Abstract
The efficiency of long-distance acoustic signalling of insects in their natural habitat is constrained in several ways. Acoustic signals are not only subjected to changes imposed by the physical structure of the habitat such as attenuation and degradation but also to masking interference from co-occurring signals of other acoustically communicating species. Masking interference is likely to be a ubiquitous problem in multi-species assemblages, but successful communication in natural environments under noisy conditions suggests powerful strategies to deal with the detection and recognition of relevant signals. In this review we present recent work on the role of the habitat as a driving force in shaping insect signal structures. In the context of acoustic masking interference, we discuss the ecological niche concept and examine the role of acoustic resource partitioning in the temporal, spatial and spectral domains as sender strategies to counter masking. We then examine the efficacy of different receiver strategies: physiological mechanisms such as frequency tuning, spatial release from masking and gain control as useful strategies to counteract acoustic masking. We also review recent work on the effects of anthropogenic noise on insect acoustic communication and the importance of insect sounds as indicators of biodiversity and ecosystem health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects were probably the first terrestrial animals to use air-borne sound signals for long-distance communication (Senter 2008): fossils of crickets and katydids from as far back as the Triassic and Jurassic periods reveal sophisticated stridulatory structures for song production (Béthoux and Nel 2002; Gorochov and Rasnitsyn 2002). Song reconstruction of a 165 my. old Jurassic fossil, Arachoboilus musicus, (Gu et al. 2012) suggests that the ancestral condition for sound production in orthopteran insects was a resonant mechanism, similar to the one in true crickets (Gryllidae). Orthopterans have thus been producing sounds probably very similar to present-day signals for at least 250 million years (Senter 2008). Much less is known about the evolutionary history of sound production in the other group of conspicuous sound-producing insects, the cicadas (Hemiptera: Cicadidae), but earliest fossil records are from the Upper Cretaceous (Senter 2008). By the end of the Cretaceous, the familiar soundscapes of chirping crickets and katydids, buzzing cicadas and calling frogs were well established (Senter 2008).
Most insect groups that produce sound signals in a sustained fashion (crickets, katydids and grasshoppers in the Orthoptera, cicadas in the Hemiptera and arctiid moths among the Lepidoptera) do so in the context of mate attraction: typically males calling to attract females of their species (Gerhardt and Huber 2002; Greenfield 2014). The diversity of long-distance acoustic signals produced by modern insects, spanning a frequency range from as low as 600 Hz (the Malaysian katydid, Tympanophyllum arcufolium: Heller 1995) up to 130 kHz produced by some katydid and moth species (Montealegre et al. 2006; Greenfield 2014), is enormous. These signals have been shaped over evolutionary time by a combination of different selection pressures, physical and physiological constraints, phylogenetic history, costs imposed by eavesdropping predators and parasitoids, sexual selection and the biotic and abiotic environment in which signalling occurs (Endler 1993; Ryan 1990). Dissecting the effects of these different selective pressures is challenging but necessary to understand how and why signals, signallers and receivers are structured the way they are in the acoustic assemblages that we see around us today.
Crickets, katydids and cicadas are the best-studied groups among acoustically signalling insects, wherein a number of species have been investigated for the physiology of sound production and reception, auditory processing and behaviour (Gerhardt and Huber 2002; Hedwig 2014; Stumpner and Nowotny 2014; Fonseca 2014). The ecological context in which signalling occurs, however, has received relatively less attention and will be the focus of this article. We do not attempt a comprehensive review of all the factors that may affect signals and signalling, such as parasitoids and predation, which are covered in depth elsewhere (Lakes-Harlan and Lehmann this issue; Hedwig and Robert 2014; Conner 2014) or of all insect groups (lepidopteran communication is covered in depth in a recent review: Greenfield 2014) but focus on recent work on the role of habitat and co-existing signallers in shaping insect signal structures, signaller behaviour and receiver physiology (see Römer 2013, for a more comprehensive and general review of insect acoustic communication and noise).
Insect acoustic signals and habitat
The natural habitat in which insects call can have significant effects on their signals and communication systems. Acoustic signals are put out in the public medium of the habitat through which they have to traverse before reaching the ‘intended’ receivers (Wiley and Richards 1982; Bradbury and Vehrencamp 2011). The medium can affect acoustic signals in different ways. Signals are attenuated due to spreading loss whereas reflection and scattering of sound by the ground and vegetation can lead to temporal pattern distortion and frequency filtering of the signal (Römer and Lewald 1992; Römer 1998) thus compromising detection and recognition by receivers. Over evolutionary time, signal structures of different species may, therefore, evolve to be adaptive to the habitats in which they call, minimising the effects of attenuation and signal distortion and, therefore, maximising transmission (acoustic adaptation hypothesis: Morton 1975).
Most studies examining acoustic adaptation of signals have been carried out on vertebrates (reviewed in Ey and Fischer 2009). There are very few studies that have examined acoustic adaptation in insects. A notable exception is the study by Couldridge and van Staaden (2004) on bladder grasshoppers (Orthoptera: Pneumoridae) in South Africa: the transmission of calls of seven pneumorid species were examined in four habitats (forests, savanna, fynbos and karoo). The results provided some support for acoustic adaptation at the macrohabitat level, since calls of forest and fynbos species tended to show less distortion and attenuation in their native habitats. This was not, however, the case for the calls of species in the other two biomes whose signals transmitted better in non-native habitats.
The habitat may also affect caller behaviour: senders of long-distance signals may be expected to select calling sites that maximise high-fidelity transmission (Endler 1993). Since the typical broadcast areas of most insect sounds are in the range of tens of metres (Paul and Walker 1979; Römer 1993; Jain and Balakrishnan 2012), calling site selection is perhaps more relevant to be examined at small spatial scales (i.e. microhabitats) rather than at macrohabitat or landscape levels (Jain and Balakrishnan 2011). Microhabitat selection in relation to calling sites was tested for two cicada species but this study did not find evidence that this was driven by call transmission (Sueur and Aubin 2003).
A number of studies have examined and found vertical stratification of species in different calling insect assemblages (Nischk and Otte 2000; Diwakar and Balakrishnan 2007a, b; Sueur 2002; Schmidt et al. 2013). Whether such vertical stratification is driven by acoustic adaptation has been tested in the cricket and katydid assemblage in the rain forests of Kudremukh National Park (KNP) in the Western Ghats of Southern India (Jain and Balakrishnan 2012). For a wide range of signal structures, the ground always emerged as the worst layer for acoustic signal propagation, illustrating that microhabitat selection by species calling from the ground is obviously driven by factors other than maximising signal transmission (Jain and Balakrishnan 2011, 2012). It is well known that calling from greater heights increases broadcast area and transmission distance (Marten and Marler 1977; Paul and Walker 1979; Römer 1993; Ellinger and Hödl 2003). The understorey (0.5–8 m) and canopy (>8 m) of tropical evergreen forests are (similar) microhabitats which differ largely in foliage density as a function of height (Jain et al. 2010; Jain and Balakrishnan 2011). In transmission experiments, the mid-understorey (2–4 m) of the forest emerges as the best layer for signal transmission for a variety of signal structures, with minimum attenuation and distortion of signals (Marten and Marler 1977; Ellinger and Hödl 2003; Jain and Balakrishnan 2012), probably because it offers the dual advantage of height above the ground and low foliage density. In spite of this advantage, only a fraction of species in an assemblage are found to call from the mid-understorey (Diwakar and Balakrishnan 2007a, b; Jain and Balakrishnan 2011) because it may offer less protection from predators. This could also explain why several species either call from within burrows or leaf litter on the ground or from the dense canopy even though sound propagation may be unfavourable. In summary, the only study testing acoustic adaptation as an explanation of vertical stratification of calling insect species in an assemblage did not find overall support for habitat acoustics as a major driver of calling assemblage structure. One should note, however, that there may be individual species for which maximising transmission range or signal fidelity may act as a major selection pressure: for example, Brochopeplus sp. (a false leaf katydid calling from the understorey) upheld all the predictions of the acoustic adaptation hypothesis in the KNP assemblage (Jain and Balakrishnan 2012).
Acoustic masking interference
Another important problem faced by acoustically communicating insects in natural environments is the possibility of acoustic masking interference. This is exemplified by the noisy dusk choruses consisting of several species of acoustically signalling insects, primarily crickets, katydids and cicadas calling at the same time and place. The fact that different species do manage to communicate successfully in these noisy conditions suggests that they have strategies to deal with the detection and recognition of relevant signals in the presence of high levels of masking noise (Römer 2013). These strategies include changes in signal structure over evolutionary time, signaller behaviour and/or receiver physiology (Endler 1992; Römer 2013). A useful framework to examine strategies involving senders is the ecological niche concept.
Sender strategies: the niche concept
In community ecology, it is an unarguable fact that available resources such as space, food and time need to be used in different ways to allow coexistence of ecologically similar species (Pianka 1973; Schoener 1974). Resource partitioning is believed to play a key role in establishing and maintaining such coexistence (MacArthur 1958; Connell 1961; Schoener 1968). For acoustically communicating species, the transmission channel for airborne sound signals can also be regarded as an ecological resource. With an increasing number of sound sources at the same time and place, the probability of interference and masking of overlapping sound signals is increased, impairing the detection or discrimination of signals and resulting in acoustic competition (Bailey and Morris 1986). Acoustic competition due to masking interference is expected to promote partitioning of the transmission channel among species in multidimensional space, consisting of the acoustic space of a signal (its spectral and temporal features), as well as when (time) and where (place) to signal.
Temporal partitioning
Hypothetically, coexisting species can partition their calling in time to avoid overlap and thereby acoustic masking interference. Such temporal partitioning could occur at different scales: at the broadest scale, species with similar signals could breed in different seasons or several weeks apart. This has not been investigated in a rigorous and quantitative manner in signalling insect assemblages. However, of the 20 species constituting the acoustic insect assemblage of the evergreen rainforests of KNP, 14 were found to overlap in their breeding season (November to March) and could be found calling together in the same patch of forest (Diwakar and Balakrishnan 2007a). Temporal partitioning of calling species might instead be achieved on a diel (circadian) scale. The trend that emerges from community studies in rain forests in India (Diwakar and Balakrishnan 2007a), Panama (Schmidt et al. 2013) and Borneo (Grant 2014) does not support diel partitioning between calling species of crickets and katydids.
In KNP, cicadas were found to be largely diurnal and were almost completely partitioned in their calling time from crickets and katydids, with little overlap even at dusk (Diwakar and Balakrishnan 2007a). Similarly, a semi-quantitative study on a dusk chorus of frogs, crickets and cicadas in Malaysia indicates fairly tight calling windows, though with some overlap, among different species (Gogala and Riede 1995). Since these were not, however, analysed at species level, it is difficult to interpret the degree of temporal partitioning. On the other hand, studies on a cicada community in the neotropical rainforests of Mexico revealed that seven out of nine species showed extensive and overlapping calling activity at dawn and dusk (Sueur 2002). This general lack of diel partitioning and extensive overlap in calling periods, especially at dusk, is interesting and begs the question why. It is possible that the benefits of signalling at dusk, which may include optimal atmospheric conditions for sound transmission (van Staaden and Römer 1997), are high relative to the costs of signalling in other time windows.
How then is intraspecific communication achieved in the noisy environment of a dusk chorus? Temporal partitioning may be occurring at finer temporal scales, over minutes or seconds, with one species calling in the silent inter-bout intervals of the other. In addition, the fact that the calls of many species are often discontinuous chirps may mean that actual overlap between calls may be low even if two species are calling together. Both of these possibilities were investigated in the KNP assemblage of crickets and katydids (Jain et al. 2014) by examining overlaps in calling probabilities between pairs of species in 5-min windows during peak calling time (gross temporal overlap or GTO) as well as probability of overlap due to chance (in simultaneously calling heterospecific individuals) based on signal temporal structures alone (fine temporal overlap or FTO). There were significant negative correlations between the distributions of GTO and FTO of the assemblage, suggesting that species pairs that had high overlap due to signal temporal structure may avoid calling together (Jain et al. 2014). This assemblage-level signature of fine temporal partitioning, however, needs to be validated by identifying and testing the relevant species pairs in behavioural experiments to check for active avoidance of overlap in time.
Spatial partitioning
One of the most straightforward ways for different species to avoid acoustic interference is to space themselves apart, making use of the fact that sounds attenuate as they move away from the source. Calling insect species could move apart in both horizontal and vertical space: whereas the former may offer more possibilities, being limited only by available habitat or microhabitat, the latter will of necessity be constrained by the canopy height. There have been very few studies relating calling activity to spatial distributions of callers of different species. In the cricket assemblage of Barro Colorado Island (BCI; Panama) horizontal spatial distribution of callers on a relatively large scale, where sampling sites examined were spaced 100 m apart, appeared to be randomly structured with no support for spatial partitioning at the community level (Schmidt et al. 2013). In the KNP assemblage of crickets and katydids, the fine spatial structure of individual multispecies choruses in the understorey was examined, with no evidence for active spacing as a mechanism to reduce interspecific acoustic interference (Jain et al. 2014).
On the other hand, vertical stratification of different calling insect species is well documented, particularly in rain forest communities of both the neotropics and paleotropics (Nischk and Otte 2000; Sueur 2002; Diwakar and Balakrishnan 2007b; Schmidt et al. 2013). Demonstrating vertical stratification is, however, only the first step, since species in different strata are not necessarily acoustically isolated from each other. Whether vertical stratification serves as a mechanism to reduce acoustic interference thus remains unclear. The use of space by signalling insects and the acoustic context and structuring of choruses in natural environments is clearly an underexplored area, with many implications for intraspecific communication, signal evolution and acoustic biodiversity monitoring (Balakrishnan et al. 2013, in press).
Spectral partitioning
The acoustic niche hypothesis indicates that different species in an assemblage should partition the available spectral space of sound signals among themselves (Krause 1987; Pijanowski et al. 2011). By inspecting spectrograms of sound recordings, particularly those obtained in tropical rainforests with high acoustic diversity, this appears as an intriguing idea because distinct frequency bands can often easily be assigned to advertisement calls of frogs, crickets, cicadas and katydids (Krause 1987; Riede 1993; Sueur 2002; Diwakar et al. 2007). However, the question arises how well separated the acoustic signals of an assemblage are in spectral space and whether these can be thought of as unique frequency niches. For insect taxa such as grasshoppers, many katydids and cicadas, with their broadband advertisement signals in the range of up to several kilohertz, frequency partitioning is not a reasonable solution (Römer 2013). Crickets, on the other hand, use almost pure-tone calling songs, usually in the range of 2–10 kHz (Elliott and Koch 1985; Riede 1993; Bennet-Clark 1998; Montealegre et al. 2011), of narrow spectral bandwidth which should allow them to separate their signals in the frequency domain. Riede (1993) provided support for this idea, showing that distinct spacing of carrier frequencies of different cricket species can be realized and suggested partitioning of the transmission channel due to acoustic competition.
In a more quantitative approach, Schmidt et al. (2013) and Jain et al. (2014) determined the degree of acoustic competition and partitioning in an entire assemblage based on extensive acoustic monitoring in the tropical rainforests of Barro Colorado (Panama) and KNP (India), respectively. Both studies demonstrated that, on average, call frequency overlap within a cricket assemblage (Schmidt et al. 2013) and an assemblage consisting of crickets and katydids (Jain et al. 2013) is extremely low, with little or no spectral overlap for the majority of species pairs. One solution to the problem of interspecific interference may thus be for each species to have its own frequency channel (Riede 1997). To test whether this is so, however, one needs to establish that the observed spectral separation in the assemblage is unlikely to be an outcome of random chance over evolutionary time scales.
In case of the cricket assemblage investigated by Schmidt et al. (2013), the spectral overlap of the observed community was significantly smaller than expected by chance when compared to a null model (Gotelli and Graves 1996). Moreover, species also tended to use a greater range of frequency channels for intraspecific communication if the frequency space was not constrained by the frequency range of other calling species, suggesting that frequency partitioning of calling songs might indeed be an evolutionary outcome of selection against interspecific frequency overlap. In the nocturnal signalling acoustic assemblage in Borneo examined by Grant (2014), consisting of cricket, katydid and frog species, the overall levels of spectral niche overlap were also low but not significantly lower than expected by chance when tested against a null model.
In principle, the competition for signal space can exert selection pressure on both senders and receivers. Thus, selection should act on signals leading to displacement of mating traits and preferences for those signals resulting in behavioural isolation and consequently to the reduction of signal interference and reduced competition in signal space (Hoskin and Higgie 2010; Mendelson and Shaw 2012).
The receiver’s perspective: strategies for hearing in noise
The niche concept discussed so far examines different axes of call structure or calling behaviour along which species may diverge and hence escape acoustic interference. From a physiological point of view masking interference is, however, ultimately a problem of the receiver and requires to be examined from a receiver’s perspective. Whereas examining the niche axes of time, space and frequency separately is convenient, the actual interference experienced by an individual receiver will depend not only on a combination of factors, which includes temporal and spectral overlap of signals, but also upon relative positions of callers and receivers, call intensities at source, call attenuation along the transmission path and hearing sensitivity and frequency response of the receivers (Jain et al. 2014). Estimating the amount of masking interference thus requires an integrated model that takes into account all the above factors. Such an integrated simulation model of 3-D acoustic active spaces was developed for a subset of cricket and katydid species of the KNP assemblage and used to estimate the probability of effective acoustic overlap (EAO) experienced by individual receivers of different species in multispecies choruses (Jain et al. 2014). Incredibly, the median and modal values of EAO were close to zero, suggesting that the levels of acoustic interference experienced by individuals in these apparently noisy environments are very low (Fig. 1; Jain et al. 2014).
The figure shows the estimated 3-dimensional active spaces of individual males of five species of understorey crickets and katydids of KNP as heard by a receiver of the Phaloria sp. tuned to its conspecific male call. The large grey sphere represents the conspecific active space and the small black spheres are the active spaces of the heterospecific male callers, showing that only a small proportion of the conspecific active space is overlapped by heterospecific signals, given tuned receivers. Callers are at the centres of the spheres. Distances on the X, Y and Z axes are in metres and inter-male distances represent natural values
The simulation models also allowed dissection of the roles of different factors such as call intensity and frequency tuning in reducing acoustic interference (Jain et al. 2014): it was found that calling louder was always a good strategy to reduce spatial masking interference, irrespective of the call structures of the interacting species. The most powerful strategy to reduce masking interference, however, was tuning receivers to match sender call frequency range (Jain et al. 2014).
Frequency tuning
A fundamental solution to enhance the detection of conspecific signals under noisy conditions was proposed by Capranica and Moffat (1983), wherein there is a match between the frequency band containing maximum energy in the signal and the frequency of maximum auditory sensitivity of the receiver (i.e. matched filters), thus improving the signal-to-noise ratio (Wehner 1987; Simmons 2013). However, not only is the match between the signaller’s call and the receiver sensitivity important but also the sharpness of the receiver tuning is crucial, particularly in environments with high acoustic noise levels. In habitats with non-overlapping acoustic signals (i.e. a setting without acoustic competition) relatively broad frequency selectivity has been observed in European field crickets (e.g. Gryllus bimaculatus and Gryllus campestris), whereas a comparative study in the rainforest of BCI revealed sharply tuned frequency selectivity in cricket species living within a large assemblage of acoustically co-active species (Kostarakos et al. 2008, 2009; Schmidt et al. 2011, 2013; Schmidt and Römer 2011).
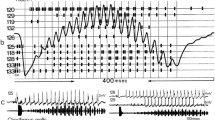
To illustrate the advantage of enhanced frequency selectivity and to quantify its quality in noise reduction, the neuronal filters of the rainforest and the European species were implemented into audio software and applied on sound recordings of natural background noise (Fig. 2). The results clearly demonstrated strongly increased noise suppression for the filter of tropical species, leading to a significantly better extraction of the species-specific song pattern and its amplitude modulation when embedded in noise (Schmidt et al. 2011).
a Comparison of standardised frequency sensitivity of AN1 in a tropical cricket species Paroecanthus podagrosus (red) and two species of field crickets Gryllus bimaculatus (green) and Gryllus campestris (blue). b The species-specific tuning properties of AN1 were implemented in audio filters and used to digitally filter mixed recordings of a P. podagrosus calling song (I) embedded in natural background noise (II). The effect of the different filter functions on the amplitude modulation (III–V) and the corresponding correlation coefficient of these filtered signals with the P. podagrosus calling song (I) are shown. Modified from Schmidt et al. (2011)
In a continuative approach the insect was placed in the nocturnal rainforest to observe directly the narrow tuned filter properties by analysing the readout of an acoustic scene encoded in the action potential activity of an auditory interneuron (AN1) under natural conditions. The results revealed highly selective coding of conspecific songs in receivers, whereas irrelevant noise mainly within the higher frequency noise band was successfully filtered out (Schmidt and Römer 2011). In the rainforest of BCI similar sharp frequency filters have been found in different members of the subfamilies Oecanthinae and Podoscirtinae, suggesting an advantageous role to cope with the high level of background noise of heterospecifics rather than being solely the outcome of phylogenetic constraint (Schmidt et al. 2011).
Frequency selectivity in receivers, coupled with matched frequency partitioning in senders, thus represents a powerful strategy for noise reduction and signal detection, enabling intraspecific communication in habitats with high acoustic diversity.
Spatial release from masking and gain control
Interestingly, co-occurring species that have been detected simultaneously in sound recordings and thus temporally overlap are not necessarily doomed to fail to communicate successfully. Signal detection against heterospecific noise and also in dense populations of conspecific individuals can be substantially improved by mechanisms known as spatial release from masking and gain control (also referred to as selective attention: Pollack 1988). Although these mechanisms have been investigated in a neurophysiological rather than behavioural context in the afferent auditory pathway of crickets and katydids, they can provide important insights into how insects solve cocktail party-like problems of natural auditory scenes.
The basic principle of spatial release from masking is that when two auditory objects (e.g. conspecific signal and masker) are spatially separated, the detection of a sound signal will be improved (Bee 2008, 2012). Owing to the directionality of the hearing system these auditory objects are selectively processed when arriving at the ear from different directions (Bradbury and Vehrencamp 2011). Such a situation will be most likely encountered in complex auditory scenes of natural environments such as nocturnal rainforests where multiple sound sources are distributed in space.
In laboratory experiments, signal detection thresholds in the cricket Paroecanthus podagrosus were significantly improved by 6–9 dB when the signal (i.e. conspecific calling song) and masker (i.e. nocturnal background noise) were spatially separated by 180° (Schmidt and Römer 2011). Similarly, in the katydid Tettigonia viridissima neuronal representation of auditory objects is strongly favoured on the ipsilateral side while strongly suppressed when coming from the contralateral side (Römer and Krusch 2000). Even a spatial separation of two concurrent sound signals of merely 15° in the frontal zone of the animal will result in a reliable representation of the ipsilateral signal in the omega neuron (an identified auditory interneuron in crickets and katydids: Römer and Krusch 2000).
In addition to spatial release from masking, gain control is another neuronal mechanism allowing crickets and katydids to accomplish the task of sound source segregation in chorus situations. In engineering, gain control describes an electronic circuit where the dynamic range of an input signal is automatically controlled to produce a constant output signal. Such a mechanism was first reported for the omega neuron in crickets, which selectively encodes the more intense signal in the presence of a signal with lower intensity, based on combined synaptic activity of inhibitory-excitatory effects (Pollack 1986, 1988; Sobel and Tank 1994; Baden and Hedwig 2007; Römer 2013). Moreover, in a neurophysiological approach mimicking a natural chorus situation, with competitive signals arriving from the same direction, an intensity difference in the range of 2–5 dB was sufficient to suppress the less intense of two signals (Römer and Krusch 2000). The authors also showed that, when presenting two stimuli from the same side, the louder signal (broadcast at 60 dB SPL) was almost exclusively represented in the omega neuron (i.e. a constant neuronal output) and the less intense signal was suppressed over a wide range of intensities (30–50 dB SPL; i.e. the dynamic range of an input signal). That such a gain control mechanism can be potentially relevant in natural auditory scenes has been shown by Schmidt and Römer (2011). In playback experiments, the AN1 activity of irrelevant sound occurring in intervals of conspecific signals was considerably decreased, leading to an enhanced contrast between signal and natural background noise. However, an open question remains as to what extent both mechanisms improve the female’s discrimination ability under real-world listening conditions.
Novelty detection
It was recently demonstrated that a small change in the spectrum of a broadband signal can have a significant impact on efficient communication. This was shown in the case of two sympatric Malaysian katydid species of the Mecopoda complex (Siegert et al. 2013) where a chirping species is heavily masked by a loud trilling species. However, Mecopoda chirper males show an extreme behavioural robustness of chorus synchrony against the background noise of the trilling species owing to the higher amount of spectral energy at 2 kHz in their chirps when compared to the song of Mecopoda triller.
The selective neuronal response of a local interneuron (TN1) to conspecific signals (“chirps”) under natural noisy conditions (“trill”) offers an elegant solution to the observed behavioural results, referred as to “novelty detection” (Schul and Sheridan 2006; Schul et al. 2012; Siegert et al. 2013). The stimulus-specific adaptation of this neuron towards the continuous trill leads to a fast and almost complete decrease in neuronal activity and enables the reliable coding of simultaneously presented Mecopoda chirps. Thus, such a neuronal novelty detector for conspecific signals can provide a useful receiver strategy under certain regimes of background noise (e.g. multi-species choruses).
Anthropogenic noise
Another form of environmental noise with impacts on acoustic communication systems is the one that emerges from human activities. Recent work has revealed the negative impact of traffic noise with its consequences on reproductive success and foraging efficiency in birds (Halfwerk et al. 2011) and bats (Siemers and Schaub 2011). In vertebrates one commonly observed effect in response to anthropogenic noise is an upward shift in call frequency (Slabbekoorn and Peet 2003; Slabbekoorn and den Boer-Visser 2006; Bermúdez-Cuamatzin et al. 2011; Hage et al. 2013).
Road traffic noise contains considerable amount of energy in the frequency range between 6 and 9 kHz and is able to mask the local frequency maximum of the courtship song of C. biguttulus males (Lampe et al. 2012), which is also highly attractive to females (von Helversen and von Helversen 1997). Males from roadside habitats call at higher local frequency maxima of 300 Hz on average compared to males from quiet habitats (Lampe et al. 2012). Moreover, nymphs that were exposed to road noise during their development also produced signals with higher frequency components as adults compared with males reared under quiet conditions, suggesting developmental plasticity as a potential mechanism for this type of signal adjustment (Lampe et al. 2014). Similarly, Shieh et al. (2012) found for the cicada species Cryptotympana takasagona an increase of song frequency in areas along roads with vehicular traffic and parking lots.
Acoustic monitoring
Insect sounds provide an ideal opportunity for monitoring biodiversity because the heterogeneity of an acoustic environment is usually correlated with biodiversity (Sueur et al. 2008). The major sound producing insect groups, namely crickets, katydids, grasshoppers and cicadas are found worldwide and over a range of different habitat and micro-habitat types (Samways and Sergeev 1997). Being small and poikilothermic, they are sensitive to and thus act as good indicators of landscape and climate change (Samways and Sergeev 1997). Many of them produce species-specific songs and calls, allowing acoustic signatures to be used as a proxy for species identification (Riede 1993). Non-invasive acoustic monitoring of species diversity and activity is thus enabled, using either psychoacoustic sampling with trained listeners (Diwakar et al. 2007) or call recordings, wherein species with known acoustic signals may be identified from spectrograms (Diwakar et al. 2007; Schmidt et al. 2013). There are also currently attempts to automate species recognition based on acoustic signals using different computational algorithms and these new technologies should allow automated long-term recording and analysis, including in remote areas (Aide et al. 2013). Given the enormous diversity and the lack of detailed taxonomic and acoustic information for many species, especially in the tropics, another approach to acoustic profiling is to identify ‘acoustic taxonomic units’ based on distinct temporal and spectral properties of calls revealed in spectrograms (Grant 2014). Even though all the species may not be physically identified, this approach allows useful comparisons of species diversity between different habitats and land-use patterns (Grant 2014).
A different approach, which does not employ species or signal pattern identification, is to use acoustic entropy or spectro-temporal heterogeneity as a measure of diversity (Sueur et al. 2008; Depratere et al. 2012; Gasc et al. 2013). This is a form of soundscape ecology (Pijanowski et al. 2011), wherein recordings of the entire soundscape are analysed as units and compared across different habitats and vegetation types. Heterogeneity of soundscapes has been found to be correlated with different habitats and vegetation types (Bormpoudakis et al. 2013) and holds promise for rapid assessment and monitoring of biodiversity: detectable acoustic gaps could serve as a ‘warning system’ for a disturbed ecosystem (Riede 1993; Pijanowski et al. 2011). Acoustic monitoring of Tettigoniidae revealed large-scale effects of anthropogenic pressure due to urbanisation and agriculture, with negative impacts on species richness, diversity and abundance (Penone et al. 2013).
Insect sounds contribute heavily to natural soundscapes, especially nocturnal ones and are thus important in the context of acoustic monitoring of biodiversity and ecosystems. Acoustic insects, with their fascinating and familiar diversity of sounds, have been an integral part of natural ecosystems and soundscapes for millions of years and deserve our respect and protection in the face of the current unprecedented rates of habitat destruction and climate change.
References
Aide TM, Corrada-Bravo C, Campos-Cerqueira M, Milan C, Vega G, Alvarez R (2013) Real-time bioacoustics monitoring and automated species identification. PeerJ 1:e103
Baden T, Hedwig B (2007) Neurite specific Ca2+-dynamics underlying sound processing in an auditory interneurone. J Neurobiol 67:68–80
Bailey WJ, Morris GK (1986) Confusion of phonotaxis by masking sounds in the bushcricket Conocephalus brevipennis (Tettigoniidae: conocephalinae). Ethology 73:19–28
Balakrishnan R, Bahuleyan J, Nandi D, Jain M (2013) Modelling the effects of chorus species composition and caller density on acoustic masking interference in multispecies choruses of crickets and katydids. Ecol Inform. doi:10.1016/j.ecoinf.2013.11.006
Bee MA (2008) Finding a mate at a cocktail party: spatial release from masking improves acoustic mate recognition in grey treefrogs. Anim Behav 75:1781–1791
Bee MA (2012) Sound source perception in anuran amphibians. Curr Opin Neurobiol 22:301–310
Bennet-Clark HC (1998) Size and scale effects as constraints in insect sound communication. Philos T Roy Soc B 353:407–419
Bermúdez-Cuamatzin E, Ríos-Chelén AA, Gil D, Garcia CM (2011) Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol Lett 7:36–38
Béthoux O, Nel A (2002) Venation pattern and revision of Orthoptera sensu nov. and sister groups. Phylogeny of palaeozoic and Mesozoic Orthoptera sensu nov. Zootaxa 96:1–88
Bormpoudakis D, Sueur J, Pantis JD (2013) Spatial heterogeneity of ambient sound at the habitat type level: ecological implications and applications. Landsc Ecol 28:495–506
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland
Capranica RR, Moffat AJM (1983) Neurobehavioral correlates of sound communication in anurans. In: Ewert J, Capranica R, Ingle D (eds) Advances in vertebrate neuroethology. Plenum, New York, pp 701–730
Connell JH (1961) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42:710–723
Conner WE (2014) Adaptive sounds and silences: acoustic anti-predator strategies in insects. In: Hedwig B (ed) Insect hearing and acoustic communication. Animal signals and communication, vol 1. Springer, Berlin, Heidelberg, pp 65–79
Couldridge VC, van Staaden MJ (2004) Habitat-dependent transmission of male advertisement calls in bladder grasshoppers (Orthoptera; Pneumoridae). J Exp Biol 207:2777–2786
Diwakar S, Balakrishnan R (2007a) The assemblage of acoustically communicating crickets of a tropical evergreen forest in Southern India: call diversity and diel calling patterns. Bioacoustics 16:113–135
Diwakar S, Balakrishnan R (2007b) Vertical stratification in an acoustically communicating ensiferan assemblage of a tropical evergreen forest in Southern India. J Trop Ecol 23:479–486
Diwakar S, Jain M, Balakrishnan R (2007) Psychoacoustic sampling as a reliable, non—invasive method to monitor orthopteran species diversity in tropical forests. Biodiv Conserv 16:4081–4093
Ellinger N, Hödl W (2003) Habitat acoustics of a neotropical lowland rainforest. Bioacoustics 13:297–321
Elliott CJH, Koch UT (1985) The clockwork cricket. Naturwissenschaften 72:150–152
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Amer Nat 139:125–153
Endler JA (1993) Some general comments on the evolution and design of animal communication systems. Phil Trans R Soc Lond B 340:215–225
Ey E, Fischer J (2009) The ‘‘Acoustic Adaptation Hypothesis’’—a review of the evidence from birds, anurans and mammals. Bioacoustics 19:21–48
Fonseca PJ (2014) Cicada acoustic communication. In: Hedwig B (ed) Insect hearing and acoustic communication. Animal signals and communication, vol 1. Springer, Berlin, Heidelberg, pp 101–121
Gasc A, Sueur J, Pavoine S, Pellens R, Grandcolas P (2013) Biodiversity sampling using a global acoustic approach: contrasting sites with microendemics in New Caledonia. PLoS One 8:e65311
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press, Chicago
Gogala M, Riede K (1995) Time sharing of song activity by cicadas in Temengor Forest Reserve, Hulu Perak, and Sabah, Malaysia. Malay Nat J 48:297–305
Gorochov AV, Rasnitsyn AP (2002) Superorder Gryllidea Laicharting, 1781. In: Rasnitsyn AP, Quicke DLJ (eds) History of insects. Kluwer Academic Publishers, Dordrecht, pp 293–303
Gotelli NJ, Graves GR (1996) Null models in ecology. Smithsonian Institution Press, Washington, DC
Grant PCB (2014) Acoustic profiling of the landscape. Ph. D thesis, Stellenbosch University, South Africa
Greenfield MD (2014) Acoustic communication in the nocturnal Lepidoptera. In: Hedwig B (ed) Insect hearing and acoustic communication. Animal signals and communication, vol 1. Springer, Berlin, Heidelberg, pp 81–100
Gu J-J, Montealegre ZF, Robert D, Engel MS, Xiao G-X, Ren D (2012) Wing stridulation in a Jurassic katydid (Insecta, Orthoptera) produced low-pitched musical calls to attract females. Proc Natl Acad Sci USA 109:3868–3873
Halfwerk W, Holleman LJM, Lessells CM, Slabbekoorn H (2011) Negative impact of traffic noise on avian reproductive success. J Appl Ecol 48:210–219
Hedwig B (2014) Towards an understanding of the neural basis of acoustic communication in crickets. In Hedwig B (ed) Insect hearing and acoustic communication. Animal signals and communication, vol 1. Springer, Berlin, Heidelberg, pp 123–141
Hedwig B, Robert D (2014) Auditory parasitoid flies exploiting acoustic communication of insects. In: Hedwig B (ed) Insect hearing and acoustic communication. Animal signals and communication, vol 1. Springer, Berlin, Heidelberg, pp 45–63
Heller KG (1995) Acoustic signalling in palaeotropical bushcrickets (Orthoptera: tettigonioidea: Pseudophyllidae): does predation pressure by eavesdropping enemies differ in the Palaeo-and Neotropics? J Zool 237:469–485
Hoskin CJ, Higgie M (2010) Speciation via species interactions: the divergence of mating traits within species. Ecol Lett 13:409–420
Jain M, Balakrishnan R (2011) Microhabitat selection in an assemblage of crickets (Orthoptera: ensifera) of a tropical evergreen forest in Southern India. Insect Conserv Div 4:152–158
Jain M, Balakrishnan R (2012) Does acoustic adaptation drive vertical stratification? A test in a tropical cricket assemblage. Behav Ecol 23:343–354
Jain M, Kuriakose G, Balakrishnan R (2010) Evaluation of methods to estimate foliage density in the understorey of a tropical evergreen forest. Curr Sci 98:508–515
Jain M, Diwakar S, Bahuleyan J, Deb R, Balakrishnan R (2014) A rain forest dusk chorus: cacophony or sounds of silence? Evol Ecol 28:1–22
Kostarakos K, Hartbauer M, Römer H (2008) Matched filters, mate choice and the evolution of sexually selected traits. PLoS One 3:e3005
Kostarakos K, Hennig MR, Römer H (2009) Two matched filters and the evolution of mating signals in four species of cricket. Front Zool 6:22
Krause BL (1987) Bioacoustics, habitat ambience in ecological balance. Whole Earth Rev 57:14–18
Lampe U, Schmoll T, Franzke A, Reinhold K (2012) Staying tuned: grasshoppers from noisy roadside habitats produce courtship signals with elevated frequency components. Funct Ecol 26:1348–1354
Lampe U, Reinhold K, Schmoll T (2014) How grasshoppers respond to road noise: developmental plasticity and population differentiation in acoustic signaling. Funct Ecol. doi:10.1111/1365-2435.12215
MacArthur RH (1958) Population ecology of some warblers of northeastern coniferous forests. Ecology 39:599–619
Marten K, Marler P (1977) Sound transmission and its significance for animal vocalization. Behav Ecol Sociobiol 2:271–290
Mendeson TC, Shaw KL (2012) The (mis)concept of species recognition. Trends Ecol Evol 27:421–427
Montealegre-Z F, Morris GK, Mason AC (2006) Generation of extreme ultrasonics in rainforest katydids. J Exp Biol 209:4923–4937
Montealegre-Z F, Jonsson T, Robert D (2011) Sound radiation and wing mechanics in stridulating field crickets (Orthoptera: gryllidae). J Exp Biol 214:2105–2117
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Nischk F, Otte D (2000) Bioacoustics, ecology and systematics of Ecuadorian rainforest crickets (Orthoptera: gryllidae: Phalangopsinae), with a description of four new genera and ten new species. J Orthopt Res 9(229–2):54
Paul RC, Walker TJ (1979) Arboreal singing in a burrowing cricket, Anurogryllusa arboreus. J Comp Physiol A 132:217–223
Penone C, Le Viol I, Pellissier V, Julien J-F, Bas Y, Kerbiriou C (2013) Use of large-scale acoustic monitoring to assess anthropogenic pressures on Orthoptera communities. Conserv Biol 27:979–987
Pianka ER (1973) The structure of lizard communities. Annu Rev Ecol Syst 4:53–74
Pijanowski BC, Gage SH, Dumyahn SL, Krause BL (2011) What is soundscape ecology? An introduction and overview of an emerging new science. Landsc Ecol 26:1213–1232
Pollack GS (1986) Discrimination of calling song models by the cricket, Teleogryllus oceanicus: the influence of sound direction on neural coding of the stimulus temporal pattern and on phonotactic behaviour. J Comp Physiol A 158:549–561
Pollack GS (1988) Selective attention in an insect auditory neuron. J Neurosci 8:2635–2639
Riede K (1993) Monitoring biodiversity: analysis of Amazonian rainforest sounds. Ambio 22:546–548
Riede K (1997) Bioacoustic diversity and resource partitioning in tropical calling communities. In: Tropical Biodiversity and Systematics, pp 275–280. Proceedings of the International Symposium on Biodiversity and Systematics in Tropical Ecosystems, Bonn
Römer H (1993) Environmental and biological constraints for the evolution of long-range signalling and hearing in acoustic insects. Philos T Roy Soc B 340:179–185
Römer H (1998) The sensory ecology of acoustic communication in insects. In: Hoy R, Popper A, Fay R (eds) Comparative hearing: insects. Handbook of auditory research. Springer, Berlin, pp 63–96
Römer H (2013) Masking by noise in acoustic insects: problems and solutions. In: Brumm H (ed) Animal communication and noise, vol 2. Springer, Berlin, Heidelberg, pp 33–63
Römer H, Krusch M (2000) A gain-control mechanism for processing of chorus sounds in the afferent auditory pathway of the bushcricket Tettigonia viridissima (Orthoptera; Tettigoniidae). J Comp Physiol A 186:181–191
Römer H, Lewald J (1992) High-frequency sound transmission in natural habitats: implications for the evolution of insect acoustic communication. Behav Ecol Sociobiol 29:437–444
Ryan MJ (1990) Sexual selection, sensory systems and sensory exploitation. Oxf Surv Evolut Biol 5:157–195
Samways MJ, Sergeev MG (1997) Orthoptera and landscape change. In: Gangwere SK, Muralirangan MC, Muralirangan M (eds) The bionomics of grasshoppers, katydids and their kin. CAB International, Oxon
Schmidt AKD, Römer H (2011) Solutions to the cocktail party problem in insects: selective filters, spatial release from masking and gain control in tropical crickets. PLoS One 6:e28593
Schmidt AKD, Riede K, Römer H (2011) High background noise shapes selective auditory filters in a tropical cricket. J Exp Biol 214:1754–1762
Schmidt AKD, Römer H, Riede K (2013) Spectral niche segregation and community organization in a tropical cricket assemblage. Behav Ecol 24:470–480
Schoener TW (1968) The Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology 49:704–726
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Schul J, Sheridan RA (2006) Auditory stream segregation in an insect. J Neurosci 138:1–4
Schul J, Mayo AM, Triblehorn JD (2012) Auditory change detection by a single neuron in an insect. J Comp Physiol A 198:695–704
Senter P (2008) Voices of the past: a review of Paleozoic and Mesozoic animal sounds. Hist Biol 20:255–287
Shieh BS, Liang SH, Chen CC, Loa HH, Liao CY (2012) Acoustic adaptations to anthropogenic noise in the cicada Cryptotympana takasagona Kato (Hemiptera: cicadidae). Acta Ethol 15:33–38
Siegert ME, Römer H, Hartbauer M (2013) Maintaining acoustic communication at a cocktail party: heterospecific masking noise improves signal detection through frequency separation. J Exp Biol 216:4655–4665
Siemers BM, Schaub A (2011) Hunting at the highway: traffic noise reduces foraging efficiency in acoustic predators. Proc R Soc Lond B 278:1646–1652
Simmons AM (2013) “To ear is human, to forgive is divine”: bob Capranica`s legacy to auditory neuroethlogy. J Comp Physiol A 199:169–182
Slabbekoorn H, den Boer-Visser A (2006) Cities change the songs of birds. Curr Biol 16:2326–2331
Slabbekoorn H, Peet M (2003) Birds sing at a higher pitch in urban noise—great tits hit the high notes to ensure that their mating calls are heard above the city’s din. Nature 424:267
Sobel EC, Tank DW (1994) In vivo Ca2+ dynamics in a cricket auditory neuron: an example of chemical computation. Science 263:823–826
Stumpner A, Nowotny M (2014) Neural processing in the bush-cricket auditory pathway. In: Hedwig B (ed) Insect hearing and acoustic communication. Animal signals and communication, vol 1. Springer, Berlin, Heidelberg, pp 143–166
Sueur J (2002) Cicada acoustic communication: potential sound partitioning in a multispecies community from Mexico (Hemiptera: cicadomorpha: Cicadidae). Biol J Linn Soc 75:379–394
Sueur J, Aubin T (2003) Is microhabitat segregation between two cicada species (Tibicina haematodes and Cicada orni) due to calling song propagation constraints? Naturwissenschaften 90:322–326
Sueur J, Pavoine S, Hamerlynck O, Duvail S (2008) Rapid acoustic survey for biodiversity appraisal. PLoS One 3:e4065
van Staaden MJ, Römer H (1997) Sexual signaling in bladder grasshoppers: tactical design for maximizing calling range. J Exp Biol 200:2597–2608
von Helversen D, von Helversen O (1997) Recognition of sex in the acoustic communication of the grasshopper Chorthippus biguttulus (Orthoptera, Acrididae). J Comp Physiol A 180:373–386
Wehner R (1987) “Matched filters”-neural models of the external world. J Comp Physiol A 161:511–531
Wiley RH, Richards DG (1982) Adaptation for acoustic communication in birds: sound transmission and signal detection. In: Kroodsma DE, Miller EH, Quellet H (eds) acoustic communication in birds. Academic, New York, pp 131–181
Acknowledgments
The authors thank the Austrian Science Foundation (FWF; P20882-B09) and the Ministry of Environment and Forests, Government of India, for supporting research projects conducted in Panama and India, respectively. They thank Jerome Sueur for help with locating interesting publications on paleobioacoustics and Diptarup Nandi for drawing the illustration in Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, A.K.D., Balakrishnan, R. Ecology of acoustic signalling and the problem of masking interference in insects. J Comp Physiol A 201, 133–142 (2015). https://doi.org/10.1007/s00359-014-0955-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-014-0955-6