Abstract
Purpose
Enhanced recovery pathways vary amongst institutions but include key components for anesthesiologists, such as haemodynamic optimization, use of short-acting drugs (and monitoring), postoperative nausea and vomiting (PONV) prophylaxis, protective ventilation, and opioid-sparing multimodal analgesia.
Methods
After critical appraisal of the literature, studies were selected with particular attention being paid to meta-analyses, randomized controlled trials, and large prospective cohort studies. For each item of the perioperative treatment pathway, available English literature was examined and reviewed.
Results
Patients should be permitted to drink clear fluids up to 2 h before anaesthesia and surgery. Oral carbohydrate loading should be used routinely. All patients may have an individualized plan for fluid and haemodynamic management that matches the monitoring needs with patient and surgical risk. Minimizing the side effects of anaesthetics and analgesics using short-acting drugs with careful perioperative monitoring should be encouraged. Protective ventilation with alveolar recruitment maneuvers is required. Preventive use of a combination with 2–3 antiemetics in addition to propofol-based total intravenous anaesthesia (TIVA) is most likely to reduce PONV. While the ideal analgesia regimen remains to be determined, it is clear that a multimodal opioid-sparing analgesic strategy has significant benefits.
Conclusion
Careful evaluation of single patient and planning of the anesthetic care are mandatory to join the ERAS philosophy. Optimal fluid management, use of short-acting drugs, prevention of PONV, protective ventilation, and multimodal analgesia are the cornerstones of the anaesthesia management within ERAS protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgery is a complex treatment method, where tissue insult is an expected part of patient care. Extended surgical interventions in abdominal surgery result in major surgical stress (including anxiety, pneumoperitoneum, tissue aggression, blood transfusion, and hypothermia) but also anaesthesia stress (opioids, ventilation, fasting, and filling). This may induce post-aggressive response with insulin resistance, increased catabolism and secretion of cortisol, catecholamines, prostaglandins, and cytokines. And this can lead to direct and/or indirect complications [1].
The enhanced recovery after surgery (ERAS) pathways reduce the delay until full recovery after major abdominal surgery by attenuating surgical stress and maintaining postoperative physiological functions. The implementation of the ERAS pathways has been shown to impact positively in reducing postoperative morbidity, and, therefore, length of stay (LOS) [2,3,4]. Such protocols are rapidly becoming the standard of care in patients undergoing gastrointestinal surgery, and evidence is growing for urological procedures, especially for radical cystectomy [5, 6].
They bring together two best practices, organization of care and clinical management, with the goal of consistent delivery of optimum care to facilitate earlier recovery after surgery. ERAS programs integrate a range of perioperative interventions, both surgical and anesthetic, and focus on minimally invasive surgery [7, 8]. The most important is the multidisciplinary teamwork that is the key of success of ERAS programs. An important step is to convince anesthesiologists of their critical role in these ERAS programs. In view of the evidence that many elements of the ERAS programs are of related to anesthetic care, it is imperative that guidelines on perioperative care include recommendations approved by an interdisciplinary team comprising anesthesiologists and surgeons. The anesthesiologist seems to be the ideal person to guide the multidisciplinary team of specialists involved in the risk assessment process through an optimal preoperative evaluation, medical optimization, and a tailor-made anaesthesia plan aiming to a fast recovery and adequate pain relief.
Methods
A systematic review of the articles published between January 1966 and April 2020 was conducted. The principal literature search utilized MEDLINE, Embase, and Cochrane databases to identify contributions related to the ERAS topic. MeSH key words included “anaesthesia”, “analgesia”, “surgery”, “enhanced recovery”, “ERAS”, “fast track”, and “urology”.
The inclusion criteria were: English , full article available, and studies dealing with one of the six key points of anesthesiology within ERAS pathway: optimal fluid management, short-acting drugs, prevention of PONV, protective ventilation, multimodal analgesia, and prehabilitation, with particular attention given to meta-analyses, randomized controlled trials, large prospective cohort studies, and systemic reviews.
The exclusion criteria were: editorials and articles assessing ERAS items in non-abdominal surgery.
After critical appraisal of the literature, titles and abstracts were screened. Reference lists of all eligible articles were examined and reviewed for other relevant studies. A total of 397 articles were checked. Editorials (n = 45) and articles assessing ERAS items in non-abdominal surgery (n = 167) were excluded. For 108 articles, full text was not available, and nine were not in English. Finally, 68 articles were selected.
Although most studies have been performed in patients undergoing colorectal surgery, these findings are considered valid for urological procedures given similarities in patient characteristics.
Figure 1 details the flowchart of article selection according to PRISMA guidelines.
Results
Fluid management
Preoperative euvolemic state
Fasting
The goal of preoperative fluid management is for the patient to arrive in the operating room in a hydrated and euvolemic state. Therefore, patients are less likely to be fluid responsive after induction of anaesthesia.
To achieve this, prolonged fasting (from midnight) is not recommended, because it worsens a postoperative metabolic response that leads to increased catabolism and state of insulin resistance. Reducing the preoperative fasting period for clear fluids to 2 h does not increase gastric volumes and may even reduce the acidity of stomach fluids [9]. Therefore, while patients are required to refrain from eating solid foods, particularly fatty meals, for at least 8 h prior to surgery, guidelines recommend intake of clear fluids until 2 h before surgery [10].
Carbohydrate loading
Patients should be encouraged to ingest a clear carbohydrate beverage 2 to 3 h before surgery. This oral carbohydrate drink should contain a relatively high concentration (12.5%) of complex carbohydrates (maltodextrins), with 100 g (800 ml) administered the night before of surgery and 50 g (400 ml) 2–3 h before induction of anaesthesia. This helps improve metabolism to an anabolic state, decrease postoperative insulin resistance, reduce anxiety, and reduce nausea and vomiting, without increasing the risk of pulmonary aspiration [11].
Within 90 min, 400 mL of a clear 12.5% carbohydrate drink empties completely from the stomach and, therefore, can safely be administered 2 h before induction of anaesthesia, except in patients with documented delayed gastric emptying or gastrointestinal motility disorders [12].
Bowel preparation
Moreover, to achieve euvolemic state, routine mechanical bowel preparation should also be avoided. Indeed, it contributes to preoperative dehydration, without decreasing anastomotic leakage, wound infection, or mortality [8, 13]. It may even have a tendency towards a higher incidence of spillage of bowel contents due to formed stool being replaced by liquid bowel contents [14].
Intraoperative hemodynamic protocol
There have been major advances in understanding the effects of fluid therapy and administration during the perioperative period. The goal of intraoperative fluid management is to ensure central euvolemia to maintain end-organ perfusion with an adequate circulating volume.
Both hypovolemia and excessive fluid administration are associated with harm. Hypovolemia can lead to an increased risk of organ hypoperfusion, sepsis, and multiorgan failure. Hypervolemia can be equally dangerous leading to a subsequent increase in intravascular hydrostatic pressure that can damage the vascular endothelial glycocalyx and shift out of the circulation into the interstitial space, inducing peripheral and pulmonary edema as well as edema of the gut wall increasing incidence of postoperative ileus, and an increased incidence of acute kidney injury after major surgery [15,16,17].
Improving fluid management during perioperative period leads to a decrease in complications, decrease in length of stay (LOS), and enhanced patient outcomes [16, 18].
Only maintenance fluid requirements are needed to replace losses from the body via urine output and insensible perspiration, to maintain preoperative body weight. This low crystalloid therapy regime is often called zero-balance fluid therapy (or restrictive approach). It allows 3–5 ml/kg/h infusion of isotonic balanced crystalloid solution (such as lactated Ringer) [19].
Patients undergoing surgery within an enhanced recovery protocol should have a personalized fluid management plan. For healthy patients undergoing low-risk surgery (prostatectomy and nephrectomy for example), a “zero-balance” approach might be sufficient. For high-risk patients and/or most patients undergoing major surgery with greater blood loss and more complex fluid shifts, such as radical cystectomy, boluses of fluid may be required to maintain euvolemia. Importantly, hemodynamic instability does not equate with volume responsiveness and does not always mean that a fluid bolus is needed [20]. Moreover, urine output that is also frequently monitored is not an reliable marker of volume status since oliguria is extremely common in the perioperative period, due to a neurohormonal response to surgical stress, and was not associated with postoperative renal failure [21]. Therefore, clinical decision to give a fluid challenge (colloid 7 ml/kg first bolus then 3 ml/kg subsequent bolus) must be made in the context of a likely volume deficit (e.g., hemorrhage) rather than a low systemic vascular resistance. As with any perioperative medication given, fluids should be titrated to the desired effect. At the opposite, if there is no reason to suspect a volume deficit, judicious use of a vasopressors to maintain appropriate hemodynamic variables may be favored [20].
Controversy remains over goal-directed fluid therapy (GDFT) using transoesophageal Doppler or pulse contour analysis [22, 23]. Several recent studies performed to test the effectiveness of GDFT within an ERAS protocol have failed to find a benefit on postoperative outcomes as that found in previous studies [24, 25]. This is not surprising, as significant improvement and change in practice in perioperative fluid management within an ERAS protocol occurred over the last 10 years, including zero-balance strategy that could minimize the impact of GDFT.
Postoperative management
In the postoperative period, when the option is available, early oral intake should be encouraged and intravenous fluid therapy should be kept at a minimum. Indeed, the best method to improve hydration is by increasing per os fluid intake (25–35 ml/kg of water per day in the recovery period). An early transition to oral hydration helps to enhance the conditions for healing and recovery from surgery, reduces the risk of infection, and is not associated with an increased risk of anastomotic dehiscence [25, 26].
If intravenous fluid therapy is required, excess salt should be avoided, as patients do not have the same ability to excrete sodium and chloride postoperatively [16]. A solution with reduced salt is required for maintenance, e.g., dextrose saline.
Within an ERAS protocol, postoperative hypotension and low urine output are common within the first 24 h. Some degree of oliguria appears to be a normal and predictable physiological response, due to the release of vasopressin in response to the stress of surgery. In a recent study, there was no significant correlation between oliguria and postoperative renal failure, but there was an increase in acute kidney injury associated with increased postoperative fluid balance [27]. Therefore, in the absence of other concerns, detrimental postoperative fluid overload is not justified. Urine output should not be the driving force for fluid administration and ‘‘permissive oliguria’’ could be tolerated.
Short-acting drugs (and monitoring)
Within ERAS protocols, efforts have to be made to minimize the impact of anaesthetic agents and techniques on organ function, and to facilitate rapid awakening from anaesthesia thus accelerating recovery. It has been shown that long-acting drugs can delay postoperative recovery [28]. Indeed, the choice of optimal molecules is crucial. Use of short-acting drugs and appropriate monitoring of depth of anaesthesia, therefore, play a pivotal role in ERAS protocols.
Total intravenous anaesthesia (TIVA)
Total intravenous anaesthesia (TIVA), is a popular choice due to its numerous benefits, including its unique ability to clear quickly out of a patient’s system for fast and easy awakening. Moreover, propofol-based total intravenous anaesthesia reduces PONV [29]. Therefore, propofol for induction (more than desflurane) combined with short-acting opioids (such as fentanyl, sufentanil, or remifentanil) seem to be the ideal combination for TIVA. Remifentanil has the best favorable pharmacokinetic/pharmacodynamic profile characterized by fast onset and very short context-sensitive half-life that varies very little regardless of infusion duration [29]. This combination undergoes rapid ester hydrolysis with a clearance in excess of 3 L/min.
Neuromuscular blocking (NMB)
Muscle relaxation facilitates tracheal intubation, mechanical ventilation, and surgical exposure. A deep NMB might be particularly useful when a laparoscopic approach is used and may allow operating at lower pressure while maintaining intra-abdominal space for surgery. At the opposite, NMB might not be always necessary for patients undergoing open abdominal surgery. Indeed, an adequate level of anaesthesia without muscle relaxants can produce a good to excellent surgical field in approximately two-third of patients undergoing radical retropubic prostatectomy [30].
At the end of surgery, it is important to restore neuromuscular function to preoperative levels and avoid residual muscle paralysis which can be responsible for respiratory insufficiency, hypoxia, and aspiration into the lungs as well as distress for the patient [31]. The incidence of residual curarization is reduced when intermediate-acting neuromuscular blocking agents, such as rocuronium, atracurium, and vecuronium, are used [32]. Rocuronium is probably the most interesting non-depolarizing neuromuscular blocking agent with a rapid to intermediate onset of action (less than 2 min), depending on dose, and with an intermediate duration of action (20–30 min), making it about six times less potent than vecuronium and faster in onset than either vecuronium or atracurium.
Monitoring
Recent focus has been on using depth of anaesthesia monitoring not just to avoid awareness during surgery but also to titrate the minimum amount of anaesthetic necessary to avoid complications. As part of an ERAS protocol, monitoring the depth of analgesia, anaesthesia, and neuromuscular blocking is recommended to give the minimum dose possible.
Bispectral index (BIS) is one of several technologies used to monitor depth of anaesthesia. It is a complex parameter calculated from the spontaneous electroencephalogram of patients under general anaesthesia (ideally between 40 and 60). Clinically, anaesthesia monitoring with the BIS is justified because it allows advantages from reducing the recovery time after waking, mainly by reducing the administration of general anesthetics as well as the risk of adverse events [33].
The Analgesia Nociception Index (ANI) allows a direct measurement of the activity of the autonomic nervous system through the analysis of its parasympathetic component via the respiratory sinus arrhythmia [34]. For an unconscious patient (e.g. under general anaesthesia), the target values are between 50 and 70. An ANI below 50 corresponds to an opioids failure and is predictive of hemodynamic response while an ANI higher than 70 encourages to conclude an opioid overdose.
Monitoring is also mandatory to manage neuromuscular block and guide reversal administration. Train-of-four (TOF) is the best way to monitor neuromuscular block and detect residual curarization. It is important to periodically determine if NMB is still needed. At the end of the surgery, a TOF ratio of 0.9 must be achieved to ensure adequate return of muscle function and thus preventing complications. Reversal of residual NMB can safely be achieved when the TOF count is 3 or greater. Acetylcholinesterase inhibitors, such as neostigmine, have traditionally been used for reversal of non-depolarizing NMB agents. However, these drugs have significant limitations, such as indirect mechanisms of reversal, limited and unpredictable efficacy, and undesirable autonomic responses. Sugammadex, a selective relaxant-binding agent, is the faster and safer reversal drug, when aminosteroids, such as rocuronium and vecuronium, have been administered, regardless of the depth of the block [35].
Postoperative nausea and vomiting (PONV) prophylaxis
Optimal management is mandatory fundamental to enhance recovery since vomiting is one of the most bothersome adverse effects of anaesthesia and surgery. It may result in dehydration, delayed return of adequate nutrition intake, increase intravenous fluid administration postoperatively, and is a limiting factor in early discharge. It is one of the most unpleasant experiences in the perioperative period [36].
The aetiology of postoperative nausea and vomiting is multifactorial and is generally divided into patient-related, anaesthesia-related and surgery-related factors.
Total intravenous anaesthesia with propofol, in addition to its ability to clear quickly out of a patient’s system for fast and easy awakening, is far superior to any other induction drug in preventing PONV. PONV are more likely with volatile anesthetics (e.g., isoflurane, desflurane) but are also common when perioperative opioids are administered [37]. Other factors like the reduction of preoperative fasting, carbohydrate loading, avoidance of nitrous oxide, adequate hydration and high inspired oxygen concentrations may influence the prevalence of PONV.
Several scoring systems have been described for the prediction of PONV, with simpler ones appearing to provide better discrimination. The most commonly used is the Apfel score using only four risk factors—female gender, a history of motion sickness or PONV, non-smoking status and the use of postoperative opioids [38, 39]. This scoring is useful to stratify patients from low-to-high risk for PONV and guide antiemetic prophylaxis.
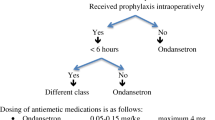
High-risk patients should be pretreated aggressively because PONV can be as frequent as 70–80% after surgery in this group [38]. Therefore, if three or more risk factors are present, it is generally recommended that patients receive at least two prophylactic pharmacologic antiemetic agents of different classes preoperatively for the prevention of nausea and vomiting. An alternative strategy employed in ERAS protocols may be to administer antiemetic prophylaxis (between one and three medications) to all patients who are having general anaesthesia and major abdominal surgery [40].
The most advised prophylactic antiemetics are corticosteroids (dexamethasone, a single 4–8 mg dose at induction [41]), serotonin receptor blockers (such as ondansetron), and dopamine antagonists (such as droperidol), both administrated at the end of surgery [42]. When these classes of drugs were given individually, they were demonstrated to contribute a relative risk reduction of about 25%, while multimodal administration of antiemetic drugs reducing PONV even further [43, 44].
Postoperatively, if rescue PONV treatment is required, despite prophylaxis, a different class of antiemetic should be administered than the one administered for prophylaxis [38].
There is also some evidence for the use of alternative therapies to reduce PONV, which include music therapy, aromatherapy, acupuncture, hypnosis, and relaxation techniques [45].
Protective ventilation
Mechanical ventilation is considered as a major risk factor for acute lung injury if not adequately managed [46].
Protective ventilation strategy is currently based on low tidal volumes (5–8 mL/kg) with a low positive end-expiratory pressure (PEEP = 6–8 cmH2O), and low FiO2 (50%). This approach aims to minimize the ventilator-induced lung injury due to mechanical, inflammatory, and oxidative stress [47]. The treatment of hypoxemia (if the tracheal tube is correctly positioned) is primarily based on optimization of lung ventilation using alveolar recruitment maneuvers (after tracheal intubation, after laparoscopy insufflation, then every hour) and setting the best PEEP level [46]. Finally, the aim of this protective ventilation (low volumes, low pressure) is to open the lung and keep it open, to decrease postoperative pulmonary complications.
Moreover, for laparoscopic surgery, intra-abdominal pressure may be maintained under 10 CmH2O as much as possible.
Opioid-sparing multimodal analgesia
The optimal management of postoperative pain is a key component of ERAS pathways. Given the importance of effective analgesia and the significance of opioid-related side effects, multimodal analgesia has emerged as a way to achieve effective postoperative analgesia while minimizing opioid use [48]. Multimodal analgesia also incorporates the idea of preemptive analgesia, or the administration of medication to reduce pain before surgery or painful stimulus has occurred. Preemptive, rather than reactive, use of analgesic medication has been shown to reduce pain, inflammation, and PONV [49].
Multimodal analgesia may involve both systemic administration of different non-opioid analgesics with separate mechanisms of action as well as regional and neuraxial techniques.
Intraoperative pain management
Intraoperative protocol usually uses the synergistic effects of intravenous glucocorticoids at induction, intravenous ketamine during the procedure and administration of regional or local anesthetics. Anticipation of analgesia 1 h before end of surgery (using intravenous analgesics such as acetaminophen, ketoprofene, nefopam, or low dose morphine) is also essential.
Glucocorticoids
Glucocorticoids (such as dexamethasone) reduce postoperative pain as well as decrease opioid requirements and side effects such as PONV [43, 50, 51]. They exert their analgesic effect via several mechanisms; they have anti-nociceptive effects at the spinal level, prevent the production of cytokines involved in inflammatory pain, and inhibit the production of inflammatory prostaglandins and leukotrienes by preventing arachidonic acid production [52].
Whereas glucocorticoids administered in high doses and for long periods have a potent immunosuppressive effect, a single prophylactic low dose of dexamethasone (up to 8 mg at induction of anaesthesia) was associated with a low risk of postoperative complications [51].
Therefore, current literature supports a single prophylactic dose of dexamethasone 4 mg at induction for PONV prophylaxis, with 8 mg providing additional opioid-sparing effects and quicker recovery without an increase in postoperative complications.
NMDA receptor antagonists
Ketamine is an N-methyl-d-aspartate (NMDA) receptor antagonist that is used for both pain control and general anaesthesia. A meta-analysis by Laskowski et al. showed that ketamine decreased postoperative opioid requirements, increased time to first rescue analgesic dose postoperatively, decreased pain scores, and decreased PONV, with minimal effects on respiratory drive [53]. Ketamine could usually be safely used at the optimal dose of 0.3 mg/kg at induction then 0.15 mg/kg/h. Other NMDA receptor antagonists include dextromethorphan, memantine, and magnesium sulfate.
Local anesthetics
There are different modalities of local anaesthesia according to each procedure. Surgical approach could be a choice criterion. In case of minimally invasive surgery, intravenous continuous lidocaïne and port infiltration may be proposed. In case of open surgery, thoracic epidural until POD 3 (midline incision), TAP block (sus-umbilical), or paravertebral block (lombotomy) could be used.
Lidocaine infusion A meta-analysis of randomized controlled trials found that an intravenous lidocaine infusion significantly reduces postoperative pain [54, 55]. This effect was most pronounced for laparoscopic abdominal surgery. Intravenous lidocaine infusion also reduced postoperative opioid consumption as well as postoperative ileus occurrence. Significant heterogeneity existed as far as duration of infusion, which ranged from intraoperative use only up to 48 h postoperatively. The standard dosing regimen in the trials reviewed by the Cochrane group was 2 mg/kg/h. Despite lack of clear data regarding optimal dosing regimen or timing, 1 mg/kg at induction then 1.5 mg/kg until the end of surgery seems to be a valid protocol.
Surgical site infiltration The incorporation of local anesthetics as part of multimodal analgesia decreases opioid requirements and side effects when used at several surgical sites [56]. Nevertheless, there is lack of evidence for effective analgesia with infiltration at laparoscopic port sites. This may be due to inadequate doses of local anesthetics and the short duration of local anesthetics in some studies [52].
Epidural analgesia Epidural analgesia improves postoperative pain scores versus parenteral opioids for a variety of surgical procedures, including abdominal surgery [57, 58]. A multimodal epidural infusion comprising drugs with different pharmacological pathways is more effective than a single-agent infusion. The combination of local anesthetics (lidocaine, ropivacaine, bupivacaine) and adjuvants (such as clonidine) provides both intraoperative and postoperative analgesia after a wide range of surgeries, including abdominal surgeries [58]. Despite the advantages of central neuraxial techniques, there are potential adverse effects, including inadvertent motor blockade, longer recovery of sensory function, postdural puncture headache, and infection. Epidural blocks may lead to a significant increase in the risk of arterial hypotension, pruritus, and urinary retention. Moreover, such blocks are resource intensive due to the need for ongoing monitoring. Surgery approach is one important consideration, as neuraxial analgesia may be more suitable for open rather than laparoscopic surgeries. Indeed, while epidural analgesia is effective for both open and laparoscopic surgeries, the level of pain after laparoscopic surgeries remains acceptable (i.e. < 4/10) and thus the risk–benefit ratio may not favour the use of epidural analgesia [59, 60].
Regional techniques Regional techniques, such as transversus abdominis plane (TAP) block and paravertebral block (PVB), are increasingly incorporated into multimodal analgesic regimens. Paravertebral block (PVB) is particularly well suited for lombotomy, with a higher safety profile and less side effects than epidural analgesia. Transversus abdominis plane (TAP) block is an analgesic technique which delivers local anesthetic to the plane between the internal oblique and transversus abdominis muscles. TAP blocks were originally described based on a landmark-guided technique but have evolved to predominantly ultrasound guided. TAP blocks for abdominal surgery reduced morphine requirements over the first 48 h postoperatively as well as increased the latency to request for first rescue analgesic dose [61]. Data for TAP blocks in laparoscopic surgery remain quite mixed but suggest a benefit in terms of postoperative opioid requirements, PONV and LOS [62, 63]. Further data are necessary to clarify which patient populations may benefit.
Postoperative pain management
Pain has adverse clinical implications on postoperative recovery, including prolonging the time to recovery milestones and length of hospital stay. Moreover, in addition to the consequences of pain in the immediate postoperative period, acute pain may trigger long-term neuronal changes that result in the development of chronic pain [52]. Postoperative pain in general continues to be undermanaged. A recent study found that more than 80% of patients still experience pain after surgery, and 75% of those have moderate to extreme pain in the immediate postoperative period. It is, therefore, hardly surprising that post-surgical pain is the patient’s greatest concern before surgery [64]. On another hand, analgesic-related side effects are a concern for patients to the extent that some patients would choose less effective analgesia as a trade-off for fewer side effects. For example, the ubiquity of opioids in postoperative analgesic regimens results in dose-related adverse effects, such as sedation, postoperative nausea and vomiting, pruritus, urinary retention, ileus, and most dangerously respiratory depression, which can delay discharge [65].
This has led to an increasing emphasis on multimodal analgesic regimens that reduce opioid demand, with opioids used as rescue analgesics when non-opioid medications are inadequate for pain control [66, 67]. Opioid-sparing treatments mainly include acetaminophen (paracetamol) and NSAID (non-steroidal anti-inflammatory drugs) and oral treatment should be given at regular times from POD 0 if possible.
Acetaminophen
Acetaminophen is a mainstay of adjuvant analgesic therapy because it is an effective analgesic for mild to moderate pain, with a very favorable safety profile. There is increasing usage of intravenous acetaminophen, which has more favorable pharmacokinetics (earlier plasma and CSF peaks) than oral formulations. Adding to the value of acetaminophen in multimodal analgesia is its apparent synergistic effect with non-steroidal anti-inflammatory drugs (NSAIDs) [68].
Non-steroidal anti-inflammatory drugs (NSAIDs)
The term NSAIDs describes a group of non-steroidal drugs that have anti-inflammatory, antipyretic, and analgesic effects, via inhibition of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), enzymes which play a role in the downstream signaling of both inflammation and pain. They reduce opioid consumption and opioid-related side effects when used in multimodal regimens [69].
Nevertheless, their use is not without risk. Some studies have raised concerns about an increased risk of anastomotic leak associated with long duration of NSAID use [70]. Considering these findings, NSAIDs should be used judiciously in patients at increased risk for anastomotic leak. In addition, with prolonged use, NSAIDs may be associated with kidney disfunction, gastrointestinal bleeding, and platelet disfunction but there is no evidence of increased postoperative risk of bleeding due to these agents [69].
In most patients, however, NSAIDs are recommended as part of the multimodal analgesic regimen. NSAIDs are probably the corner stone of multimodal pain strategy, with good safety if not used more than 3 days and when renal function is correct (MDRD > 30 ml/min).
Other strategies
Other pharmacological modalities include gamma-aminobutyric acid analogues (gabapentin and pregabalin), beta-blockers (such as esmolol), alpha-2 agonists (clonidine and dexmedetomidine).
Adjunctive non-pharmacological techniques include acupuncture, music therapy, and hypnosis. There is mixed evidence regarding such techniques, although a lack of harm is associated with their use. Clinical opinion on their efficacy remains divided. A randomized sham-controlled trial found that electroacupuncture, a variant of acupuncture involving the addition of electric current, resulted in reduced postoperative analgesic requirements at 45 min when added to a multimodal regimen for radical prostatectomy [71].
Prehabilitation
Prehabilitation is defined as ‘‘A process in the continuum of care that occurs between diagnosis and the beginning of treatment”. It includes preoperative physical, nutritional, and psychological conditioning to enhance the functional and physiological capacity of an individual which may help recovery after surgery [72]. This concept seems particularly well suited to radical cystectomy given the comorbidities of patients and a long preoperative period in case of neoadjuvant chemotherapy providing an opportunity to increase the physiological reserve in anticipation of surgery.
Preoperative assessment and optimization of comorbidities
Pre‐operative optimization of the high‐risk elective surgical patient includes both lifestyle modification and medical improvement of comorbidities [73, 74]. The first step is the identification of impairments of the patient who is being considered for major surgery. This accurate assessment should be proposed as soon as possible. Indeed, the time before surgery is seen as a ‘teachable moment’, as patients are more amenable to lifestyle modifications [75]. The second step is to provide interventions that improve general health of the patient to reduce the incidence and/or severity of these impairments. These interventions include physical fitness training, improving nutritional status and psychological robustness, but also smoking cessation [76,77,78,79].
Maintenance of physical exercise
Most practitioners are familiar with the concept of physical rehabilitation after surgery, but the idea of preoperative rehabilitation is gaining recognition. The impact of the inevitable muscle loss and joint stiffness is then minimized postoperatively. Older et al. has shown for the first time that poor physical fitness, as demonstrated by cardiopulmonary exercise testing, was associated with adverse outcomes following surgery [80]. More recent publications corroborate this observation [81,82,83], especially for patients undergoing radical cystectomy [84].
Another point to facilitate safe anaesthesia is incentive spirometry for the prevention of postoperative pulmonary complications after major abdominal surgery. However, evidence regarding the effectiveness of incentive spirometry on postoperative pulmonary outcomes after thoracic, cardiac, and abdominal surgery remains inconclusive [85]. This is attributed to various methodological issues in the different trials.
Patient blood management
Patient blood management improves patient outcomes by having a direct impact on perioperative fluid management and avoidance of transfusions of donated blood components, thus avoiding transfusion-associated complications. This requires a multidisciplinary team approach. The three pillars of patient blood management are:
-
Optimizing hematopoiesis, including early detection and treatment of underlying preoperative anemia before elective surgery associated with high risk of transfusion. Management of preoperative anemia may require treatment of iron stores or iron deficiency as well as treatment of other hematinic deficiencies and refer to further evaluation if necessary. Erythropoiesis-stimulating agents (ESA) could be considered if nutritional anemia is ruled out.
-
Minimizing blood loss (by favoring minimally invasive approach) and intensifying the use of blood conserving measures to allow a rational and adequate use of allogenic blood products.
-
Optimizing the physiological tolerance of anemia based on the patient’s physiological reserve and risk factors. This requires comparing estimated blood loss with patient-specific tolerable blood loss and formulating a patient-specific management plan using appropriate blood conservation modalities.
Perioperative blood transfusion has been shown to be associated with an increased cancer specific and overall mortality [86]. Reducing the risk of blood transfusion, therefore, remains one of the major ERAS objectives.
Conclusion
Our article summarizes six major elements of anaesthesia management in patients involved in ERAS pathway and the putative direct application in routine practice for urologic procedures.
By design, ERAS protocols comprise many elements, and these many factors make it difficult to carry out randomized trials controlling for each specific modifiable intervention. Hence, it is difficult to determine the relative importance of any one component of the multimodal drugs and elements of ERAS. Anyway, the success of an ERAS program is strongly associated with the number of implemented measures, as demonstrated recently for radical cystectomy [87, 88]. The adhesion of all the medical and paramedical teams involved is crucial to reduce variability of care.
References
Kehlet H (1997) Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 78(5):606–617
Muller S et al (2009) Zurich Fast Track Study Group. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology 136:842–847
Giannarini G et al (2019) Impact of enhanced recovery after surgery protocols versus standard of care on perioperative outcomes of radical cystectomy: a systematic review and meta-analysis of comparative studies. Minerva Urol Nefrol 71(4):309–323
Tan WS et al (2018) Intracorporeal robot-assisted radical cystectomy, together with an enhanced recovery programme, improves postoperative outcomes by aggregating marginal gains. BJU Int 121(4):632–639
Cerantola Y et al (2013) Guidelines for perioperative care after radical cystectomy for bladder cancer: enhanced recovery after surgery (ERAS(®)) society recommendations. Clin Nutr 32(6):879–887
Pang KH et al (2018) Prospective implementation of enhanced recovery after surgery protocols to radical cystectomy. Eur Urol 73(3):363–371
Brodner G et al (2001) Multimodal perioperative management—combining thoracic epidural analgesia, forced mobilization, and oral nutrition—reduces hormonal and metabolic stress and improves convalescence after major urologic surgery. Anesth Analg 92(6):1594–1600
Xu R et al (2010) No advantage is gained by preoperative bowel preparation in radical cystectomy and ileal conduit: a randomized controlled trial of 86 patients. Int Urol Nephrol 42(4):947–950
American Society of Anesthesiologists Committee (2011) Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology 114:495–511
Brady M et al (2003) Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev 4:CD004423
Smith MD et al (2014) Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev 08:CD009161
Awad S et al (2013) A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr 32:34–44
Jung B et al (2007) Mechanical Bowel Preparation Study Group. Multicentre randomized clinical trial of mechanical bowel preparation in elective colonic resection. Br J Surg 94:689–695
Mahajna A et al (2005) Bowel preparation is associated with spillage of bowel contents in colorectal surgery. Dis Colon Rectum 48:1626–1631
Becker BF et al (2010) Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol 105:687–701
Lobo DN et al (2002) Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 359:1812–1818
Kambhampati G et al (2012) Perioperative fluid balance and acute kidney injury. Clin Exp Nephrol 16:730–738
Gan TJ et al (2002) Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 97:820–826
Chappell D et al (2008) A rational approach to perioperative fluid management. Anesthesiology 109:723–740
Marik PE et al (2014) Fluid responsiveness: an evolution of our understanding. Br J Anaesth 112:617–620
Kheterpal S et al (2007) Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 107:892–902
Roche AM et al (2009) Goal-directed fluid management with trans-oesophageal Doppler. Best Pract Res Clin Anaesthesiol 23:327–334
Hamilton MA et al (2011) A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg 112:1392–1402
Srinivasa S et al (2013) Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br J Surg 100:66–74
Miller TE et al (2015) Fluid management and goal-directed therapy as an adjunct to Enhanced Recovery after Surgery (ERAS). Can J Anaesth 62:158–168
Han-Geurts IJ et al (2007) Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br J Surg 94:555–561
Kambhampati G et al (2010) Perioperative fluid balance and acute kidney injury. Clin Exp Nephrol 16:730–738
Walker KJ et al (2009) Premedication for anxiety in adult day surgery. Cochrane Database Syst Rev 4:CD002192
Mandel JE (2014) Considerations for the use of short-acting opioids in general anaesthesia. J Clin Anesth 26:S1–7
King M et al (2000) Requirements for muscle relaxants during radical retropubic prostatectomy. Anesthesiology 93:1392–1397
Fortier LP et al (2015) The RECITE study: a canadian prospective, multicenter study of the incidence and severity of residual neuromuscular blockade. Anesth Analg 121(2):366–372
Donati F (2013) Residual paralysis: a real problem or did we invent a new disease? Can J Anaesth 60:714–729
Oliveira CR et al (2017) Benefit of general anaesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta-analysis. Braz J Anesthesiol 67(1):72–84
Boselli E et al (2015) Prediction of hemodynamic reactivity during total intravenous anaesthesia for suspension laryngoscopy using Analgesia/Nociception Index (ANI): a prospective observational study. Minerva Anestesiol 81(3):288–297
Hristovska AM et al (2017) Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev 8:CD012763
Gan TJ (2002) Postoperative nausea and vomiting–can it be eliminated? JAMA 287:1233–1236
Apfel CC et al (2012) Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology 117:475–486
Gan TJ et al (2014) Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 118:85–113
Apfel CC et al (2002) Comparison of predictive models for postoperative nausea and vomiting. Br J Anaesth 88:234–240
Chandrakantan A et al (2011) Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth 107:i27–40
DREAMS Trial Collaborators and West Midlands Research Collaborative (2017) Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS Trial). BMJ 357:j1455
Carlisle JB et al (2006) Drugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev 3:CD004125
Apfel CC et al (2004) A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 350(24):2441–2451
Matsota P et al (2015) Ondansetron-droperidol combination vs. ondansetron or droperidol monotherapy in the prevention of postoperative nausea and vomiting. Arch Med Sci 11(2):362–370
Stoicea N et al (2015) Alternative therapies for the prevention of postoperative nausea and vomiting. Front Med (Lausanne) 2:87
Della Rocca G et al (2013) Acute lung injury in thoracic surgery. Curr Opin Anaesthesiol 26:40–46
Lohser J et al (2015) Lung injury after one-lung ventilation: a review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth Analg 121:302–318
Kehlet H et al (1993) The value of ‘‘multimodal’’ or ‘‘balanced analgesia’’ in postoperative pain treatment. Anesth Analg 77:1048–1056
Pandazi A et al (2010) Preincisional versus postincisional administration of parecoxib in colorectal surgery: effect on postoperative pain control and cytokine response. A randomized clinical trial. World J Surg 34(10):2463–2469
Henzi I et al (2000) Efficacy, dose-response, and adverse effects of droperidol for prevention of postoperative nausea and vomiting. Can J Anesth 47:537–551
Waldron NH et al (2013) Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth 110(02):191–200
Pyati S et al (2007) Perioperative pain management. CNS Drugs 21:185–211
Laskowski K et al (2011) A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth 58(10):911–923
Vigneault L et al (2011) Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anesth 58:22–37
Kranke P et al (2015) Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst Rev 7(07):CD009642
Moiniche S et al (2000) Local anesthetic infiltration for postoperative pain relief after laparoscopy: a qualitative and quantitative systematic review of intraperitoneal, port-site infiltration and mesosalpinx block. Anesth Analg 90:899–912
Block BM et al (2003) Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA 290(18):2455–2463
Jorgensen H et al (2000) Epidural local anaesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev 4:CD001893
Joshi GP et al (2013) Evidence-based postoperative pain management after laparoscopic colorectal surgery. Colorect Dis 15:146–155
Hübner M (2015) Randomized clinical trial on epidural versus patient-controlled analgesia for laparoscopic colorectal surgery within an enhanced recovery pathway. Ann Surg 261(04):648–653
Charlton S et al (2010) Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev 12(12):CD007705
Johns N et al (2012) Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: a systematic review and meta-analysis. Colorect Dis 14:e635–e642
Pedrazzani C et al (2016) Local wound infiltration plus transversus abdominis plane (TAP) block versus local wound infiltration in laparoscopic colorectal surgery and ERAS program. Surg Endosc 30(11):5117–5125
Gan TJ et al (2014) Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin 30:149–160
Oderda GM et al (2013) Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother 27:62–70
American Society of Anesthesiologists Task Force on Acute Pain Management (2012) Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 116:248–273
Tan M et al (2015) Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can J Anaesth 62(2):203–218
Ong CK et al (2010) Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg 110:1170–1179
Maund E et al (2011) Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side effects after major surgery: a systematic review. Br J Anaesth 106(03):292–297
Gorissen KJ et al (2012) Risk of anastomotic leakage with non-steroidal anti-inflammatory drugs in colorectal surgery. Br J Surg 99(05):721–727
Ntritsou V et al (2014) Effect of perioperative electroacupuncture as an adjunctive therapy on postoperative analgesia with tramadol and ketamine in prostatectomy: a randomised sham-controlled single-blind trial. Acupunct Med 32:215–222
Carli F et al (2017) Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin N Am 28:49–64
Levy N et al (2019) Patient optimisation before surgery: a clear and present challenge in peri-operative care. Anaesthesia 74(Suppl 1):3–6
Engel D et al (2020) Surgical safety in radical cystectomy: the anesthetist's point of view-how to make a safe procedure safer. World J Urol 38(6):1359–1368
Grocott MPW et al (2017) Re-designing the pathway to surgery: better care and added value. Peri-Oper Med (Lond) 6:9
Scheede-Bergdahl C et al (2019) Multi-modal prehabilitation: addressing the why, when, what, how, who and where next? Anaesthesia 74(Suppl. 1):20–26
Lumb AB (2019) Pre-operative respiratory optimisation: an expert review. Anaesthesia 74(Suppl. 1):43–48
Gillis C et al (2019) Pre-operative nutrition and the elective surgical patient: why, how, and what? Anaesthesia 74(Suppl. 1):27–35
Levett DZH et al (2019) Psychological factors, prehabilitation and surgical outcomes: evidence and future directions. Anaesthesia 74(Suppl. 1):36–42
Older P et al (1999) Cardiopulmonary exercise testing as a screening test for peri-operative management of major surgery in the elderly. Chest 116:355–362
Moran J et al (2016) Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: a systematic review. Br J Anaesth 116:177–191
West MA et al (2016) Peri-operative exercise testing and training society. Validation of pre-operative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg 103:744–752
Wijeysundera DN et al (2018) Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 391:2631–2640
Rammant E et al (2018) A systematic review of exercise and psychosocial rehabilitation interventions to improve health-related outcomes in patients with bladder cancer undergoing radical cystectomy. Clin Rehabil 32(5):594–606
Narayanan ALT et al (2016) Evidence regarding patient compliance with incentive spirometry interventions after cardiac, thoracic and abdominal surgeries: a systematic literature review. Can J Respir Ther 52(1):17–26
Furrer MA et al (2018) Impact of packed red blood cells and fresh frozen plasma given during radical cystectomy and urinary diversion on cancer-related outcome and survival: an observational cohort study. Eur Urol Focus 4:916–923
Wessels F et al (2020) Early recovery after surgery for radical cystectomy: comprehensive assessment and meta-analysis of existing protocols. World J Urol. https://doi.org/10.1007/s00345-020-03133-y(Epub ahead of print)
Williams SB et al (2020) Reporting radical cystectomy outcomes following implementation of enhanced recovery after surgery protocols: a systematic review and individual patient data meta-analysis. Eur Urol. https://doi.org/10.1016/j.eururo.2020.06.039(Online ahead of print)
Author information
Authors and Affiliations
Contributions
GP: protocol/project development, data collection, and manuscript writing. CB: data analysis. MT: data analysis. FL: data collection and manuscript writing. SF: data collection. SR: data collection. TM: manuscript editing. MP: data analysis. JW: manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pignot, G., Brun, C., Tourret, M. et al. Essential elements of anaesthesia practice in ERAS programs. World J Urol 40, 1299–1309 (2022). https://doi.org/10.1007/s00345-020-03410-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03410-w