Abstract
Daily changes in the content of phytohormones accumulating in the hypocotyls of young sunflower seedlings that grow under a 14/10-h light-to-dark (LD) photoperiod were followed using LC–MS. The objective of the work was to investigate whether the presence of light and light transitions lead to visible changes visible in phytohormone levels on the sixth day after the onset of germination. The LC–MS technique used allowed the simultaneous detection of more than one hundred individual phytohormone derivatives, of which the daily accumulation patterns were determined for nearly fifty members. The daily changes in phytohormone levels followed specific patterns for each phytohormone. There were differences between the daytime and nighttime levels, most likely reflecting the effects of light on phytohormone metabolism. A significant difference was found between cytokinins (CKs) and all other phytohormones, so that CK group of phytohormones may be considered to have a separate role in hypocotyl elongation. Prolonged daytime (postponed dusk) resulted in a rapid disruption of rhythmic hypocotyl elongation and it triggered an acute light stress response that was evident in increased levels of a number of important phytohormones from all groups except CKs. However, this light stress, termed ARELD (Acute Response to Extended Light Duration), was not present in control plants grown under continuous light conditions. The data suggest that sunflower has means to adapt to the potentially stressful conditions of continuous light (LL). The qPCR-RT study of the isoforms of circadian clock-associated genes HaLHY, HaTOC1, HaELF3, and HaPIF3 revealed diurnal rhythms under both LD and LL conditions in which the peaks were synchronized and shifted toward the subjective dawn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant hormones (phytohormones), regulators of plant growth and development are defined as naturally occurring organic substances that affect physiological processes at low concentrations (Davies 2004). Based on their chemical structure and biological functions, phytohormones are divided into several groups, including cytokinins (CKs), auxins, abscisic acid (ABA), gibberellins, ethylene, brassinosteroids, jasmonates, salicylic acid (SA), and strigolactones. The mode of action of plant hormones has been studied using a variety of techniques and approaches, with most data currently coming from biochemical and functional genomic studies.
Analytical liquid chromatography–mass spectrometry (LC-MC) is a technique suitable for quantitative determination of endogenous plant hormones. Critical advantage of this technique is the simultaneous determination of large groups of phytohormone moieties. Thus, by tracking changes in phytohormone levels at well-selected time points during the day, we can gain insight into the events that precede visible manifestations of plant growth and development. Here, we combined quantitative analyses of phytohormone levels and qPCR-RT expressions of circadian clock-associated genes and related them to changes in hypocotyl elongation influenced by the presence of light.
Hypocotyl elongation is a process that allows germinated seedlings to reach full daylight conditions and develop autotrophic metabolism. Seedlings that grow in light follow a developmental program called photomorphogenesis, in which light is the primary energy source. The key events in this program are the rapid development of cotyledons and maturation with the transition to autotrophy. Seedlings that remain in the dark after germination follow an unsustainable, skotomorphogenetic developmental program with accelerated hypocotyl elongation.
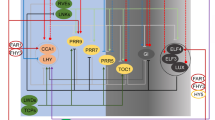
Thus, light acts as a switch that controls the direction of seedling development. Plants use the light transitions at dawn and dusk to detect the presence of light, which is registered by specialized pigments, mostly phytochromes and cryptochromes (Sommers et al. 1998; Galvão and Fankhauser 2015). A number of light-sensitive transcription factors (TFs) is also involved in recognition of light presence. Pigments and TFs together form a complex, interconnected network of light signal transduction pathways (Lau and Deng 2010).
Light perceived by phytochromes can be relayed to phytochrome-interacting factors (PIFs), which, once activated are localized in the nucleus where they have direct access to the genome (Ni et al. 1999; Quail 2002). In darkness, PIFs accumulate and prevent the onset of photomorphogenesis. In light, PIF levels decrease as they are proteolytically degraded (Leivar et al 2008). The HY5 protein is a TF that, unlike PIFs, is a positive regulator of light signaling (Nawkar et al. 2017). It regulates the expression of nearly one third of all genes present in Arabidopsis (Xiao et al. 2022). HY5 targets both light signaling components and genes related to the circadian clock (Gangappa and Botto 2016).
COP1 is a TF that accumulates in the dark, where it can bind to the transcription factors HY5 and PIF3 (Jing and Lin 2020). HY5 and COP1 proteins act antagonistically in regulation of the seedling development (Osterlund et al. 2000), as COP1 negatively modulates the activity of HY5 (Ang et al. 1998). The COP1/HY5 regulatory pathway is the central hub of photomorphogenesis (Bhatnagar et al. 2020), with HY5 affecting the activity of most phytohormones, including ABA, auxin, gibberellins, brassinosteroids and ethylene. In addition to COP1, the large COP9 signalosome, which performs proteolytic degradation, also participates in light signaling and controls plant development in the dark toward skotomorphogenesis (Deng et al. 1991; Chamowitz et al. 1996; Ma et al. 2002; Qin et al. 2020). Thus, pigments, TFs, circadian clock genes, and phytohormones are united in a common light signaling network.
The light signaling pathways of individual phytohormones often share the same light-induced TFs, indicating that light and phytohormones can establish some degree of mutual interactions (Alabadi et al. 2004; Casal et al. 2004). Their signaling pathways may converge, as in the case of auxin (Huq 2018). Auxin and light signaling pathways are highly integrated (Halliday et al. 2009) and share the same light-induced TFs that allow different phytohormone groups to converge and respond simultaneously to the same light signal. In general, most previous studies on phytohormones in sunflower have focused on auxins (Bruinsma et al. 1975). However, CK changes were also studied as in plants grown under different ratios of red to far-red light ratios (Kurepin et al. 2007).
The main objective of our study was to follow the light-induced changes in phytohormones and to verify the functioning of the circadian clock by qPCR-RT analysis, controlling for hypocotyl elongation as the output of the clock. We previously observed (Vinterhalter and Vinterhalter 2021), that all light-containing photoperiods, both in diurnal and constant light conditions, support similar hypocotyl elongation rates, implying that darkness considered as the absence of light is not a critical factor for hypocotyl elongation. We will show here that unexpectedly prolonged daytime duration in plants grown in diurnal photoperiods rapidly changes the elongation pattern by abolishing rhythmicity and significantly increasing the levels of some phytohormones. However, plants are able to adapt to this light stress keeping a constant rate of hypocotyl elongation, by resetting the high expression of circadian clock-associated genes.
For all responsive phytohormones, we will provide data on their daily levels and also on changes following light transitions. Two control points will provide data for extended daytime duration under LD photoperiod (CP1) and continuous irradiation (CP2) conditions. The qPCR-RT expression of circadian clock-associated genes HaLHY, HaTOC1, HaELF3, and HAPIF3 will show the functioning of circadian clock in plants grown under LD and LL photoperiods.
Materials and Methods
Seed Germination and Growth Conditions
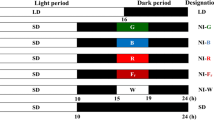
Sunflower cv Kondi (Syngenta) plants were grown under diurnal14/10-h light-to-dark (LD) or continuous light (LL) photoperiods in growth chambers under 70 µmol m−2 s−1 irradiance generated by cool white light LED panels, with temperature set at 24 ± 0.5 °C. After a short 1—2 h water imbibition, seeds were germinated for 24–36 h under layers of moist paper tissue. Germinated seeds, selected to be the same size, were planted individually in 50 ml PVC tubes filled up to the rim with peat-based substrate. Irradiance in the growth chamber was measured using Li 250A light meter (Li-Cor, Lincoln, NE, USA) with a quantum sensor.
Under both LD and LL conditions, imbibition was initiated at dawn and counted as (ZT0) when illumination was turned on in later days. In the LL treatments, lights were turned on continuously from the time of imbibition.
The relative hypocotyl length of plantlets was measured as previously described (Vinterhalter et al. 2022), using the test tube rim as the lower and the suture between cotyledon bases as the upper hypocotyl position marker. Seedling development was monitored and documented by time-lapse imaging at 10-min intervals. Material for qRT- PCR and LC–MS analysis was collected from well-established plants with fully open cotyledons, starting at dawn 120 h from the beginning of germination. Sampling was done in 3-h increments. Material for the LD control point CP1 and the CP2 control point of LL grown plants was collected at the same time, 144 h from the onset of imbibition, which corresponds to dawn (ZT0) in LD and subjective dawn in LL conditions. Prior to sampling, the material for CP1 was exposed to an extended daytime period of 24 h instead of 14 h. The material for CP2 point was collected from plants that had been continuously exposed to light since the start of germination.
Comparison parameters for light/dark effects
For each phytohormone, the time points with the highest and lowest daily (24-h) phytohormone levels were determined, as well as their daily amplitude (% change) and average daily levels. The effects of light and darkness were evaluated by two additional rounds of comparison. The first round provides data on the percent increase in phytohormone levels following the light-to-dark transition at dusk, comparing phytohormone levels at ZT13 (one hour before dusk) and ZT16 (2 h in the nighttime). In the second round, phytohormones at ZT 7 (in the middle of the day) and ZT19 (in the middle of the night) were compared to show how their levels were affected by the longer presence or absence of light.
Circadian Clock-Associated Genes—RNA Isolation and Gene Expression
Total RNA was extracted from approximately 150 mg of 3 cm long apical hypocotyl segments following the procedure of Gašić et al. (2004). To eliminate genomic DNA, samples were treated at 37 °C for 30 min with DNase I (Thermo Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Reverse transcription (RT) reaction was performed in a 20 μl reaction mixture containing 200 ng of total RNA, using the GeneAmp®Gold RNA PCR Core Kit (Applied Biosystems, Foster City, CA, USA).
The sunflower genome is fully sequenced, but the genes involved in circadian regulation are not yet fully annotated. Searching the NCBI database for these genes, the following sequences were found: HaTOC1 (Two component response regulator-like APRR1; NCBI ID: 110873837 (X1), 110917737 (X2)), HaPIF3 (Transcription factor PIF3-like; NCBI ID: 110885065 (X1), 110915563 (X2)), HaELF3 (ELF3-like protein2; NCBI ID: 110921032 (X1), 110921674 (X2), 110937420 (X3), 110943034 (X4)), HaLHY (Protein LHY-like; NCBI ID: 110879133 (X1), 110879135 (X2), 110912436 (X3), 110912437 (X4)). Since the predicted sequences and multiple gene isoforms are available, we designed primers for all the mentioned isoforms. For easier monitoring, the designation "X" was added for each gene with the number of possible variants in the subscript (X1, X2 …). Primers for all isoforms of the genes were designed using the Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast; (Ye et al. 2012) and are listed in Suppl. Table S1.
Quantitative real-time PCR (qRT-PCR) was performed in a QuantStudio™ 3 Real-Time PCR System (Applied Biosystems) in a reaction mixture (10 μl) containing Maxima SYBR Green/Rox qPCR Master Mix (Thermo Scientific), 300 nM primers, and 1 μl cDNA (equivalent to 10 ng) template. Thermal cycling conditions for amplification were: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 1 min, and extension at 72 °C for 1 min. Changes in gene expression were calculated using the ΔΔCt method (Livak and Schmittgen 2001). Expression of all tested genes was normalized to the expression of (Ha pi-TUB, GenBank™ accession number AF401481.1) and controls at ZT0 (light on) served as calibration samples. Each experiment was performed in three biological replicates, each with three technical replicates. Statistical significance between expression levels determined at different time points was tested by one-way ANOVA, and means were separated by Fisher’s LSD post hoc test for P ≤ 0.05.
Phytohormone Analysis
Sampling for phytohormone analysis was done using well-developed plantlets with fully open cotyledons. The cotyledons were removed and the apical 2–3 cm long portions of the hypocotyls were collected, frozen in liquid nitrogen, and stored at -80 °C. Each replicate contained apical hypocotyl portions from five plantlets. The lyophilized material was ground and analyzed for hormone content.
Plant hormones were determined as described previously (Prerostova et al. 2021), and three biological replicates were used for each analysis. Phytohormones were extracted from lyophilized samples (ca 1–2.5 mg dry weight (DW) by 100 μl 50% acetonitrile solution in water. Isotope labeled standards (10 pmol/sample) were added to the samples: [13C6]IAA, [2H4]OxIAA, and [2H4]OxIAA-GE (Cambridge Isotope Laboratories, Tewksbury, MA, USA); [2H4]SA and [2H2]GA19 (Sigma-Aldrich, St. Louis, MO, USA); [2H3]PA and [2H3]DPA (NRC-PBI); and [2H6]ABA, [2H5]JA, [2H5]tZ, [2H5]tZR, [2H5]tZRMP, [2H5]tZ7G, [2H5]tZ9G, [2H5]tZOG, [2H5]tZROG, [15N4]cZ, [2H3]DZ, [2H3]DZR, [2H3]DZ9G, [2H3]DZRMP, [2H7]DZOG, [2H6]iP, [2H6]iPR, [2H6]iP7G, [2H6]iP9G, and [2H6]iPRMP (Olchemim, Olomouc, Czech Republic) (see List of abbreviations, Table 1). Samples were homogenized with zirconia beads (1.5 mm diameter) in the FastPrep-24TM 5G Instrument (MP Biomedicals) for 40 s at 6 m s−1. The extracts were centrifuged at 4 °C and 30 000 rpm. The supernatant was applied to the SPE Oasis HLB 96-well column plate (10 mg/well; Waters, Milford, MA, USA) activated with 100 ml of methanol and eluted with 100 ml of 50% acetonitrile using a Pressure + 96 manifold (Biotage, Uppsala, Sweden). The sediment was re-extracted in 100 mlL of 50% acetonitrile, centrifuged, and reapplied to the column plate.
Phytohormones were separated on a Kinetex EVO C18 column (2.6 µm, 150 × 2.1 mm, Phenomenex, Torrance, CA, USA). Mobile phases consisted of (A) 5 mM ammonium acetate in water and (B) 95:5 acetonitrile/water (v/v). The following gradient programme was applied: 5% B in 0 min, 7% B in 6 s to 5 min, 10 to 35% in 5.1 to 12 min, and 35 to 100% in 12 to 13 min, 100% B in 13 to 14 min, and 5% B in 14.1 min. Hormone analysis was performed using the LC–MS system consisting of UHPLC 1290 Infinity II (Agilent, Santa Clara, CA, USA) coupled to 6495 Triple Quadrupole mass spectrometer (Agilent). MS analysis was performed in MRM mode, using the isotope dilution method. Methylthiolated tZ- and cZ-type CKs (ribosides of tZ and cZ) could not be distinguished by HPLC–MS because of identical mass spectra and are therefore considered as one substance, referred to as MeSZR in the text and graphs. Data acquisition and processing were carried out using Mass Hunter software B.08 (Agilent). Phytohormone concentrations were calculated as amount (in picomoles) per 1 g DW of plant material. Phytohormone abbreviations are listed in Table 1.
Results
Rhythmicity in Diurnal and Free-Running Photoperiods
Hypocotyl elongation of sunflower plantlets grown in diurnal photoperiods is rhythmic and shows a distinct difference in elongation kinetics for the light and dark periods (Fig. 1a). During the daytime, the cumulative increase in hypocotyl length follows a sigmoid curve with a peak elongation rate at midday. In the nighttime, the peak elongation rate is reached 1 to 2 h after the onset of dusk (Fig. 1b), after which it decreases until the end of the night.

Adapted from Vinterhalter et al. (2022).Periodicity and Spectral Composition of light in the regulation of Hypocotyl Elongation of Sunflower Seedlings. Plants 11, 1982,1–17
a Rhythmic hypocotyl elongation in diurnal 14/10 h LD photoperiod compared with uniform elongation in continuous light, nLL; b elongation rates in the diurnal 14/10 h LD photoperiod, c – transition from rhythmic into uniform elongation in extended (prolonged) daytime duration.
In constant light applied from the beginning of germination, hypocotyl elongation is uniform (arrhythmic) as in the much faster elongation of etiolated shoots grown in constant darkness.
Plants grown in a diurnal 14/10 h of light-to-darkness are light entrained from the sixth day after germination, as they anticipate the daytime and nighttime peaks of hypocotyl elongation and the advance of light transitions. When light transitions are delayed (prolonged duration of either daytime or nighttime), plants quickly slip from a rhythmic to a uniform (arrhythmic) elongation pattern. The elongation following a prolonged daytime can be considered a de novo onset of growth in LL conditions (Fig. 1c). Unlike the elongation of plants that have grown under LL conditions from the beginning of imbibition, there is no stage of de-etiolation in this renewed LL elongation.
In Arabidopsis, as in most other plants, circadian regulation extends well into free-running conditions (Dawson-Day and Millar 1999), and this prolonged rhythmicity under constant conditions is considered a fundamental feature of circadian regulation.
The change in the elongation pattern in sunflower plantlets occurs approximately 1–2 h after the onset of the unanticipated delay in the light-to-dark transition. The change is best seen in graphs of hypocotyl lengths measured from serial time-lapse images.
Expression of Circadian Clock-Associated Genes
The change in the elongation pattern of hypocotyls during prolonged daytime can be considered as a change in the functioning of the circadian clock. We therefore examined circadian clock function in plants grown under diurnal and continuous light photoperiods.
The expression of the transcription factor genes LHY, PIF3, ELF3, and TOC1 was followed, and each of these TFs is present in several different isoforms according to the NCBI database. In both LD and LL (Fig. 3), photoperiods the isoforms exhibited considerable variability, although most of them showed the expected patterns of daily rhythmicity, well-known from case studies in Arabidopsis (Alabadi et al. 2001; Covington et al. 2001; Viczián et al. 2005) and expression of LHY-like and TOC1-like genes in sunflower (Atamian et al. 2016).
In diurnal (LD) conditions the LHY isoforms showed strong induction in the early morning hours. The PIF isoforms also had a morning maximum, 4 h after dawn. The ELF3 isoforms were variable, with HaELF3 (X1, X2, and X3) showing high expression at night time points, in contrast to the X4 isoform, which had unusual daily expression. Both TOC1 isoforms HaTOC1 X1 and X2 had increased expression at the end of the day and during the night.
In continuous light (LL) conditions, expression was rhythmic, but the patterns in LL differed from those recorded in diurnal conditions. A common feature of all isoforms except HaELF3X4 was high expression at the end of the subjective night. The LHY and PIF3 isoforms also exhibited morning peaks that were not present in the ELF3 and HaTOC1X2 isoforms. The HaELF3X3 and HaTOC1X1 isoforms showed no expression in LL. Taken together, the results presented in Fig. 2 show that under LL conditions there is a synchronization of gene expression that leads to a prominent maximum at the end of the subjective night in addition to the morning maxima. This is the time of the day when according to studies of phase response curves (Millar 2003) circadian clock is resetted. The expression delay, which resembles a synchronization of daily peaks in LL conditions, obviously disrupts the expected sequence of daily expression events found in diurnal photoperiods.
Phytohormone Changes in Diurnal Photoperiod
In plants grown in the diurnal 14/10-h LD photoperiod, phytohormones showed significant daily concentration changes, with peaks falling in the daytime or nighttime or even in both daily light periods. In general, significant differences in distribution patterns of peeks between the phytohormone groups were found only for CKs, which tended to have peaks both during the day and night. ABA, phenolics, and jasmonates had peak values mostly in the nighttime, while auxins peaked mainly during the day.
The highest daily concentrations of phytohormones expressed in picomoles/g DW are shown in Fig. 3a, together with phytohormone daily amplitudes (Fig. 3f), showing the percentage increase from lowest to highest daily levels, measured for each phytohormone. Daily amplitudes although high were rather conserved, as daily concentration differences for most of them ranged from 0.5 to 2.5 times. Exceptionally high daily amplitudes were observed in only four phytohormones including, the auxin metabolite 5-hydroxy-indole-3-acetic acid (5OH-IAA), the active jasmonate derivative jasmonic acid-isoleucine (JA-Ile), and two CKs—trans-zeatin riboside (tZR) and dihydrozeatin 9-glucoside (DZ9G). This increased daily amplitude values did not appear to be associated with some other characteristics.
A clear connection between the phytohormone peaks and presence/absence of light was difficult to establish. Most of the phytohormones (26 of 47) peaked during the daytime, while 21 phytohormones peaked in the dark period. The light transition (change at dusk) was indecisive, as only 22 phytohormones showed some concentration changes between ZT13 and ZT16 (fast light responses), which covers the period of dusk. Fast light responses are presented in Fig. 4a with data for Cks provided separately (Fig. 4b).
Second comparison covers adjusted light responses showing level differences between the middle of the daytime at ZT 7 and the middle of the nighttime at ZT 17 (Fig. 4a). Data for Cks are also presented separately (Fig. 4b).
Among the fast light responses, only the increase in OxIAA-Asp levels was indicative as it progressed and manifested extended increase from fast into the adjusted light responses. A similar increase could be observed in iPRMP.
Adjusted light response provided significant light affected differences in a number of phytohormones. Those with high levels achieved in the middle of the nighttime include OxIAA-Asp, BzA, cis-OPDA, iP, iPRMP, Mes-iP, and Mes-Z. Plant hormones with levels decreasing at night included OxIAA, IAA-GE, OxIAA-GE, IAA-Glu, SinAC, tZ, and DZ9G. The nighttime increase in OxIAA-Asp was aparently associated with a decrease in other auxin types, and also cZ, wich is abundant during the day, is replaced by tZ at night. In general, CKs seem to be more abundant in the nighttime in contrast to auxins. The only exeptions are OxIAA-Asp and tZ.
CP1 and CP2 Control Points
The considerable daily variation in phytohormone concentrations raised the question if there was a time of day that could be considered representative. The average daily phytohormone level values are a usefull indicator for comparing daily events. It is interesting that early morning levels at ZT1 are fairly similar to the average daily values. ZT1 values are presented together with control points (CP1 and CP2) for which they provide a good contrast (Fig. 5 a, b) Phytohormones with concentrations somewhat higher at the ZT1 morning compared to the daily average included mainly auxins, such as IAA-Asp with levels increased by 40% up, some CKs such as all trans-zeatin (tZ)-types (tZ 15%, tZRMP 41%, tZROG 49%, tZR 66%), iPR with 13% and iPRMP with 310% increase. Phytohormones with concentrations lower than the daily average were more numerous, including other CKs such as all cis-zeatin (cZ)-types, non-ribosylated N6-(∆2-isopentenyl)adenine (iP)-types, and methylthiolated (MeS)-types, and all stress phytohormones except phenylacetamide (PAAM) which showed a 20% increase.
Control points CP1 and CP2 were selected to compare how extended light duration and continuous light affected phytohormone concentrations in tissues (Fig. 5a,b). Corresponding to the time of dawn (ZT0), the phytohormone concentrations of the control points were compared with the average daily values.
At the CP1 point, most phytohormones (32 of 47) showed increased levels. A group of phytohormones that exhibited a single, very high daily peak at the CP1 point was termed ARELD (Acute Response to Extended Light Duration) phytohormones, and the extended light duration is therefore considered here as light stress. The daily changes of all ARELD phytohormones in the LD photoperiod show exactly the same pattern and have a prominent peak at the CP1 point. ARELD phytohormones include (percent increase at CP1 over daily average in parentheses): IAA (+ 353%), SA (+ 417%), BzA (+ 221%), ABA (+ 674%), PA (+ 150%), 9OH-ABA (+ 150%), JA (+ 566%), JA-Me (+ 978%), and JA-Ile (+ 1045%). CKs are not listed among the ARELD-type phytohormones because their concentrations at CP1 are lower, with the exception of iPR and iP 9-glucoside (iP9G), which have additional daily concentration peaks besides the CP1 time point. Nine typical ARELD-type phytohormones are shown in Fig. 6.
ARELD type phytohormones. Concentration (pmol g−1DW ± SE) measured in apical portions of sunflower hypocotyls grown under a 14/10-h light-to-dark photoperiod (eight time points) and in two control points shown 144 h from the onset of germination. CP1 is the value of the extended day and CP2 is the value at subjective dawn under continuous light conditions. All the ARELD type phytohormones have an exceptionally high concentration at CP1 point
Phytohormones with very low concentrations at the CP1 point include IAA-Asp (2.1%), 2-oxindole-3-acetic acid aspartate (OxIAA-Asp; 6.5%), PAAM (23.7%), and DZ9G (6.7%.). Percentages are expressed relative to the daily mean, which was calculated as 100%.
At the CP2 control point, phytohormone levels were lower and resembled those characteristic of the morning (ZT1) time point of the diurnal photoperiod. All nine ARELD-type phytohormones had significantly lower concentrations at the CP2 point, including the highest positioned JA, whose concentration decreased from + 566% at CP1 to only + 125% above the daily average. Comparison of phytohormone concentrations measured at CP1 and CP2 points indicates that continuous light treatment (CP2) is much less stressful than the unexpected prolonged light irradiation at CP1. This also suggests that plants have mechanisms enabling them to adapt and overcome light stress when growing under LL conditions.
Auxins
Among auxins only IAA, the active auxin form, showed an ARELD pattern that was not visible in the other members of the group.
Inactivated, oxidative products of IAA predominated at all daily time points, reaching levels almost 10-times higher than those of the active and conjugated forms. The high levels of inactivated auxin forms reflect their slow metabolic degradation in cells and tissues, in contrast to the active and conjugated forms, which have rapid turnover and require small metabolic pools. Inactivated forms are therefore excellent indicators of the current state of auxin metabolism. A sudden decrease in the level of inactivated auxins observed under ARELD stress conditions (CP1) was accompanied by a marked increase in the level of active auxin forms. This suggests a kind of metabolic arrest in which active auxin forms are synthesized but not utilized. Interestingly, both auxin conjugates with aspartate (IAA-Asp and OxIAA-Asp) were close to zero under ARELD conditions. On the other hand, OxIAA-Asp content is greatly increased in the absence of light, beginning shortly after the transition to dusk.
At the CP2 control point, the situation was back to normal, with very high levels of all and inactivated IAA members. Auxin levels at CP2 (subjective LL morning) were similar to those at (ZT1) diurnal morning, as shown in Fig. 5a.
The light transition at dusk under the 14/10-h LD photoperiod is subject to fully functional circadian regulation. Such a light-to-dark transition results in a rapid but transient increase in the level of inactivated oxidative IAA forms, with a nighttime peak 2 h after the onset of darkness. In the middle of the night, only the level of OxIAA-Asp remains high.
A graphical presentation of phytohormone levels measured at all time points for members of the auxin group is provided in Suppl. Fig. S1.
Phenolics
The two active phenolic forms, SA and BzA, show typical ARELD response patterns. Other phenolic phytohormones show some unique ARELD-like daily level patterns. PAA, for example, exhibits an ARELD-like pattern, but with a sharp nighttime peak. PAAM exhibits a similar ARELD-like pattern, but with a peak at the end of the daytime. This peak may be, however, an artifact due to the large standard error for this time point. Sinapic acid (SinAc) partially resembles the typical ARELD pattern, but has a strong peak at midday.
Phenolics other than PAAM, as well as most ABA derivatives, jasmonates, GA19, and some CKs, show lower levels at the ZT1 diurnal morning point than the daily average. Thus, for most phytohormones, with the exception of auxins and some CKs, the diurnal morning appears to be a fresh start of the day. At the CP1 point, all phenolics except PAAM, as well as all ABA derivatives, jasmonates, and GA19, reach exceptionally high values, so we can classify them as stress phytohormones.
A graphical presentation of phytohormone levels measured at all time points for members of the phenolic group can be found in Suppl. Fig. S1.
Gibberellins (GA)
Although the samples were analyzed for a number of different gibberellins, only GA19, which is considered a precursor of the active form, was detected at significant levels. Neither the inactive nor the active GA forms were found. GA19 was among the phytohormones that respond with an ARELD-type response to extended daytime, similar to IAA and stress phytohormones (Fig. 2; Suppl. Fig. S2). The daily amplitude change of GA19 was otherwise small in the 14/10-h LD photoperiod.
Abscisic Acid and Derivatives
Four ABA-type compounds were detected in the sunflower samples: ABA itself, 9OH-ABA, PA and dihydrophaseic acid (DPA), see Suppl. Fig. S2. Thus, in addition to the active form (ABA), there were also oxidized and cyclic forms, 9OH-ABA and PA, as well as end-inactivated DPA, which is the most abundant fraction of the ABA-type group.
Under the diurnal 14/10-h LD photoperiod, all ABA-type compounds showed flat changes in daily values. There were no pronounced differences between light and dark periods and no changes after the light-to-dark dusk transition. ABA and PA showed typical ARELD response patterns, indicating that they recognize persistent light as stress. DPA showed a low ARELD-like response, but with a morning minimum. 9OH-ABA showed no changes during the prolonged day, but at a subjective LL dawn, its levels were significantly increased, in contrast to ABA and PA. To summarize, ABA and PA showed increased levels in response to the prolonged day, in contrast to 9OH-ABA, which showed a flat daily response with elevated levels only at LL dawn.
Jasmonates
The jasmonates in the sunflower samples were represented by five compounds: JA, JA-Ile, Ja-Me, dihydrojasmonic acid (DiH-JA), and cis-( +)-12-oxo-phytodienoic acid (cisOPDA). The changes in their daily concentrations are shown in Suppl. Fig. S2. All three most abundant jasmonate forms, JA, JA-Me, and DiH-JA, show typical ARELD response patterns with minor daily changes under the 14/10-h LD photoperiod, except for a very sharp increase toward the ARELD point. JA-Ile also shows a pronounced ARELD-like response, but with a peak at night, five hours from dusk. cisOPDA, a precursor of JA, shows a pattern that differs from that of other jasmonates. The ARELD response is absent in cisOPDA, and there is a sharp increase toward the end of the day and beginning of the night.
Cytokinins
The large group of sunflower CKs shows patterns of daily changes that differ markedly from those of other phytohormone groups (Suppl. Figs. S3, S4). None of the 22 CKs listed shows a typical ARELD pattern, although four members of the iP group (iP itself, its 9-riboside, and the 7- and 9-glucosides) exhibit concentrations that are higher during the extended daytime than at any other time during the 14/10-h LD photoperiod. Similarly, cis-zeatin 9-riboside-5’-monophophate (cZRMP) approaches the definition of an ARELD-type phytohormone but has a high peak at midday.
Most CKs have a maximum at midday, 7 h after dawn, followed by a sharp decline before dusk and/or a nighttime maximum 2 h after dusk. With a frequent minimum in the early morning, the CK daily patterns resemble the daily changes in hypocotyl elongation rates, indicating a possible involvement of CKs in hypocotyl elongation.
CKs can be subdivided according to their chemical structure (tZ-, cZ-, DZ-, iP- and MeS-types) or physiological function (bioactive, inactive, transport, storage, and phosphorylated forms representing immediate biosynthetic precursors).
It appears that sunflower cells maintain homeostasis of CKs by replenishing and deplating a large pool of CK storage forms. The most abundant CK form in sunflower is cis-zeatin O-glucoside (cZOG), a storage form present in amounts of about 1600–1800 pmol/g DW. Apparently, cZOG is strongly depleted at the beginning of the daytime, 4 h after dawn, and then during the night, peaking 2 h after dusk (Suppl. Fig. S3). Transferring this observation to shoot elongation, it appears that cZOG depletion precedes increased hypocotyl elongation during the day whereas at night it corresponds to the gradual nighttime decline in hypocotyl elongation rate.
After the CK O-glucosides, the second most abundant CK forms are the transport ribosides, with tZR being the leading form. They show peaks in the middle of the day and then 2 h after dusk at night, implying that they coincide with the maxima of hypocotyl elongation rates (Suppl. Fig. S4). Deactivation CK forms, N7- and N9-glucosides, are present at very low concentrations (not exceeding 2.7 pmol/g DW) and their occurrence is restricted to iP7-glucoside (iP7G), iP9G and DZ9G. They show three daily peaks with lower values at other time points. Two peaks are identical to the midday and early night maxima of transport ribosides mentioned earlier, and the third is a peak at dawn (Suppl. Fig. S4).
The group of CK phosphate precursors dominated by cZRMP is more or less flat during the day with a single prominent peak in the middle of the day (Suppl. Fig. S3).
The methylthiolated forms of CKs show a simple diurnal pattern with gradually increasing values during the day and more or less constant values at night (Suppl. Fig. S4). The dominant CK form in this group is 2-methylthiozeatin 9-riboside (MeS-ZR).
Finally, in the group of bioactive CK-free bases, iP is the predominant form, increasing during the day and night, with a marked decrease 10 h from dusk and at dawn (Suppl. Fig. S4). The other two bioactive forms, tZ and cZ, resemble phosphate precursors and both peak at midday, whereas dihydrozeatin (DZ) was not detected. All tZ-type CKs are elevated at the ZT1 morning time compared with the cZ-types.
Discussion
The collected data on the expression of clock TF genes showed that the circadian clock is functional in LL. Therefore, the change in hypocotyl elongation pattern induced by LL can be considered as a change in the output of the circadian clock rather than a failure of its rhythmic function. The really important question here is what drives the rhythmicity of the clock in LL when plants have never experienced light-to-dark period shifts. It is usually neglected today that besides hypocotyl elongation, there are other processes in sunflower seedlings, driven by strong diurnal rhythms. One of them is the upward water translocation (root to shoot) driven by root pressure (Vaadia 1960), which does not require light to start.
The shift in peak gene expression in LL conditions toward the end of the subjective night is not a new observation. A similar observation was reported for potato shoot cultures grown in LL conditions (Vinterhalter et al. 2019). The rhythmicity of the phytohormone daily levels in LL was maintained, but the peaks were shifted into the late subjective night.
Since sunflower exhibits dual (separate) elongation kinetics for day and night in LD photoperiods, the rapid slide into a uniform elongation pattern under free-running conditions simply means that one of the two effector pathways is temporarily out of use.
A direct relationship between circadian regulation and changes in the patterns of hypocotyl elongation and phytohormone production could not be established. The change in the elongation pattern occurred after a very short delay in the advance of nighttime, too short to expect a significant phytohormone change. However, the dramatic increase in the level of ARELD-type phytohormones recorded in the CP1 point after 10 h of extended daytime far exceeded any recorded values.
It is obvious that some phytohormones are adjusted by their metabolism to prefer certain light conditions, light or darkness, but high levels cannot be maintained indefinitely. Very high phytohormone levels are adjusted either upon return of expected but absent light conditions or by activation of the circadian clock resetting mechanism late in the subjective night.
Synchronization affecting the expected order of gene expression of clock components could be the result of adaptation to LL conditions based on convergence between light signal transduction pathways and circadian regulation, as shown in the case of Arabidopsis (Soy et al. 2016). These results fit well with the coincidence mechanism proposed by Nozue et al. (2007) to explain why the hypocotyl elongation of Arabidopsis growing under short-day conditions is directed toward dawn. A similar mechanism could operate in sunflower, but the problem is that the peaks of hypocotyl elongation in sunflower, unlike those of Arabidopsis, are at different times, one at midday of the daytime and the other at nighttime, two hours from dusk.
Convergence of light signal transduction in different plant species has been demonstrated for a number of phytohormones, including GAs (Achard et al. 2007), auxin (Xu et al. 2018), ABA (Xu et al. 2014; Bulgakov and Koren 2022) and stress phytohormones (ABA, SA, JA, BzA, ethylene), the members of which are strongly linked in their effects (Yang et al. 2019; Liu and Timko 2021).
The convergence of light signaling pathways of the major sunflower phytohormones that we present here may provide a better understanding of the internal hierarchical organization of phytohormones in future studies.
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Code Availability
Not applicable.
References
Achard P, Liao L, Jiang C, Desnos T, Bartlett FuX, Harberd NP (2007) DELLAs contribute to plant photomorphogenesis. Plant Physiol 143:1163–1172. https://doi.org/10.1104/pp.106.092254
Alabadí D, Oyama T, Yanovsky MJ, Harmon FG, Más P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293:880–883. https://doi.org/10.1126/science.1061320
Alabadi D, Gil J, Blázquez MA, García-Martínez JL (2004) Gibberellins repress photomorphogenesis in darkness. Plant Physiol 134:1050–1057. https://doi.org/10.1104/pp.103.035451
Ang L-H, Chattopadhyay S, Wei N et al (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1:213–222
Atamian HS, Creux NM, Brown EA, Garner AG, Blackman BK, Harmer SL (2016) Circadian regulation of sunflower heliotropism, floral orientation and pollinator visits. Plant Sci 353:587–590. https://doi.org/10.1126/science.aaf9793
Bhatnagar A, Singh S, Khurana JP, Burman N (2020) HY5-COP1: the central module of light signaling pathway. J Plant Biochem Biotechnol 29:590–610. https://doi.org/10.1007/s13562-020-00623-3
Bruinsma J, Karssen CM, Benschop M, Van Dort JB (1975) Hormonal regulation of phototropism in the light grown sunflower seedling, Helianthus annuus L.: Immobility of endogenous indoleacetic acid and inhibition of hypocotyl growth by illuminted cotyledons. J Exp Bot 26:411–441. https://doi.org/10.1093/jxb/26.3.411
Bulgakov VP, Koren OG (2022) Basic protein modules combining abscisic acid and light signaling in Asabidopsis. Front Plant Sci 12:808960. https://doi.org/10.3389/fpls.2021.808960
Casal JJ, Fankhauser C, Coupland G, Blazquez MA (2004) Signaling for developmental plasticity. Trends Plant Sci 9:309–314. https://doi.org/10.1016/j.tplants.2004.04.007
Chamowitz DA, Wei N, Osterlund MT, von Arnim AG, Staub JM, Matsui M, Deng XW (1996) The COP9 complex. A novel multisubunit nuclear regulator involved in light control of a plant developmental switch. Cell 86:115–121. https://doi.org/10.1016/s0092-8674(00)80082-3
Covington MF, Panda S, Liu XL, Strayer CA, Wagner DR, Kay SA (2001) ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell 13:1305–1315. https://doi.org/10.1105/tpc.13.6.1305
Davies PJ (2004) Plant hormones: biosynthesis, signal transduction, action! Davies PJ (ed) Kluwer Academic Publishers, Dordrecht, Netherlands. ISBN: 1–4020–2684–6 (hardcover) & 1–4020–2685–7 https://doi.org/10.1007/978-1-4020-2686-7_1
Dawson-Day MJ, Millar AJ (1999) Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J 17:63–71. https://doi.org/10.1046/j.1365-313x.1999.00353
Deng XW, Caspar T, Quail PH (1991) cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev 5:1172–1182. https://doi.org/10.1101/gad.5.7.1172
Galvão VC, Fankhauser C (2015) Sensing the light environment in plants: photoreceptors and early signaling steps. Curr Opin Neurobiol 34:46–53
Gangappa SN, Botto JF (2016) The multifaceted roles of HY5 in plant growth and development. Mol Plant 9:1353–1365. https://doi.org/10.1016/j.molp.2016.07.002
Gašić K, Hernandez A, Korban S (2004) RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol Biol Rep 22:437–437. https://doi.org/10.1007/BF02772687
Halliday KJ, Martinez-Garcia JF, Josse EM (2009) Integration of light and auxin signaling. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a001586
Huq E (2018) Direct convergence of light and auxin signaling pathways in Arabidopsis. Mol Plant 11:515–517. https://doi.org/10.1016/j.molp.2018.02.002
JingY LR (2020) Transcriptional regulatory network of the light signaling pathways. New Phytol 227:683–697. https://doi.org/10.1111/nph.16602
Kurepin LV, Walton LJ, Reid DM (2007) Interation of red to far red light ratio and ethylene in regulating stem elongation of Helianthus annuus. Plant Growth Regul 51:53–61
Lau OS, Deng XW (2010) Plant hormone signaling lightens up: integrators of light and hormones. Curr Opin Plant Biol 13:571–577. https://doi.org/10.1016/j.pbi.2010.07.001
Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, Quail PH (2008) Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol 18(23):1815–1823. https://doi.org/10.1016/j.cub.2008.10.058
Liu H, Timko MP (2021) Jasmonic acid signaling and molecular crosstalk with other phytohormones. Int J Mol Sci 22(6):2914. https://doi.org/10.3390/ijms22062914
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Ma L, Gao Y, Qu L, Chen Z, Li J, Den ZH, XW, (2002) Genomic evidence for COP1 as a repressor of light-regulated gene expression and development in Arabidopsis. Plant Cell 14:2383–2398. https://doi.org/10.1105/tpc.004416
Millar AJ (2003) Input signals to the plant circadian clock. J Exp Bot 55:277–283. https://doi.org/10.1093/jxb/erh034
Nawkar GM, Kang CH, Maibam P et al (2017) HY5, a positive regulator of light signaling, negatively controls the unfolded protein response in Arabidopsis. Proc Natl Acad Sci USA 114:2084–2089. https://doi.org/10.1073/pnas.1609844114
Ni M, Tepperman JM, Quail PH (1999) Binding of phytochrome B to its nuclear signaling partner PIF3 is reversibly induced by light. Nature 400:781–784. https://doi.org/10.1038/23500
Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN (2007) Rhythmic growth explained by coincidence between internal and external cues. Nature 448(7151):358–361. https://doi.org/10.1038/nature05946
Osterlund MT, Wei N, Deng XW (2000) The roles of photoreceptor systems and the COP1-targeted destabilization of HY5 in light control of Arabidopsis seedling development. Plant Physiol 124:1520–1524. https://doi.org/10.1104/pp.124.4.1520
Prerostova S, Černý M, Dobrev PI, Motyka V, Hluskova L, Zupkova B, Gaudinova A, Knirsch V, Janda T, Brzobohaty B, Vankova R (2021) Light regulates the cytokinin-dependent cold stress responses in Arabidopsis. Front Plant Sci 11:608711. https://doi.org/10.3389/fpls.2020.608711
Qin NX, Xu DQ, Li JG, Deng XW (2020) COP9 signalosome: discovery, conservation, activity, and function. J Integr Plant Biol 62:90–103. https://doi.org/10.1111/jipb.12903
Quail PH (2002) Phytochrome photosensory signaling networks. Nat Rev Mol Cell Biol 3:85–93. https://doi.org/10.1038/nrm728
Somers DE, Devlin PF, Kay SA (1998) Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282:1488–1490
Soy J, Leivar P, Gonzalesz-Schain N, Mertin G, Diaz C, Sentandreu M, Al-Sady B, Quail PH, Monte E (2016) Molecular convergence of clock and photosensory pathways through PIF3-TOC1 interaction and co-occupancy of target promoters. PNAS 113:4870–4875. https://doi.org/10.1073/pnas.1603745113
Vaadia Y (1960) Autonomic diurnal fluctuations in rate of exudation and root pressure of decapitated sunflower plants. Physiol Plant 13:701–717. https://doi.org/10.1111/j.1399-3054.1960.tb08092.x
Viczián A, Kircher S, Fejes E, Millar AJ, Schäfer E, Kozma-Bognár L, Nagy F (2005) Functional characterization of phytochrome interacting factor 3 for the Arabidopsis thaliana circadian clockwork. Plant Cell Physiol 46:1591–1602. https://doi.org/10.1093/pcp/pci175
Vinterhalter D, Savić J, Stanišić M et al (2019) Diurnal rhythmicity of endogenous phytohormones and phototropic bending capacity in potato (Solanum tuberosum L.) shoot cultures. Plant Growth Regul 90:151–161. https://doi.org/10.1007/s10725-019-00561-8
Vinterhalter D, Vinterhalter B (2021) Diurnal photoperiods and rhythmicity of the phototropic bending response in hypocotyls of sunflower, Helianthus annuus L. seedlings. Arch Biol Sci 73:237–246. https://doi.org/10.2298/ABS210205018V
Vinterhalter D, Vinterhalter B, Motyka V (2022) Periodicity and spectral composition of light in the regulation of hypocotyl elongation of sunflower seedlings. Plants 11(15):1982. https://doi.org/10.3390/plants11151982
Xiao Y, Chu L, Zhang Y et al (2022) HY5: A pivotal regulator of light-dependent development in higher plants. Front Plant Sci 12:800989. https://doi.org/10.3389/fpls.2021.800989
Xu DQ, Li JG, Gangappa SN, Hettiaracchchi C, Lin F, Andersson MX, Jiang Y, Den XW, Holm M (2014) Convergence of light and ABA signaling on the ABI5 promoter. PLoS Genet 10(2):e1004197. https://doi.org/10.1371/journal.pgen.1004197
Xu F, He SB, Zhang JG, Mao ZL, Wang WX, Li T, Hua J, Du S, Xu PB, Li L, Lian HL, Yanh H-Q (2018) Photoactivated CRY1 and phyB interact directly with AUX/IAA proteins to inhibit auxin signaling in Arabidopsis. Mol Plant 11:523–541. https://doi.org/10.1016/j.molp.2017.12.003
Yang J, Duan G, Li C, Liu L, Han G, ZaWang YC (2019) The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stress. Front Plant Sci 10:1349. https://doi.org/10.3389/fpls.2019.01349
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden T (2012) Primer–BLAST: a tool to design target–specific primers for polymerase chain reaction. BMC Bioinforms 13:134–145
Acknowledgements
We wish to thank Jelena Savić for suggestions related to qPCR-RT studies and Rade Stanisavljević for help in obtaining plant material.
Funding
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic from European Regional Development Fund-Project “TowArds Next GENeration Crops' (CZ.02.01.01/00/22_008/0004581) and by Serbian Ministry of Science, Technological Development and Innovations, grant 451-03-66/2024-03/200007.
Author information
Authors and Affiliations
Contributions
Development of the research concept and basic design—DV and VM; final manuscript preparation—DV and VM; project management—BV; plant material cultivation and growth analysis—DV and BV; gene expression studies—MB and JM; quantitative phytohormone analysis—PID, RF, VM. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
All authors worked on the final manuscript.
Consent for Publication
All authors agreed to publish the final manuscript.
Additional information
Handling Editor: Branka Salopek Sondi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vinterhalter, D., Vinterhalter, B., Milojević, J. et al. Light-Induced Changes in Phytohormone Levels of Developing Sunflower (Helianthus annuus L.) Seedlings. J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11321-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11321-y