Abstract
Background
The current study aimed to carry out a comprehensive meta-analysis on the existing evidence to quantify and compare the oncological, surgical and functional outcomes following radical prostatectomy between TURP group and Non-TURP group.
Methods
A systematic literature search was conducted using EMBASE, PubMed and Cochrane databases to identify relevant studies published in English up to March 2019. A meta-analysis was conducted using Review Manager.
Results
There were 13 studies included in the present study. Our results suggest that TURP group demonstrates a significantly higher positive surgical margin rate, bladder neck reconstruction rate and overall complication rate compared with Non-TURP group (OR = 1.31, 95% CI 1.09–1.58, P = 0.004, I2 = 0%; OR = 14.36, 95% CI 2.93–70.45, P = 0.001, I2 = 81%; OR = 2.63, 95% CI 1.87–3.71, P < 0.00001, I2 = 0%); whereas TURP group demonstrates a significantly lower nerve sparing rate compared with Non-TURP group (OR = 0.30, 95% CI 0.22–0.43, P < 0.00001, I2 = 40%); the operation time, blood loss and 1-year urinary continence rate are same between TURP group and Non-TURP group (MD = 4.25, 95% CI − 0.13 to 8.63, P = 0.06, I2 = 34%; MD = 27.29, 95% CI − 10.31 to 64.90, P = 0.15, I2 = 39%; OR = 0.68, 95% CI 0.43–1.06, P = 0.09, I2 = 0%).
Conclusion
This meta-analysis demonstrates that Non-TURP group may have a great advantage over TURP group in terms of positive surgical margin rate, bladder neck reconstruction rate, overall complication rate and sparing rate. The operation time, blood loss and 1-year urinary continence rate are comparable between TURP group and Non-TURP group. Therefore, important information should be given to those patients at risk of prostate cancer that TURP procedure may increase perioperative complications in case of a following radical prostatectomy. In the meantime, our meta-analysis found that each of these four subgroups (RARP, LRP, ORP and RARP/ORP) has its own advantages or disadvantages in every pool results. So when radical prostatectomy is performed on patients with TURP history, the appropriate operation method should be selected as per the conditions of patients, doctors and hospitals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign prostatic hyperplasia and prostate cancer are common causes of lower urinary tract symptoms in elderly men. Therefore, it is common for men to be diagnosed with prostate cancer on transurethral resection of prostate (TURP) chips or to develop prostate cancer after having undergone TURP for benign prostatic hyperplasia [1]. Prostate cancer is found in 3–16% of specimens from TURP [2, 3]. Radical prostatectomy is an effective treatment for prostate cancer. The presence of peripheral venous fibrosis, scar tissue and inflammation following previous TURP may contribute to poor outcomes in radical prostatectomy [4]. In order to minimize the impact of TURP on radical prostatectomy, the interval between TURP and radical prostatectomy is of paramount importance, but no consensus has been reached with regards to the specific interval up until now. Radical prostatectomy was recommended in the first month after TURP or until 4 months after TURP [5]. However, some studies recommend waiting at least 3 months between TURP and radical prostatectomy [6, 7]. In the past few decades, robot-assisted radical prostatectomy (RARP), laparoscopic radical prostatectomy (LRP) and open radical prostatectomy (ORP) have been used for radical prostatectomy. However, these operative methods showed different oncological, surgical and functional outcomes on radical prostatectomy compared with patients who had not TURP. To our knowledge, there is no meta-analysis to compare the effects of a history of TURP on radical prostatectomy. Therefore, we conduct a meta-analysis to compare the oncological, surgical and functional outcomes of patients who had previous TURP prior to radical prostatectomy and patients who had no TURP prior to radical prostatectomy.
Methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and meta-analysis (PRISMA) statement [8].
Data sources and searches
We conducted a systematic literature search in the EMBASE, PubMed and Cochrane databases by two independent reviewers, from their inception to March 2019. The Medical Subject Heading (MeSH) terms and/or key words and/or free words were prostate cancer AND radical prostatectomy AND Transurethral resection of prostate. Then, we performed additional manual searches of references in key studies to retrieve additional papers relevant to our topic.
Study selection
Two reviewers (X.D and XX.M) independently reviewed all the full texts of the included studies. If the following inclusion criteria were met, the studies were included in the meta-analysis: (1) patients were diagnosed with prostate cancer based on their pathological data; (2) patients in experimental group had a history of TURP, while those in control group had no history of TURP; (3) the outcome indicators included at least one or more of the following, positive surgical margin, nerve sparing, operation time, blood loss, bladder neck reconstruction, overall complications and 1-year urinary continence; (4) study that had a prospective cohort design or a retrospective case–control design; (5) studies that were published in English. Any study that did not meet the above criteria was excluded.
Data extraction and quality assessment
A standardized data extraction form collecting information on the year of publication, country, study design, prostate specific antigen (PSA), prostate volume, Gleason Score, operative method, number of TURP group and Non-TURP group, positive surgical margin (PSM) rate, nerve sparing (NS) rate, operation time (OT), blood loss (BL), bladder neck reconstruction (BNR) rate, overall complication (OC) and 1-year urinary continence (1 year UC). Each included article was appraised by two independent reviewers (X.D. and XX.M). According to the different methods of radical prostatectomy, we divided them into four subgroups (RARP, LRP, ORP and RARP/ORP). Two reviewers (X.D. and XX.M) independently assessed the methodological quality of the included studies based on the risk of bias in non-randomized studies of interventions (ROBINS-I). Seven domains were assessed based on signaling questions tailored to either cohort or case–control study designs: bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes and bias in selection of the reported result. Risk of bias was assigned as low, moderate, serious or critical in each domain.
Data synthesis and meta-analysis
We used Review RevMan 5.3 Software (The Cochrane Collaboration, Oxford, UK) and Stata v.12.0 Software (StataCorp, College Station, TX, USA) to conduct our data analysis. Four subgroups (RARP, LRP, ORP and RARP/ORP) were set in this meta-analysis, and the results were presented in the forest plots. For continuous variables, mean difference (MD) and 95% confidence interval (95% CI) were used. The odds ratio (OR) and its 95% confidence interval (95% CI) were used to represent the dichotomous variables. The heterogeneity was classified as low (I2 ≤ 50%) and high (I2 > 50%). According to whether the homogeneity was low or high, we used the fixed or the random effect model in our meta-analysis [9]. If high heterogeneity (I2 > 50) was still found, subgroup analysis or sensitivity analysis was used to find the sources of heterogeneity and exhaust them. If heterogeneity still exists, we used the random effect model (REM) in our meta-analysis. Subgroup analysis was carried out by the operative method in the meta-analysis. For all statistical analyses, a two-sided P < 0.05 was considered statistically significant. Publication bias was tested by Begg’s tests.
Results
Literature search and study election
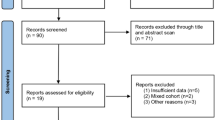
A PRISMA [8] flowchart of screening and selection results is shown in Fig. 1. Using our pre-specified search strategy, we retrieved 505 extracts and obtained 12 additional citations by other sources. From 43 studies initially identified, 34 were considered potentially suitable. After a full-text review, 13 studies [4, 6, 7, 10,11,12,13,14,15,16,17,18,19] with 1163 patients with TURP history and 5587 patients without TURP history met inclusion criteria and were included in the final analysis. Among all 13 studies, three studies were prospective case series studies, ten studies were retrospective case–control studies. Table 1 provides the basic information. Table 2 provides the oncological, surgical and functional outcomes of included studies. Supplemental Figure 1 provides the quality assessment results based on ROBINS-I.
Results of meta-analyses
Oncological outcomes
Positive surgical margin rate
Positive surgical margin rate was reported in 12 studies. TURP group provided a significantly higher positive surgical margin rate compared with Non-TURP group (OR = 1.31, 95% CI 1.09–1.58, P = 0.004, I2 = 0%). Subgroup analysis stratified based on the operation method was also carried out, and the RARP subgroup analysis showed a higher positive surgical margin rate in TURP group (OR = 1.71, 95% CI 1.16–2.52, P = 0.006, I2 = 0%), whereas both groups shared the same positive surgical margin rate as demonstrated by LRP, ORP and RARP/ORP subgroup analysis (OR = 1.62, 95% CI 0.98–2.69, P = 0.06, I2 = 0%; OR = 1.30, 95% CI 0.82–2.07, P = 0.26, I2 = 0%; OR = 1.09, 95% CI 0.83–1.4, P = 0.53) (Fig. 2).
Nerve sparing rate
As for the nerve sparing rate, TURP group provided a significantly lower nerve sparing rate compared with Non-TURP group (OR = 0.30, 95% CI 0.16–0.54, P < 0.0001, I2 = 83%). Furthermore, analysis of RARP, LRP and ORP subgroups showed that the nerve sparing rate in TURP group was low (OR = 0.39, 95% CI 0.20–0.76, P = 0.006; OR = 0.35, 95% CI 0.22–0.54, P < 0.00001, I2 = 0%; OR = 0.12, 95% CI 0.07–0.20, P < 0.00001, I2 = 0%). However, RARP/ORP subgroup analysis suggested no statistically significant difference in the nerve sparing rate between TURP group and Non-TURP group (OR = 0.77, 95% CI 0.55–1.10, P = 0.15) (Fig. 3). Due to high heterogeneity (I2 = 83%), sensitivity analysis was performed by Stata. After removing the studies by Pompe [13] and Colombo [7] as the sample that was “left out”, the pooled results did not change substantially and the heterogeneity was significantly reduced (OR = 0.30, 95% CI 0.22–0.43, P < 0.00001, I2 = 40%). In addition, no change was observed in the pooled results of RARP, LRP and ORP subgroup analysis (OR = 0.39, 95% CI 0.20–0.76, P = 0.006; OR = 0.35, 95% CI 0.22–0.54, P < 0.00001, I2 = 0%; OR = 0.11, 95% CI 0.04–0.28, P < 0.00001) (Supplemental Figure 1).
Surgical outcomes
Operation time
Referring to the operation time, TURP group offered a significantly longer operation time compared with Non-TURP group (MD = 11.40, 95% CI 2.25–20.55, P = 0.01, I2 = 67%) (Fig. 4). Sensitivity analysis was also carried out owing to the high heterogeneity (I2 = 67%). After removing the study by Yazici [19] as the sample that was “left out”, the heterogeneity was significantly reduced. The meta-analysis showed the same operation time in both groups (MD = 4.25, 95% CI − 0.13 to 8.63, P = 0.06, I2 = 34%). Furthermore, the two groups also shared the operation time as demonstrated by the RARP, ORP and RARP/ORP subgroup analysis (MD = 12.60, 95% CI − 39.00 to 64.20, P = 0.63; MD = 3.17, 95% CI − 6.34 to 12.67, P = 0.51, I2 = 0%; MD = 1.50, 95% CI − 4.33 to 7.33, P = 0.61). However, TURP group offered a significantly longer operation time compared with Non-TURP group in LRP subgroup analysis (MD = 12.26, 95% CI 2.83–21.69, P = 0.01, I2 = 71%) (Supplemental Figure 2).
Blood loss
The blood loss was the same in both TURP group and Non-TURP group (MD = 90.06, 95% CI − 7.09 to 187.21, P = 0.07, I2 = 81%) (Fig. 5). Sensitivity analysis was employed due to the high heterogeneity (I2 = 81%). When study of Colombo [7] was removed from our meta-analysis, the heterogeneity was lower. The meta-analysis showed the same blood loss in both groups (MD = 27.29, 95% CI − 10.31 to 64.90, P = 0.15, I2 = 39%). It was also the same in both groups after subgroup analysis (MD = − 38.20, 95% CI − 117.33 to 40.93, P = 0.34; MD = 49.43, 95% CI − 40.20 to 139.06, P = 0.44, I2 = 73%; MD = 44.20, 95% CI − 7.73 to 96.13, P = 0.10) (Supplemental Figure 3).
Bladder neck reconstruction rate
As for the bladder neck reconstruction rate, TURP group provided a significantly higher bladder neck reconstruction rate compared with Non-TURP group (OR = 14.36, 95% CI 2.93–70.45, P = 0.001, I2 = 81%). In addition, RARP and LRP subgroup analysis came to the same results between TURP group and Non-TURP group (OR = 22.68, 95% CI 2.26–228.05, P = 0.008, I2 = 86%; OR = 4.78, 95% CI 1.47–15.53, P = 0.009) (Fig. 6).
Overall complication rate
With regard to the overall complication rate, TURP group had a markedly increased overall complication rate relative to that in Non-TURP group (OR = 2.09, 95% CI 1.26–3.46, P = 0.004, I2 = 70%) (Fig. 7). Sensitivity analysis was carried out to account for high heterogeneity (I2 = 70%). We found that the heterogeneity was significantly reduced after study of Pompe [13] was removed. Our meta-analysis suggested higher overall complication rate in TURP group (OR = 2.63, 95% CI 1.87–3.71, P < 0.00001, I2 = 0%), and that was the same between the two groups in the RARP and LRP subgroup analysis (OR = 2.92, 95% CI 1.45–5.90, P = 0.003, I2 = 0%; OR = 2.55, 95% CI 1.72–3.77, P < 0.00001, I2 = 30%) (Supplemental Figure 4).
Functional outcomes
One-year urinary continence rate
As for the 1-year urinary continence rate, TURP group provided no significantly higher or lower 1-year urinary continence rate compared with Non-TURP group (OR = 0.68, 95% CI 0.43–1.06, P = 0.09, I2 = 0%). Furthermore, there was no statistically significant difference in the 1-year urinary continence rate between the two groups upon RARP, LRP and ORP subgroup analysis (OR = 0.62, 95% CI 0.24–1.58, P = 0.31, I2 = 0%; OR = 0.61, 95% CI 0.33–1.13, P = 0.12, I2 = 0%; OR = 0.90, 95% CI 0.36–2.22, P = 0.82) (Fig. 8).
Sensitivity analysis and publication bias
The nerve sparing rate, operation time, blood loss, bladder neck reconstruction rate and overall complication rate had high heterogeneity (I2 > 50%) in our meta-analysis. Sensitivity analysis was performed for high heterogeneity. When one or two studies were removed from our meta-analysis, lowered heterogeneity was observed in the nerve sparing rate, operation time, blood loss and overall complication rate. For the sensitivity analysis of bladder neck reconstruction rate, we found that the heterogeneity was significantly reduced after removing studies of Zugor [6] and Hung [12]. However, when these two studies were excluded, only two studies were included in this meta-analysis. Finally, these two articles were not eliminated considering the small scope of studies included; we did not delete these two articles. Publication bias was tested by Begg’s tests. Begg’s funnel plot showed no substantial asymmetry and the regression tests indicated no significant publication bias for PSM (pBegg = 0.837, Supplemental Figure 5a), NS (pBegg = 0.806, Fig. 5b), OT (pBegg = 0.452, Fig. 5c), BL (pBegg = 0.260, Fig. 5d), BNR (pBegg = 1.000, Fig. 5e), OC (pBegg = 0.452, Fig. 5f) and 1-year UC (pBegg = 1.000, Fig. 5g).
Discussion
This meta-analysis reviewed and analyzed 13 published studies to investigate and compare the oncological, surgical and functional outcomes of prostate cancer patients undergoing radical prostatectomy between TURP group and Non-TURP group. Ten published studies used a match-paired analysis. The results revealed that TURP group had higher the positive surgical margin rate, overall complication rate and bladder neck reconstruction rate, and lower nerve sparing rate.
Positive surgical margin is commonly accepted that the most affected sites are the prostatic apex and posterolateral location [20]. However, radical prostatectomy in patients undergoing TURP accounts for a difficulty in precisely identifying the prostatic apex and prostatic margins [21]; due to peripheral venous fibrosis, the anatomy of patients after TURP is technically difficult [22]; capsular perforation and fluid absorption during TURP can lead to fibrotic post-inflammatory reaction, this eventually leads to more difficult bladder neck dissection, which negatively affects the surgical margins in this area [7]; TURP can lead to surgical plane distortion [23]. Taken together, these reasons may increase the positive surgical margin rate and bladder neck reconstruction rate, whereas decreasing the nerve sparing rate in patients undergoing TURP after radical prostatectomy. These results are consistent with the results of this meta-analysis. However, the results of some subgroup analyses were inconsistent with the overall results, which may be due to the lack of adequate included studies in subgroups.
As suggested in most studies, TURP will add to the difficulty in radical prostatectomy. However, no consensus is reached about whether radical prostatectomy increases the operation time and blood loss in patients with previous TURP history. To be specific, some studies [4, 7, 12, 15, 19] suggested that radical prostatectomy might prolong the operation time in patients with previous TURP history. However, some studies [13, 18] had the opposite results, the result was the same as this meta-analysis. Three [12, 13, 15] of the six studies [4, 12, 13, 15, 18, 19] indicated that the blood loss in the two groups has statistical significance. However, this meta-analysis produced the opposite results. The results of meta-analysis may be attributed to the improvement of surgical techniques, the greater maturity of surgical methods and the more experienced operators, which may offset the negative effects of TURP. The need for bladder neck reconstruction is increased because preservation of the bladder neck after TURP was difficult.
Our meta-analysis on the overall complications in both groups showed that TURP group had a markedly higher overall complication rate than that in Non-TURP group. Such finding may be explained as follows: scar and fibrosis of bladder neck after TURP can make the healing of anastomosis more difficult; posterior dissection is difficult, leading to an increase risk of rectal injury; bladder neck thickening, fibrosis and stiffness after transurethral resection of prostate can increase anastomotic leakage; patients with tumors discovered on TURP chips may increase seminal vesiculitis, because tumors in the transition zone may be more easily transmitted through the ejaculatory duct; during TURP, there is an increased risk of stricture due to urethral manipulation and catheterization, which may lead to anterior urethral stricture.
Maintaining the quality of life is an important secondary goal after radical prostatectomy [24]. According to numerous studies, urinary incontinence is one of the most common factors affecting the quality of life following radical prostatectomy [25, 26]. The cause of urinary incontinence after radical prostatectomy remains unknown. A higher body mass index (BMI) is considered as an independent predictor of urinary incontinence following radical prostatectomy [27]. Besides, surgical technique and surgeon experience are also associated with postoperative urinary incontinence [28,29,30]. Preservation of the membranous urethral length, preservation of the neurovascular supply, reconstruction of urethral and urethrovesical support were beneficial to urinary continence [31]. In our meta-analysis, the 1-year urinary continence rate was the same in both TURP group and Non-TURP group. Such results indicated that radical prostatectomy after TURP might have no significant effect on patients’ functional results. However, we found that the urinary continence rate of TURP group was worsened in our clinical center, which contradicted our meta-analysis results. It might be ascribed to the fact that only five studies were included in this meta-analysis to analyze the urinary continence rate, and the sample size in TURP group was small. On the other hand, although there was no statistical significance in the urinary continence rate in this meta-analysis, the Non-TURP group was better than the TURP group in terms of the inclusion of the studies. It can be considered that the urinary continence rate in the two groups had clinical significance. Therefore, more well designed and randomized high-quality studies with large sample size should be carried out in the future to explore the effect of TURP on the urinary continence rate after radical prostatectomy.
According to our meta-analysis, prostate cancer patients with a history of TURP had poorer oncological outcomes, as well as higher bladder neck reconstruction rate and overall complication rate after radical prostatectomy. Through our clinical observation, it could be due to the fact that the age and prostate volume of patients in the TURP group may be larger. However, this might not be the case for the 13 studies included in this present study. Because there were only three studies showing different ages in the two groups, among which two studies showed significant differences in the prostate volume between the two groups. We should exclude the possibility of prostate cancer before operating on patients with TURP in clinical work, to avoid the diagnosis of prostate cancer after TURP as much as possible. Currently, surgery has become the primary approach to effectively relieve the lower urinary tract symptoms in patients with benign prostatic hyperplasia. However, currently TURP, vaporization of the prostate and endoscopic enucleation of the prostate can be selected as the current surgical methods [32], and each of above-mentioned methods has its own advantages and disadvantages. In the future, a better surgical method should be explored to reduce peripheral venous fibrosis, scar tissue and inflammation. As also shown in this meta-analysis, there was no difference in the operation time, blood loss and 1-year urinary continence rate between the two groups. Therefore, radical prostatectomy is still a good choice for prostate cancer patients with a history of TURP. In order to reduce the influence of TURP on radical prostatectomy, the interval time between TURP and radical prostatectomy is an important factor. However, currently no consensus has been reached in this regard. Consequently, more relevant studies are warranted to identify the optional interval, so that prostate cancer patients with a TURP history can have a better recovery after radical prostatectomy.
Several limitations of the meta-analysis should be noted. Firstly, the samples size in the TURP group was relatively small in some studies; secondly, the differences in surgical equipment and techniques might affect the outcomes since the studies were conducted in different countries and hospitals. Thirdly, the meta-analysis in terms of the nerve sparing rate, operation time, blood loss, bladder neck reconstruction rate and overall complication rate had high heterogeneity. After sensitivity analysis, the heterogeneities of this meta-analysis in terms of the nerve sparing rate, operation time, blood loss bladder neck reconstruction and overall complication rate were significantly reduced. At last, patients with a history of TURP might be older or have a late stage of prostate cancer, leading to poorer oncological and functional outcomes, which might lead to inherent biases. In the future, studies with well-designed retrospective or prospective case controls are needed.
Conclusions
This meta-analysis demonstrates that the TURP group provides a significantly higher positive surgical margin rate, overall complication rate, bladder neck reconstruction rate and lower nerve sparing rate compared with Non-TURP group. The operation time, blood loss and 1-year urinary continence rate are same between TURP group and Non-TURP group.
References
Mustafa M, Davis JW, Gorgel SN, Pisters L (2017) Robotic or open radical prostatectomy in men with previous transurethral resection of prostate. Urol J 14:2955
Rassweilera J, Kuntz R, Hofmann R (2006) Complications of transurethral resection of the prostate (TURP)—incidence, management, and prevention. Eur Urol 50:969–980
Merrill RM, Wiggins CL (2002) Incidental detection of population-based prostate cancer incidence rates through transurethral resection of the prostate. Urol Oncol 7:213–219
Menard J, de la Taille A, Hoznek A et al (2008) Laparoscopic radical prostatectomy after transurethral resection of the prostate: surgical and functional outcomes. Urology 72:593–597
Elder JS, Gibbons RP, Correa RJ, Brannen GE (1984) Morbidity of radical perineal prostatectomy following transurethral resection of the prostate. J Urol 132:55–57
Zugor V, Labanaris AP, Porres D, Witt JH (2012) Surgical, oncologic, and short-term functional outcomes in patients undergoing robot-assisted prostatectomy after previous transurethral resection of the prostate. J Endourol 26:515–519
Colombo R, Naspro R, Salonia A et al (2006) Radical prostatectomy after previous prostate surgery: clinical and functional outcomes. J Urol 176:2459–2463
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Gupta NP, Singh P, Nayyar R (2011) Outcomes of robot-assisted radical prostatectomy in men with previous transurethral resection of prostate. BJU Int. 108:1501–1505
Hampton L, Nelson RA, Satterthwaite R, Wilson T, Crocitto L (2008) Patients with prior TURP undergoing robot-assisted laparoscopic radical prostatectomy have higher positive surgical margin rates. J Robot Surg 2:213–216
Hung C, Yang C, Ou Y (2014) Robotic assisted laparoscopic radical prostatectomy following transurethral resection of the prostate: perioperative, oncologic and functional outcomes. Prostate Int 2:82–89
Pompe RS, Leyh-Bannurah S, Preisser F et al (2018) Radical prostatectomy after previous TUR-P: oncological, surgical, and functional outcomes. Urol Oncol Semin Original Investig 36:521–527
Su Y, Katz BF, Sehgal SS et al (2015) Does previous transurethral prostate surgery affect oncologic and continence outcomes after RARP? J Robot Surg. 9:291–297
Jaffe J, Stakhovsky O, Cathelineau X, Barret E, Vallancien G, Rozet F (2007) Surgical outcomes for men undergoing laparoscopic radical prostatectomy after transurethral resection of the prostate. J Urol 178:483–487
Teber D, Cresswell J, Ates M et al (2009) Laparoscopic radical prostatectomy in clinical T1a and T1b prostate cancer: oncologic and functional outcomes—a matched-pair analysis. Urology 73:577–581
Yang Y, Luo Y, Hou G et al (2015) Laparoscopic radical prostatectomy after previous transurethral resection of the prostate in clinical T1a and T1b prostate cancer: a matched-pair analysis. Urol J. 12:2154
Palisaar JR, Wenske S, Sommerer F, Hinkel A, Noldus J (2009) Open radical retropubic prostatectomy gives favourable surgical and functional outcomes after transurethral resection of the prostate. BJU Int. 104:611–615
Yazici S, Inci K, Yuksel S, Bilen CY, Ozen H (2009) Radical prostatectomy after previous prostate surgery: effects on surgical difficulty and pathologic outcomes. Urol 73:856–859
Gettman MT, Blute ML (2010) Radical prostatectomy: does surgical technique influence margin control? Urol Oncol. 28:219–225
Pastore AL, Palleschi G, Silvestri L et al (2015) Laparoscopic radical prostatectomy after previous transurethral resection of prostate using a catheter balloon inflated in prostatic urethra: oncological and functional outcomes from a matched pair analysis. Int J Urol 22:1037–1042
Bandhauer K, Senn E (1988) Radical retropubic prostatectomy after transurethral prostatic resection. Eur Urol 15:180–181
Ramon J, Rossignol G, Leandri P, Gautier JR (1994) Morbidity of radical retropubic prostatectomy following previous prostate resection. J Surg Oncol 55:14–19
Magheli A, Gonzalgo ML, Su LM et al (2011) Impact of surgical technique (open vs laparoscopic vs robotic-assisted) on pathological and biochemical outcomes following radical prostatectomy: an analysis using propensity score matching. BJU Int. 107:1956–1962
Walsh PC (1998) Patient-reported impotence and incontinence after nerve-sparing radical prostatectomy. J Urol 159:308–309
Stanford JL, Feng Z, Hamilton AS et al (2000) Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the prostate cancer outcomes study. JAMA 283:354–360
Wolin KY, Luly J, Sutcliffe S, Andriole GL, Kibel AS (2010) Risk of urinary incontinence following prostatectomy: the role of physical activity and obesity. J Urol 183:629–633
Cooperberg MR, Odisho AY, Carroll PR (2012) Outcomes for radical prostatectomy: is it the singer, the song, or both? J Clin Oncol 30:476–478
Hu JC, Gu X, Lipsitz SR et al (2009) Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 302:1557–1564
Talcott JA, Rieker P, Propert KJ et al (1997) Patient-reported impotence and incontinence after nerve-sparing radical prostatectomy. J Natl Cancer Inst 89:1117–1123
Kania P, Wośkowiak P, Salagierski M (2019) Preservation of continence in radical prostatectomy patients: a laparoscopic surgeon’s perspective. Central Eur J Urol 72:32
Rieken M, Herrmann T, Fullhase C (2019) Surgical treatment of benign prostatic hyperplasia-resection, vaporization or enucleation? Urol A 58:263–270
Acknowledgements
This work was supported by the Natural Science Foundation of Sichuan Provincial Department of Education (16ZB0227), Scientific Research Foundation of Health and Family Planning Commission of Sichuan Province (17PJ155) and City of Nanchong Strategic Cooperation with Local Universities Foundation of technology (NSMC20170421, NSMC20170111, 18SXHZ0581, 18SXHZ0128).
Author information
Authors and Affiliations
Contributions
SC, TW: project development, public funding. XD, XXM, THH, TC, JBL: data collection. HL, XD: data analysis, manuscript writing/editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liao, H., Duan, X., Du, Y. et al. Radical prostatectomy after previous transurethral resection of the prostate: oncological, surgical and functional outcomes—a meta-analysis. World J Urol 38, 1919–1932 (2020). https://doi.org/10.1007/s00345-019-02986-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02986-2