Abstract
Purpose
This study aimed at evaluating the potential of CT-calculometry (CT-CM) as a novel method to determine mineralisation, composition, homogeneity and volume of urinary calculi based on preoperative non-contrast-enhanced computed tomography (NCCT) scans.
Materials and methods
CT-CM was performed in preoperative NCCTs of 25 patients treated for upper tract urinary calculi by ureterorenoscopy or percutaneous nephrolithotomy. Absolute mineralisation values were achieved by use of quantitative CT-osteoabsorptiometry and compared to Fourier infrared spectroscopy as a reference for stone composition. Homogeneity was assessed by advanced software-based NCCT post-processing and visualised by using a maximum intensity projection algorithm. Volumetric measurement was performed by software-based three-dimensional reconstruction.
Results
CT-CM was feasible in all of the 25 NCCTs. Absolute mineralisation values calculated by quantitative CT-OAM might be used to identify the most frequent stone types. High levels of inhomogeneity could be detected even in pure component stones. Volumetric measurement could be performed with minimal effort.
Conclusions
CT-CM is based on advanced NCCT post-processing software and represents a novel and promising approach to determine mineralisation, composition, homogeneity and volume of urinary calculi based on preoperative NCCT. CT-CM could provide valuable information to predict outcome of different stone treatment methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-contrast-enhanced computed tomography (NCCT) represents the gold standard examination for diagnosis and management of urinary calculi [1]. Besides patient’s symptoms, comorbidities and preferences, treatment strategies are mainly based on stone size, location and composition.
While stone location and diameter can be accurately assessed by NCCT [2, 3], prediction of stone composition is still limited [4]. As highly mineralised stones (e.g. calcium oxalate monohydrate) are well known to show treatment failure after extracorporeal shock wave lithotripsy (SWL) more frequently [5], preoperative determination of stone composition seems to be of major importance for decision making in many patients.
Various studies attempted to predict stone composition and outcome of SWL by NCCT attenuation values [6,7,8,9]. However, it has been shown that this parameter is inadequate due to material overlap and multiple confounding factors [4, 10, 11]. Dual-energy CT seems to overcome some of these drawbacks with a reported accuracy in predicting stone composition of 80.2–97.5% [11, 12]. But as this method is costly, time-consuming and associated with additional radiation exposure, it is rarely implemented in daily clinical practice.
Recently, stone homogeneity and volume emerged as predictors of SWL outcome and improved predictive values compared to stone composition and CT-derived attenuation values have been described [3, 10, 13, 14]. Moreover, these factors might provide important information about stone disintegration during retrograde intrarenal surgery (RIRS). Thus, they could drive the decision between dusting and basketing of renal stones and help to avoid residual fragments, which still represent a major problem of RIRS [15].
In this study we introduce CT-calculometry (CT-CM), a novel method, which incorporates advanced NCCT post-processing, and the well-established method of CT-osteoabsorptiometry (CT-OAM). CT-OAM is a method that was initially developed to investigate the subchondral bone plate based on NCCT and was recently validated in orthopaedic surgery [16]. Using maximum intensity projection and three-dimensional (3D) reconstruction, mineralisation patterns can be visualized resulting in a precise mapping of density values. It has been shown that these mineralisation patterns correlate with material strength [17]. The implementation of this time- and cost-effective method into the field of urolithiasis is a novel and promising approach to assess urinary tract stones and guide treatment decisions.
The objective of this proof-of-concept study was to evaluate applicability and potential of CT-CM as a novel method to determine mineralisation, composition, homogeneity and volume of urinary calculi based on preoperative NCCT.
Materials and methods
Study design
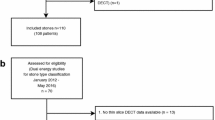
The study was approved by the local Ethics Committee (EKOS 17/051). Twenty-five patients treated at our institution by URS or PCNL for urolithiasis between January 2016 and February 2017 were retrospectively included. Inclusion criteria were as follows: preoperative NCCT performed at our hospital, stone analysis (Fourier infrared spectroscopy) available, pure stone composition, minimum stone size of 4 mm.
NCCT and CT-calculometry
All patients were scanned using a multidetector row helical CT scanner (Siemens, Definition Flash, Forchheim, Germany). Standard dose non contrast CT was performed at reference settings of 120 kV and 100 quality reference mAs using automated attenuation-based tube current modulation (CAREDose4D; Siemens Healthcare) and automated attenuation-based tube potential selection (CAREkV; Siemens Healthcare) with a collimation of 128 × 0.6. CT images were reconstructed using a slice thickness of 2 mm with an increment of 1.5 mm.
For CT-CM analysis, datasets were transferred into an image analysing system (3D Slicer Version 4.2.1, http://www.slicer.org). After isolation of the stone from the surrounding tissue by Hounsfield-units (HU) threshold adaption, 3D-reconstruction of urinary stones was performed (Fig. 1).
Grade of stone mineralisation was achieved by quantitative CT-OAM, determining the number of volumetric elements (voxels) for each density (HU). Using a calcium hydroxyapatite phantom model of a known degree of mineralisation as reference, HU were correlated with the degree of stone mineralisation. Like described in previous studies, this allows to determine the grade of mineralisation as mineral salt mass per volume (mg/ml) [18, 19]. Mineralisation values were then compared to Fourier infrared spectroscopy as a reference for stone composition using descriptive statistics (median and range).
Homogeneity was assessed by advanced NCCT post-processing software using the extension package HeterogeneityCAD for 3D slicer (3D Slicer Version 4.2.1, http://www.slicer.org). Using a voxel-based grey value assessment, the sum of the squares of each discrete value in the image ROI was quantified resulting in a dimensionless measure of homogeneity, where higher values indicate higher grades of inhomogeneity.
Visualisation of inhomogeneity was achieved by using a maximum intensity projection algorithm. Thus, the highest density value in each voxel is recorded and projected onto the surface of the stone. For better visualisation, using the open source software Paraview 3.8, density values were transferred to a colour scale and superimposed to the stones surface to enable visualisation of homogeneity as illustrated in Figs. 2 and 3. In addition, density values of all specific pixels were plotted to illustrate number and distribution of pixels per density value as shown in Fig. 5.
Calculation of stone volume was performed semi-automatically by 3D based volumetric rendering (3D Slicer Version 4.2.1, http://www.slicer.org). Using the functions “threshold painting” and “model maker”, a 3D model of each stone was achieved and the volume and surface area of each stone were calculated.
Results
CT-CM was feasible in all investigated NCCTs. Even small calculi could be isolated easily using threshold adjustment.
Highest grades of mineralisation as determined by quantitative CT-OAM were detected for ca-ox-mono (median 993 mg/ml; range 907–1204) and brushit calculi (959 mg/ml; 710–1222). Intermediate mineralisation grades were found for ca-ox-di (705 mg/ml; only one stone investigated), cystine (686 mg/ml; 602–694) and carbonatapatite (656 mg/ml; 504–690), while urinary acid stones showed markedly lower concentrations of mineral salts (374 mg/ml; 255–533). Table 1 summarizes the results of all stones assessed in the study. Figure 4 shows absolute mineralisation values and composition for all investigated stones.
Homogeneity measurement of stone density was performed by assessment of the sum of the squares of each discrete value in the image ROI. Thus, high homogeneity scores correspond to low stone homogeneity and vice versa. Homogeneity analyses revealed huge differences between the 25 stones (range 37–715,385) (Table 1). Though all included stones were pure component stones, a very high inhomogeneity was found for some of them. Remarkably, apparent variations of homogeneity were even found between stones of the same composition and a similar size.
Homogeneity could be illustrated easily using CT-OAM voxel-based maximum intensity projection and allowed to separate stones with high inhomogeneity (Fig. 2) from rather homogenous stones (Fig. 3a) at a glance. Plotting the density values for all specific pixels could further objectify this phenomenon and allowed for a swift estimation of range and distribution patterns of different densities (Fig. 5).
Number of pixels per density values (HU) detected in two stones composed of pure urinary acid (same stones as displayed in Fig. 3). a Density value plotting for a more homogenous stone; b density plotting of an inhomogeneous appearing stone
Stone volume and surface could be calculated easily using 3D-based volumetric rendering as described above. Remarkable discrepancies of stone volumes were found between stones of similar maximum diameters as shown in Table 1. These discrepancies were especially high in stones with a non-spherical shape.
Discussion
In the present study, we evaluated different novel methods to assess urinary stones using advanced software-based NCCT post-processing summarised by the term “CT-calculometry (CT-CM)”. All of them (i.e. for the examination of mineralisation, composition, homogeneity and volume of urinary calculi) proved to be feasible for NCCTs as performed in every day clinical practice and, thus, can be performed in the majority of stone patients without exposing them to additional examinations. CT-CM is mainly based on CT-OAM, which has been implemented in anatomy and orthopaedic surgery to investigate the subchondral bone plate as a marker for long-term stress distribution [16, 17, 19] and has been proven to be time- and cost-effective.
Measurement of absolute mineralisation values by quantitative CT-OAM, using a calcium hydroxyapatite phantom model as reference, has been shown to be precise to investigate absolute mineralisation values previously [18]. Considering the limitations of recent approaches to predict stone composition and response to treatment [4, 6,7,8,9,10,11,12], CT-OAM might provide additional information to serve as an independent predictor or improve existing prediction models. Larger scaled studies to assess the role of CT-OAM mineralisation grades as a predictor of stone composition and response to stone therapy are already in progress.
It has been shown recently that stone inhomogeneity might influence the outcome of stone treatment much more than mean density values [10]. In this study, we could clearly demonstrate that CT-CM has the potential to improve stone homogeneity assessment substantially. To the best of our knowledge it is the first method to quantify and visualise homogeneity in vivo based on NCCT. Complete mass was assessed by Fourier infrared spectroscopy for all of the stones in our study. Though all of them were pure-component stones, we could show remarkable differences in homogeneity, which might be caused by changes in the crystalline microstructure as stated recently [20] and which might influence the outcome of SWL as well as the choice between vaporisation and lithotripsy in endoscopic stone treatment.
Beside excellent visualisation of homogeneity, that might allow for ad hoc support of treatment decisions, CT-CM based quantification of inhomogeneity is a promising field that should be subject of further experimental and clinical trials.
In addition to composition and homogeneity of stones, CT-CM provides further information like stone surface or volumetric 3D-reconstruction, which have been shown to be predictors of the outcome after SWL [3, 21]. Instead of the widely used approximation of stone size by measurement of the diameters, CT-CM can easily provide precise computed volumetric data, which seems to be of special interest within the framework of clinical trials.
Our recent work represents a proof-of-concept study and, therefore, is associated with several limitations. A rather small group of patients was assessed in a retrospective manner. Only patients with a pure stone composition were included and we did not assess clinical outcome parameters.
Conclusion
In conclusion, our study introduces CT-CM as a novel and promising method to determine mineralisation, composition, homogeneity and volume of urinary calculi based on preoperative NCCT. Further studies assessing the clinical impact and potential of CT-CM are already on the way.
References
Ziemba JB, Matlaga BR (2015) Guideline of guidelines: kidney stones. BJU Int 116(2):184–189. doi:10.1111/bju.13080
Dalrymple NC, Verga M, Anderson KR, Bove P, Covey AM, Rosenfield AT, Smith RC (1998) The value of unenhanced helical computerized tomography in the management of acute flank pain. J Urol 159(3):735–740
Bandi G, Meiners RJ, Pickhardt PJ, Nakada SY (2009) Stone measurement by volumetric three-dimensional computed tomography for predicting the outcome after extracorporeal shock wave lithotripsy. BJU Int 103(4):524–528. doi:10.1111/j.1464-410X.2008.08069.x
Stewart G, Johnson L, Ganesh H, Davenport D, Smelser W, Crispen P, Venkatesh R (2015) Stone size limits the use of Hounsfield units for prediction of calcium oxalate stone composition. Urology 85(2):292–295. doi:10.1016/j.urology.2014.10.006
Renner C, Rassweiler J (1999) Treatment of renal stones by extracorporeal shock wave lithotripsy. Nephron 81(Suppl 1):71–81
Ouzaid I, Al-qahtani S, Dominique S, Hupertan V, Fernandez P, Hermieu JF, Delmas V, Ravery V (2012) A 970 Hounsfield units (HU) threshold of kidney stone density on non-contrast computed tomography (NCCT) improves patients’ selection for extracorporeal shockwave lithotripsy (ESWL): evidence from a prospective study. BJU Int 110(11 Pt B):E438–E442. doi:10.1111/j.1464-410X.2012.10964.x
Nakasato T, Morita J, Ogawa Y (2015) Evaluation of Hounsfield units as a predictive factor for the outcome of extracorporeal shock wave lithotripsy and stone composition. Urolithiasis 43(1):69–75. doi:10.1007/s00240-014-0712-x
Mullhaupt G, Engeler DS, Schmid HP, Abt D (2015) How do stone attenuation and skin-to-stone distance in computed tomography influence the performance of shock wave lithotripsy in ureteral stone disease? BMC Urol 15:72. doi:10.1186/s12894-015-0069-7
Marchini GS, Remer EM, Gebreselassie S, Liu X, Pynadath C, Snyder G, Monga M (2013) Stone characteristics on noncontrast computed tomography: establishing definitive patterns to discriminate calcium and uric acid compositions. Urology 82(3):539–546. doi:10.1016/j.urology.2013.03.092
Yamashita S, Kohjimoto Y, Iguchi T, Nishizawa S, Iba A, Kikkawa K, Hara I (2017) Variation coefficient of stone density: a novel predictor of the outcome of extracorporeal shockwave lithotripsy. J Endourol. doi:10.1089/end.2016.0719
Zhang GM, Sun H, Xue HD, Xiao H, Zhang XB, Jin ZY (2016) Prospective prediction of the major component of urinary stone composition with dual-source dual-energy CT in vivo. Clin Radiol 71(11):1178–1183. doi:10.1016/j.crad.2016.07.012
Hidas G, Eliahou R, Duvdevani M, Coulon P, Lemaitre L, Gofrit ON, Pode D, Sosna J (2010) Determination of renal stone composition with dual-energy CT: in vivo analysis and comparison with X-ray diffraction. Radiology 257(2):394–401. doi:10.1148/radiol.10100249
Sorokin I, Cardona-Grau DK, Rehfuss A, Birney A, Stavrakis C, Leinwand G, Herr A, Feustel PJ, White MD (2016) Stone volume is best predictor of operative time required in retrograde intrarenal surgery for renal calculi: implications for surgical planning and quality improvement. Urolithiasis 44(6):545–550. doi:10.1007/s00240-016-0875-8
Patel SR, Wells S, Ruma J, King S, Lubner MG, Nakada SY, Pickhardt PJ (2012) Automated volumetric assessment by noncontrast computed tomography in the surveillance of nephrolithiasis. Urology 80(1):27–31. doi:10.1016/j.urology.2012.03.009
Ghani KR, Wolf JS Jr (2015) What is the stone-free rate following flexible ureteroscopy for kidney stones? Nat Rev Urol 12(5):281–288. doi:10.1038/nrurol.2015.74
Zumstein V, Kraljevic M, Wirz D, Hugli R, Muller-Gerbl M (2012) Correlation between mineralization and mechanical strength of the subchondral bone plate of the humeral head. J Shoulder Elbow Surg 21(7):887–893. doi:10.1016/j.jse.2011.05.018
Kraljevic M, Zumstein V, Wirz D, Hugli R, Muller-Gerbl M (2011) Mineralisation and mechanical strength of the glenoid cavity subchondral bone plate. Int Orthop 35(12):1813–1819. doi:10.1007/s00264-011-1308-5
Meirer R, Muller-Gerbl M, Huemer GM, Schirmer M, Herold M, Kersting S, Freund MC, Rainer C, Gardetto A, Wanner S, Piza-Katzer H (2004) Quantitative assessment of periarticular osteopenia in patients with early rheumatoid arthritis: a preliminary report. Scand J Rheumatol 33(5):307–311. doi:10.1080/03009740410005890
Muller-Gerbl M (1998) The subchondral bone plate. Adv Anat Embryol Cell Biol 141(III–XI):1–134
Zhang M, Zhang X, Zhang B, Wang D (2016) Composition, microstructure and element study of urinary calculi. Microsc Res Tech 79(11):1038–1044. doi:10.1002/jemt.22739
Finch W, Johnston R, Shaida N, Winterbottom A, Wiseman O (2014) Measuring stone volume—three-dimensional software reconstruction or an ellipsoid algebra formula? BJU Int 113(4):610–614. doi:10.1111/bju.12456
Author information
Authors and Affiliations
Contributions
VZ: Project development, data collection, data analysis, manuscript writing. PB: Project development, manuscript writing. LH: Data collection, data analysis, manuscript editing. HPS: Project development, manuscript editing. DA: Project development, data collection, data analysis, manuscript writing. MMG: Project development, data collection, data analysis, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
V. Zumstein, P. Betschart, L. Hechelhammer, H. P. Schmid, D. Abt and M. Müller-Gerbl have nothing to disclose according to the ICMJE conflict of interest form.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Zumstein, V., Betschart, P., Hechelhammer, L. et al. CT-calculometry (CT-CM): advanced NCCT post-processing to investigate urinary calculi. World J Urol 36, 117–123 (2018). https://doi.org/10.1007/s00345-017-2092-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-017-2092-7