. 2014; Endlein and Federle 2015).
Role of different attachment devices on substrates with different roughness
In the traction force experiments, (1) intact insects and (2) insects with ablated pulvilli, as well as hairs and claws on artificial substrates with different roughness were compared. It is shown that the ablation of pulvilli had an important effect on the friction force: the safety factor was significantly lower in comparison with the intact insects on all the tested surfaces. Such a force decrease was reduced on the most rough surfaces (G, Ra = 73.94 µm, Fig. 3) due to the presence of claws. Indeed, it is known that insect tarsi equipped only with claws can attach to a vertical surface only at a substrate roughness comparable or larger than the diameter of the claw tip (Dai et al. 2002; Song et al. 2016). Considering that in N. viridula, claw tip diameter is about 7.9 µm (Salerno et al. 2017), the role of claws can be taken into account only on the surface with the dimension of asperities larger than that (G, Fig. 3). Surprisingly, the claws ablation caused significant reduction in the friction force in comparison with intact insects not only on G but also on all the other tested surfaces (except F, Fig. 3), and the claw ablation effect on the safety factor was not significantly different on different surfaces. Different papers reported the effect of claws ablation on insect attachment ability to substrates with different roughness. The removal of claws in beetles resulted in a decrease of forces generated on rough surfaces, such as plant leaves, filter paper or cloth, but not on smooth surfaces (Stork 1980; Betz 2002). Attachment forces for the beetle G. viridula recorded on epoxy casts of surfaces with different roughness using a centrifuge device showed that the claw removal led to a significant reduction in force on rough substrates with asperity sizes ≥ 12 µm (Bullock and Federle 2011). A previous study on the Colorado potato beetle Leptinotarsa decemlineata reported that friction forces of claw-amputated beetles tested with a centrifuge device were smaller than those of intact beetles on most studied surfaces (smooth, micro-rough and coarse-rough) (Voigt et al. 2008). This result was explained by the authors as a consequence of possible damage to the unguitractor tendon complex during claw amputation (Voigt et al. 2008). Moreover the authors did not test the substrates with the asperity sizes higher than 12 µm. In the present investigation on N. viridula, the tarsus was left intact and only the claws were clipped, as proved by the SEM analysis of some tested individuals (Fig. 1). The strong impact of ablated claws on all the tested surfaces in N. viridula insects could be due to a possible effect of the claws ablation on the pulvilli functioning. We can hypothesize that, in consideration that the insect generally touches the surface with the claws even on smooth surfaces (Rebora et al. 2018), the removal of claws could affect the ability to apply additional load to the pulvilli, which are in an initial contact with the substrate. This must be the case on all surfaces independently on roughness. This hypothesis is in agreement with our observations on the contact area of N. viridula: insects with ablated claws during pulling show fore legs maximum contact area contributed by pulvilli lower than in intact insects. A functional synergism between the claws and the tarsal tenent setae was suggested by Betz (2002) in a Staphylinidae beetle in which the removal of claws (leaving the setae intact) and the neutralization of tenent setae (leaving the claws intact) resulted in a significant decrease in the pulling forces during vertical upward climbing.
The hair ablation had a less important effect on the insect attachment ability on flat surfaces, if compared with pulvilli, as suggested by its effect on the safety factor which was not different from intact insects on different tested surfaces. The ablation of hairs caused a significantly lower force in comparison with intact insects on the most smooth surface (A, Ra = 0.08 µm, Fig. 3) and on the surface with an intermediate roughness (D, Ra = 1.12 µm, Fig. 3), while on micro-rough surfaces (B,C, Ra ranging from 0.24 to 0.54 µm, Fig. 3) and high roughness surfaces (Ra ranging from 2.47 to 73.94 µm, Fig. 3), friction force was not significantly different in comparison with intact insects. These results showing an important role of the setae of the hairy pads especially on smooth surfaces or on intermediate roughness, but not on micro-rough surfaces, could be in agreement with what was previously observed for other insects, such as the fly Musca domestica (Peressadko and Gorb 2004), the beetles G. viridula (Zurek et al. 2017) and L. decemlineata (Voigt et al. 2008) and the spider Philodromus dispar (Wolff and Gorb 2012). Tarsal setae of these animals are able to generate sufficient contact mainly with the smooth substrate or smooth islands within rough substrates, whereas there is a reduction of the contact area with small surface irregularities (Gorb 2001; Peressadko and Gorb 2004; Voigt et al. 2008; Wolff and Gorb 2012; Zhou et al. 2014; Zurek et al. 2017). On the other hand, in N. viridula, the reduction of attachment forces on micro-rough surfaces (B, C, Fig. 3) and its increase on intermediate roughness (D, Fig. 3) is clearly visible in intact insects (Salerno et al. 2017) similar to what was recorded in the above reported species. Interestingly, these species (fly, beetles and spider) are equipped solely with hairy pads which make their attachment system different from that of N. viridula having also smooth pulvilli. Adhesion and/or friction on substrata with varied roughness in insects with smooth pads has been so far investigated in Locusta migratoria (Orthoptera) (Gorb 2008), C. morosus (Scholz et al. 2010; Bußhardt et al. 2012), C. impigra (Bußhardt et al. 2012) (both Phasmatodea) and Vespa crabro (Hymenoptera) (Frantsevich and Gorb 2004). In L. migratoria, a different behaviour of smooth pads in comparison with hairy pads has been observed: indeed in this species adhesion force did not increase with an increasing substrate roughness after reaching the minimum on micro-rough surfaces. However, in this experiment, only pull-off forces of euplantulae were considered (Gorb 2008), without an influence of claws and without influence of shear forces, which can be potentially important in bringing thin membranous structures, such as superficial layer of the smooth pad (Gorb et al. 2000), in contact (Filippov et al. 2011). In centrifugal tests on a series of micro-rough artificial surfaces, the stick insect C. morosus showed the lowest force on the finest roughness as compared to smooth and other micro-rough substrates (Scholz et al. 2010). As it has been shown in our previous investigation on N. viridula (Salerno et al. 2017), differently from what was reported for the other tested species with hairy pads, but similarly to L. migratoria, intact individuals, insects with ablated hairs and claws demonstrated surprisingly low friction force at higher Ra values (F, 2.74 µm). The reason of such a decrease could be due to the presence of a high amplitude roughness (as it appears from white light interferometer images, see Fig. 3) impairing the adhesive properties of pulvilli. We think that this topic should be further investigated in different insects with the same techniques and substrates, in order to better clarify the role of smooth pads on substrates with different roughness.
Attachment ability on underwater substrates and role of the attachment devices
In our experiments, aiming at evaluation of attachment ability of intact and ablated N. viridula on two substrates with different surface energies immersed in water, we observed that in intact insects, friction force on hydrophilic substrates (glass) under water was significantly lower than that on the same substrate in air, while the friction force on hydrophobic surfaces (plastic Petri dish) under water did not change as compared with that in air. The same features have been observed in another terrestrial insect, the chrysomelid beetle G. viridula. These beetles have adhesive setae on their feet that are able to trap air bubbles and freely walk on flooded substrata or even under water. Bubbles in contact with the hydrophobic substrate de-wet the substrate and produce capillary adhesion (Hosoda and Gorb 2012). That is why, in the case of N. viridula, the plausible explanation for underwater adhesive capabilities should be the contribution of the tarsal hairy pads. Which attachment devices are used by N. viridula to walk on underwater hydrophobic surfaces? The present experiments with N. viridula with different ablated leg structures, walking on plastic Petri dish in air or under water, showed an important involvement of the hairy pad, whose absence reduces significantly the attachment force, if compared with intact insects. Only in case of experiments on the plastic Petri dish, there is significant difference between the force produced by insects without hairy pads in air or in water. The ablation of pulvilli also causes a reduction of attachment ability on underwater hydrophobic surfaces, but this reduction is not statistically different between the experiments performed in air or under water.
Conclusions
Numerous insects belonging to different orders (Blattodea, Heteroptera, Hymenoptera, Orthoptera, Phasmatodea, Plecoptera and Mantophasmidae) bear two attachment pads on the same leg: tarsal and pretarsal ones (Beutel and Gorb 2001, 2006; Eberhard at al. 2009). It is also the case for the pentatomid bug N. viridula. Usually distal pads are characterized by strong adhesion and proximal pads serve mainly friction according to the reports in different papers (Roth and Willis 1952; Clemente and Federle 2008; Bullock and Federle 2009; Labonte and Federle 2013; Labonte et al. 2014; Endlein and Federle 2015). Even if the above mentioned division of labor is a widespread phenomenon across arthropods, the distal pulvilli of N. viridula revealed to be very important not only in adhesion but seem to have a fundamental role also in producing friction forces on substrates with different roughness. The ventral pulvillus side, which contains strong proportion of resilin in its distal area (the area in contact with the substrate) (Rebora et al. 2018), is constituted of a very thin epicuticle, underlain by a thick exocuticle with cuticular rods branching into thinner fibres towards the pad surface and oriented at an angle of 40° to the surface plane. The exocuticle is much thicker in the two lateral parts of the pulvillus, if compared to the central portion of the pad. This feature probably helps the pulvillus to better deform and replicate the surface profile and, also due to the flexibility and elastic properties of resilin, thereby reversibly increases contact area on rough surfaces (Rebora et al. 2018).
The proximal hairy pad of N. viridula has a role in producing some friction forces on smooth surfaces or surfaces with intermediate roughness and in producing adhesion under water or on flooded substrates. Adhesion to hydrophobic substrates under water could be very relevant for N. viridula insects in consideration that many plant leaves have hydrophobic surfaces based on their lipophilic chemistry (Koch and Barthlott 2009) and in nature plants may be covered by water for quite a long period of time, especially after heavy rain.
Our data show the role of distal and proximal attachment pads on horizontal flat surfaces. In consideration that N. viridula is a polyphagous species able to walk on vertical and horizontal stems with different dimensions and features, we cannot exclude a role of the proximal hairy pad in controlling the ability of the insect to walk on surfaces with a different geometry such as curved surfaces. To further investigate these aspects in N. viridula is particularly interesting in consideration that, to our knowledge, they have been studied so far only in insects with hairy attachment devices (Voigt et al. 2017). Moreover, in consideration that both attachment devices of N. viridula release fluid on the substrate and leave traces of such fluid at each step (Ghazi-Bayat and Hasenfuss 1980; Rebora et al. 2018), studies regarding the physical properties of such fluid and its role in the adhesion to the substrate are in progress.
The acquired knowledge on the attachment ability of N. viridula is important not only to clarify the function and performance of an attachment organ combining smooth and hairy attachment structures but can be useful also to develop in the future methods to control the southern green stink bug in the crops.
References
Betz O (2002) Performance and adaptive value of tarsal morphology in rove beetles of the genus Stenus (Coleoptera, Staphylinidae). J Exp Biol 205:1097–1113
Beutel RG, Gorb SN (2001) Ultrastructure of attachment specializations of hexapods (Arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny. J Zool Syst Evol Res 39:177–207
Beutel RG, Gorb SN (2006) A revised interpretation of the evolution of attachment structures in Hexapoda with special emphasis on Mantophasmatodea. Arthropod Syst Phylogeny 64:3–25
Beutel RG, Gorb SN (2008) Evolutionary scenarios for unusual attachment devices of Phasmatodea and Mantophasmatodea (Insecta). Syst Entomol 33:501–510
Bullock JMR, Federle W (2009) Division of labour and sex differences between fibrillar, tarsal adhesive pads in beetles: effective elastic modulus and attachment performance. J Exp Biol 212:1876–1888
Bullock JMR, Federle W (2011) The effect of surface roughness on claw and adhesive hair performance in the dock beetle Gastrophysa viridula. Insect Sci 18(3):298–304
Bullock JMR, Drechsler P, Federle W (2008) Comparison of smooth and hairy attachment pads in insects: friction, adhesion and mechanisms for direction-dependence. J Exp Biol 211(20):3333–3343
Bußhardt P, Wolf H, Gorb SN (2012) Adhesive and frictional properties of tarsal attachment pads in two species of stick insects (Phasmatodea) with smooth and nubby euplantulae. Zoology 115(3):135–141
Clemente CJ, Federle W (2008) Pushing versus pulling: division of labour between tarsal attachment pads in cockroaches. Proc R Soc Lond B Biol Sci 275:1329–1336
Dai Z, Gorb SN, Schwarz U (2002) Roughness-dependent friction force of the tarsal claw system in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). J Exp Biol 205:2479–2488
Dirks J-H, Federle W (2011) Fluid-based adhesion in insects—principles and challenges. Soft Matter 7:11047
Eberhard MJB, Pass G, Picker MD, Beutel R, Predel R, Gorb SN (2009) Structure and function of the arolium of Mantophasmatodea (Insecta). J Morphol 270(10):1247–1261
Endlein T, Federle W (2015) On heels and toes: How ants climb with adhesive pads and tarsal friction hair arrays. PLoS One 10:1–16
Federle W (2006) Why are so many adhesive pads hairy? J Exp Biol 209:2611–2621
Federle W, Riehle M, Curtis AS, Full RJ (2002) An integrative study of insect adhesion: mechanics and wet adhesion of pretarsal pads in ants. Integr Comp Biol 42:1100–1106
Filippov A, Popov VL, Gorb SN (2011) Shear induced adhesion: contact mechanics of biological spatula-like attachment devices. J Theor Biol 276:126–131
Frantsevich L, Gorb SN (2004) Structure and mechanics of the tarsal chain in the hornet, Vespa crabro (Hymenoptera: Vespidae): implications on the attachment mechanism. Arthropod Struct Dev 33:77–89
Ghazi-Bayat A, Hasenfuss I (1980) Zur Herkunft der Adhäsionsflüssigkeit der tarsalen Haftlappen bei den Pentatomidae (Heteroptera). Zool Anz 204:13–18
Gorb SN (2001) Attachment devices of insect cuticle. Kluwer Academic Publishers, Dordrecht
Gorb SN (2005) Uncovering insect stickiness: structure and properties of hairy attachment devices. Am Entomol 51:31–35
Gorb SN (2007) Visualisation of native surfaces b 414 y two-step molding. Microsc Today 15:44–46
Gorb SN (2008) Smooth attachment devices in insects: Functional morphology and biomechanics. In: Casas J, Simpson SJ (eds) Advances in insect physiology: insect mechanics and control, vol 34. Academic Press, London, pp 81–115
Gorb EV, Gorb SN (2009) Functional surfaces in the pitcher of the carnivorous plant Nepenthes alata: a cryo-SEM approach. In: Gorb SN (ed) Functional surfaces in biology—adhesion related phenomena, vol 2. Springer, Dordrecht, pp 205–238
Gorb SN, Jiao Y, Scherge M (2000) Ultrastructural architecture and mechanical properties of attachment pads in Tettigonia viridissima (Orthoptera, Tettigoniidae). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 186:821–831
Gorb EV, Hosoda N, Miksch C, Gorb SN (2010) Slippery pores: anti-adhesive effect of nanoporous substrates on the beetle attachment system. J R Soc Interface 7:1571–1579
Heepe L, Kovalev AE, Gorb SN (2014) Direct observation of microcavitation in underwater adhesion of mushroom-shaped adhesive microstructure. Beilstein J Nanotechnol 5:903–909
Hosoda N, Gorb SN (2012) Underwater locomotion in a terrestrial beetle: combination of surface de-wetting and capillary forces. Proc R Soc Lond B Biol Sci 279:4236–4242
Koch K, Barthlott W (2009) Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials. Philos Trans A Math Phys Eng Sci 367:1487–1509
Labonte D, Federle W (2013) Functionally different pads on the same foot allow control of attachment: Stick insects have load-sensitive “heel” pads for friction and shear-sensitive “toe” pads for adhesion. PLoS One 12:e81943
Labonte D, Williams JA, Federle W (2014) Surface contact and design of fibrillar “friction pads” in stick insects (Carausius morosus): mechanisms for large friction coefficients and negligible adhesion. J R Soc Interface 11(94):20140034
Panizzi AR, McPherson JE, James DG, Javaheri M, McPherson RM (2000) Stink bugs (Pentatomidae). In: Schaefer CW, Panizzi AR (eds) Heteroptera of economic importance. CRC Press, Boca Raton, pp 421–474
Peressadko AG, Gorb SN (2004) Surface profile and friction force generated by insects. In: Boblan I, Bannasch R (eds) First International Industrial Conference Bionik. VDI Verlag, Düsseldorf, pp. 257–261
Ploem JS (1975) Reflection-contrast microscopy as a tool for investigation of the attachment of living cells to a glass surface. In: van Furth R (ed) Mononuclear phagocytes in immunity, infection and pathology. Blackwell, Oxford, pp 405–421
Rebora M, Michels J, Salerno G, Heepe L, Gorb E, Gorb S (2018) Tarsal attachment devices of the southern green stink bug Nezara viridula (Heteroptera: Pentatomidae). J Morphol. https://doi.org/10.1002/.jmor.20801
Roth LM, Willis ER (1952) Tarsal structure and climbing ability of cockroaches. J Exp Biol 119:483–517
Salerno G, Rebora M, Gorb EV, Kovalev A, Gorb SN (2017) Attachment ability of the southern green stink bug Nezara viridula (Heteroptera: Pentatomidae). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 203(8):1–11
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature methods 9(7):671–675
Scholz I, Bückins L, Dolge L, Erlinghagen T, Weth A, Hischen F, Mayer J, Hoffmann S, Riederer M, Riedel M, Baumgartner W (2010) Slippery surfaces of pitcher plants: nepenthes wax crystals minimize insect attachment via microscopic surface roughness. J Exp Biol 213:1115–1125
Sokal RR, Rohlf FJ (1998) Biometry. W. E. Freeman and Company, New York
Song Y, Dai Z, Wang Z, Ji A, Gorb SN (2016) The synergy between the insect-inspired claws and adhesive pads increases the attachment ability on various rough surfaces. Sci Rep 6: N 26219
StatSoft I (2001) Statistica (Data Analysis Software System), Version 6. StatSoft Italia S.r.l., Italy
Stork NE (1980) Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae: Coleoptera) on a variety of surfaces. J Exp Biol 88:91–107
Todd JW (1989) Ecology and behavior of Nezara viridula. Annu Rev Entomol 34:273–292
Voigt D, Schuppert JM, Dattinger S, Gorb SN (2008) Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) to rough substrates. J Insect Physiol 54:765–776
Voigt D, Takanashi T, Tsuchihara K, Yazaki K, Kuroda K, Tsubaki R, Hosoda N (2017) Strongest grip on the rod: tarsal morphology and attachment of Japanese pine sawyer beetles. Zool Lett 3(1):16
Wolff JO, Gorb SN (2012) Surface roughness effects on attachment ability of the spider Philodromus dispar (Araneae, Philodromidae). J Exp Biol 215:179–184
Zhou Y, Robinson A, Steiner U, Federle W (2014) Insect adhesion on rough surfaces: analysis of adhesive contact of smooth and hairy pads on transparent microstructured substrates. J R Soc Interface 11(98):20140499
Zurek DB, Gorb SN, Voigt D (2015) Locomotion and attachment of leaf beetle larvae Gastrophysa viridula (Coleoptera, Chrysomelidae). Interface Focus 5:20140055
Zurek DB, Gorb SN, Voigt D (2017) Changes in tarsal morphology and attachment ability to rough surfaces during ontogenesis in the beetle Gastrophysa viridula (Coleoptera, Chrysomelidae). Arthropod Struct Dev 46:130–137
Acknowledgements
This work was supported by the European Cooperation in Science and Technology, EMBA COST Action CA15216, STSM Grant (ECOST-STSM-CA15216-220617-088785) at the Zoological Institute, Functional Morphology and Biomechanics, Christian-Albrechts-Universität, Kiel, Germany.
Author information
Authors and Affiliations
Contributions
The study was designed by all the authors. SG performed the cryo-scanning electron microscopy investigations. GS and MR performed the traction force experiments. EG and AK characterized the tested surfaces. The manuscript was written by GS, MR and EG. All authors discussed the analysis and interpretation of the results and participated in the final editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Salerno, G., Rebora, M., Kovalev, A. et al. Contribution of different tarsal attachment devices to the overall attachment ability of the stink bug Nezara viridula. J Comp Physiol A 204, 627–638 (2018). https://doi.org/10.1007/s00359-018-1266-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-018-1266-0