Abstract
Reproductive and behavioural specialisations characterise advanced social insect societies. Typically, the honey bee (Apis mellifera) shows a pronounced reproductive division of labour between worker and queen castes, and a clear division of colony roles among workers. In a queenless condition, however, both of these aspects of social organisation break down. Queenless workers reproduce, forage and maintain their colony operating in a manner similar to communal bees, rather than as an advanced eusocial group. This plasticity in social organisation provides a natural experiment for exploring physiological mechanisms of division of labour. We measured brain biogenic amine (BA) levels and abdominal fat body vitellogenin gene expression levels of workers in queenright and queenless colonies. Age, ovary activation and social environment influenced brain BA levels in honey bees. BA levels were most influenced by ovary activation state in queenless bees. Vitellogenin expression levels were higher in queenless workers than queenright workers, but in both colony environments vitellogenin expression was lower in foragers than non-foragers. We propose this plasticity in the interacting signalling systems that influence both reproductive and behavioural development allows queenless workers to deviate significantly from the typical worker bee reaction norm and develop as reproductively active behavioural generalists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A key reason for the success of the social insect lifestyle is a marked division of labour between members of the society (Oster and Wilson 1978). The degree of individual specialisation is considered one of the features that distinguish advanced from primitive societies (Michener 1974; Page et al. 2006) such that in the advanced eusocial insects the reproductive role is limited to one, or a few, highly specialised individuals supported by a mostly sterile worker caste. This segregates investment in reproduction, colony growth, and maintenance into different individuals, which increases the total reproductive output of the group (Wilson 1985; Richards et al. 2005).
In advanced social insect societies, colony efficiency is boosted further by specialisation of roles within the worker caste (Oster and Wilson 1978). In some species (particularly some species of ants and termites) there are morphologically specialised worker castes, but it is more common for worker castes to be behaviourally and physiologically specialised in association with age; a process known as temporal polyethism (Michener 1974; Robinson 1992).
The honey bee (Apis mellifera) has emerged as an important model system for exploring the development and evolution of the worker caste, and behavioural specialisation between workers (Page and Amdam 2007; Toth and Robinson 2007; Kucharski et al. 2008; Toth and Robinson 2009; Amdam and Page; Bloch and Grozinger 2011). From this research an influential family of “ground-plan” and “tool kit” hypotheses (Amdam et al. 2004; Page and Amdam 2007; Toth and Robinson 2007, 2009; Amdam and Page 2010; Bloch and Grozinger 2011) has developed inspired by West-Eberhard’s (2003) theories of phenotypic evolution. These theories differ slightly in detail, but all propose that the social traits of the worker caste and temporal polyethism evolved by selection acting on physiological mechanisms of phenotypic plasticity in a solitary or subsocial ancestor. Selection for specialisation between individuals in a society is imagined to have driven the evolution of alternative phenotypes as different reaction norms from the same genome by canalisation of physiological mechanisms enabling behavioural and reproductive plasticity in the ancestor.
While it is possible the worker honey bee caste evolved by canalisation of behavioural plasticity in an ancestral species, a great deal of phenotypic plasticity remains within the extant worker caste. The typical worker reaction norm is sterile, but in queenless (QL) colonies workers are able to activate their ovaries and lay viable male eggs (Boomsma 2009). Worker sterility in queenright (QR) colonies is interpreted as a form of adaptive self-restraint by workers (Ratnieks and Visscher 1989; Visscher 1996). In QR colonies worker ovary activation is extremely rare (Ruttner and Hesse 1981; Visscher 1996; Miller and Ratnieks 2001). In QR colonies worker-laid eggs are destroyed by worker policing, but in QL colonies worker policing breaks down and very high levels of worker ovary activation and worker laying have been reported (Miller and Ratnieks 2001).
Recent studies have emphasised the marked differences in both physiology and behaviour between the ‘typical’ workers from QR colonies and the reproductive workers that develop in QL colonies (Naeger et al. 2013). The degree of reproductive and behavioural specialisation of workers in QL colonies is far less than in QR colonies (Naeger et al. 2013). QL workers combine periods of foraging with egg laying and attending brood (Naeger et al. 2013), and forage with activated ovaries, wax glands and brood food producing glands (Naeger et al. 2013). QR workers atrophy these glands before commencing foraging, and once foraging has begun they do not continue other tasks (Seeley 1982, 1995; Winston 1987; Seeley and Kolmes 1991). QL honey bee collective behaviour is reminiscent of subsocial communal bees that cooperate to maintain and defend a nest while engaging in direct reproduction (Michener 1974; Naeger et al. 2013). A similar phenomenon that has been noted in QL A. cerana colonies also (Tan et al. 2015).
The variation in worker behavioural and reproductive phenotypes between QR and QL conditions provides a natural experiment which can be used to examine the physiological mechanisms of specialisation in worker honey bees. Here we compared the physiology of QL and QR worker bees to examine how specialisations in worker honey bees develop. We examined two signalling systems in QL workers that have been causally related to reproductive and behavioural division of labour in bees: the storage protein vitellogenin (Vg) (Amdam et al. 2004, 2006; Page et al. 2006) and the biogenic amine (BA) neurochemicals (Wagener-Hulme et al. 1999; Barron et al. 2002).
BAs have been shown to be involved both in reproductive pathways and worker behavioural specialisation in honey bees (Harris and Woodring 1995; Barron and Robinson 2005). Workers with activated ovaries have increased dopamine (DA) levels in the brain (Harris and Woodring 1995; Sasaki and Nagao 2001) and workers fed with DA show increased ovary activation (Dombroski et al. 2003). In two-day-old bees, DA levels are reduced when bees are treated with synthetic queen mandibular pheromone (Beggs et al. 2007), suggesting that queen presence may reduce DA levels and inhibit ovary activation in very young bees. Behavioural specialisations are also influenced by brain BA levels. Older foraging bees have higher brain levels of octopamine (OA), serotonin (5HT) and DA than non-foragers (Taylor et al. 1992; Schulz and Robinson 1999; Wagener-Hulme et al. 1999). Brain OA levels are higher in foragers, regardless of age (Schulz and Robinson 1999; Wagener-Hulme et al. 1999; Schulz et al. 2003). Further, OA treatment causes an earlier onset of foraging in young bees (Schulz and Robinson 2001).
Vg has also been causally related to both reproduction and division of labour. Vg typically functions in egg formation in insects (Amdam et al. 2004), but in honey bees the onset of foraging in workers is driven in part by a reciprocal inhibitory interaction between Vg and juvenile hormone. An increase in juvenile hormone levels and a decrease in Vg levels triggers the onset of foraging (Amdam et al. 2006; Page and Amdam 2007; Bloch and Grozinger 2011). Peripheral knockdown of the Vg gene by RNAi resulted in precocious foraging (Nelson et al. 2007; Antonio et al. 2008), elevated juvenile hormone titres (Guidugli et al. 2005), and extensive gene expression changes in the honey bee brain that are also regulated by juvenile hormone (Wheeler et al. 2013).
In typical QR honey bee workers the regulatory systems described above organise an inhibition of worker reproduction and clear temporal polyethism with distinct hive work and foraging phases. These features are canonical of honey bee society, and yet reproductive workers in QL colonies deviate markedly from this pattern. Reproductive QL workers combine foraging with reproduction, and act as task generalists. Here we compared brain BA levels, and mRNA expression of Vg in the abdominal fat body of QL and QR workers displaying different behaviour and levels of ovary activation to explore how QL workers can deviate so markedly from the typical worker pattern. Our study highlights the extent of the flexibility in honey bee behavioural and physiological development, and how bees can adapt to changes in their social environment.
Methods
Colony establishment
This study took place at Macquarie University in North Ryde, NSW Australia and the University of Illinois at Urbana, IL, USA. To establish the colonies, we took regular commercial colonies housed in a Langstroth hive box holding eight frames of honeycomb and split the colony into two smaller four-frame nucleus hive boxes (one will be QL the other QR) balancing numbers of bees, brood and food stores across the two nucleus colonies. In the QR colonies, we allowed the bees in one nucleus hive to naturally raise a new queen from the eggs transferred to the nucleus hive from the original colony. In the QL colonies, queen cells were destroyed, preventing the bees from rearing a new queen. This process continued for the QL colonies until no new queen cells, no eggs in the regular pattern laid by queens were observed, and repeated inspection found no queen in the colony. In this state, the colony was considered to be hopelessly queenless.
Once this stage was reached, cohorts of 1000 newly emerged bees were obtained from honeycomb frames of emerging brood taken from at least five colonies. The frames were held overnight in an incubator (34 °C, 65 % humidity), and the newly emerged adult bees were marked with Tamiya enamel paint on the thorax. 1000 marked bees of this age-matched cohort were added to each of the nucleus colonies.
BA analyses were conducted initially in 2011, with one QL and one QR colony (which were also used for behavioural analyses (Naeger et al. 2013)). In 2013, we repeated the study with two QL colonies and two QR colonies. BA analyses on whole brains were conducted on the 2011 and 2013 samples. BA analyses on brain regions were conducted only on the 2013 samples. For Vg qPCR we used a pair of QL and QR colonies established using the same method at the University of Illinois apiary in 2012 (Naeger et al. 2013).
Forager identification and marking
The entrances of experimental colonies were observed when the age-matched focal cohort was between 8–21 days old in the 2011 cohorts [for behavioural observations (Naeger et al. 2013)], and when the focal cohort of bees were 13–14 days old and 20–21 days old in the 2012 and 2013 cohorts. Bees returning with pollen in their corbiculae were identified as pollen foragers, and bees returning with distended abdomens were identified as non-pollen foragers. When a bee from the marked focal cohort was noted foraging it was marked with a second paint mark on the abdomen so focal foragers could be identified later.
Bee collection
Forager and non-forager bees from the focal cohorts were sampled when 14 and 21 days of age. Ovary activation takes at least 2 weeks to complete in worker bees, and in a typical colony bees usually transition from in-hive tasks to foraging when >14 days old. Hence with this sampling strategy we hoped to sample bees with a range of ages, behaviour and reproductive condition from both colonies. Marked worker bees were sampled arbitrarily from inside the hive at dusk (when most foragers had returned to the hive for the night), with foragers being identified by the extra paint mark on their abdomens. Non-foragers were bees that had not been observed foraging. Workers were sampled in the same manner for all of the experiments. Bees were immediately frozen in liquid nitrogen (thus preserving physiological state at the time of collection), before being placed in Eppendorf tubes (pre-chilled in dry ice) and stored at −80 °C until dissection. Heads were separated from abdomens so that brains could be taken for BA analysis and abdomens were dissected to assess level of ovary activation.
Analysis of worker ovary activation
Bee abdomens were dissected to determine the level of ovary activation. Worker ovaries were scored according to the criteria established by Hess (1942). For analysis purposes, ovaries scored 1 or 2 were considered ‘inactivated’ if they lacked yolk (stages 1 and 2 from Hess 1942) and ‘activated’ if they contained yolk (stages 3–5 from Hess 1942).
Brain dissection
Frozen bee heads were partially lyophilised at 300 mTorr and −40 °C for 45 min prior to brain dissection (Schulz and Robinson 1999). Whole bee brains, including the optic lobes and gnathal ganglion (GNG), were dissected from the head capsule on dry ice so they remained frozen. In 2011, amine content of the whole brain was analysed. In 2013, brain samples were further dissected into four distinct regions: optic lobes (OLs), antennal lobes, the central brain and the GNG (see Fig. 3 inset for a schematic diagram). Dissected brains and brain regions were returned to storage at −80 °C until analysis of BA content with high-pressure liquid chromatography (HPLC).
Biogenic amine analysis
The BA content of individual brains was measured using HPLC coupled to a coulometric electrochemical detector. HPLC methods followed those of Søvik et al. (2013). In brief, brains were centrifuged at 15 G for 2 min at 4 °C, and homogenised by ultrasonication in 60 µl of 0.2 M perchloric acid containing the HPLC standard dihydroxybenzylamine (DHBA). Samples were incubated for 20 min at 0 °C protected from light, then centrifuged at 15 G and 4 °C for 14 min to pellet cellular debris. Ten microlitre of the supernatant of each sample was analysed using an Agilent 1200 Series HPLC system. Samples were separated across a BDS Hypersil column (Thermo Scientific) with 0.2 micron octadecylsilane packing. BA content was quantified using an ESA Coulochem III electrochemical detector with an ESA 5011A high-sensitivity dual-electrode analytical cell. All amines were quantified relative to DHBA on one channel running at 1100 mV. Brain regions were homogenised in 20 µl (not 60 µl) of 0.2 M perchloric acid containing the HPLC standard DHBA. To obtain whole brain BA levels for individual bees sampled in 2013, we summed the amounts for all brain regions.
Vg quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Abdomens from the frozen samples were incubated in RNA Later ICE for 16 h at −20 °C. The internal organs were removed with the exception of the fat body on the dorsal abdominal plates. The mRNA preservation method used for the Vg sampling rendered worker ovarioles both transparent and brittle. Regrettably, it proved impossible to accurately assess state of ovary activation for these samples. Tergites 2–6 were then homogenised in a bead beater with 500 mL of Trizol®, and nucleotides were then extracted with a Qiagen RNEasy kit. The samples were treated with DNase (Qiagen) while bound to the column. Transcription levels were assessed with qRT-PCR. We performed 10 μL reactions using FastStart Universal SYBR Green Master (Roche). Primers used for Vg were: forward: 5′-AGTTCCGACCGACGACG-3′; reverse 5′-TTCCCTCCCACGGAGTCC-3′. The housekeeping gene AmeIF.S8 (eukaryotic initiation factor S8) was used as a control AmeIF.S8, GenBank accession no: GB41874. Primers used for AmeIF.S8 were: forward: 5′-TGAGTGTCTGCT ATGGATTGCAA-3′; reverse 5′-TCGCGGCTCGTGGTAAA-3′.
Statistical analysis
We examined several factors that could potentially explain the observed variance in BA levels: ovary activation (active or inactive ovaries), age (14 or 21 days of age), foraging status (forager or non-forager), colony queen status (QR or QL). We used a general linear mixed modelling approach to examine which of the aforementioned factors could best explain the observed variation in levels of each of the BAs (OA, DA and 5HT). Year, colony, and HPLC batch were included as random factors. The variable ‘colony’ referred to the specific replicate colony the bee was collected from. As HPLC machine performance varies over time, we included the variable ‘HPLC batch’, which referred to the day that we ran the samples through the HPLC machine. Analyses were conducted on BA levels of whole brains, or brain regions. Since there is no significant variation in brain protein content between adult bees of different ages or performing different roles (Schulz and Robinson 1999) we considered any variation in brain size with age or behaviour to be insignificant. For simplicity our analyses were performed on BA levels of each brain, or each brain region rather than expressing brain BA levels relative to brain protein content as has been done previously (Schulz and Robinson 1999).
The following procedure was used for all modelling. Maximal models attributing variance to all explanatory variables with all their possible interactions were generated. Interactions with a p value >0.1 were removed from the model, one level at the time starting with the highest order and re-running the model after each removal until only interactions with a p value <0.1 remained. From this reduced model all non-significant (p > 0.05) main effects not necessary for the interactions remaining in the model were removed one at a time. This was done in a step-wise manner, starting with the least significant and working downwards to the minimum adequate model containing factors explaining significant variation in the data only.
Results
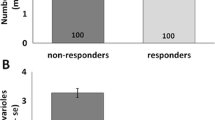
The proportion of bees with active ovaries was much higher in the QL colonies than the QR colonies in both 2011 and 2013 (Fig. 1). Furthermore, in both the QL and QR colonies, there was no significant difference in the proportion of bees with active ovaries between foragers and non-foragers at either 14 and 21 days of age (Fig. 1 depicts differences for 21 day old bees).
Proportion of 21-day-old foraging and non-foraging workers with activated ovaries in the queenright (QR) and queenless (QL) colonies in the experiment. In both the QL and QR colonies, there was no significant difference in ovary activation between foragers and non-foragers in each colony. Sample sizes are listed above the bars. n.s. = no significant difference in ovary activation between foragers and non-foragers in the colony of interest (Chi-square tests)
Analysis of biogenic amine content of whole brains
The combined analyses of whole brain BA were conducted on samples collected in 2011 and 2013 and are presented below, with year of collection considered as a random factor in the statistical model. Bee age, level of ovary activation and queen status affected brain BA levels (Figs. 2, 3, 4). A colony’s queen status (QR or QL) and individual ovary activation (active or inactive) were highly confounded because most workers from QL colonies possessed activated ovaries, and most workers from QR colonies did not (Fig. 1). We considered both queen status and ovary activation as possible factors in the statistical modelling, but the two factors are biologically related and statistically difficult to separate. Significant variation in OA levels was explained by bee age—older bees had higher brain OA levels (Figs. 2, 4; Table 1). By contrast, variation in 5HT brain levels was more related to colony queen status and ovary activation. In our general linear mixed model both queen status and ovary activation explained significant variation in 5HT levels (Table 1; Fig. 4). 5HT levels were higher in both bees with activated ovaries and in QL colonies. Variation in 5HT levels between ages and sampling years was noticed (Figs. 2, 4) but this was not significant (Table 1). Significant variation in DA levels was explained by age, ovary activation, and the interaction between these terms. DA levels in whole brains were higher in 21 day old bees than 14 day old bees (Figs. 2, 4). Bees with activated ovaries had higher DA levels compared to bees with inactive ovaries when 14 days old, but this difference was not seen in 21 day old bees (Figs. 2, 4).
Whole brain biogenic amine levels (mean ± SEM) in honey bee workers from QR and QL colonies sampled at 14 and 21 days of age in 2011 and 2013 in Sydney, Australia. QR = queenright colony, QL = queenless colony, DA = dopamine, OA = octopamine, 5-HT = serotonin. Sample sizes are listed inside the bars
Biogenic amine levels (mean ± SEM) in brain regions of workers sampled at 21 and 14 days old pooled from the two QR and two QL colonies sampled in 2013 in Sydney, Australia. QR = queenright colony, QL = queenless colony, DA = dopamine, OA = octopamine, 5-HT = serotonin, GNG = gnathal ganglion. Sample sizes are listed inside the bars
Minimum adequate models of relationship between brain biogenic amines and predictors (Tables 1, 2). Top row shows the relationship between DA, OA, and 5-HT (first, second, and third column, respectively) in whole bee brains as summarised by the minimum adequate models. Ovary status is shown in red (activated), and grey (not activated), while age is shown in yellow (14 day olds) and blue (21 day olds). Second row shows the same, but for central brains. The third row displays results for optic lobes, while the bottom row show results for gnathal ganglia. DA was not detected at high enough levels to analyse in optic lobes or gnathal ganglia, OA was not present at high enough levels in gnathal ganglia. QR = queenright colony, QL = queenless colony, DA = dopamine, OA = octopamine, 5-HT = serotonin
Analysis of variation in biogenic amine levels of brain regions
The analyses of brain biogenic amines in brain regions were conducted on samples collected in 2013. Unfortunately, we were not able to accurately resolve BA levels in the antennal lobe samples with our current HPLC set-up as most levels were below detection threshold. Similarly we were unable to accurately quantify levels of DA in the OLs; and both OA and DA in the GNG as levels of these BA were at or below detection threshold in these brain regions.
Central brain
In the central brain, significant variance in DA levels was explained by age, and the interaction between age and ovary activation (Table 2; Fig. 4). DA levels were higher in 21-day-old bees than 14-day-old bees, and this difference was greater for bees with inactivated ovaries than activated ovaries (Figs. 3; 4). Variance in OA levels was explained by ovary activation (Table 2; Fig. 4) in that OA levels were lower in bees with activated ovaries (Figs. 3, 4). Queen status explained significant variance in 5HT levels (Table 2), with higher levels of 5HT in the central brain of QR than in QL bees (Fig. 3).
Optic lobes
Ovary activation explained significant variance in OA levels in OLs (Table 2), whereas for 5HT queen status explained significant variance (Table 2; Fig. 4). In both cases, levels were higher in the QR colony, which contained the majority of the bees with inactivated ovaries (Fig. 3).
Gnathal ganglion
In the GNG significant variance in 5HT levels were explained by queen status and ovary activation (Table 2; Fig. 4), similar to results for whole brain analyses.
Vg expression levels
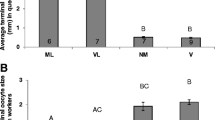
Vg analysis was conducted on samples collected in 2012. Vg expression varied with both colony type and behaviour (Fig. 5). Vg expression levels (relative to expression levels of a housekeeping gene, S8) were far higher in QL bees than QR bees. In both colonies Vg levels were lower in foragers than non-foragers (Fig. 5).
Level of Vg mRNA in fat body of foragers and non-forager bees sampled from QL and QR colonies sampled in 2012 in Urbana, Illinois. The level is not an absolute scale, but ΔΔCT calculated relative to S8 expression levels. Values are plotted on a log2 scale to increase resolution at the lower range of measurement. In boxplots the central line marks the median value, boxes extend to quartiles and whiskers extend to 1.5 × interquartile distance. Sample size was 20 independent replicates for each group. Expression levels differed between groups (Kruskal–Wallis test: KW = 43.82, P < 0.0001). Significant differences were observed between foragers and non-foragers in both QL and QR colonies (Dunns’ multiple comparisons test: P < 0.05)
Discussion
Our data detail the very high degree of phenotypic plasticity within the honey bee worker caste. Several physiological pathways influence the development of the ‘reaction norm’ of a sterile worker bee in a QR colony: most notably Vg (interacting with juvenile hormone) and the BAs (Bloch and Grozinger 2011). By contrast, QL workers deviate markedly from this reaction norm. In QL colonies most workers have active ovaries (Fig. 1), combine foraging with egg laying and show far less individual behavioural specialisation than is typical of QL workers (Naeger et al. 2013). Here we have shown marked differences in brain BA levels and abdominal Vg expression in workers from QL and QR colonies. Brain levels of DA, OA and 5HT in workers were strongly influenced by the queen status of the colony and/or worker ovary activation (Figs. 2, 3, 4). Abdominal Vg expression was influenced by both queen status and worker behaviour (Fig. 5). We propose the changes in Vg and BA systems seen in QL colonies are causally related to the unusual reproductive worker phenotype.
In our samples there was an unavoidable statistical colinearity between the degree of ovary activation of individual bees and the colony environment in which bees developed. Ovary activation can only be determined after sampling by dissection, and in both the 2011 and 2013 collections almost all bees collected from the QL colonies had activated ovaries, and conversely almost all bees collected from the QR colonies had inactive ovaries. Worker ovary activation can occur in QR colonies, but it is extremely rare (Ruttner and Hesse 1981; Visscher 1996; Miller and Ratnieks 2001). This is interpreted as adaptive self-restraint by QR workers in response to destruction of worker laid eggs by other workers (worker policing) (Ratnieks and Visscher 1989; Visscher 1996), but worker policing breaks down in QL colonies and in this environment very high levels of worker laying are seen (Miller and Ratnieks 2001). The statistical consequence of this colinearity for our modelling was that any variance in BA levels attributable to either colony type or ovary activation was almost entirely confounded.
Plasticity in brain BA levels with age and social environment
Despite this confound, brain levels of 5HT were influenced by both ovary activation and queen status (Table 1; Figs. 2, 4). Modelling suggested variance in 5HT levels in the central brain was influenced by queen status, whereas variance in 5HT levels in the GNG was influenced by both queen status and ovary activation. (Table 2; Fig. 4). We do not wish to over-speculate, but it is possible that different 5HT systems in different regions of the brain are involved in different social or physiological responses. Workers upregulate expression of two serotonin receptor genes in their ovaries in a QL environment (Vergoz et al. 2012), implying a possible role of 5HT signalling in worker ovary activation. There may be greater 5HT release to the periphery from the GNG (Rehder et al. 1987) in workers with active ovaries in QL colonies.
Brain levels of both OA and DA varied with both ovary activation and age (Figs. 2, 3, 4). Brain DA levels were influenced by both age and ovary activation; highest in older bees, and in bees with active ovaries. DA has previously been shown to be causally linked to ovary activation in bees (Dombroski et al. 2003), and mRNA expression for two DA receptors is increased in active worker ovaries (Vergoz et al. 2012). DA signalling systems may even interact with Vg signalling to influence worker physiology. Nuñes et al. (2013) recently showed that experimental manipulation of Vg expression was linked to changes in two microRNAs potentially modifying DA receptor expression in the brain.
Here we documented variation in brain OA levels with both age and ovary activation (Figs. 2, 3, 4). Previously, differences between forager and nurse bees (bees observed caring for the brood) in brain OA levels have been documented for same age bees (Schulz and Robinson 1999; Barron et al. 2002; Barron and Robinson 2005), but no differences between forager and non-forager bees in brain BA levels were detected here. This may have been due to our sampling strategy. We could not collect nurse bees from QL colonies because there was not enough drone brood in the QL colonies at the times of sampling to collect bees caring for brood. We were limited to collect age-matched foragers and bees that had not been observed foraging for at least 2 days prior to sampling. Our non-forager samples likely contained bees performing a range of in-hive tasks. Given that we could not collect nurse bees in this study it is not contradictory of earlier studies data that we did not observe differences in BA levels between foragers and non-foragers here (Schulz and Robinson 1999). While OA levels increased with age in the brain overall, they decreased in the central brain with ovary activation (Figs. 2, 3, 4). As a consequence, in QL colonies the increase in brain OA with age typically seen in worker bees was reduced (Fig. 2).
Plasticity in Vg expression
In this study abdominal Vg expression varied with both worker ovary development and behaviour (Fig. 5). Our data support the findings of Lin et al. (1999) and Nakaoka et al. (2008) who documented that workers with developed ovaries have higher Vg expression levels than bees with inactivated ovaries. But our data also shows that Vg levels decline in foragers (Amdam and Omholt 2003; Guidugli et al. 2005; Nelson et al. 2007; Antonio et al. 2008). In this study Vg expression levels were far higher overall in QL than QR colonies, but in both colony environments Vg expression levels were lower in foragers than non-foragers (Fig. 5).
Amdam et al. (Amdam and Omholt 2003; Guidugli et al. 2005) have proposed that Vg levels regulate the onset of foraging by interaction with juvenile hormone in a reciprocally inhibitory dynamic such that Vg inhibits juvenile hormone and vice versa. The onset of foraging is caused by both a decrease in Vg and/or an increase in juvenile hormone. Our data would be consistent with this model (Amdam and Omholt 2003) if it is the decrease in Vg expression level that stimulates foraging onset rather than an absolute level of Vg expression level. This hypothesis is reconcilable with our observations of foragers in QL colonies that had Vg expression levels far higher than the non-foragers in QR colonies (Fig. 5). A signalling system organised in this way could allow reproductive workers in QL colonies to combine active foraging with supporting active ovaries and hypopharyngeal glands (Naeger et al. 2013). Similarly, plasticity in brain BA systems that influence both behaviour and reproductive physiology could enable the co-occurrence of reproduction and foraging, and the task generalism seen in reproductive QL workers.
Conclusion and implications for the development of the worker caste
To summarise our findings; we have reported many differences in neuroendocrine systems involved in reproduction and task specialisation between QL and QR bees. We argue these differences may in part explain the unusual reproductive worker phenotype which combines active laying with active foraging and brood feeding (Ohashi et al. 2000; Naeger et al. 2013). We cannot conclude from these data whether differences in BA levels and Vg expression were caused by the different social environments of the QL and QR colonies directly or indirectly by effects of the social and pheromonal environment on ovary activation or other aspects of physiology in workers. Differentiating between these factors could be very difficult since ovary activation in a QR colony is extremely rare (Visscher 1989, 1996), and workers react physiologically very rapidly to the absence of the queen (Grozinger et al. 2003). However, our data clearly speak to the very high levels of reproductive plasticity within the honey bee worker caste and the sensitivity of worker physiology and behaviour to their social environment.
BA and Vg signalling systems are involved in the control of reproduction in so many insect groups that a reproductive function for these systems is considered rather basal for insects (West-Eberhard 1987; Toth and Robinson 2007; Bloch and Grozinger 2011). These systems also regulate the derived features of reproductive caste formation and division of labour. This supports the genetic tool kit and ground-plan family of hypotheses (West-Eberhard 1987, 1996; Amdam et al. 2004; Toth and Robinson 2007), which propose that these advanced social traits evolved by exaptation and modification of mechanisms of reproductive and behavioural plasticity in solitary or subsocial ancestors (Bloch and Grozinger 2011). This resulted in the evolution of divergent reaction norms from the same genotype yielding reproductive and worker castes (Toth and Robinson 2007; Bloch and Grozinger 2011). The ground-plan theories have been extended to propose that behavioural specialisations within the worker caste evolved by evolutionary modification of signalling systems that ancestrally regulated reproductive phases to yield new functions for them in the control of worker temporal polyethism (Amdam et al. 2004, 2006; Page et al. 2006; Page and Amdam 2007). While evolution may have acted on ancestral phenotypic plasticity to form the worker caste, our study emphasises the high degree of physiological and behavioural plasticity that exists within the extant honey bee worker caste. Reproductive QL workers deviate markedly from the worker reaction norm. They combine traits, which are normally distinct, supporting active egg laying with active foraging and colony maintenance tasks. We propose extant plasticity in the interacting systems that regulate both behaviour and reproductive physiology in bees enables this unusual combination of traits to coexist in reproductive worker bees.
Abbreviations
- 5HT:
-
Serotonin
- BA:
-
Biogenic amine(s)
- DA:
-
Dopamine
- OA:
-
Octopamine
- QR:
-
Queenright
- QL:
-
Queenless
- Vg:
-
Vitellogenin
- Vg :
-
Vitellogenin gene
References
Amdam GV, Omholt SW (2003) The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. J Theor Biol 223:451–464
Amdam GV, Page RE (2010) The developmental genetics and physiology of honeybee societies. Anim Behav 79(5):973–980. doi:10.1016/j.anbehav.2010.02.007
Amdam GV, Norberg K, Fondrk MK, Page RE (2004) Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc Nat Acad Sci USA 101:11350–11355
Amdam GV, Csondes A, Fondrk MK, Page RE (2006) Complex social behaviour derived from maternal reproductive traits. Nature 439:76–78
Antonio DSM, Guidugli-Lazzarini KR, do Nascimento AM, Simões ZLP, Hartfelder K (2008) RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissen 95(10):953–961
Barron AB, Robinson GE (2005) Selective modulation of task performance by octopamine in honey bee (Apis mellifera) division of labor. J Comp Physiol A 191:659–668
Barron AB, Schulz DJ, Robinson GE (2002) Octopamine modulates responsiveness to foraging-related stimuli in honey bees (Apis mellifera). J Comp Physiol A 188:603–610
Beggs KT, Glendining KA, Marechal NM, Vergoz V, Nakamura I, Slessor KN, Mercer AR (2007) Queen pheromone modulates brain dopamine function in worker honey bees. Proc Nat Acad Sci USA 104:2460–2464
Bloch G, Grozinger CM (2011) Social molecular pathways and the evolution of bee societies. Phil Trans Roy Soc B 366:2155–2170
Boomsma JJ (2009) Lifetime monogamy and the evolution of eusociality. Phil Trans Roy Soc B 364(364):3191–3207
Dombroski T, Simões Z, Bitondi M (2003) Dietary dopamine causes ovary activation in queenless Apis mellifera workers. Apidologie 34(3):281–289
Grozinger CM, Sharabash NM, Whitfield CW, Robinson GE (2003) Pheromone-mediated gene expression in the honey bee brain. Proc Nat Acad Sci USA 100:14519–14525
Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Omholt S, Simões ZLP, Hartfelder K (2005) Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett 579(12):4961–4965
Harris JW, Woodring J (1995) Elevated brain dopamine levels associated with ovary development in queenless worker honey bees (Apis mellifera L.). Comp Biochem Physiol A 111C:271–279
Hess G (1942) Über den Einfluß der Weisellosigkeit und des Fruchtbarkeitvitamines auf die Ovarien der Bienenarbeiterin. Schweiz Bienen Zeitung 2:33–110
Kucharski R, Maleszka J, Foret S, Maleszka R (2008) Nutritional control of reproductive status in honeybees via DNA methylation. Science 319:1827–1830
Lin H, Winston ML, Haunerland NH, Slessor KN (1999) Influence of age and population size on ovarian development, and of trophallazis on ovarian development and vitellogenin titres of queenless worker honey bee (Hymenoptera: Apidae). Can Entomol 131:695–706
Michener CD (1974) The social behaviour of the bees. Harvard University Press, Cambridge Mass
Miller DG, Ratnieks FLW (2001) The timing of worker reproduction and breakdown of policing behaviour in queenless honey bee (Apis mellifera L.) societies. Ins Soc 48(2):178–184. doi:10.1007/pl00001762
Naeger NL, Peso M, Even N, Barron AB, Robinson GE (2013) Altruistic behavior by egg-laying worker honeybees. Curr Biol 23(16):1574–1578
Nakaoka T, Takeuchi H, Kubo T (2008) Laying workers in queenless honeybee (Apis mellifera L.) colonies have physiological states similar to that of nurse bees but opposite that of foragers. J Insect Phys 54(5):806–812. doi:10.1016/j.jinsphys.2008.02.007
Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam GV (2007) The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol 5(3):e62. doi:10.1371/journal.pbio.0050062
Nuñes FMF, Ihle KE, Mutti NS, Simões ZLP, Amdam GV (2013) The gene vitellogenin affects microRNA regulation in honey bee (Apis mellifera) fat body and brain. J Exp Biol 216(19):3724–3732
Ohashi K, Sasaki M, Sasagawa H, Nakamura J, Natori S, Kubo T (2000) Functional flexibility of the honey bee hypopharyngeal gland in a dequeened colony. Zool Sci 17(8):1089–1094
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton
Page RE, Amdam GV (2007) The making of a social insect: developmental architectures of social design. BioEssays 29:334–343
Page RE, Scheiner R, Erber J, Amdam GV (2006) The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.). Curr Topics Dev Biol 74:253–286
Ratnieks FLW, Visscher PK (1989) Worker policing in the honeybee. Nature 342:796–797
Rehder V, Bicker G, Hammer M (1987) Serotonin-immunoreactive neurons in the antennal lobes and suboesophageal ganglion of the honeybee. Cell Tissue Res 247(1):59–66
Richards MH, French D, Paxton RJ (2005) It’s good to be queen: classically eusocial colony structure and low worker fitness in an obligately social sweat bee. Mol Ecol 14(13):4123–4133
Robinson GE (1992) Regulation of division of labour in insect societies. Annu Rev Entomol 37:637–665
Ruttner F, Hesse B (1981) Rassenspezifische Unterschiede in Ovarentwicklung und Eiablage von weisellosen Arbeiterinnen der Honigbiene. Apidologie 12:159–183
Sasaki K, Nagao T (2001) Distribution and levels of dopamine and its metabolites in brains of reproductive workers in honeybees. J Insect Phys 47(10):1205–1216
Schulz DJ, Robinson GE (1999) Biogenic amines and division of labor in honey bee colonies: behaviorally related changes in the antennal lobes and age related changes in the mushroom bodies. J Comp Physiol A 184:481–488
Schulz DJ, Robinson GE (2001) Octopamine influences division of labor in honey bee colonies. J Comp Physiol A 187:53–61
Schulz DJ, Elekonich MM, Robinson GE (2003) Biogenic amines in the antennal lobes and the initiation and maintenance of foraging behavior in honey bees. J Neurobiol 54:406–416
Seeley TD (1982) Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol 11:287–293
Seeley TD (1995) The wisdom of the hive: the social physiology of honey bee colonies. Harvard University Press, Cambridge
Seeley TD, Kolmes SA (1991) Age polyethism for hive duties in honey bees—illusion or reality. Ethology 87:287–297
Søvik E, Cornish JL, Barron AB (2013) Cocaine tolerance in honey bees. PLoS One 8(5):e64920. doi:10.1371/journal.pone.0064920
Tan K, Wang Y, Dong S, Liu X, Zhuang D, Chen W, Oldroyd BP (2015) Associations between reproduction and work in workers of the Asian hive bee Apis cerana. J Insect Phys 82:33–37
Taylor DJ, Robinson GE, Logan BJ, Laverty R, Mercer AR (1992) Changes in brain amine levels associated with the morphological and behavioural development of the worker honeybee. J Comp Physiol A 170:715–721
Toth AL, Robinson GE (2007) Evo-devo and the evolution of social behavior. Trends Genet 23:334–341
Toth AL, Robinson GE (2009) Evo-devo and the evolution of social behavior: brain gene expression analyses in social insects. Cold Spring Harb Symp Quant Biol. doi:10.1101/sqb.2009.74.026
Vergoz V, Lim J, Oldroyd BP (2012) Biogenic amine receptor gene expression in the ovarian tissue of the honey bee Apis mellifera. Ins Mol Biol 21(1):21–29
Visscher PK (1989) A quantitative study of worker reproduction in honey bee colonies. Behav Ecol Sociobiol 25:247–254
Visscher PK (1996) Reproductive conflict in honey bees: a stalemate of worker egg-laying and policing. Behav Ecol Sociobiol 39:237–244
Wagener-Hulme C, Kuehn JC, Schulz DJ, Robinson GE (1999) Biogenic amines and division of labor in honey bee colonies. J Comp Physiol A 184:471–479
West-Eberhard MJ (1987) Flexible strategy and social evolution. In: Itô Y, Brown JL, Kikkawa J (eds) Animal societies: theories and facts. Japan Science Society Press, Tokyo, pp 35–51
West-Eberhard MJ (1996) Wasp societies as microcosms for the study of development and evolution. In: West-Eberhard MJ, Turillazzi S (eds) Natural history and evolution of paper wasps. Oxford University Press, Oxford, pp 290–317
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford
Wheeler MM, Ament SA, Rodriguez-Zas SL, Robinson GE (2013) Brain gene expression changes elicited by peripheral vitellogenin knockdown in the honey bee. Insect Mol Ecol 22(5):562–573
Wilson EO (1985) The sociogenesis of insect colonies. Science 28:1489–1495
Winston ML (1987) The biology of the honey bee. Harvard University Press, Cambridge
Acknowledgments
We wish to thank Louise Crépeau and Branden Dunbar for sorting samples and two anonymous reviewers for their comments that significantly improved this manuscript. This research was supported by iMQRES scholarships awarded to M. Peso and N. Even, and an NIH Pioneer Award DP1 OD006416 (GER). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peso, M., Even, N., Søvik, E. et al. Physiology of reproductive worker honey bees (Apis mellifera): insights for the development of the worker caste. J Comp Physiol A 202, 147–158 (2016). https://doi.org/10.1007/s00359-015-1061-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-015-1061-0