Abstract
Purpose
Comparing the accuracy of MRI/ultrasound-guided target-biopsy by transrectal biopsy (TRB) with elastic versus rigid image fusion versus transperineal biopsy (TPB) with rigid image fusion in a standardized setting.
Methods
Target-biopsy of six differently sized and located lesions was performed on customized CIRS 070L prostate phantoms. Lesions were only MRI-visible. After prior MRI for lesion location, one targeted biopsy per lesion was obtained by TRB with elastic image fusion with Artemis™ (Eigen, USA), TRB with rigid image fusion with real-time virtual sonography (Hitachi, Japan) and TPB with rigid image fusion with a brachytherapy approach (Elekta, Sweden), each on a phantom of 50, 100 and 150 ml prostate volume. The needle trajectories were marked by contrast agent and detected in a postinterventional MRI.
Results
Overall target detection rate was 79.6% with a slight superiority for the TPB (83.3 vs. 77.8 vs. 77.8%). TRB with elastic image fusion showed the highest overall precision [median distance to lesion center 2.37 mm (0.14–4.18 mm)], independent of prostate volume. Anterior lesions were significantly more precisely hit than transitional and basal lesions (p = 0.034; p = 0.015) with comparable accuracy for TRB with elastic image fusion and TPB. In general, TRB with rigid image fusion was inferior [median 3.15 mm (0.37–10.62 mm)], particularly in small lesions.
Conclusion
All biopsy techniques allow detection of clinically significant tumors with a median error of 2–3 mm. Elastic image fusion appears to be the most precise technique, independent of prostate volume, target size or location.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
MRI/ultrasound-fusion prostate biopsy improves detection of clinically significant prostate cancer compared to conventional systematic biopsy [1–3]. Recent analysis provides evidence for a superiority of a combined targeted and systematic biopsy regime compared to an exclusive targeted biopsy according to cancer detection [4]. Fusion of multiparametric MRI (mpMRI) and ultrasound images can be carried out by cognitive overlaying of the MRI and live ultrasound (visual estimation), by direct MRI/MRI-fusion (in-bore) or by software co-registered MRI/ultrasound-fusion.
Compared to a systematic biopsy, visual estimation shows higher detection rates of significant cancer [5]. However, the technique is operator dependent and requests a considerable learning curve.
Despite its high accuracy, in-bore biopsy is not widespread due to its limited availability, high cost and expenditure of time. Further, the reduced number of cores without systematic sampling and up to 10% MRI-invisible significant cancers can lead to a substantial rate of underdetection [6–8].
Therefore, software co-registered MRI/ultrasound-fusion is the most promising technique in target-biopsies at present. The biopsy devices primarily differ in the way of probe tracking and image fusion. Probe tracking can be performed by co-registration within an electromagnetic field or by sensors mounted at the probe. Elastic and rigid image fusion with or without consideration of prostate deformation have to be distinguished [9]. Further, fusion biopsy is feasible via transrectal and via transperineal access and with freehanded or mechanically assisted needle guidance. Many publications demonstrated increasing detection rates of significant cancers in contrast to systematic transrectal or transperineal mapping biopsies [9, 10]. However, only a few studies compared different target-biopsy techniques. In-bore and software co-registered MRI/ultrasound-fusion seem to improve overall cancer detection and detection of clinically significant cancer compared to visual estimation [11, 12]. Another study demonstrated no significant differences between rigid image fusion and visual estimation [5]. Delongchamps et al. [12] constituted that elastic image fusion retrieved more cancer than rigid image fusion.
Commonly, quality of target-biopsies is determined in terms of cancer detection rates compared to systematic biopsy. Previous studies have investigated the precision of newly introduced biopsy systems on prostate phantoms [13–15]. It remains unclear which technique of image fusion and access renders highest accuracy. Patient movement, anatomical differences or mpMRI techniques can further bedevil results. Thus, the intention was to compare the accuracy of three different approaches in a standardized ex vivo setting on prostate phantoms with only MRI-visible lesions.
Materials and methods
Prostate phantoms
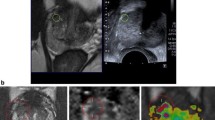
Nine customized prostate phantoms (CIRS 070L, CIRS Inc., Norfolk, USA) were used for fusion biopsy. Anatomical structures consist of a tissue equivalent gel. A perineal membrane and rectal wall allows access for both techniques. All phantoms consist of six lesions that are only visible in MRI and are isoechoic in ultrasound. Each lesion of 5 and 10 mm diameter was placed at base, in transitional zone and anterior, respectively (Fig. 1). For each biopsy system, intervention was performed on a phantom of 50, 100 and 150 ml prostate volume.
Pre- and postinterventional MRI
In clinical routine, patients undergo a state-of-the-art multiparametric MRI of the prostate using a clinically established whole-body 3T scanner (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany) prior biopsy. High-resolution T2-weighted turbospin echo sequences [repetition time (TR): 3590 ms; echo time (TE): 108 ms; slice thickness: 3 mm] are then utilized for real-time US-fusion in MR-guided biopsies.
Phantoms were imaged using the same scanner and an isotropic 3D T2 space sequence (TR: 1400 ms; TE: 140 ms; slice thickness: 0.7 mm) allowing for 3D reconstructions and precise angulation along biopsy core channels (Fig. 2).
Biopsy systems
Transrectal biopsies were carried out with both elastic and rigid MRI/ultrasound-fusion. Artemis™ (Eigen, CA, USA) is a biopsy device that enables 3D-reconstruction of the ultrasound images and elastic fusion with prior delineated contours of the prostate and regions of interest (ROI) in transversal sections of the MRI. The ultrasound probe is attached to a semi-robotic arm that supports needle navigation controlled by real-time sonography. Probe movements are tracked by encoders in the mechanical arm. All biopsy sites are registered and visualized in the 3D model for exact tumor localization and re-biopsies in case of active surveillance [16].
Real-time virtual sonography (Hitachi Medical Systems, Tokyo, Japan) uses sensor-based tracking of the transrectal ultrasound probe in an electromagnetic field. T2-weighted transversal MRI sequences are loaded on the HiVision Preirus US-device. The lesions have to be circled, and the plane image sections are fused afterward with the live ultrasound image in a rigid form without adaption of the prostate borders. Biopsy is performed freehand thereafter [17].
Brachytherapy equipment is used for transperineal biopsy (TPB) (Nucletron®, Elekta, Sweden) where the biplane transrectal probe is placed on a mechanical stepper. After scanning the prostate in transversal and sagittal view, a 3D model is reconstructed and fused rigidly with the MRI images. A grid on the stepper is mounted near the perineal membrane for needle placement. Live ultrasound allows insertion of the needle into prior marked lesions.
Biopsy sampling
First, for each system preinterventional MRI-visible lesions were delineated in the particular software. We performed one target-biopsy per lesion with a 18 G coaxial needle and a core length of 22 mm. Needle pathway was always planned to cross-lesion center in transversal view. The inner sheet of the needle was removed afterward, and an MRI contrast agent (Gadoteric acid 0.5 mmol/ml, Dotarem, Guerbet) was injected through the indwelling outer sheet to fill out the needle cylinder. The urologists who performed biopsies on the phantoms were experienced in the specific procedure.
Geometrical and statistical analysis
The postinterventional MRI sequences were evaluated in OsiriX DICOM viewer (Pixmeo, Bernex, Switzerland). To increase accuracy, the three-dimensional coordinates of the center of the lesions were determined by arithmetic means of the outermost points of the lesions. The three-dimensional coordinates of the start and end of the needle pathway were determined. The first 2 cm of the needle pathway starting from the tip was defined as the biopsy core. A target hit (and thus hit length) was defined by geometrically calculating the intersection of the biopsy core and target lesion using the aforementioned parameters. Wilcoxon rank sum test was used to compare precision of the systems and target localization.
Results
Overall precision
Among all 54 biopsy cores, 43 hit a lesion (79.6%). Overall TPB revealed one more hit than Artemis and Hitachi RVS (Table 1).
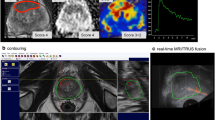
The median precision of a system to guide the needle into a lesion center was 2.37 mm (0.14–4.18 mm) for Artemis, 2.54 mm (0.22–6.88 mm) for TPB and 3.15 mm (0.37–10.62 mm) for Hitachi RVS for all lesions, irrespective of target localization, size and prostate volume (Fig. 3a). No statistical significance could be found.
Target localization
In general, the anterior located lesions were significantly hit more precisely than transitional zone and basal lesions (anterior vs. transitional: p = 0.034; anterior vs. basal: p = 0.015) (Fig. 3b). Only one of 18 anterior biopsies missed the target (Table 2). TPB and Artemis showed almost the same accuracy in anterior lesions, whereas Hitachi RVS was inferior to both systems. Further Hitachi RVS had higher variance and more spikes in transitional and basal lesions (Fig. 3c).
Target size
Except the anterior 5 mm lesion, detection rates for the 10 mm lesions outbalanced the smaller ones over all systems. TPB and Hitachi RVS biopsies missed more small lesions than Artemis (33.3 and 44.4 vs. 11.1%). Target-biopsies most frequently missed the 5 mm basal lesions (Table 1).
Prostate volume
Target-biopsies achieved highest detection rates (88.9%) for all biopsy systems in the 50-ml prostate phantom. In this small prostate volume, only Artemis accurately targeted the lesions in all six biopsies. TPB and Hitachi RVS were less precise (TPB in 150 ml, Hitachi RVS in 100 ml) with increasing prostate volumes, whereas Artemis yielded a constant distance irrespective of the prostate volume (Fig. 3d).
Discussion
Prostate imaging prior to biopsy became an emerging tool for cancer screening during the past years. Thereby mpMRI accurately detects prostate cancer and index lesions, which has been proven in prostatectomy specimens [7]. Target-biopsy leads to increased detection rates of clinically significant cancer beside a reduced overdetection of insignificant low-risk cancers. Recent data provide correlation for the detection of prostate cancer in general and clinically significant cancer in particular with the PI-RADS score that has been introduced and recently updated for standardization of mpMRI interpretation [18]. Further, mpMRI enables accurate risk stratification in patients with low-risk cancer undergoing active surveillance. However, there are still up to 10% clinically significant cancers missed by mpMRI in combination with a target-biopsy only [8]. Thus, a combination of target and systematic biopsy is recommended. Due to this MRI-interpretation error the intention of this study was to evaluate and compare the precision of target-biopsy systems head-to-head and independent of radiologic inaccuracy.
We analyzed the ability of a biopsy system to detect the center of a target. Biopsy with Artemis™ revealed high precision and the shortest median errors. Two main characteristics of this biopsy platform might explain these advantages. First, the software-based co-registration and elastic fusion of mpMRI and live ultrasound images take into account the differing prostate contours. These occur due to TRUS insertion and deformation of the prostate. Additionally, TRUS probe leads to substantial prostate displacement. Elastic fusion and motion compensation mode allows for continuous adjustment of the fusion, even during biopsy. In contrast, rigid image fusion, as with Hitachi RVS, has a higher mismatch potential. A shift of the fused slices during the TRUS scanning is not mentioned by patient movements or prostate deformations [9].
In addition, the Artemis™ platform supports biopsy navigation with the TRUS probe mounted on a semi-robotic mechanical arm. The arm has four degrees of freedom around the fixed tip of the probe. This leads to reduced probe movement during the scanning and navigation phase to a minimum. By 360° rotation of the probe around its fixed longitudinal axis, scanning of the prostate in 2D slices renders dataset for 3D-reconstruction. This allows exact biopsy planning and has been investigated formerly by Han et al. [19]. They demonstrated suboptimal freehand biopsy sampling, while robotic assistance optimized accuracy (error 9.0 vs. 1.0 mm) [20].
In our study, TPB hits one more target than TRB with both fusion techniques but showed minimally inferior biopsy precision compared to TRB with Artemis™. Median error was 2.54 mm (0.22–6.88 mm). In basal lesions and high volume prostate of 150 ml TPB demonstrated higher variation and less accuracy. Several other clinical studies compared cancer detection of TRB and TPB with controversial results. Guo et al. [21] found a cancer detection rate of 35.3% for 8–12 core TPB vs. 31.9% for 12 core TRB in a prospective randomized trial. In the series of Scott et al. [22], there was no significant difference between conventional TRUS-guided biopsy and transperineal mapping biopsy in terms of upgrading in whole mount pathology (33.22 vs. 30.41%). The analysis of 1.132 prostatectomy specimens by Hossack et al. [23] showed that TPB and TRB are similar in tumor size, stage and significance identification.
Biopsy precision was significantly influenced by the location of the lesions. As previously published, a reduced ability of TRB in detection of anterior tumors is based on the apparent difficult biopsy angle. Thus, several studies proposed a separate additional anterior biopsy sampling to avoid underdetection [24, 25]. Whether TPB is superior to TRB in anterior lesions is still being discussed. However, MRI/US-target-biopsy of anterior lesions has been shown to significantly detect more cancer than systematic biopsy [26]. Here, we demonstrate that anterior lesions can be accurately detected by MRI/US-target-biopsy without a difference between TPB and TRB with elastic image fusion.
Except for TRB with elastic image fusion the accuracy and target detection rate decreased with enlarged prostate volumes. It could be hypothesized that in large prostates with TPB the needle has to be guided through more tissue, especially for basal lesions which harbors a higher risk of deviance. In patients with large prostates, the symphysis often barricades transperineal access to anterior. In a prospective comparison study, target-biopsy and systematic biopsy had decreasing cancer detection rates for prostate volumes from >30 to 160 ml. However, in more than 40 ml volumes target-biopsy found significantly more cancer than systematic biopsy [27].
TRB with elastic image fusion and robotic assistance hits small targets of 5 mm diameter most accurately. This comes along with previous results of Wysock et al. [11] in their comparison of visual estimation and TRB with elastic image fusion.
To our best knowledge there exists only one study that compares different co-registration systems. Delongchamps et al. [12] based their results in favor of elastic image fusion on population and MRI differences and overall cancer detection rates. It is likely that our findings confirm their suggestion of a higher mismatch when fusing MRI and ultrasound images with rigid nondeformable registration.
This study has some shortcomings. First, the analysis of the biopsy trajectory in the post-biopsy MRI sequence depends on the visual analysis that might harbor some inaccuracies. Further in some biopsy cores the contrast agent diffused around the exact pathway. To rule out these individual deviations, we analyzed the trajectory in three different views in the three-dimensional MRI sequence within a coordinate plane. Secondly, there were some outliers for each biopsy system not conforming to the normal interquartile range. This demonstrates that minor biopsy inaccuracies can lead to substantial deviances among all biopsy systems despite their well-established clinical use. Consequently, the urologist has to focus on an unmitigated exact image fusion and biopsy sampling. Thirdly, this phantom-based study differs from real patient biopsy in two relevant aspects: Whereas the phantom is completely “immobile”, patient movement during biopsy in local anesthesia as well as prostate movement due to breathing can reduce accuracy. Although the phantoms consist of tissue equivalent material and realistic anatomical structures, accuracy of TPB in large prostate volumes is often reduced because of anatomical difficulties caused by the symphysis which is not included in the phantoms. Hence, this harbors the potential risk of a worse target detection and precision for TPB in large prostates than demonstrated in this study.
Summarizing, this evaluation of biopsy precision in an ex vivo setting generated four key findings: (1) elastic image fusion combined with transrectal access permits the highest accuracy to detect an MRI-suspect target, neither affected by prostate volume nor by target size. (2) Anterior localized targets are detected significantly more precisely than transitional zone and basal targets. TRB with elastic image fusion and TPB do not differ in anterior precision crucially. (3) TRB with rigid image fusion in a freehand fashion shows inferiority in overall precision, in anterior precision as well as small target detection. It seems to be prone to deviation. (4) Median distance to a lesion’s center is small enough to allow detection of clinically significant tumors according to the definition of a cancer volume of more than 0.5 ml with all target-biopsy systems [28].
Conclusion
This ex vivo study demonstrates that all biopsy techniques are applicable to detect targets with approximately 80% sensitivity and median precision of 2–3 mm. Thus, detection of clinically significant cancer might not be influenced by the choice of biopsy system when an experienced urologist performs the procedure with an exact image fusion and biopsy sampling. However, patients with large prostate volumes might benefit from a TRB with elastic image fusion. When MRI reveals small or anterior localized lesions, TRB with elastic image fusion or TPB seems to be superior in detection compared to TRB with rigid image fusion. Further studies will be necessary to compare the accuracy in a clinical setting.
References
Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, Hoang AN, Walton-Diaz A, Shuch B, Weintraub M, Kruecker J, Amalou H, Turkbey B, Merino MJ, Choyke PL, Wood BJ, Pinto PA (2013) Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 64(5):713–719. doi:10.1016/j.eururo.2013.05.059
Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, Mozer P, Rastinehad AR, Ahmed HU (2014) Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol. doi:10.1016/j.eururo.2014.10.026
Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG (2015) Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 68(3):438–450. doi:10.1016/j.eururo.2014.11.037
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM, Merino MJ, Simon RM, Choyke PL, Wood BJ, Pinto PA (2015) Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 313(4):390–397. doi:10.1001/jama.2014.17942
Puech P, Rouviere O, Renard-Penna R, Villers A, Devos P, Colombel M, Bitker MO, Leroy X, Mege-Lechevallier F, Comperat E, Ouzzane A, Lemaitre L (2013) Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy—prospective multicenter study. Radiology 268(2):461–469. doi:10.1148/radiol.13121501
Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, Vergunst H, Sedelaar JP, Futterer JJ, Barentsz JO (2012) Three-tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol 62(5):902–909. doi:10.1016/j.eururo.2012.01.047
Baco E, Ukimura O, Rud E, Vlatkovic L, Svindland A, Aron M, Palmer S, Matsugasumi T, Marien A, Bernhard JC, Rewcastle JC, Eggesbo HB, Gill IS (2015) Magnetic resonance imaging-transrectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol 67(4):787–794. doi:10.1016/j.eururo.2014.08.077
Delongchamps NB, Lefevre A, Bouazza N, Beuvon F, Legman P, Cornud F (2015) Detection of significant prostate cancer with magnetic resonance targeted biopsies—should transrectal ultrasound-magnetic resonance imaging fusion guided biopsies alone be a standard of care? J Urol 193(4):1198–1204. doi:10.1016/j.juro.2014.11.002
Cornud F, Brolis L, Delongchamps NB, Portalez D, Malavaud B, Renard-Penna R, Mozer P (2013) TRUS–MRI image registration: a paradigm shift in the diagnosis of significant prostate cancer. Abdom Imaging 38(6):1447–1463. doi:10.1007/s00261-013-0018-4
Radtke JP, Kuru TH, Boxler S, Alt CD, Popeneciu IV, Huettenbrink C, Klein T, Steinemann S, Bergstraesser C, Roethke M, Roth W, Schlemmer HP, Hohenfellner M, Hadaschik BA (2015) Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol 193(1):87–94. doi:10.1016/j.juro.2014.07.098
Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, Melamed J, Taneja SS (2014) A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol 66(2):343–351. doi:10.1016/j.eururo.2013.10.048
Delongchamps NB, Peyromaure M, Schull A, Beuvon F, Bouazza N, Flam T, Zerbib M, Muradyan N, Legman P, Cornud F (2013) Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol 189(2):493–499. doi:10.1016/j.juro.2012.08.195
Kuru TH, Roethke M, Popeneciu V, Teber D, Pahernik S, Zogal P, Schlemmer HP, Hadaschik BA, Hohenfellner M (2012) Phantom study of a novel stereotactic prostate biopsy system integrating preinterventional magnetic resonance imaging and live ultrasonography fusion. J Endourol /Endourol Soc 26(7):807–813. doi:10.1089/end.2011.0609
Bax J, Cool D, Gardi L, Knight K, Smith D, Montreuil J, Sherebrin S, Romagnoli C, Fenster A (2008) Mechanically assisted 3D ultrasound guided prostate biopsy system. Med Phys 35(12):5397–5410
Ukimura O, Desai M, Palmer S, Valencerina S, Rodrigues H, Berger A, Brandina R, Aron M, Gill I (2010) Accuracy of 3D elastic registration of prostate biopsy trajectory by real-time 3D Trus guidance with Mr/Trus image fusion: pilot prostate phantom study. J Endourol 24:A80–A81
Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, Dorey FJ, Marks LS (2013) Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol 189(1):86–91. doi:10.1016/j.juro.2012.08.095
Miyagawa T, Ishikawa S, Kimura T, Suetomi T, Tsutsumi M, Irie T, Kondoh M, Mitake T (2010) Real-time Virtual Sonography for navigation during targeted prostate biopsy using magnetic resonance imaging data. Int J Urol Off J Jpn Urol Assoc 17(10):855–860. doi:10.1111/j.1442-2042.2010.02612.x
Vargas HA, Hotker AM, Goldman DA, Moskowitz CS, Gondo T, Matsumoto K, Ehdaie B, Woo S, Fine SW, Reuter VE, Sala E, Hricak H (2015) Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. doi:10.1007/s00330-015-4015-6
Kaye DR, Stoianovici D, Han M (2014) Robotic ultrasound and needle guidance for prostate cancer management: review of the contemporary literature. Curr Opin Urol 24(1):75–80
Han M, Chang D, Kim C, Lee BJ, Zuo Y, Kim HJ, Petrisor D, Trock B, Partin AW, Rodriguez R, Carter HB, Allaf M, Kim J, Stoianovici D (2012) Geometric evaluation of systematic transrectal ultrasound guided prostate biopsy. J Urol 188(6):2404–2409. doi:10.1016/j.juro.2012.07.107
Guo LH, Wu R, Xu HX, Xu JM, Wu J, Wang S, Bo XW, Liu BJ (2015) Comparison between ultrasound guided transperineal and transrectal prostate biopsy: a prospective, randomized, and controlled trial. Sci Rep 5:16089. doi:10.1038/srep16089
Scott S, Samaratunga H, Chabert C, Breckenridge M, Gianduzzo T (2015) Is transperineal prostate biopsy more accurate than transrectal biopsy in determining final Gleason score and clinical risk category? A comparative analysis. BJU Int 116(Suppl 3):26–30. doi:10.1111/bju.13165
Hossack T, Patel MI, Huo A, Brenner P, Yuen C, Spernat D, Mathews J, Haynes AM, Sutherland R, del Prado W, Stricker P (2012) Location and pathological characteristics of cancers in radical prostatectomy specimens identified by transperineal biopsy compared to transrectal biopsy. J Urol 188(3):781–785. doi:10.1016/j.juro.2012.05.006
Bott SR, Young MP, Kellett MJ, Parkinson MC, Contributors to the UCLHTRPD (2002) Anterior prostate cancer: is it more difficult to diagnose? BJU Int 89(9):886–889
Wright JL, Ellis WJ (2006) Improved prostate cancer detection with anterior apical prostate biopsies. Urol Oncol 24(6):492–495. doi:10.1016/j.urolonc.2006.03.003
Volkin D, Turkbey B, Hoang AN, Rais-Bahrami S, Yerram N, Walton-Diaz A, Nix JW, Wood BJ, Choyke PL, Pinto PA (2014) Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int 114(6b):E43–E49. doi:10.1111/bju.12670
de Gorski A, Roupret M, Peyronnet B, Le Cossec C, Granger B, Comperat E, Cussenot O, Renard-Penna R, Mozer P (2015) Accuracy of magnetic resonance imaging/ultrasound fusion targeted biopsies to diagnose clinically significant prostate cancer in enlarged compared to smaller prostates. J Urol 194(3):669–673. doi:10.1016/j.juro.2015.03.025
Stamey TA, Yemoto CM, McNeal JE, Sigal BM, Johnstone IM (2000) Prostate cancer is highly predictable: a prognostic equation based on all morphological variables in radical prostatectomy specimens. J Urol 163(4):1155–1160
Acknowledgements
Prostate phantoms were funded by the Viktor-and-Sigrid-Dulger-foundation (Heidelberg, Germany). We thank Dr. Volker Seitz for his critical review of the manuscript.
Authors’ contribution
NW and MR were involved in project development, data collection and data analysis, manuscript writing; FPS collected and analyzed the data; DH and MP collected the data; JvH and MSM edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Westhoff, N., Siegel, F.P., Hausmann, D. et al. Precision of MRI/ultrasound-fusion biopsy in prostate cancer diagnosis: an ex vivo comparison of alternative biopsy techniques on prostate phantoms. World J Urol 35, 1015–1022 (2017). https://doi.org/10.1007/s00345-016-1967-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1967-3