Abstract
Purpose
Recently, several randomized controlled trials (RCTs) explored the effects of α-blockers with or without phosphodiesterase type 5 inhibitors (PDE5-Is) for lower urinary tract symptoms secondary to benign prostatic hyperplasia (LUTS/BPH). However, the results were inconsistent. We performed this meta-analysis to evaluate the role of combination therapy (α-blockers and PDE5-Is) in patients with LUTS/BPH.
Materials and methods
Databases including PubMed, Cochrane library, Web of Science, and Embase were searched for qualified RCTs. Pooled mean differences (MDs) and odds ratios (ORs) were calculated to measure the effects and adverse events in combination therapy. Moreover, subgroup analyses of ethnicity, dosage of PDE5-Is, treatment duration, and severity of LUTS/BPH were performed. In addition, trial sequential analyses (TSAs) were used to assess whether the evidence for the results was sufficient.
Results
Overall, this study identified 11 eligible RCTs, including 855 LUTS/BPH patients. Patients receiving combination therapy had better improvement in international prostate symptom score (IPSS: MD: 1.66, 95% CI − 3.03 to − 0.29), maximum urinary flow rate (Qmax: MD: 0.94, 95% CI 0.24–1.64), and international index of erectile function (IIEF: MD: 4.73, 95% CI 2.95–6.51), comparing those without PDE5-Is. Besides, subgroup analyses indicated that the effects of combination treatment were associated with ethnicity, treatment duration, and severity of LUTS/BPH. By TSA, the findings in the current study were based on sufficient evidence.
Conclusions
Our results indicated that combination therapy can significantly improve IPSS, Qmax, and IIEF in patients with LUTS/BPH. Combination therapy might be more suitable for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lower urinary tract symptoms (LUTS) are a common urologic disorder in adult male. Symptoms of LUTS can be divided into three parts: filling (storage) or irritative symptoms, voiding or obstructive symptoms, and post-micturition symptoms [1]. LUTS can significantly impair the quality of life of patients, increase their substantial economic burden, and has become a great challenge to the world. Various factors were reported associated with LUTS, including benign prostatic hyperplasia (BPH), bladder dysfunction, prostatic inflammation, and other non-urological conditions [2, 3]. Among these factors, histologic condition of BPH, which can result in prostatic enlargement and subsequently bladder outlet obstruction, is a traditional and important cause [4].
Pharmacological therapies are widely used in patients with LUTS/BPH [5,6,7]. α-Blockers are the first-line drugs for LUTS/BPH [8, 9]. They can reduce prostate tone and bladder outlet obstruction by inhibiting endogenously released noradrenaline on smooth muscle cells in the prostate and ultimately alleviate the symptoms [10]. Phosphodiesterase type 5 inhibitors (PDE5-Is) also showed advantages on treatment of LUTS/BPH by increasing intracellular cyclic guanosine monophosphate, reducing smooth muscle tone of the detrusor, prostate, and urethra. Besides, PDE5-Is might also alter reflex neurotransmission, increase blood perfusion and oxygenation, and reduce chronic inflammation in urinary system [11,12,13]. Moreover, various studies also showed that PDE5-Is can improve the international index of erectile function (IIEF) and international prostate symptom score (IPSS) in patients with LUTS/BPH [14,15,16], and tadalafil has been licensed for treating LUTS/BPH in America and European Union.
Recently, several randomized controlled trials (RCTs) have compared the effects of combination therapy of PDE5-Is and α-blockers versus α-blockers alone in patients with LUTS/BPH [17,18,19,20,21,22,23,24,25,26,27]. In most studies, compared with α-blockers alone, combination therapy has shown advantages on improving IPSS, IIEF, maximum urinary flow rate (Qmax), and postvoid residual volume (PVR) without severe adverse events (AEs) [19,20,21,22,23,24,25,26,27]. However, contradictory or non-significant results were also demonstrated in some studies. For instance, two recently published studies concerning Asian populations showed non-significant improvements in IPSS in patients receiving combination therapy [17, 18]. Moreover, three parts of IPSS including IPSS storage-system score, IPSS voiding-system score, and IPSS quality of life score were analyzed, respectively, in several recent publications. However, the results also varied from different trials [17, 18, 20, 21, 24, 25].
Meta-analysis is a powerful tool in explaining controversial conclusions by pooling all relevant qualified data. Besides, further analyses in different subgroups, for instance the ethnicity, can provide more detailed information in LUTS/BPH treatment. Accordingly, we performed such meta-analysis by including all eligible RCTs to obtain a more comprehensive conclusion.
Methods
This study was strictly reported according to the preferred reporting items for systematic review and meta-analyses (PRISMA) statement [28] (Table S1).
Search strategies
Online databases including PubMed, Cochrane library, Web of Science, and Embase were comprehensively searched to identify eligible articles. The search was restricted to randomized controlled studies before March 2018. The following search items were used in this meta-analysis: “phosphodiesterase type 5 inhibitor”, “PDE5 inhibitor”, “tadalafil”, “sildenafil”, “vardenafil”, “udenafil”, “α-blockers”, “alfuzosin”, “tamsulosin”, “doxazosin”, “terazosin”, “lower urinary tract symptom”, “LUTS”, “benign prostatic hyperplasia”, “BPH”, and “randomized controlled trials”. In addition to electronic search results, we searched reference lists of the original articles and reviews manually to obtain more relevant studies. Besides, a search of the website: www.clinicaltrials.gov was performed to identify completed but still unpublished trials. If the data were unclear or not available in relative studies, we would contact the corresponding author to obtain desired information.
Studies fulfilled the following criteria were involved in this meta-analysis: (1) RCTs; (2) English literature; and (3) studies comparing the effects of combination of α-blockers and PDE5-Is with α-blockers in LUTS/BPH patients; to maintain the quality of the meta-analysis, the studies were excluded when: (1) no clear definitions of the population, diagnosis of the LUTS and BPH, type and dosage of PDE5-Is, type and dosage of α-blockers and outcome assessment; (2) duplicated data of previous publication; (3) without placebo groups or no-treatment groups; and (4) without sufficient data to estimate the outcome.
Quality assessment
The enrolled studies were evaluated by a 25-item CONSORT (Consolidated Standards of Reporting Trials) checklist [29] (Table S2), a method facilitating critical appraisal and interpretation of RCTs. The score of each RCT was determined by how many of the 25 items reported, which is positively associated with the quality of the study. A RCT of high quality will report all the items.
Data extraction
Data were extracted by two individual investigators (JZ Zhang and B Yang). If there were some uncertain data, a third investigator (X Li) would reassess the data and participated in discussion to solve the problem. All data in the current study were recorded in a standardized form. The following basic characteristics of each study were extracted: first author’s name, year of publication, origin of country, ethnicity, study design, type and dosage of PDE5-Is, type and dosage of α-blockers, treatment duration, mean age, mean body mass index (BMI), baseline IPSS, IIEF, Qmax, PVR, and prostate volume. The aforementioned basic characteristics are detailed in Table 1. The primary outcomes after treatment were extracted as follows: IPSS, IPSS storage-system score, IPSS voiding-system score, IPSS quality of life score, Qmax, PVR, and IIEF. The outcomes mentioned above are listed in Table 2. Besides, the incidence of AEs including dizziness, flushing, gastrointestinal disorders (diarrhea or dyspepsia or abdominal pain), headache, myalgia, and nasopharyngitis were extracted and are listed in Table 3.
Trial sequential analysis
Trial sequential analysis (TSA) was conducted to verify the results of meta-analyses by controlling the risk of random error and estimation of required sample size with an adjusted threshold for statistical significance [30,31,32]. In the current TSA, type I error and type II error were set at 5 and 20% (power was 80%), respectively. Moreover, 20% relative risk increase was predetermined according to the required information size. The cumulative Z-curve (the blue line) was constructed, and crossing of Z = 1.96 (p = 0.05) (vertical red line) or the monitoring boundaries (sloping red line) were assessed. If the cumulative Z-curve was crossed by the monitoring boundaries or exceeds the required sample size, then the result was considered firm. The TSA software (TSA, version 0.9; Copenhagen Trial Unit, Copenhagen, Denmark, 2011) was used in this study.
Statistical analysis
Continuous data including IPSS, IPSS storage-system score, IPSS voiding-system score, IPSS quality of life score, Qmax, PVR, and IIEF were presented as mean and standard deviation (SD). If only standard error (SE) or the 95% confidence interval of the mean difference was available, SD value will be transformed. Weighted mean difference (MD) was calculated for the primary assessment of the efficacy of the addition of PDE5-Is in LUTS patients with BPH. Besides, AEs were calculated by the pooled odds ratio (OR) with corresponding 95% confidence interval (CI). Higgins I2 statistic and Cochrane Q test were used to assess the heterogeneity of the enrolled studies [33]. If the heterogeneity was not significant (p > 0.05 or I2 < 50%), a fixed model by inverse-variance method was used, otherwise, a random model by DerSimonian-Laird method was applied [34, 35]. A pooled MD value lower than 0 or a pooled OR lower than 1 indicated that combination therapy was associated with decrease of specific parameters. The result was considered statistical significant when the 95% CI did not include 0 (MD) or 1 (OR). Moreover, subgroup analyses were further carried out by ethnicities, dosage of PDE5-Is, treatment duration, and severity of LUTS/BPH. When the dosage is higher than 5 mg per day in tadalafil or 25 mg per day in sildenafil, a relative larger dosage was defined. In addition, the severity of LUTS/BPH was roughly estimated by baseline total IPSS. LUTS/BPH was considered severe when IPSS was more than 19. Besides, a longer therapy was defined when the treatment period exceeded 3 months. Sensitivity analysis was carried out by repeating the meta-analysis by omitting one study each time. Publication bias was calculated by Egger’s linear regression test with a funnel plot [36]. The meta-analysis was performed by Stata version 12 (StataCorp LP, College Station, TX, USA).
Results
Summary of the enrolled RCTs
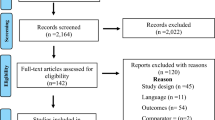
The flow chart of the study selection process is shown in Fig. 1. A total of eleven RCTs including 855 LUTS/BPH patients were finally enrolled in the current meta-analysis [17,18,19,20,21,22,23,24,25,26,27]. Among the 11 studies, 8 focused on Caucasian [19, 20, 22,23,24,25,26,27], 2 focused on Asian [17, 18], and the last 1 is a multi-ethnicity study [21]. Five researches were randomized, placebo-controlled studies [17, 19, 21, 23, 26] and six were no-treatment controlled trials [18, 20, 22, 24, 25, 27]. Noticeably, the primary regimen of PDE5-Is varied from the enrolled studies. Tadalafil, vardenafil, and sildenafil were used in five [17, 18, 20, 21, 26], one [23], and five [19, 22, 24, 25, 27] studies, respectively. Dosage of tadalafil or sildenafil was relative higher in five studies [18, 20, 22, 24, 26], the other five trials used a lower dosage [17, 19, 21, 25, 27]. In regard to the treatment period, eight studies exceeded 3 months [18,19,20, 22,23,24, 26, 27], two trials were 2 months [17, 25], and one trial was 1 month [21]. Besides, severity of LUTS/BPH differed in the involved studies. The baseline IPSS was higher than 19 in six studies [18, 19, 21, 23, 24, 26], the other five have lower baseline IPSS [17, 20, 22, 25, 27]. The detailed information of basic characteristics of the 11 RCTs is listed in Table 1.
Effects on IPSS
A total of ten studies compared the effects on total IPSS [17,18,19,20,21,22,23,24, 26, 27]. Overall, the pooled MD was − 1.66 (95% CI − 3.03 to − 0.29) in a random-effect model, which indicated a significant decrease in IPSS in patients received combination therapy (Fig. 2a). In addition, to further evaluate the specific changes in IPSS, pooled MD of IPSS storage-system score, IPSS voiding-system score, and quality of life were calculated. All these indicators showed non-significant improvement with combination therapy (IPSS storage-system score: MD: − 0.18, 95% CI − 0.56 to 0.20, Fig. 2b; IPSS voiding-system score: MD: − 0.63, 95% CI − 1.56 to 0.31, Fig. 2c; Quality of life score: MD: − 0.32, 95% CI − 0.84 to 0.20, Fig. 2d).
For subsequent subgroup analyses, a significant improvement in total IPSS was detected in Caucasian population in combination therapy (Caucasian population: MD: − 1.94, 95% CI − 3.47 to − 0.41; Asian population: MD: − 0.12, 95% CI − 0.94 to 0.70) (Figure S1A). Besides, a longer treatment period and higher total IPSS (severe LUTS/BPH symptoms) at baseline were significantly associated with better IPSS improvement (longer treatment period: MD: − 1.74, 95% CI − 3.20 to − 0.28; shorter treatment period: MD: − 1.43, 95% CI − 5.03 to 2.17, Figure S1B; severe symptoms: MD: − 2.34, 95% CI − 4.07 to − 0.60; moderate symptoms: MD: − 0.36, 95% CI − 1.15 to 0.44, Figure S1C). When the RCTs were stratified by dosage of PDE5-Is, both lower and higher dose of PDE5-Is showed non-significant improvement in IPSS (higher dosage: MD: − 0.62, 95% CI − 1.47 to 0.22; lower dosage: MD: − 2.18, 95% CI − 4.83 to 0.47, Figure S1D).
Effects on Q max, PVR and IIEF
Nine studies compared the effects on Qmax between combination therapy and α-blockers alone [18,19,20,21,22,23,24,25, 27]. The pooled MD was 0.94 (95% CI 0.24–1.64) in a random-effect model, which indicated a significant increase in Qmax in patients received combination therapy (Fig. 3a). For subsequent subgroup analyses, a longer treatment period and lower total IPSS at baseline were significantly associated with better Qmax improvement (longer treatment period: MD: 0.95, 95% CI 0.31–1.59; shorter treatment period: MD: 1.37, 95% CI − 3.03 to 5.78, Figure S2A; severe symptoms: MD: 0.72, 95% CI − 0.20 to 1.65; moderate symptoms: MD: 1.37, 95% CI 0.08–2.66, Figure S2B). When the RCTs were stratified by dosage of PDE5-Is, both groups showed non-significant improvement in Qmax (higher dosage: MD: 0.30, 95% CI − 0.25 to 0.84; lower dosage: MD: 1.38, 95% CI − 0.24 to 3.00, Figure S2C).
Seven studies compared the effects on PVR between combination therapy and α-blockers alone [18, 20, 22,23,24,25, 27]. The pooled MD was − 2.97 (95% CI − 7.62 to 1.68) in a random-effect model, indicating a moderate but not significant decrease in PVR in combination therapy (Fig. 3b). For subsequent subgroup analyses of severity of LUTS/BPH and dosage of PDE5-Is, non-significant decrease of PVR was detected (severe symptoms: MD: − 7.35, 95% CI − 15.09 to 0.40; moderate symptoms: MD: − 0.56, 95% CI − 4.83 to 3.72, Figure S3A; higher dosage: MD: − 1.02, 95% CI − 5.40 to 3.36; lower dosage: MD: − 1.52, 95% CI − 7.28 to 4.24, Figure S3B).
Seven studies compared the effects on IIEF between combination therapy and α-blockers alone [18,19,20, 22,23,24, 27]. The pooled MD was 4.73 (95% CI 2.95–6.51) in a random-effect model, indicating a significant increase in IIEF in combination therapy (Fig. 3c). For subsequent subgroup analyses of severity of LUTS/BPH and dosage of PDE5-Is, results also showed significant increase in IIEF (severe symptoms: MD: 5.50, 95% CI 3.14–7.87; moderate symptoms: MD: 3.26, 95% CI 1.18–5.34, Figure S4A; higher dosage: MD: 4.14, 95% CI 2.27–6.02; lower dosage: MD: 6.99, 95% CI 5.22–8.76, Figure S4B).
Trial sequential analyses results
TSA was conducted in this study for the first time to obtain a more comprehensive assessment of the effects of combination therapy. Results showed sufficient evidence that combination therapy can decrease IPSS (Fig. 4a) and increase Qmax (Fig. 4b) and IIEF (Fig. 4c).
Adverse events
No serious AEs were reported in all enrolled studies. Common AEs of the enrolled patients included dizziness, flushing, gastrointestinal disorders, headache, myalgia, and nasopharyngitis. Detailed incidences of these AEs are summarized in Table 3. Moreover, meta-analyses results indicated that combination therapy was associated with a higher incidence of gastrointestinal disorders (OR 3.43, 95% CI 1.16–10.13) and headache (OR 9.33, 95% CI 3.40–25.62) (Table 4).
Sensitivity analyses
Sensitivity analysis results showed non-significant alterations in pooled MDs when one individual study was excluded (Figure S5). Sensitivity analyses indicated that our results were dependable.
Publication bias
Egger’s tests and the funnel plots of the meta-analysis indicated no potential publication bias (IPSS: p = 0.124; Qmax: p = 0.549; PVR: p = 0.965; IIEF: p = 0.694) (Figure S6).
Discussion
To date, PDE5 isoenzymes have been widely identified in the smooth muscle cells of the lower urinary tract, including bladder, prostate, and urethra [37]. PDE5-Is can inhibit PDE5 isoenzymes in the lower urinary tract and affect NO/cGMP signaling, which leads to calcium efflux and relaxation of the smooth muscle cell [38]. Meanwhile, α-blockers can inhibit endogenously released noradrenaline on the same smooth muscle targets [39, 40]. Accordingly, a hypothesis that α-blockers could enhance the NO/cGMP signaling was established. In 2007, Kaplan et al. [27] first compared the effects of combination of PDE5-Is and α-blockers versus α-blockers only in patients with LUTS/BPH. Since then, numerous RCTs were performed to explore the role of combination therapy.
Results of this meta-analysis and TSA showed that combination therapy has significant improvements in IPSS, Qmax, and IIEF in patients with LUTS/BPH, which were consistent with most published RCTs. To investigate the alterations in IPSS in combination therapy, we further analyzed IPSS storage-system score, IPSS voiding-system score and quality of life in patients with LUTS/BPH. Non-significant improvements were found in these three parts, which suggested a trend that combination therapy can improve IPSS storage-system score, IPSS voiding-system score and quality of life in these patients, and ultimately improve total IPSS.
By subgroup analyses, significant improvements in IPSS were only detected in Caucasian populations, patients receiving longer treatment and patients with severe LUTS. In addition, Qmax significantly increased by combination therapy in patients receiving longer treatment and patients with moderate LUTS. It seems that longer treatment is more efficient in these patients, which can significantly improve both the IPSS and Qmax. Interestingly, IPSS was improved only in patients with severe LUTS, who still had plenty of room to upgrade the urinary symptoms and subjective feelings. Combination therapy can result in a larger enhanced degree in IPSS in these patients. On the other side, the effects of combination treatment were not obvious in patients with moderate LUTS, whose symptoms were not severe. These results indicated that ethnicity, treatment period, and severity of LUTS may influence the effects of combination therapy. More studies are required for further investigation of the specific roles of these factors in combination therapy.
By subgroup analyses of the dosage of PDE5 inhibitors, our results indicated non-significant improvements in IPSS and Qmax and significant improvements in IIEF in both larger and smaller dosage. Noticeably, the improvements are greater in IPSS, Qmax and IIEF in patients receiving smaller dosage of PDE5 inhibitors. Smaller dosage of PDE5 inhibitors can be enough for combination therapy and can lessen the economic burden of these patients.
Combination therapy is well tolerated and none severe AEs were reported in all enrolled studies. Dizziness, flushing, gastrointestinal disorders, headache, myalgia, and nasopharyngitis are common side effects in both patients receiving a-blockers with or without PDE5-Is. Noticeably, patients receiving combination therapy had a higher incidence of gastrointestinal disorders and headache according to existing data.
Indirect comparisons and limited direct comparisons between α-blockers demonstrate that all α-blockers have a similar efficacy in appropriate doses in treating LUTS [9]. A recent published meta-analysis further demonstrated that tamsulosin 0.2 mg had similar efficacy compared with other α-blockers as an initial treatment strategy for men with LUTS; however, the prevalence of AEs was different [41]. Noticeably, the type and dosage of α-blockers varied among the enrolled 11 trials in this meta-analysis (Table 1), which could result in potential bias in analyses of AEs.
In the current meta-analysis, we have several advantages: (1) A total of eleven RCTs were enrolled in the current meta-analysis; the sample size is much larger than any single study, making our results convinced; (2) further stratified analyses by ethnicity, treatment period, severity of LUTS, and dosage of PDE 5 inhibitors can provide more detailed information in LUTS/BPH treatment; (3) the funnel plots and Egger’s tests indicated no publication bias; (4) sensitivity analyses showed non-significant alterations, suggesting our results were dependable; and (5) TSA was conducted for the first time in this study and the results verified that combination therapy can decrease IPSS and increase Qmax and IIEF.
Although the evidence was overall sufficient statistical according to aforementioned analyses, several limitations should also be stressed. (1) Only few RCTs focused on Asian or African populations, and more studies are required in future research to comprehensively evaluate the role of ethnicity in patients with LUTS/BPH; (2) only four RCTs analyzed IPSS storage-system score, IPSS voiding-system score, and IPSS quality of life in several recent publications; limited sample size in these subgroups might result in potential inaccuracy; (3) adjusted estimates were not analyzed due to insufficient data for the adjustment by other covariates such as age, BMI, PVR, and prostate volume at baseline; (4) although subgroup analyses by dosage of PDE5-Is were performed, primary regimens for instance the type of α-blockers, were different in enrolled studies, which might induce potential bias; (5) the type and dosage of α-blockers varied among the enrolled 11 studies and could result in potential bias in meta-analysis. Studies with large sample of cases are required to obtain more precise results; and (6) although TSA results showed that combination therapy has a firm improvement in IPSS, Qmax, and IIEF, more RCTs of high quality are recommended to offer more detailed individual data.
Conclusion
Compared with a-blockers alone, combination of PDE5-Is and a-blockers can significantly improve IPSS, Qmax and IIEF in patients with LUTS/BPH. Combination therapy can be well tolerated and are recommended for these patients.
References
Gratzke C, Bachmann A, Descazeaud A, Drake MJ, Madersbacher S, Mamoulakis C, Oelke M, Tikkinen KAO, Gravas S (2015) EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 67:1099–1109. https://doi.org/10.1016/j.eururo.2014.12.038
Martin SA, Haren MT, Marshall VR, Lange K, Wittert GA (2011) Prevalence and factors associated with uncomplicated storage and voiding lower urinary tract symptoms in community-dwelling Australian men. World J Urol 29:179–184. https://doi.org/10.1007/s00345-010-0605-8
Alawamlh OAH, Goueli R, Lee RK (2018) Lower urinary tract symptoms, benign prostatic hyperplasia, and urinary retention. Med Clin N Am 102:301–311. https://doi.org/10.1016/j.mcna.2017.10.005
Ficarra V, Rossanese M, Zazzara M, Giannarini G, Abbinante M, Bartoletti R, Mirone V, Scaglione F (2014) The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr Urol Rep. 15:463. https://doi.org/10.1007/s11934-014-0463-9
Yuan JQ, Mao C, Wong SY, Yang ZY, Fu XH, Dai XY, Tang JL (2015) Comparative effectiveness and safety of monodrug therapies for lower urinary tract symptoms associated with benign prostatic hyperplasia: a network meta-analysis. Medicine (Baltimore). 94:e974. https://doi.org/10.1097/md.0000000000000974
Gacci M, Sebastianelli A, Spatafora P, Corona G, Serni S, De Ridder D, Gravas S, Abrams P (2018) Best practice in the management of storage symptoms in male lower urinary tract symptoms: a review of the evidence base. Ther Adv Urol. 10:79–92. https://doi.org/10.1177/1756287217742837
MacDonald R, Brasure M, Dahm P, Olson CM, Nelson VA, Fink HA, Risk MC, Rwabasonga B, Wilt TJ (2018) Efficacy of newer medications for lower urinary tract symptoms attributed to benign prostatic hyperplasia: a systematic review. Aging Male. https://doi.org/10.1080/13685538.2018.1434503
Thomas D, Chughtai B, Kini M, Te A (2017) Emerging drugs for the treatment of benign prostatic hyperplasia. Expert Opin Emerg Drugs. 22:201–212. https://doi.org/10.1080/14728214.2017.1369953
Djavan B, Chapple C, Milani S, Marberger M (2004) State of the art on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 64:1081–1088. https://doi.org/10.1016/j.urology.2004.07.031
van Dijk MM, de la Rosette JJ, Michel MC (2006) Effects of alpha(1)-adrenoceptor antagonists on male sexual function. Drugs 66:287–301
Giuliano F, Uckert S, Maggi M, Birder L, Kissel J, Viktrup L (2013) The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol 63:506–516. https://doi.org/10.1016/j.eururo.2012.09.006
Taoka R, Kakehi Y (2017) The influence of asymptomatic inflammatory prostatitis on the onset and progression of lower urinary tract symptoms in men with histologic benign prostatic hyperplasia. Asian J Urol. 4:158–163. https://doi.org/10.1016/j.ajur.2017.02.004
Morelli A, Sarchielli E, Comeglio P, Filippi S, Mancina R, Gacci M, Vignozzi L, Carini M, Vannelli GB, Maggi M (2011) Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J Sex Med. 8:2746–2760. https://doi.org/10.1111/j.1743-6109.2011.02416.x
Wang XH, Wang X, Shi MJ, Li S, Liu T, Zhang XH (2015) Systematic review and meta-analysis on phosphodiesterase 5 inhibitors and alpha-adrenoceptor antagonists used alone or combined for treatment of LUTS due to BPH. Asian J Androl. 17:1022–1032. https://doi.org/10.4103/1008-682x.154990
Gacci M, Corona G, Salvi M, Vignozzi L, McVary KT, Kaplan SA, Roehrborn CG, Serni S, Mirone V, Carini M, Maggi M (2012) A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with alpha-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol 61:994–1003. https://doi.org/10.1016/j.eururo.2012.02.033
Oelke M, Shinghal R, Sontag A, Baygani SK, Donatucci CF (2015) Time to onset of clinically meaningful improvement with tadalafil 5 mg once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: analysis of data pooled from 4 pivotal, double-blind, placebo controlled studies. J Urol 193:1581–1589. https://doi.org/10.1016/j.juro.2014.11.094
Takeda M, Yokoyama O, Yoshida M, Nishizawa O, Hirata K, Nakaoka R, Takita Y, Murakami M (2017) Safety and efficacy of the combination of once-daily tadalafil and alpha-1 blocker in Japanese men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized, placebo-controlled, cross-over study. Int J Urol 24:539–547. https://doi.org/10.1111/iju.13357
Karami H, Hassanzadeh-Hadad A, Fallah-Karkan M (2016) Comparing monotherapy with tadalafil or tamsulosin and their combination therapy in men with benign prostatic hyperplasia: a randomized clinical trial. Urol J. 13:2920–2926
Fawzi A, Kamel M, Salem E, Desoky E, Omran M, Elgalaly H, Sakr A, Maarouf A, Khalil S (2017) Sildenafil citrate in combination with tamsulosin versus tamsulosin monotherapy for management of male lower urinary tract symptoms due to benign prostatic hyperplasia: a randomised, double-blind, placebo-controlled trial. Arab J Urol. 15:53–59. https://doi.org/10.1016/j.aju.2016.11.001
Kumar S, Kondareddy C, Ganesamoni R, Nanjappa B, Singh SK (2014) Randomized Controlled trial to assess the efficacy of the combination therapy of alfuzosin and tadalafil in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Low Urin Tract Symptoms. 6:35–40. https://doi.org/10.1111/luts.12016
Regadas RP, Reges R, Cerqueira JB, Sucupira DG, Josino IR, Nogueira EA, Jamacaru FV, de Moraes MO, Silva LF (2013) Urodynamic effects of the combination of tamsulosin and daily tadalafil in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: a randomized, placebo-controlled clinical trial. Int Urol Nephrol 45:39–43. https://doi.org/10.1007/s11255-012-0317-7
Abolyosr A, Elsagheer GA, Abdel-Kader MS, Hassan AM, Abou-Zeid AM (2013) Evaluation of the effect of sildenafil and/or doxazosin on Benign prostatic hyperplasia-related lower urinary tract symptoms and erectile dysfunction. Urol Ann. 5:237–240. https://doi.org/10.4103/0974-7796.120293
Gacci M, Vittori G, Tosi N, Siena G, Rossetti MA, Lapini A, Vignozzi L, Serni S, Maggi M, Carini M (2012) A randomized, placebo-controlled study to assess safety and efficacy of vardenafil 10 mg and tamsulosin 0.4 mg vs. tamsulosin 0.4 mg alone in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Sex Med. 9:1624–1633. https://doi.org/10.1111/j.1743-6109.2012.02718.x
Ozturk MI, Kalkan S, Koca O, Gunes M, Akyuz M, Karaman MI (2012) Efficacy of alfuzosin and sildenafil combination in male patients with lower urinary tract symptoms. Andrologia. 44(Suppl 1):791–795. https://doi.org/10.1111/j.1439-0272.2011.01268.x
Tuncel A, Nalcacioglu V, Ener K, Aslan Y, Aydin O, Atan A (2010) Sildenafil citrate and tamsulosin combination is not superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. World J Urol 28:17–22. https://doi.org/10.1007/s00345-009-0484-z
Bechara A, Romano S, Casabe A, Haime S, Dedola P, Hernandez C, Rey H (2008) Comparative efficacy assessment of tamsulosin vs. tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J Sex Med. 5:2170–2178. https://doi.org/10.1111/j.1743-6109.2008.00940.x
Kaplan SA, Gonzalez RR, Te AE (2007) Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol 51:1717–1723. https://doi.org/10.1016/j.eururo.2007.01.033
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269, W64
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340:c869. https://doi.org/10.1136/bmj.c869
Zhang J, Yang B, Xiao W, Li X, Li H (2018) Effects of testosterone supplement treatment in hypogonadal adult males with T2DM: a meta-analysis and systematic review. World J Urol. https://doi.org/10.1007/s00345-018-2256-0
Li X, Shen M, Cai H, Liu K, Liu Y, Huang Z, Liang C, Deng X, Ye J, Zou Q, Li J (2016) Association between manganese superoxide dismutase (MnSOD) polymorphism and prostate cancer susceptibility: a meta-analysis. Int J Biol Markers 31:e422–e430. https://doi.org/10.5301/jbm.5000188
Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, Gluud LL, Als-Nielsen B, Gluud C (2009) Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 38:276–286. https://doi.org/10.1093/ije/dyn179
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials. 7:177–188
DerSimonian R, Kacker R (2007) Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 28:105–114. https://doi.org/10.1016/j.cct.2006.04.004
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ. 315:629–634
Uckert S, Kuthe A, Jonas U, Stief CG (2001) Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol. 166:2484–2490
Francis SH, Busch JL, Corbin JD, Sibley D (2010) cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62:525–563. https://doi.org/10.1124/pr.110.002907
Yokoyama O, Igawa Y, Takeda M, Yamaguchi T, Murakami M, Viktrup L (2015) Tadalafil for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a review of clinical data in Asian men and an update on the mechanism of action. Ther Adv Urol. 7:249–264. https://doi.org/10.1177/1756287215589238
Kolontarev K, Govorov A, Kasyan G, Priymak D, Pushkar D (2016) Current drug therapy of patients with BPH-LUTS with the special emphasis on PDE5 inhibitors. Cent Eur J Urol 69:398–403. https://doi.org/10.5173/ceju.2016.879
Shim SR, Kim JH, Chang IH, Shin IS, Hwang SD, Kim KH, Yoon SJ, Song YS (2016) Is tamsulosin 0.2 mg effective and safe as a first-line treatment compared with other alpha blockers? A meta-analysis and a moderator focused study. Yonsei Med J 57:407–418. https://doi.org/10.3349/ymj.2016.57.2.407
Acknowledgements
This work is supported by the grant from the National Natural Science Foundation of China (81671488) and the Beijing Natural Science Foundation (Grant No. 7162152).
Funding
This study was funded by the National Natural Science Foundation of China (81671488) and the Beijing Natural Science Foundation (Grant no. 7162152).
Author information
Authors and Affiliations
Contributions
HJL: project development and manuscript writing. JZZ: data collection and manuscript writing. XL: data collection and data analysis. BY: data collection and data analysis. CW: data collection. YHF.: data analysis.
Corresponding author
Ethics declarations
Conflict of interest
Hongjun Li has received research grants from the National Natural Science Foundation of China (81671488) and the Beijing Natural Science Foundation (Grant no. 7162152).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals
For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
345_2018_2370_MOESM1_ESM.tif
Figure S1 Subgroup analyses of effects of combination therapy versus a-blockers alone by assessment of IPSS. A–D Subgroup analyses by ethnicity, treatment period, severity of LUTS, and dosage of PDE5-Is, respectively (TIFF 1091 kb)
345_2018_2370_MOESM2_ESM.tif
Figure S2 Subgroup analyses of effects of combination therapy versus a-blockers alone by assessment of Qmax. A–C Subgroup analyses by treatment period, severity of LUTS, and dosage of PDE5-Is, respectively (TIFF 438 kb)
345_2018_2370_MOESM3_ESM.tif
Figure S3 Subgroup analyses of effects of combination therapy versus a-blockers alone by assessment of PVR. A, B Subgroup analyses by treatment period and dosage of PDE5-Is, respectively (TIFF 476 kb)
345_2018_2370_MOESM4_ESM.tif
Figure S4 Subgroup analyses of effects of combination therapy versus a-blockers alone by assessment of IIEF. A, B Subgroup analyses by treatment period and dosage of PDE5-Is, respectively (TIFF 500 kb)
345_2018_2370_MOESM5_ESM.tif
Figure S5 Sensitivity of each included study in this meta-analysis. A–D indicated sensitivity analyses of IPSS, Qmax, PVR, and IIEF, respectively (TIFF 550 kb)
345_2018_2370_MOESM6_ESM.tif
Figure S6 Funnel plots of the publication bias. A–D indicated funnel plots of IPSS, Qmax, PVR, and IIEF, respectively 6 (TIFF 265 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Li, X., Yang, B. et al. Alpha-blockers with or without phosphodiesterase type 5 inhibitor for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review and meta-analysis. World J Urol 37, 143–153 (2019). https://doi.org/10.1007/s00345-018-2370-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2370-z