Abstract
Objectives
To evaluate the predictive value of advanced non-contrasted computed tomography (NCCT) post-processing using novel CT-calculometry (CT-CM) parameters compared to established predictors of success of shock wave lithotripsy (SWL) for urinary calculi.
Materials and Methods
NCCT post-processing was retrospectively performed in 312 patients suffering from upper tract urinary calculi who were treated by SWL. Established predictors such as skin to stone distance, body mass index, stone diameter or mean stone attenuation values were assessed. Precise stone size and shape metrics, 3-D greyscale measurements and homogeneity parameters such as skewness and kurtosis, were analysed using CT-CM. Predictive values for SWL outcome were analysed using logistic regression and receiver operating characteristics (ROC) statistics.
Results
Overall success rate (stone disintegration and no re-intervention needed) of SWL was 59% (184 patients). CT-CM metrics mainly outperformed established predictors. According to ROC analyses, stone volume and surface area performed better than established stone diameter, mean 3D attenuation value was a stronger predictor than established mean attenuation value, and parameters skewness and kurtosis performed better than recently emerged variation coefficient of stone density. Moreover, prediction of SWL outcome with 80% probability to be correct would be possible in a clearly higher number of patients (up to fivefold) using CT-CM-derived parameters.
Conclusions
Advanced NCCT post-processing by CT-CM provides novel parameters that seem to outperform established predictors of SWL response. Implementation of these parameters into clinical routine might reduce SWL failure rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-contrasted computed tomography (NCCT) represents the gold standard examination for diagnosis and further treatment of urinary calculi [1]. Shockwave lithotripsy (SWL) is still recommended as first-line treatment for the most stone scenarios [1, 2]. SWL is safer and less invasive compared to other established techniques such as ureterorenoscopy (URS) or percutaneous nephrolithotomy (PCNL) but associated with lower stone free rates [2]. Therefore, many attempts to reduce SWL failure rates by identification of appropriate SWL outcome predictors have been made.
Several parameters, such as skin-to-stone distance (SSD), body mass index (BMI), and established mean attenuation values (in Hounsfield units; HU) have been shown to be significantly associated with SWL outcome and are, therefore, considered by leading urological guidelines [1, 2].
Recent studies found that exact volumetric measurements might predict SWL outcome more precisely than widely used planar stone diameter [3]. However, this fact is not yet part of the leading urological guidelines, probably due to its technically demanding application in daily clinical routine.
Moreover, stone homogeneity and microstructure have been recently introduced as promising predictors of SWL success in vitro [4,5,6]. So far, clinical studies assessing the impact of 3D-greyscale measurements and homogeneity parameters in vivo are lacking.
CT-Calculometry (CT-CM) was lately introduced as an advanced NCCT post-processing method in a proof-of-concept study and facilitates precise 3D-analyses of size and shape of urinary calculi as well as analyses of internal structural homogeneity with a small effort [7].
Therefore, the aim of this study was to investigate the predictive value of novel 3D greyscale measurements, homogeneity parameters such as skewness and kurtosis, stone size and shape metrics, assessed by CT-CM, and to compare it to established predictors of SWL success.
Materials and methods
Study design
Totally 312 consecutive patients suffering from urolithiasis, who were treated by SWL between March 2012 and October 2017, were retrospectively assessed. Patients were included in this study if preoperative NCCT was available and if SWL had been performed for a single renal or ureteral stone of ≥ 0.4 cm (largest diameter in NCCT). The study was approved by the local ethics committee (EKOS 17/051).
Shock wave lithotripsy
SWL was performed with a SLX-F2 (Storz Medical, Tägerwilen, Switzerland) under X-ray monitoring [8]. Each session consisted of a maximum of up to 4000 shocks applied to the stone according to best practices in SWL [8]. All interventions were conducted by three experienced technicians and supervised by an urologist. Treatment was performed until complete stone fragmentation occurred, including a maximum of three subsequent applications during one in-patient stay. If after three SWL sessions, stones showed no disintegration or disintegration was insufficient (i.e. fragments ≥ 0.4 cm present) and/or there was a need for a re-intervention (URS, PCNL or SWL), the patient was considered as treatment failure. Disintegration was assessed by kidney, ureter and bladder X-ray (KUB) and ultrasound after each SWL session and 6 weeks postoperatively.
NCCT and established parameters
For all patients, diagnosis and planning of treatment was based on a pre-interventional NCCT, performed by a multidetector row helical CT scanner (Siemens, Sensation 64; Somatom Definition; Definition Flash; Definition Force; Forchheim, Germany). Standard dose non-contrast CT was performed at a reference setting of 120 kV and 100 quality reference mAs using automated attenuation-based tube current modulation (CAREDose4D; Siemens Healthcare) with a slice collimation of 0.6-mm CT images were reconstructed using a slice thickness of 2 mm with an increment of 1.5 mm.
SSD was calculated by measuring the distance from the stone to the skin in three angles (0°, 45° and 90°) and determining the mean distance as described elsewhere [9]. Body mass index was assessed by dividing the patient’s weight (kg) by the square of the height (m2). Conventional stone size was measured by taking the largest diameter of the stone in NCCT in coronal and axial planes. Established mean attenuation values (in HU) were measured in axial NCCT scans using a region of interest radiographic calliper slightly smaller than the stone to be measured [7, 10]. As described previously [11], the variation coefficient of stone density (VCSD) is calculated by [(Standard deviation of mean attenuation value)/(mean attenuation value)]. For practical reasons and to improve accuracy, this parameter was calculated using the software mentioned below. Stone location was classified in five groups including upper, middle and lower calices and proximal and distal ureter.
CT-Calculometry (CT-CM)
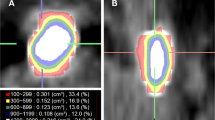
CT-CM was performed by advanced NCCT post-processing using the software 3D Slicer Version 4.6.2 (http://www.slicer.org) as recently described [7]. 3D Slicer is a free open-source application similar to a radiology workstation. It supports versatile visualizations, but also provides advanced functionality such as automated segmentation and reconstruction [12]. The data were imported from Digital Imaging and Communications in Medicine (DICOM), afterwards the stone was isolated from the neighbouring soft tissue by HU threshold adaption [13]. A 3D model of each stone resulted, which was the basis for further analyses using the extension OpenCAD for Slicer 4.6.2.
Established parameters and CT-CM parameters that were analysed are described in Table 1.
Statistical analysis
Our sample size (312 patients) was chosen such as to include the full range of variation in each of the predictors to properly compare their importance, and to observe at least 10 successes and failures per predictor.
The predictive impact of established parameters such as SSD, BMI, mean attenuation or stone diameter on SWL success was compared to that of novel parameters generated by CT-CM in three ways. First, logistic regression was used to determine whether and how each parameter was related to the probability of treatment success (no re-intervention needed). The slope coefficient b of the regression model indicated whether high values (b > 0) or low values (b < 0) of the parameter predict treatment success. The significance of the relationship was tested by likelihood-ratio tests, and p-values < 0.05 were considered significant. The X2-test statistic additionally measured the strength of the association.
Second, the predictive values of the different parameters were further evaluated with receiver operating characteristic (ROC) curve statistics. The area under the curve (AUC) was determined as a second overall measure of association between predictors and SWL success. An optimal cutpoint for the distinction between patients with high and low probability of SWL success was determined as the value maximising the sum of sensitivity and specificity. Sensitivity and specificity, positive predictive and negative predictive values and percentage of patients for whom success is predicted were calculated for these cutpoints.
Finally, because the ROC analysis showed that a single cutpoint would not allow for prediction with high accuracy with any of these parameters, we additionally determined for how many patients either SWL success or SWL failure could be predicted reliably using two cutpoints. We sorted parameter values in increasing order and identified two cutpoints such that values above and below these were associated with ≥ 80% probability of success or failure, respectively, depending on the slope of the logistic regression. We then counted the number of patients with such values.
Results
Overall success (disintegration and no re-intervention needed) of SWL was 59% (184 patients). At least slight stone disintegration in KUB after the last SWL session was reported in 90% of the patients (n = 279). CT-CM analysis failed in one patient due to DICOM importing problems into the slicer application package. Patient characteristics are reported in Table 2.
All assessed parameters except for BMI and VCSD were found to be significantly associated with SWL success in logistic regression analyses. Results of logistic regression and ROC curve statistics for all parameters are reported in Table 3.
Regarding shape metrics, stone volume (AUC 0.67) and surface (0.68) performed slightly better than maximum and 3D diameter (AUC 0.66). CT-CM derived mean 3D attenuation value (AUC 0.70) outperformed established mean attenuation value (AUC 0.66). Skewness (AUC 0.64) and kurtosis (AUC 0.65) were significant predictors whereas VCSD showed an almost random association with SWL outcome (AUC 0.53). ROC curves grouped to “patient characteristics”, “stone size and shape metrics”, “stone density” and “stone homogeneity” are shown in Fig. 1.
Classification of patients into two groups, for which relatively reliable predictions can be made (i.e. either “SWL success” or “SWL failure”, with a probability of at least 80% to be correct) revealed, that such reliable predictions of SWL success can be made in more patients using selected novel, CT-CM-based parameters (e.g., volume, mean 3D attenuation) compared to related established parameters (e.g., stone diameter, mean attenuation) (Table 4).
Discussion
To our knowledge, this work represents the first clinical study evaluating the predictive value of novel 3D-greyscale and homogeneity parameters. Advanced NCCT post-processing techniques, as recently described in ex vivo and model studies [4,5,6,7], have been shown to be able to accurately characterise the stones’ geometry, mean attenuation and homogeneity. A recent proof-of-concept study showed that these techniques can be easily implemented into clinical routine [7]. Our study shows that besides stone volume and surface area, mean 3D attenuation and homogeneity parameters skewness and kurtosis are significant predictors of SWL outcome. These novel parameters mainly outperform related established parameters.
Several predictors of SWL outcome have been established in the past to reduce SWL failure rates.
SSD has been shown to be a strong factor predicting failure of SWL [10, 14, 15] and is known to be significantly associated with SWL failure with cut-off values varying between 100 and 119 mm, which is supported by the results of our study.
In strong relation to SSD, BMI is an established predictor as well [9, 10, 14]. In our study BMI performed slightly weaker compared to SSD, which is in line with previously published results [16].
Our study confirmed the results of Bandi et al. [3] pointing at superior prediction of exact measurement of stone volume compared to simple stone diameters, which are usually used in clinical practice. In addition, our data show that the exact surface area of a stone might predict the outcome of SWL as well. In contrast, the shape compactness of a stone was not significantly correlated with SWL outcome, which seems to be surprising as shape irregularities seem to be likely to make stones more prone to fragmentation compared to more compact formed stones. The fact that marked shape irregularities are more often found in large stones, in which SWL success as defined in our study is generally lower [3], might be a possible explanation for this finding.
Mean stone attenuation expressed in HU, a measure for mean stone density, is also known to be significantly linked to SWL success [9, 17, 18]. In our study, this NCCT-derived established parameter performed clearly better compared to SSD and BMI, and prediction could even be improved using CT-CM-based mean 3D attenuation values, which might be explained by a more accurate determination by 3D analysis compared to the use of exemplary regions of interest.
Recent in vitro studies suggested, that the homogeneity and structural integrity of urinary calculi might predict disintegration after SWL [6, 19]. In an attempt to describe the homogeneity of calculi by the variation coefficient of stone density (VCSD, the stone’s standard HU deviation divided by the mean HU), a recent work concluded that this parameter might be a novel predictor of SWL success [11]. In contrast, VCSD was not significantly related to SWL success in our study.
Cui et al. tried to characterise the structural homogeneity of urinary calculi more accurately and introduced the parameters skewness and kurtosis in an ex vivo study [4]. Both parameters could be confirmed as statistically significant predictors of SWL outcome in vivo in our study. Nevertheless, it should be kept in mind that these parameters represent a simplified approach to calculate microstructural homogeneity based on the frequency distribution of grey values and that the spatial pattern of grey levels may actually be more relevant for SWL success. For example, the size and the shape of “breaking zones” with reduced density may be relevant for disintegration of generally dense stones, and this aspect is not captured by simple measures of variation. Therefore, further efforts should be directed towards appropriate measures of stone texture in vivo.
In an attempt to combine information of all the above-mentioned categories (stone size, mean intensity and variation) in one single CT-CM parameter, the so-called stone “Energy” was assessed. Though “Energy” represents a further novel significant predictor of SWL outcome, the combination of the three included parameters did not exceed predictive accuracy of the best single CT-CM parameter mainly due to inherent opposing effects of the combined categories.
Reliable predictions (i.e. ≥ 80% accuracy) of SWL success could be made in more patients (up to fivefold) using selected novel, CT-CM-based parameters compared to related established parameters. Nevertheless, the number in whom SWL outcome could be predicted with high accuracy based on a single parameter was still rather low (maximum 26% of the patients met the thresholds allowing for prediction with ≥ 80% accuracy). However, as CT-CM allows for semi-automatic determination of all of the parameters assessed in our study at once with a reasonable expenditure of time (i.e. approximately 4 min per patient), the combination of CT-CM-derived parameters to prognostic models might further improve NCCT-based prediction of SWL outcome, which was beyond the scope of the present study.
The study has some limitations that have to be addressed. In particular, this was a retrospective analysis performed at a single centre. Assessment of disintegration was performed by KUB films supplemented by ultrasound and not by more accurate NCCT. Since it has been shown that KUB-based assessment of disintegration might overestimate the effect of SWL [20], only clinically relevant stone disintegration (i.e., no further treatment necessary) was defined as successful SWL. This approach is supported by an SWL success rate of 59%, which is in line with a recent study assessing SWL success by post-interventional NCCT [11].
Conclusion
Parameters derived by advanced NCCT post-processing are significantly associated with SWL outcome and even outperform some of the established predictors. These novel parameters can be assessed with a small effort and, therefore, can be implemented into daily clinical decision-making.
References
Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck AC, Gallucci M, Knoll T, Lingeman JE, Nakada SY, Pearle MS, Sarica K, Türk C, Wolf JS, American Urological Association Education and Research Ic, Urology EAo (2007) 2007 Guideline for the management of ureteral calculi. Eur Urol 52(6):1610–1631
Turk C, Petrik A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T (2016) EAU guidelines on interventional treatment for urolithiasis. Eur Urol 69(3):475–482. https://doi.org/10.1016/j.eururo.2015.07.041
Bandi G, Meiners RJ, Pickhardt PJ, Nakada SY (2009) Stone measurement by volumetric three-dimensional computed tomography for predicting the outcome after extracorporeal shock wave lithotripsy. BJU Int 103(4):524–528. https://doi.org/10.1111/j.1464-410X.2008.08069.x
Cui HW, Devlies W, Ravenscroft S, Heers H, Freidin AJ, Cleveland RO, Ganeshan B, Turney BW (2017) CT texture analysis of ex vivo renal stones predicts ease of fragmentation with shockwave lithotripsy. J Endourol 31(7):694–700. https://doi.org/10.1089/end.2017.0084
Mannil M, von Spiczak J, Hermanns T, Alkadhi H, Fankhauser CD (2017) Prediction of successful shock wave lithotripsy with CT: a phantom study using texture analysis. Abdom Radiol. https://doi.org/10.1007/s00261-017-1309-y
Zarse CA, Hameed TA, Jackson ME, Pishchalnikov YA, Lingeman JE, McAteer JA, Williams JC Jr (2007) CT visible internal stone structure, but not Hounsfield unit value, of calcium oxalate monohydrate (COM) calculi predicts lithotripsy fragility in vitro. Urol Res 35(4):201–206. https://doi.org/10.1007/s00240-007-0104-6
Zumstein V, Betschart P, Hechelhammer L, Schmid HP, Abt D, Muller-Gerbl M (2017) CT-calculometry (CT-CM): advanced NCCT post-processing to investigate urinary calculi. World J Urol. https://doi.org/10.1007/s00345-017-2092-7
Brown RD, De S, Sarkissian C, Monga M (2014) Best practices in shock wave lithotripsy: a comparison of regional practice patterns. Urology 83(5):1060–1064. https://doi.org/10.1016/j.urology.2014.01.017
El-Nahas AR, El-Assmy AM, Mansour O, Sheir KZ (2007) A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: the value of high-resolution non-contrast computed tomography. Eur Urol 51(6):1688–1693. https://doi.org/10.1016/j.eururo.2006.11.048 (discussion 1693-1684)
Müllhaupt G, Engeler DS, Schmid HP, Abt D (2015) How do stone attenuation and skin-to-stone distance in computed tomography influence the performance of shock wave lithotripsy in ureteral stone disease? BMC Urol 15:72. https://doi.org/10.1186/s12894-015-0069-7
Yamashita S, Kohjimoto Y, Iguchi T, Nishizawa S, Iba A, Kikkawa K, Hara I (2017) variation coefficient of stone density: a novel predictor of the outcome of extracorporeal shockwave lithotripsy. J Endourol 31(4):384–390. https://doi.org/10.1089/end.2016.0719
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30(9):1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Yoshida S, Hayashi T, Ikeda J, Yoshinaga A, Ohno R, Ishii N, Okada T, Osada H, Honda N, Yamada T (2006) Role of volume and attenuation value histogram of urinary stone on non-contrast helical computed tomography as predictor of fragility by extracorporeal shock wave lithotripsy. Urology 68(1):33–37. https://doi.org/10.1016/j.urology.2006.01.052
Pareek G, Hedican SP, Lee FT Jr, Nakada SY (2005) Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology 66(5):941–944. https://doi.org/10.1016/j.urology.2005.05.011
Wiesenthal JD, Ghiculete D, DAH RJ, Pace KT (2010) Evaluating the importance of mean stone density and skin-to-stone distance in predicting successful shock wave lithotripsy of renal and ureteric calculi. Urol Res 38(4):307–313. https://doi.org/10.1007/s00240-010-0295-0
Yazici O, Tuncer M, Sahin C, Demirkol MK, Kafkasli A, Sarica K (2015) Shock wave lithotripsy in ureteral stones: evaluation of patient and stone related predictive factors. Int Braz J Urol 41(4):676–682. https://doi.org/10.1590/S1677-5538.IBJU.2014.0330
Massoud AM, Abdelbary AM, Al-Dessoukey AA, Moussa AS, Zayed AS, Mahmmoud O (2014) The success of extracorporeal shock-wave lithotripsy based on the stone-attenuation value from non-contrast computed tomography. Arab J Urol 12(2):155–161. https://doi.org/10.1016/j.aju.2014.01.002
Ouzaid I, Al-qahtani S, Dominique S, Hupertan V, Fernandez P, Hermieu JF, Delmas V, Ravery V (2012) A 970 Hounsfield units (HU) threshold of kidney stone density on non-contrast computed tomography (NCCT) improves patients’ selection for extracorporeal shockwave lithotripsy (ESWL): evidence from a prospective study. BJU Int 110(11 Pt B):E438–E442. https://doi.org/10.1111/j.1464-410x.2012.10964.x
Kim SC, Burns EK, Lingeman JE, Paterson RF, McAteer JA, Williams JC Jr (2007) Cystine calculi: correlation of CT-visible structure, CT number, and stone morphology with fragmentation by shock wave lithotripsy. Urol Res 35(6):319–324. https://doi.org/10.1007/s00240-007-0117-1
Kupeli B, Gurocak S, Tunc L, Senocak C, Karaoglan U, Bozkirli I (2005) Value of ultrasonography and helical computed tomography in the diagnosis of stone-free patients after extracorporeal shock wave lithotripsy (USG and helical CT after SWL). Int Urol Nephrol 37(2):225–230. https://doi.org/10.1007/s11255-004-7975-z
Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006. https://doi.org/10.1038/ncomms5006
Author information
Authors and Affiliations
Contributions
JL and PB data collection, manuscript writing, manuscript editing. LH data collection, manuscript editing. SG project development, statistical analysis, manuscript writing, manuscript editing HPS, DSE, DA project development, manuscript editing. VZ project development, data collection, manuscript writing, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Informed consent
General consent for data usage was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the local research committee and with the 1964 Helsinki declaration and its later amendments.
Support/financial disclosures
None.
Rights and permissions
About this article
Cite this article
Langenauer, J., Betschart, P., Hechelhammer, L. et al. Advanced non-contrasted computed tomography post-processing by CT-Calculometry (CT-CM) outperforms established predictors for the outcome of shock wave lithotripsy. World J Urol 36, 2073–2080 (2018). https://doi.org/10.1007/s00345-018-2348-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2348-x