Abstract
The switch from within-hive activities to foraging behavior is a major transition in the life cycle of a honeybee (Apis mellifera) worker. A prominent regulatory role in this switch has long been attributed to juvenile hormone (JH), but recent evidence also points to the yolk precursor protein vitellogenin as a major player in behavioral development. In the present study, we injected vitellogenin double-stranded RNA (dsVg) into newly emerged worker bees of Africanized genetic origin and introduced them together with controls into observation hives to record flight behavior. RNA interference-mediated silencing of vitellogenin gene function shifted the onset of long-duration flights (>10 min) to earlier in life (by 3–4 days) when compared with sham and untreated control bees. In fact, dsVg bees were observed conducting such flights extremely precociously, when only 3 days old. Short-duration flights (<10 min), which bees usually perform for orientation and cleaning, were not affected. Additionally, we found that the JH titer in dsVg bees collected after 7 days was not significantly different from the controls. The finding that depletion of the vitellogenin titer can drive young bees to become extremely precocious foragers could imply that vitellogenin is the primary switch signal. At this young age, downregulation of vitellogenin gene activity apparently had little effect on the JH titer. As this unexpected finding stands in contrast with previous results on the vitellogenin/JH interaction at a later age, when bees normally become foragers, we propose a three-step sequence in the constellation of physiological parameters underlying behavioral development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transition from within-hive activities to foraging represents a major step in the life cycle of a honeybee worker and is accompanied by a suite of behavioral and physiological changes (Rösch 1925; Lindauer 1952; Free 1961). Although this transition typically becomes fully expressed when a worker bee is about 3 weeks old (Winston 1987), it is not strictly related to age (Robinson 1992). In temperate climates, for example, bees reared in autumn may forego for several months this transition from intranidal activities to foraging and form a worker population that survives until the following spring. Furthermore, drastic changes in the age demography of a honeybee colony, such as the loss of a large portion of the forager or the hive bee population can either advance this transition or cause a reversal in the ontogeny of tasks performed by a bee (Rösch 1930; Robinson and Huang 1998).
This behavioral plasticity requires the presence of globally acting regulators capable of integrating the entire suite of behavioral–physiological changes. The first regulator shown to fulfill such a role was juvenile hormone (JH), which, when applied to newly emerged adult bees, induced them to forage at an earlier age (Jaycox 1976). The role of JH in the transition from a hive bee to becoming a forager and its relation to age has subsequently been dissected by analyses of JH biosynthesis dynamics and JH titer changes in natural colonies, in hives with an experimentally altered age demography (Huang and Robinson 1996), and also in allatectomized bees (Sullivan et al. 2003), all suggesting that JH plays a pacemaker role in behavioral development.
The close interaction of JH with a second major pacemaker component, the yolk protein precursor vitellogenin, was first postulated on theoretical grounds and fitted into a mathematical model termed the “double repressor hypothesis,” which proposed that vitellogenin and JH could mutually repress each other (Amdam and Omholt 2003). After the honeybee vitellogenin gene was cloned and sequenced (Piulachs et al. 2003), this hypothesis could be tested using RNA interference (RNAi)-mediated silencing of vitellogenin gene function (Amdam et al. 2003). And as predicted by the model, injection of vitellogenin double-stranded RNA (dsRNA) resulted in a stable increase in the JH titer in honeybees independent of their geographic origin and social setting (Guidugli et al. 2005).
An intrinsic and desirable property of such double repressor circuitries is that, at a critical concentration of either component, the system quickly shifts to a new state, which, in the case of the honeybee worker, makes possible a rapid hive bee-to-forager transition and subsequent fixation in the new behavioral task. As the high vitellogenin titer found in nurse bees should repress the increase of the JH titer, behavioral development would then essentially become contingent on the level of vitellogenin in the hemolymph. This apparent evolutionary co-option of a female-specific reproductive protein into the role of a systemic regulator of behavior and aging has expanded its functions into that of a pleiotropically acting major life cycle regulator in honeybees (Amdam and Page 2007). The role of vitellogenin in redirecting the behavior of a bee has recently been evidenced by Nelson et al. (2007) who showed that injection of vitellogenin dsRNA accelerates the transition of a hive bee to become a forager and, concomitantly, decreases its lifespan.
In the present study, we specifically investigated the effect of vitellogenin depletion on JH titer and the onset of foraging. We focused on young hive bees because previous data suggested that critical decisions for the determination of a bee’s life history may be taken during the first few days of adult life (Jassim et al. 2000; Amdam et al. 2007; Nelson et al. 2007). Being the first direct correlative analysis between vitellogenin-dependent behavioral change and endogenous hormone levels, it was designed to reveal the coupling strength of the vitellogenin/JH circuitry in these young bees.
Materials and methods
Silencing of vitellogenin expression, behavioral analysis, and sampling of bees
Double-stranded honeybee vitellogenin RNA was prepared as previously described (Amdam et al. 2003) following the protocol of the Promega RiboMax™ T7 system (Promega). After phenol-chloroform extraction and heat treatment, the dsRNA was diluted with nuclease-free water to a final concentration of 5 μg/μl for injection.
Bees emerging from a donor-colony frame within a 12-h period were randomly assigned to one of three treatments. In the experimental group, newly emerged workers were injected intra-abdominally with 1 μl vitellogenin dsRNA (dsVg group, N = 230 in four experiments). The second group (sham group, N = 140 in four experiments) received a 1-μl injection of nuclease-free water to control for handling effects, which is an established protocol for vg RNAi experiments (Amdam et al. 2003; Guidugli et al. 2005). The third group of bees was left untreated (control group, N = 125 in four experiments). Bees showing signs of hemolymph leakage after withdrawal of the injection needle were discarded. All bees were tagged with numbered Opalith plates (Graze, Germany), reserving a different color for each of the three groups.
These groups of focal bees were then introduced together (common garden setup) into an observation hive consisting of 1,000–2,000 worker bees of all age classes and an egg-laying queen. The observation hive was located in a darkened observation facility, and the bees had access to the outside via an inspection ramp. The ramp contained a section that could be closed when a marked bee departed for or returned from a flight, so that its individual number could be registered. Observations were made daily for 2 h (10:30–11:30 and 13:30–14:30) during seven to ten consecutive days. During these observations, we registered the behavior of tagged bees both inside the flight ramp and on the comb. At days 10 (experiments 1–3) or 7 (experiment 4), the observation hive was opened, and all tagged bees were sampled. Hemolymph retrieved by capillary suction from a lateral incision in the bee’s abdomen was used for protein electrophoresis and for JH quantification by radioimmunoassay. Subsequently, the intestine was removed, and the abdomen was used for RNA extraction.
The four repetitions of the experiment were all performed in the winter/spring season in São Paulo State, Brazil, which offers ideal warm and dry weather conditions for honeybee flight studies. In each experiment, we introduced a total of 100–120 bees, with a predominance of dsVg bees over the controls to compensate for the higher initial mortality rates of dsVg bees observed in preliminary trials.
Validation of knockdown phenotype by sodium dodecyl sulfate polyacrylamide gel electrophoresis and reverse transcriptase polymerase chain reaction
Protein analyses were performed using samples of 1 μl hemolymph that were run on sodium dodecyl sulfate (SDS)–polyacrylamide gels (7.5% gel), subsequently stained with Coomassie Brilliant Blue. Gels were scanned (Kodak EDAS 290) for quantification of the vitellogenin and apolipoprotein-I bands (Kodak 1D program, version 3.6.2). Apolipoprotein-I, which is expressed at constant levels throughout the adult life cycle, was used as protein loading control for normalization of vitellogenin levels in the 1-μl hemolymph samples (Guidugli et al. 2005).
RNA was extracted from abdominal carcasses kept in Trizol reagent (Invitrogen). After treatment with RNase-free DNase (Promega), 1.5 μg RNA were reverse transcribed using an oligo(dT)12–18 primer (Invitrogen) in a standard protocol for SuperScript II (Invitrogen). These first-strand complementary DNA samples were then subjected to quantitative real-time polymerase chain reaction (qPCR) using primers specific for honeybee vitellogenin (AJ517411; vgF: 5′-GCAGAATACATGGACGGTGT-3′, vgR: 5′-GAACAGTCTTCGGAAGCTTG-3′). As endogenous control, we used a constitutively expressed β-actin gene of A. mellifera (AB023025; actF: 5′-TGCCAACACTGTCCTTTCTG-3′, actR: 5′-AGAATTGACCCACCAATCCA-3′). Amplifications were carried out in a real-time PCR system (7500 Real Time PCR System, Applied Biosystems) using SYBR®Green Master Mix (Applied Biosystems) at the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Relative copy numbers were calculated by the comparative C T method (Pfaffl 2001); Applied Biosystems, User bulletin 2). Each sample was analyzed in triplicate.
Juvenile hormone titer analysis
For JH extraction, we followed an established protocol for honeybee hemolymph (Huang et al. 1994). Briefly, 1 ml NaCl (0.9%) and 1 ml hexane were added to each sample (1–2 μl hemolymph in 500 μl acetonitrile). After vigorous mixing and phase separation by centrifugation (1,000×g) the hexane phase was removed, and the extraction was repeated twice. The pooled hexane phases were dried, and the residue was redissolved in 50 μl toluene. After transfer to 1 ml glass vials, the solvent was again removed by vacuum centrifugation before adding the radioimmunoassay (RIA) components.
A JH-specific antiserum generated in rabbit was diluted to 1:1,250 in phosphate buffer supplemented with bovine serum albumin (0.1%) and rabbit immunoglobulin G (0.1%). The assays were performed with [10–3H(N)]-juvenile hormone III (spec. activity 19.4 Ci/mmol, NEN Life Science Products), diluted in the phosphate buffer to 6,000–6,500 cpm per 50 μl. Racemic juvenile hormone III (Fluka) was used as non-radioactive ligand. The RIA procedure followed the protocol established by (Goodman et al. 1990). Standard curves were set up to cover a 50-pg to 10-ng range and transformed into a linear log/logit regression model for calculation of the JH content of unknown samples (expressed as JH-III equivalents in pg/μl hemolymph).
Data analysis
Vitellogenin and JH titers were analyzed by non-parametric analysis of variance (ANOVA; Kruskal–Wallis test). Dunn’s post hoc tests were performed for comparisons between groups. The results on number of flights, age at onset of potential foraging flights, and on duration of flights were analyzed by two-way ANOVA (treatment and age). In all cases, the adequacy of ANOVA assumptions was verified by Bartlett’s tests.
Results
Validation of RNAi-mediated vitellogenin depletion
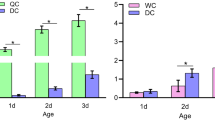
As an expected result of the vitellogenin dsRNA injection, vitellogenin transcript levels (Fig. 1a) were significantly lower than those of the control groups (Kruskal–Wallis, H 2,24 = 13.10, P = 0.0014; Dunns multiple comparisons: control vs. sham F 1,14 = 3.29, P > 0.05; control vs. dsVg, F 1,17 = 11.93, P < 0.01; sham vs. dsVg F 1,17 = 8.64, P < 0.05). This reduction in vitellogenin gene function resulted in a concordant reduction in vitellogenin protein. In most of the control and the sham group samples, vitellogenin bands were clearly visible, and their densitometric values (Fig. 1b) were significantly higher than those of the dsRNA-injected group (Kruskal–Wallis, H 2,89 = 21.22, P < 0.0001; Dunns multiple comparisons: control vs. sham, F 1,53 = 8.49, P > 0.05; control vs. dsVg, F 1,66 = 28.53, P < 0.001; sham vs. dsVg, F 1,59 = 20.03, P < 0.05). In terms of mean values, the dsVg group showed an approximately 50% reduction compared to the untreated controls and a 30% reduction compared to the sham group in this major hemolymph protein.
Vitellogenin transcript (a) and vitellogenin protein (b) levels in 7- to 10-day-old honeybee workers. Newly emerged workers were individually marked and intra-abdominally injected with 1 μl (5 μg/μl) vitellogenin dsRNA (dsVg), whereas controls were injected with 1 μl nuclease-free water (sham) or were left untreated (control). These bees were introduced into observation hives and were sampled after 7 (experiment 4) or 10 days (experiments 1–3). Represented are mean values and their standard errors, sample size and the results of statistical analyses (Kruskal–Wallis, Dunn’s multiple comparisons, see text; different letters indicate significant differences between groups, P < 0.05)
Effect of vitellogenin knockdown on onset, number, and duration of flights
Observations on flight activity were initiated 1 day after release of the focal bees and were conducted during two 1-h periods per day. In the subsequent analyses, we separated the observed flights into two groups. Flights that lasted less than 10 min were considered as orientation or cleaning flights, whereas flights lasting more than 10 min were considered as potential foraging events. Foraging flights are generally longer than 5 min (Winston and Katz 1982), and this flight duration criterium has been used in previous studies (Giray et al. 1999). Other studies were more stringent and took repeated flights of 15 min as an indication of foraging activity (Sullivan et al. 2000). The 10-min flight criterium that we adopted here is an intermediate one, accounting for the fact that we had our focus on young bees.
The observed flight events were first analyzed separately for the four experiments. In all four experiments, the bees that had received an injection of vitellogenin double-stranded RNA (dsVg group) initiated flights earlier in life, generally followed by the water-injected bees (sham group) and then the untreated tagged bees (control group). It was only in one experiment (experiment 3) that the control bees and the dsVg bees overlapped in their flight activity curves. As, however, there was no statistical difference for each treatment across the four experiments (Kruskal–Wallis controls, H 3,40 = 3.23, P = 0.36; sham, H 3,52 = 0.49, P = 0.92; dsVg, H 3,65 = 1.16, P = 0.76), we pooled the data to increase the power of statistical analyses. Figure 2a shows the cumulative number of flights during the observation period, summing up all flights, independent of whether they were of short (<10 min) or long duration (>10 min) and also whether a bee performed more than one flight. In contrast, Fig. 2b shows the onset of long-duration flights for individual bees, and consequently, in this latter graph, each marked bee is represented only once; that is, it shows the age when she was first seen performing a flight of at least 10-min duration.
Daily records of all flights, onset of long-duration flights, and duration of individually registered flights performed by focal bees kept in observations hives. a Cumulative numbers of flights representing all flight events registered over seven to 10 consecutive days during daily two 1-h observation periods show a slightly earlier onset of flight activity for the vitellogenin dsRNA-injected bees (dsVg, 47 bees total), compared to the sham-injected (43 bees) and the untreated control group (34 bees). b Day at which the first potential foraging flight (flight duration, >10 min) was performed by an individual bee. For this parameter, the difference between the dsVg group (22 bees total) and controls (16 bees for sham and nine bees for untreated control) is more pronounced. c Plotting age vs. duration of flights performed by the three groups of focal bees in the four experiments further illustrates the earlier onset of long-term flight activity in vitellogenin-depleted bees
The dsVg bees differed from the sham and the control groups in both attributes, and in particular, they were seen to perform potential foraging flights already 2 days after they were introduced into the observation hives. In the sham and the control group, only very few bees showed an early onset of potential foraging flight activity, and this occurred only 4–5 days after the first long-duration flight event was registered for the vitellogenin knockdown group (Fig. 2b). The onset of long-duration flight activity for bees of the vitellogenin-depleted group differed significantly from the controls (two-way ANOVA: treatment, F 2,46 = 6.39, P = 0.0107; days, F 7,46 = 10.38, P = 0.001); LSD tests: dsVg vs. control, t 7,29 = 5.45, P = 0.0005; dsVg vs. sham, t 7,39 = 5.22, P = 0.0038; sham vs. control, t 7,26 = 1.47, P = 0.092).
A similar picture emerged when we plotted all flights registered during the observation periods in the four experiments according to their respective duration (Fig. 2c). This graph shows that most flights were of short duration, lasting less than 10 min, and that all three groups were almost equally represented in this class of flights. As already evident from Fig. 2b, longer flights were only performed by dsVg bees during the first 5 days. Especially for flights that lasted 30 min and more, the predominance of dsVg bees during the entire observation period is noteworthy (12 flights vs. four for sham and two for control bees). These results corroborate those of a recently published study (Nelson et al. 2007), demonstrating in an independent setup that depletion of hemolymph vitellogenin levels has a dramatic effect on a worker bee’s life history, effectively turning it into an extremely precocious forager.
Effect of vitellogenin knockdown on the juvenile hormone titer
From the set of focal bees bled at termination of the last two experiments, we selected a total of 63 hemolymph samples for radioimmunoassay analyses of their JH titers. These samples were selected based on prior SDS–polyacrylamide gel electrophoresis (SDS-PAGE) analyses of the vitellogenin titer to make sure that the dsVg bees were authentic knockdowns. The results of the JH analyses (Fig. 3) were surprising because they did not reveal significant differences between the three groups (Kruskal–Wallis, H 2,66 = 5.22, P = 0.073). A closer look at individual bees, however, showed a possible break point in the distribution of JH titer values for the dsVg bees and also for the sham group. In both of these groups, we found a considerable number of bees (eight out of 30 in dsVg and seven out of 20 in sham) presenting titers above 200 pg/μl hemolymph, whereas in the control group, only one hemolymph sample (out of 16) reached this level. This threshold level is not arbitrary but has already appeared in a previous study as a separator for dsVg bees from the control and sham bees in a vitellogenin RNAi experiment with caged workers (Guidugli et al. 2005).
Hemolymph juvenile hormone titer of 7-day-old worker honeybees (experiment 4). Bees injected at emergence with vitellogenin dsRNA (dsVg), with nuclease-free water (sham), or untreated bees (control) were kept in an observation hive and were all sampled at the end of the experiment. Values shown are for individual bees; the horizontal bars representing group mean values. There were no statistical differences for the treatment, but especially the dsVg bees appear to fall into two distinct groups, bees above and below a 200-pg JH/μl threshold
Discussion
Our results confirm recently published data of an independent study (Nelson et al. 2007), revealing a very pronounced effect of vitellogenin depletion on a worker bee’s propensity to initiate foraging flights. Some of the vitellogenin dsRNA-injected bees initiated long-duration flights approximately 4 days before the earliest flights were observed in the control groups, and they did so possibly as soon as their flight machinery was sufficiently matured (Fielding et al. 1980; Harrison and Fewell 2002; Roberts and Elekonich 2005; Schippers et al. 2006). As foraging loads of pollen or nectar were not collected, we cannot affirm that these early long-duration flights were actually foraging flights, but the fact that several bees were seen to perform more than one long-duration flight during the same or on consecutive days suggests that these bees were actually visiting flowers (Sullivan et al. 2000). For comparison with other studies, it is important to note that we do not report in this study mean values for the onset of flight in populations of focal bees, but rather, we terminated the experiment after seven (experiment 4) or 10 days (experiments 1–3), that is, well before all bees could have initiated foraging. This end point was chosen because we were primarily interested in the early effects on behavior and physiology resulting from the depletion of vitellogenin.
The young age at onset of flight activity observed in our study is extraordinary, even for African genotype bees. It is commonly accepted that, on the one hand, African or Africanized worker bees initiate foraging earlier than European honeybees (Winston and Katz 1982) and, on the other, that an altered age demography in a colony can lead to precocious foraging (Huang and Robinson 1996; Jassim et al. 2000). But neither of such genotype nor environment effects have ever been reported to drive a worker into foraging activity as early as observed here in vitellogenin-depleted Africanized honeybees.
Even though in general terms our results confirm those obtained by Nelson et al. (2007), the onset of flight in our set of Africanized bees was earlier, in both the experimental and the control groups. Furthermore, the separation between the experimental group and the controls for the parameter onset of flight activity was wider in our study than in their study performed on honeybees kept in Davis, CA, USA. This difference may be attributed to a genotype effect evidencing a stronger penetration of African alleles in the Brazilian bee population, when compared to bee genotypes in the South of the USA, where the Africanization process has only recently begun (Whitfield et al. 2006). Other reasons for this stronger separation between the experimental and control groups could lie in the experimental design and subsequent statistical analysis. While Nelson et al. (2007) performed their study with two colonies and observed the bees and their survival for 40 days, which permitted a cumulative hazard data analysis, we ran four trials and interrupted them at 7–10 days because we were interested in vitellogenin RNAi effects on young bees. This required data analysis by two-way ANOVA. It is also important to emphasize environmental conditions; we performed our study under weather conditions that permitted unrestricted daily flight activity.
It may seem quite surprising that we did not see a stronger response in the JH titer in the dsVg group, as such a connection was clearly evident in a previous study performed on caged worker bees (Guidugli et al. 2005). It is, however, important to note that, among the marked bees assayed for their Vg and JH titers at the end of this current experiment, there were only a few ones for which we had obtained flight records. Especially the most active bees, the early fliers, could not be retrieved, and we assume that they had already died before the end point of the experiment. This could imply that the bees selected for JH analysis, based on their previously determined vitellogenin titer, were mainly from a group that behaviorally responded to a lesser extent to vitellogenin depletion than the population average. A closer look at the distribution pattern of individual JH titers, as shown in Fig. 3, indeed revealed that only few of the dsVg bees actually had JH titers above the 200 pg/μl threshold, which we typically saw in caged dsVg bees (Guidugli et al. 2005).
It is actually this unpredicted result—a strong downregulation of vitellogenin after dsVg injection that is not firmly coupled to an upregulation of the JH titer in all of these bees—that raises the interesting question of coupling strength between these two major regulators of division of labor in worker honeybees, especially in young bees. The study by Jassim et al. (2000) had already shown that, in single-cohort colonies, up to 50% of the focal bees started to forage once they were 7–13 days old and that this was accompanied but not preceded by an increase in their hemolymph JH titer.
The emerging picture is that vitellogenin may figure as the initial factor that determines the onset of foraging, while JH would come in secondarily to definitively lock a worker’s behavior and physiology into the forager state. This proposed sequence of physiological events underlying the transition from hive bee to forager explains earlier, apparently problematic findings on the association between JH and the onset of foraging. First of all, the doses of JH or JH analogs required to turn worker bees into precocious foragers are extremely high (applications of 250 μg JH-III or methoprene: Jaycox 1976; Robinson 1985, 1987; Sullivan et al. 2000) when compared with the dose of pyriproxyfen required to suppress the vitellogenin titer (Pinto et al. 2000) or to JH-III doses required for queen induction in late larval instars (Rembold et al. 1974; Copijn et al. 1979). Even though considering that pyriproxyfen is a biologically more active JH analog than methoprene (Cusson et al. 1994; Wyatt et al. 1995), these differences in dose are quite striking. Second, advances in the hive bee-to-forager transition induced by such hormone treatments were only apparent at a relatively late age, that is, bees never became foragers within the first few days of adult life. Third, a high JH titer is apparently not always required for continued foraging, as shown by JH titer analyses of bees foraging in early spring or late fall (Huang and Robinson 1995). Unfortunately, vitellogenin titers have not been measured in these bees.
From the current results, we now propose a three-step model for behavioral development in honeybee workers. In the first step, which coincides with the maturation phase of young honeybee workers, the gradual increase of the vitellogenin titer would be required to prevent these bees from initiating foraging flights. The JH titer level should not be critical because small JH and ecdysteroid peaks were observed during this phase (Jassim et al. 2000; Hartfelder et al. 2002), and these probably promote maturation of the newly emerged bees, especially of their flight machinery (Fielding et al. 1980; Harrison and Fewell 2002; Roberts and Elekonich 2005; Schippers et al. 2006). The second phase would then cover the hive bee stage. The duration of this stage is highly plastic and appears to be primarily contingent on the repression of JH synthesis by an elevated vitellogenin titer, as predicted in the double repressor model for honeybee life cycle regulation (Amdam and Omholt 2003). When this balance is tipped by downregulation of vitellogenin synthesis (Guidugli et al. 2005), a gradual increase in the JH titer then accompanies the hive bee-to-forager transition (Fahrbach and Robinson 1996; Huang and Robinson 1996; Robinson and Huang 1998). The repression of vitellogenin synthesis by an elevated JH titer (Pinto et al. 2000) would subsequently guarantee that workers become firmly (but not irreversibly) locked into the forager state.
Logically reasoning, a second point that will have to be addressed experimentally is whether maintaining higher-than-normal vitellogenin levels would delay the onset of foraging in bees. While it is not yet feasible to analyze behavioral development in transgenic bees overexpressing vitellogenin, it is possible to interfere with genes involved in JH synthesis or metabolism and to see how this may affect the vitellogenin titer and behavior. One such study, where a JH esterase encoding gene was functionally silenced by RNAi, showed that the JH titer was increased, as expected, but that this only had a minor effect on the vitellogenin titer (Makert et al. 2008). Furthermore, while there is firm evidence for genotype influence on rates of behavioral development (Giray et al. 1999; Elekonich et al. 2003), it has also become clear that the absolute level of the vitellogenin titer is less critical for determining the age at first foraging than its rates of change during the first 15 days of adult life (Amdam et al. 2007). This latter study was designed to investigate coupling strength between vitellogenin and JH titers and used two strains of bees selected for differences in pollen hoarding behavior. Five-day-old bees of the strong pollen hoarding phenotype had not only a higher vitellogenin titer than those of the low pollen hoarding strain but also experienced a stronger modulation in the hemolymph titer of this protein in the subsequent days, showed an earlier onset of foraging, and had a tendency to a decreased life span. Worker bees of the high strain also showed a higher propensity to activate their ovaries under queenless condition (Amdam et al. 2006) and exhibited a more pronounced response in the JH titer to a reduction in vitellogenin gene function (Amdam et al. 2007). Obviously, this suite of traits constitutes a complex syndrome within which vitellogenin and JH are important players, and what appears to be really critical is their genotypic coupling strength.
The determinants of this coupling strength are still unknown yet can become accessible via quantitative trait locus analysis of complex behavioral traits followed by identification of candidate genes on the background of the honeybee genome sequence (Hunt et al. 2007). Once such determinants come to light, it should be possible to investigate whether and how the coupling strength between vitellogenin and JH may change during the life cycle of a worker bee, as proposed above in the three-step sequence for the interaction between these two factors. It is especially in young bees (1–5 days of age) where much of the relationship between JH and vitellogenin is still unclear in terms of coupling strength of the double repressor hypothesis (Amdam and Omholt 2003). It is during this maturation phase that the vitellogenin titer increases in both queens and workers (Engels 1974). It is also the phase when the JH titer increases in queens, coinciding with the onset of mating flights (Robinson et al. 1991), and when it goes through a small peak in workers (Jassim et al. 2000), coincident with a small ecdysteroid peak (Hartfelder et al. 2002).
Currently, our understanding of signaling through vitellogenin is still limited because this protein has only recently been upgraded from a downstream factor in female reproductive physiology to a major life cycle regulator in the highly eusocial honeybees (Amdam and Omholt 2003; Amdam et al. 2006). In this context, the detection of vitellogenin expression in fat body cells surrounding the brain is of interest (Corona et al. 2007). However, whether this represents a feasible pathway for vitellogenin signaling to the neuroendocrine axis still needs corroboration from localization studies of vitellogenin receptor.
New links among vitellogenin, JH, longevity, and behavioral development are currently emerging from studies on insulin/insulin-like growth factor signaling (IIS) related to longevity in queens (Corona et al. 2007) and division of labor in honeybees (Ament et al. 2008). The expression patterns observed for an insulin-like peptide (AmILP-1) and for two genes encoding insulin receptors (AmInR-1 and AmInR-2) in brains and abdomens of honeybee workers indicate that the evolution of social behavior in corbiculate bees involved not only rewiring in the vitellogenin/JH circuitry but also the appearance of atypical IIS responses (Ament et al. 2008), when compared to solitary insects (Tatar et al. 2003; Flatt et al. 2005). In view of our results on apparent change in coupling strength between vitellogenin and JH, which is possibly mediated by an atypical IIS signature, we fully ascribe to the conclusion of Ament et al. (2008): ‘that interactions among insulin signaling, nutrition, JH, Vg, and the environment are more complicated than had previously been imagined.’ The factor constellation during the maturation phase early in adult life, which emerged as of interest during this study, should definitely receive more attention in future more direct investigations, as it may be during this phase where much of the life history characteristics of a worker bee become established.
References
Amdam GV, Omholt SW (2003) The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. J Theor Biol 223:451–464
Amdam GV, Page RE (2007) The making of a social insect: developmental architectures of social design. BioEssays 29:334–343
Amdam GV, Simoes ZLP, Guidugli KR, Norberg K, Omholt SW (2003) Disruption of vitellogenin gene function in adult honeybees by intra-abdominal injection of double-stranded RNA. BMC Biotechnol 3:1–8
Amdam GV, Csondes A, Fondrk MK, Page RE (2006) Complex social behaviour derived from maternal reproductive traits. Nature 439:76–78
Amdam GV, Nilsen K-A, Norberg K, Fondrk MK, Hartfelder K (2007) Variation in endocrine signaling underlies variation in social life history. Am Nat 170:37–46
Ament SA, Corona M, Pollock HS, Robinson GE (2008) Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci USA 105:4226–4231
Copijn GM, Beetsma J, Wirtz P (1979) Queen differentiation and mortality after application of different juvenile hormone analogues to worker larvae of the honey bee (Apis mellifera L.). Proc Kon Ned Akad Wetensch 82C:295–302
Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE (2007) Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Natl Acad Sci USA 104:7128–7133
Cusson M, Yu CG, Carruthers K, Wyatt GR, Tobe SD, McNeill JN (1994) Regulation of vitellogenin production in armyworm moths, Pseudaletia unipunctata. J Insect Physiol 40:129–136
Elekonich MM, Jez K, Ross AJ, Robinson GE (2003) Larval juvenile hormone treatment affects pre-adult development, but not adult age at onset of foraging in worker honey bees (Apis mellifera). J Insect Physiol 49:359–366
Engels W (1974) Occurrence and significance of vitellogenins in female castes of social Hymenoptera. Am Zool 14:1229–1237
Fahrbach SE, Robinson GE (1996) Juvenile hormone, behavioral maturation, and brain structure in the honey bee. Dev Neurosci 18:102–114
Fielding K, Hepburn HR, Chandler HD (1980) On the development of flight competence in worker honeybees. Comp Biochem Physiol Part A Mol Integr Physiol 654:129–133
Flatt T, Tu MP, Tatar M (2005) Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bio Essays 27:999–1010
Free JB (1961) Hypopharyngeal gland development and division of labor in honey bee (Apis mellifera L.) colonies. Proc R Entomol Soc Lond A 36:5–8
Giray T, Huang ZY, Guzman-Novoa E, Robinson GE (1999) Physiological correlates of genetic variation for rate of behavioral development in the honeybee, Apis mellifera. Behav Ecol Sociobiol 47:17–28
Goodman WG, Coy DC, Baker FC, Xu L, Toong YC (1990) Development and application of a radioimmunoassay for the juvenile hormones. Insect Biochem 20:357–364
Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Omholt S, Simoes ZLP, Hartfelder K (2005) Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett 579:4961–4965
Harrison JF, Fewell JH (2002) Environmental and genetic influences on flight metabolic rate in the honey bee, Apis mellifera. Comp Biochem Physiol Part A Mol Integr Physiol 133:323–333
Hartfelder K, Bitondi MMG, Santana WC, Simões ZLP (2002) Ecdysteroid titer and reproduction in queens and workers of the honey bee and of a stingless bee: loss of ecdysteroid function at increasing levels of sociality? Insect Biochem Mol Biol 32:211–216
Huang ZY, Robinson GE (1995) Seasonal changes in juvenile hormone titers and rates of biosynthesis in honey bees. J Comp Physiol B Sens Neural Behav Physiol 165:18–28
Huang ZY, Robinson GE (1996) Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol 39:147–158
Huang ZY, Robinson GE, Borst DW (1994) Physiological correlates of division of labor among similarly aged honey bees. J Comp Physiol A Sens Neural Behav Physiol 174:731–739
Hunt GJ, Amdam GV, Schlipalius D, Emore C, Sardesai N, Williams CE, Rueppell O, Guzmán-Novoa E, Arechavaleta-Velasco M, Chandra S, Fondrk MK, Beye M, Page RE (2007) Behavioral genomics of honeybee foraging and nest defense. Naturwissenschaften 94:247–267
Jassim O, Huang ZY, Robinson GE (2000) Juvenile hormone profiles of worker honey bees, Apis mellifera, during normal and accelerated behavioural development. J Insect Physiol 46:243–249
Jaycox ER (1976) Behavioral changes in worker honey bees (Apis mellifera) after injection with synthetic juvenile hormone (Hymenoptera: Apidae). J Kans Entomol Soc 49:165–170
Lindauer M (1952) Ein Beitrag zur Arbeitsteilung im Bienenstaat. Z Vergl Physiol 34:299–345
Makert A, Nascimento AM, Bitondi MMG, Hartfelder K, Simões ZLP (2008) Identification of a juvenile hormone esterase-like gene in the honey bee, Apis mellifera L.—expression analysis and functional assays. Comp Biochem Physiol B Biochem Mol Biol 150:33–44
Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam GV (2007) The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol 5:e62
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Pinto LZ, Bitondi MMG, Simoes ZLP (2000) Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J Insect Physiol 46:153–160
Piulachs MD, Guidugli KR, Barchuk AR, Cruz J, Simoes ZLP, Belles X (2003) The vitellogenin gene of the honey bee, Apis mellifera: structural analysis of the cDNA and expression studies. Insect Biochem Mol Biol 33:459–465
Rembold H, Czoppelt C, Rao PJ (1974) Effect of juvenile hormone treatment on caste differentiation in the honey bee Apis mellifera. J Insect Physiol 20:1193–1202
Roberts SP, Elekonich MM (2005) Muscle biochemistry and the ontogeny of flight capacity during behavioral development in the honey bee, Apis mellifera. J Exp Biol 208:4193–4198
Robinson GE (1985) The effect of a juvenile hormone analogue on honey bee foraging behaviour and alarm pheromone production. J Insect Physiol 31:277–282
Robinson GE (1987) Regulation of honey bee age polyethism by juvenile hormone. Behav Ecol Sociobiol 20:329–338
Robinson GE (1992) Regulation of division of labor in social insects. Annu Rev Entomol 37:637–665
Robinson GE, Huang ZY (1998) Colony integration in honey bees: genetic, endocrine and social control of division of labor. Apidologie 29:159–170
Robinson GE, Strambi C, Strambi A, Feldlaufer MF (1991) Comparison of juvenile hormone and ecdysteroid hemolymph titers in adult worker and queen honey bees (Apis mellifera). J Insect Physiol 37:929–935
Rösch GA (1925) Untersuchungen über die Arbeitsteilung im Bienenstaat, I. Teil: Die Tätigkeiten im normalen Bienenstaate und ihre Beziehungen zum Alter der Arbeitsbienen. Z Vergl Physiol 2:571–631
Rösch GA (1930) Untersuchungen über die Arbeitsteilung im Bienenstaat, II. Teil: Die Tätigkeiten der Arbeitsbienen unter experimentell veränderten Bedingungen. Z Vergl Physiol 12:1–71
Schippers MP, Dukas R, Smith RW, Wang J, Smolen K, McClelland GB (2006) Lifetime performance in foraging honeybees: behaviour and physiology. J Exp Biol 209:3828–3836
Sullivan JP, Jassim O, Fahrbach SE, Robinson GE (2000) Juvenile hormone paces behavioral development in the adult worker honey bee. Horm Behav 37:1–14
Sullivan JP, Fahrbach SE, Harrison JF, Capaldi EA, Fewell JH, Robinson GE (2003) Juvenile hormone and division of labor in honey bee colonies: effects of allatectomy on flight behavior and metabolism. J Exp Biol 206:2287–2296
Tatar M, Bartke A, Antebi A (2003) The endocrine regulation of aging by insulin-like signals. Science 299:1346–1351
Whitfield CW, Behura SK, Berlocher SH, Clark AG, Johnston JS, Sheppard WS, Smith DR, Suarez AV, Weaver DB, Tsutsui ND (2006) Thrice out of Africa: ancient and recent expansions of the honey bee, Apis mellifera. Science 314:642–645
Winston ML (1987) The biology of the honey bee. Harvard University Press, Cambridge, MA
Winston ML, Katz SJ (1982) Foraging differences between cross-fostered honeybee workers (Apis mellifera) of European and Africanized races. Behav Ecol Sociobiol 10:125–129
Wyatt GR, Braun RP, Zang J (1995) Priming effect in gene activation by juvenile hormone in locust fat body. Arch Insect Biochem Physiol 32:633–640
Acknowledgment
We thank Walter Goodman (University of Wisconsin, Madison) for kindly providing the JH-specific antiserum and Gro V. Amdam (Arizona State University, Tempe, and Agricultural University of Norway, Aas) for her comments on a previous version of this manuscript. We acknowledge financial support by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2004/10836-0 and 2005/03926-5). The experiments performed in the present study comply with the current laws of Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
David Santos Marco Antonio and Karina Rosa Guidugli-Lazzarini contributed equally to the present study.
Rights and permissions
About this article
Cite this article
Marco Antonio, D.S., Guidugli-Lazzarini, K.R., do Nascimento, A.M. et al. RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissenschaften 95, 953–961 (2008). https://doi.org/10.1007/s00114-008-0413-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-008-0413-9