Abstract
Postharvest senescence of cut flowers is a stumbling impediment in harnessing their commercial potential. Consequently, the postharvest quality preservation of cut flowers is a crucial factor to allure buyers and maximize economic gains. Flower senescence being final phase of organ development is a key factor triggering postharvest quality deterioration. The process of flower senescence is closely regulated by developmental and environmental cues. The perception of these signals subsequently involves loss of membrane integrity, decreased activity of antioxidant enzymes, and upregulation of proteases and nucleases, which are key signatures of senescence and culminate in the death of petal tissues. Moreover, the developmental and environmental cues are synchronized by considerable turnover in different growth regulators, particularly cytokinins, abscisic acid, ethylene, and gibberellic acid, which act both antagonistically and synergistically to coordinate the senescence process in flowers. Among these growth regulators, ethylene has a crucial role in orchestrating petal senescence in ethylene-responsive systems, while, abscisic acid regulates petal senescence in ethylene-independent systems. Recent research on ethylene-sensitive flowers revealed that the crosstalk of ethylene with sugars and other growth regulators plays a crucial role in modulating senescence by affecting the expression of ethylene-responsive genes. Despite the plethora of postharvest studies conducted so far, considerable miss links still persist in understanding the intricacies of senescence regulating mechanisms, mainly in ethylene-responsive flowers. To this end, it is imperative to critically re-evaluate our current understanding of ethylene-dependent flower senescence to gain intricate inputs regarding the underlying senescence mechanisms, particularly in ornamental families like Ranunculaceae. This constitutes the pivotal gateway toward deciphering the enigmatic complexities governing senescence regulatory mechanisms, thereby forging a path for postharvest researchers to craft pioneering methodologies aimed at accentuating the longevity of commercially significant flowers, thereby yielding substantial economic ramifications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flowers are incredibly intricate organs that have developed to improve the reproductive fitness of angiosperms. Petals being an integral part of flowers facilitate reproduction through pollination and eventually undergo senescence leading to the death of these tissues (Ma et al. 2018). Senescence is an active and crucial aspect of flower development, designed to maximize nutrient recycling from senescing petals to young parts of a flower. Flowers offer excellent model systems for senescence investigations as they possess uniform tissue, short lifespan, and easily manipulable chemically without suffering significant damage (Wagstaff et al. 2002). Petals, originated from leaves through an evolutionary process, manifest analogous physiological and biochemical characteristics during senescence, entailing disintegration of intracellular structures and degradation of membranes and macromolecules, along with salvage of essential substances. (Xu and Hanson 2000; Friedman et al. 2004). However, petal senescence varies from leaf senescence in certain aspects. Firstly, petal senescence is irreversible and progresses at different rates in the components of the flower, while still interconnected to each other. Leaf senescence on the other hand is reversible up to the “point of no return.” In contrast to leaf senescence which is strictly regulated by environmental cues, petal senescence is particularly regulated by developmental signals, like pollination, fertilization, and fruit formation (Dar et al. 2014; Ahmad and Tahir 2015). Secondly, nutrient remobilization is of less importance in petals as they act as sinks, whereas nutrient remobilization is of key importance in leaves being active source organs performing photosynthesis (Chapin and Jones 2007a, b; Jones 2013; Maillard et al. 2015). Thirdly, petal senescence is relatively rapid as compared to leaf senescence, even in some species abscission of petals occur while still fresh (Woltering and van Doorn 1988; Yamada and Ichimura. 2007). The onset of petal senescence encompasses a multitude of physiological and biochemical changes, like dehydration of senescent tissues, increase in membrane fluidity, generation of reactive oxygen species (ROS), lipid peroxidation, and degradation of proteins and carbohydrates. (Tripathi and Tuteja 2007). These changes are orchestrated by turnover in different plant growth regulators especially, ethylene, abscisic acid, cytokinins, and gibberellic acid. Among these, ethylene and abscisic acid act synergistically to accentuate senescence (Costa et al. 2016; Dar et al. 2021), while cytokinins and gibberellic acid retard this process (Kumar et al. 2014; Lone et al. 2021a, b). Depending on their response to exogenous ethylene, the flowers may exhibit ethylene-sensitive or ethylene-insensitive senescence. Treatment of ethylene-responsive flowers with ethylene antagonists like silver thiosulfate has been found to delay the instigation of senescence in Clarkia pulchella, likewise, application of GA3, an ABA antagonist reduced the impact of ABA action in Gladiolus grandiflora (Kumar et al. 2014; Dar and Tahir 2018). Besides hormonal regulation, several marker genes like ETR1, DAD1, and SAG12 act as key regulatory switches during the process of flower senescence. The accumulation of SAG12 transcripts in senescent tissues of Nicotiana mutabilis accelerates senescence by facilitating protein degradation (Jones et al. 2005). Meanwhile, DAD1 and ETR1 genes were upregulated during the open stage of flower development in Petunia hybrida flowers. DAD1 is anti-senescent which encourages the glycosylation of nascent proteins to prevent their breakdown (Jeong et al. 2018). DAD1 transcripts accumulate at higher levels in fresh petal tissues as observed in Petunia hybrida flowers, whereas higher transcript levels of ETR1 signpost its potential role in the perception of ethylene signals (Nisar et al. 2021a, b). Modulating the expression levels of important genes such as cysteine protease and DAD1 through postharvest treatments and biotechnological intervention may be an effective approach to enhance the longevity of significant cut flowers. Application of CaCl2 retarded the expression of the cysteine protease gene, while the concomitant upregulation of the DAD1 gene considerably improved the longevity in Gladiolus flowers (Sairam et al. 2012). Furthermore, altering ethylene output by reducing the expression levels of ACC synthase and ACC oxidase can act as a breakthrough in augmenting the postharvest longevity of commercially important ornamentals as realized in Dianthus caryophyllus (Savin et al. 1995). In this perspective, the current review focuses on understanding hormonal crosstalk during flower senescence and sheds light on recent developments in the modulation of senescence mechanisms through postharvest treatments and biotechnological approaches. All these strategies have considerable future technological implications for improving the postharvest longevity of commercially important ornamentals, often judged an extremely important parameter in assessing their quality.
From Order to Organized Disorder: How the Collapse of Structural Integrity Triggers Petal Senescence?
Petal senescence encompasses subtle changes in the structure of petal tissues. Modes of petal senescence vary widely between species. In some species, the petal senescence involves wilting followed by the abscission of floral tissues, while in others, the petals abscise while still turgid. The petals that abscise without undergoing wilting are generally sensitive to ethylene, whereas the petals that first undergo wilting and are eventually shed off, are usually non-responsive to ethylene. However, in some ethylene-insensitive flower systems, such as tulips, petals fall off before wilting (Van Doorn 2001). Senescence in petal cells includes both structural and metabolic transformations, along with the activation of catalytic machinery, which fosters nutrient recovery amassed during the growth phase (Rogers 2013; Shibuya and Ichimura 2016). The maintenance of the structural integrity of cells is vital for their survival and performance of metabolic processes (Ahmad and Tahir 2016a, b). Cell wall degeneration besides turgor loss and tonoplast rupture could be one of the factors contributing to cell death (Shibuya et al. 2016). In ethylene-sensitive flowers, ethylene and auxin mediate the abscission of petal tissues, while in ethylene-insensitive systems, INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) peptide and HAE-HSL2 kinases mediate abscission of petal tissues. These genes encode for cell wall-degrading enzymes which lead to the abscission of floral organs (Meir et al. 2019). Studies on senescing petals of Dianthus and Ipomea have reported a decrease in cellulose and hemicellulose content in cell walls (Wiemken-Gehrig et al. 1974; De Vetten and Huber 1990). Cell wall degeneration has been attributed to the discharge of hydrolytic enzymes into the cell wall, before cell death. In Iris and Dendrobium flowers, swelling of cell walls has been observed in the senescing mesophyll cells. This swelling is ascribed to vesicle formation between the plasma membrane and cell wall, which secrete hydrolytic enzymes, resulting in cell wall degradation (Kamdee et al. 2015). After cell wall degradation, the integrity of the membrane is compromised, which is unraveled through the microscopic investigations of senescent cells. This loss of integrity impairs their prime functions (Yamada et al. 2009; Shahri and Tahir 2011a). Senescence causes an upsurge in the activity of phospholipases and acyl hydrolases and replaces phospholipids with neutral lipids thereby increasing the membrane outflow. The loss of membrane phospholipids is a crucial index of lipid metabolism in senescing petals (Rubinstein 2000; Van Doorn and Woltering 2008). The loss of membrane integrity is followed by subtle changes in cytoplasm, which undergoes structural alterations characterized by the accumulation of electron-dense globules on the surface of the plastids. These globules are thought to originate from plastoglobules that form within the plastid (Mulisch and Krupniska 2013). These globules consisting of proteins and lipids increase in size toward the senescent stage as observed in the epidermal cells of Iris petals. Reportedly, these enlarged globules participate in the degradation of plastids by breaking down proteins in the proteasome and lipids in the peroxisome (Shibuya et al. 2016). In addition, intercellular connections, such as plasmodesmata, involved in the movement of RNA, carbohydrates, and phytohormones also get closed before the onset of flowering. The closure of plasmodesmata is the primary structural modification as noticed in the petal cells of Iris, which prevents the transport of sugars into cells, ultimately causing their demise due to the exhaustion of ATP (Van Doorn et al. 2003). In addition, autophagic structures have been noticed in petal cells of Ipomea, Dianthus and Hemerocallis, which contain acid phosphatases and other hydrolytic substances and function as lytic vacuoles. (Phillips and Kenede 1980; Smith et al. 1992; Stead and Van Doorn 1994; Marty 1999). These vacuoles increase in size by combining with other vacuoles and participate in cytoplasmic degradation. In addition plastids and mitochondria of senescing petals also function as lytic vacuoles (van Doorn et al. 2011). The plastids of Dendrobium petals plastids have been found to degrade the engulfed portion of cytoplasm owing to their intrinsic proteolytic and lipolytic activity (Kato et al. 2005; Barsan et al. 2010). The nucleus is the sole cell organelle that survives till the last stage of flower senescence. It undergoes various morphological changes, like nuclear fragmentation and chromatin condensation. Chromatin condensation is followed by DNA degradation due to the depolymerization of F-actin, which is the most commonly assessed criterion during petal senescence. The process of petal senescence has been linked to DNA fragmentation (Wagstaff et al. 2003; Arora and Singh 2006; Yamada et al. 2006). In case of pea petals, DNA degradation is characterized by a laddering pattern of DNA fragments on the agarose gel. Moreover, the application of Ca2+ has been found to upregulate Dnase activity and hence DNA laddering. DNA degradation being a hallmark of PCD is also imitated by the emancipation of Cyt c from the inner membrane space of mitochondria since the timing of this release of Cyt c is coupled with the initiation of DNA laddering (Xu and Hanson 2000).
What Causes a Flower to Die?
Flower senescence is a complex tripartite process involving initiation, disassembly, and nutrient remobilization. The disassembly phase entails the breakdown of structural integrity, giving way to prominent biochemical transformations, including proteolysis, nucleic acid degradation, carbohydrate catabolism, lipid peroxidation, and compromised activity of antioxidant enzymes. These biochemical alterations collectively facilitate the efficient transfer of nutrients from petal tissues to sink tissues. The key biochemical events are described as follows:
Senescence and Proteolysis: Two Processes, Single Destiny
Proteolysis being a hallmark of petal senescence fosters protein degradation and enables the transfer of amino acids to the sink (Solomon et al. 1999). The protein content of petal tissue acts as a decisive factor in flower senescence, as the decrease in protein concentration truncates the vase life of cut flowers (Rezvanypour and Osfoori 2011). Proteins not only improve relative water content but also act as a substitute energy source during sugar depletion (Shan and Zaho 2015; Hirota et al. 2018). Protein enrichment improves the performance of antioxidant enzymes and encourages the production of stress-specific proteins that stimulate defense systems, leading to an increased ability to resist postharvest deterioration and thus extending the postharvest longevity (Doganlar et al. 2010; Promyou et al. 2012). The characteristic feature of senescence is elevated proteolytic activity due to an upsurge in the expression of the cysteine protease gene (Jones et al. 2005). Earlier studies linked the cysteine protease gene with the petal senescence of ethylene-sensitive flowers (Jones et al 1995). However, later findings confirmed its role in insensitive flowers as well (Wagstaff et al. 2002; Arora and Singh 2004; Pak and Van Doorn 2005). Proteases being abundant and best characterized cell death proteins hydrolyze proteins by internal peptide bonds (Beers et al. 2000). Protein degradation takes place in multiple organelles, such as proteasomes, vacuoles, mitochondria, and the nucleus, but the majority of it ensues within vacuoles. Proteasomal-based degradation involves the breakdown of misfolded proteins via ubiquitination (Van Doorn and Woltering 2008). Delay of senescence signs via silencing of the Ring domain of E3 protein in petunia and chemical inhibition of proteases in Iris indicates the implication of Proteasomal action in petal senescence (Xu et al. 2008). Proteins are also degraded independent of Proteasomal action in vacuoles, mitochondria, plastids, and the nucleus. However, the bulk of protein degradation occurs in vacuoles as most of the proteases are localized in them (Van Doorn and Woltering 2008).
Sugar Starvation and Flower Senescence
Sugars act as an important energy source to maintain cellular homeostasis and regulate water levels by elevating the concentration of osmotic solutes (Van Doorn 2004; Van Doorn and Woltering 2008). Depletion of sugars is regarded as the key feature of senescent petal tissues, orchestrating petal senescence either by regulating metabolites or by modulating the action of ethylene Van Doorn 2004; Van Doorn and Woltering 2008; Shahri et al. 2010). Sugars are recognized for their ability to enhance the postharvest quality of cut flowers and prolong their vase life (van Doorn 2004; Eason 2006). Detachment of flowers from their mother plant truncates their carbohydrate supply, subsequently triggering physiological alterations that eventually lead to cell death. (Halevy and Mayak 1979). Also, cut flowers cannot assimilate sufficient carbon as they receive light intensity below the compensation point, hence leading to fast depletion of carbohydrate reserves (Pun et al. 2016). Sugar starvation fosters protein and lipid breakdown which act as alternate respiratory substrates for sustaining key physiological processes. Low sugar content also induces expression of senescence-associated genes which are otherwise suppressed by sugars (Morkunas et al. 2012). Sugars and sugar-metabolizing enzymes effectively counter oxidative stress (Bolouri-Moghaddam et al. 2010). The synergistic interaction of sugars and sugar-like compounds including phenols form an important part of the redox system, which quenches ROS and therefore confers stress tolerance. Sugars being important signaling molecules perform differently in different flower systems. In ethylene-sensitive flowers, like Dianthus, sucrose treatment delays the climacteric rise in ethylene by reducing the expression of ACC synthase and ACC oxidase, thereby suppressing ethylene biosynthesis (Pun et al. 2016). Likewise, glucose and mannitol treatments prevented the abscission of flowers in Delphinium (Ichmura et al. 2000). Thus, sugars serve as important energy substrates besides modulating the ethylene pathway, thereby regulating petal senescence.
From Bright to Bleach: Anthocyanin Degradation
Discoloration and color fading are core causes of quality deterioration in cut flowers. The primary pigments contributing to the vibrant color of the flowers include anthocyanins, carotenoids, and betalains. Anthocyanins being the largest class of plant pigments form red, violet, and blue colors. The accumulation of anthocyanins is determined by the degree of expression of various biosynthetic genes, like Chalcone synthase (CHS), chalcone isomerase (CHI), phenylalanine ammonia-lyase (PAL), and flavonoids 3-hydroxylase (F3H). Studies conducted on Petunia, Malus, Antirrhinum, and Rosa have revealed that the expression of these genes peaks in the initial stages of flower development and declines toward advanced stages (Cavaiuolo et al. 2013). The color formation by anthocyanins is under the regulation of pH. Under low pH, anthocyanins produce red color while at high pH, anthocyanins produce blue color (Avila-Rostant et al. 2010). In Ipomea tricolor, flower petals are characterized by low pH upon bud opening, but as the development proceeds the pH increases resulting in the blue color of petals (Yoshida et al.1995). Besides pH, high temperatures and low light also reduce the pigment level in petals as a result of degeneration and downregulation of anthocyanin biosynthetic genes (Gonzalez 2009). In ethylene-sensitive flowers, exogenous ethylene inclusion induced color fading in Vanda flowers. Ethylene-induced color bleaching was due to an increase in peroxidase activity, responsible for anthocyanin degradation. Pretreatment of spikes with 1-MCP (1-Methylcyclopropene) prevented color blenching and degradation of anthocyanins. Moreover, MCP-treated inflorescences exhibited vibrant color of petal tissues relative to control (Khunmuang et al. 2019).
Lipid Peroxidation and Loss of Membrane Integrity
Petal senescence involves significant structural and biochemical changes in the cell membrane. The key biochemical changes include loss of membrane phospholipids and increase in neutral lipids and sterol to phospholipid ratio, as well as an increase in the ratio of saturated to unsaturated fatty acids (Lesham 1992; Thompson et al. 1998). This alteration in membrane constituents are induced by membrane-degrading enzymes, like phospholipase C, Lipolytic acyl hydrolase, and Lipoxygenase, which are localized in membrane microsomes. Membrane lipids particularly polyunsaturated fatty acids are prone to oxidation by enzymatic means, like lipoxygenase (LOX). Lipoxygenase activity is positively correlated with membrane damage and promotes senescence in flowers, like Gladiolus (Peary and Prince 1990; Hossain et al. 2006). Membrane degradation causes ion leakage and the release of hydrolytic enzymes from the vacuoles to induce the breakdown of cellular components and hampers the exchange of metabolites as signaling molecules between neighboring cells. Thus, the collapse of the tonoplast and subsequent execution of cell death as a result of mass lipid degradation during senescence may be caused by membrane degradation. Besides degrading membrane lipids, lipid peroxidation produces many toxic aldehydes and ketones resulting in the breakdown of macromolecules (Wilhelmova et al. 2006). Membrane breakdown is directly linked to free radical production induced by ethylene (Carlos et al. 1996). Studies conducted in Dianthus revealed that inhibition of ethylene production improves vase life by preventing lipid peroxidation, indicating a link between ethylene production and free radical generation (Brochov et al. 1997). Previous investigations demonstrated that treatment of cut carnations with sodium benzoate (free radical scavenger) can improve their vase life by inhibiting ethylene burst (Baker et al. 1977). In addition, alteration in membrane fluidity of microsomes is linked with an increase in superoxide radical production, wherein exogenous application of free radical scavenger, propyl gallate prevented such changes in microsomes (Mayak et al. 1983). Thus, ethylene is a potent inducer of membrane degradation in ethylene-sensitive flowers.
Loss of Synchronization Between ROS Production and Antioxidant Machinery
The aging process in plants, including their individual parts, often involves oxidative stress caused by the abundant production of reactive oxygen species (ROS such as superoxides (O2–) and hydrogen peroxide (H2O2) by the cells (Saeed et al. 2014). The primary sources of ROS in petals are believed to be peroxisomes, mitochondria, and the apoplast. To manage ROS levels during senescence, plants produce antioxidant compounds and increase the activity of antioxidant enzymes. Non-enzymatic antioxidants such as ascorbic acid and tocopherol are vital ROS regulators. Studies have demonstrated that ascorbic acid plays a crucial role in regulating genes associated with senescence. A reduction in ascorbic acid levels has been found to induce senescence by increasing the expression of senescence-associated genes (Barth et al. 2004). Tocopherols, which are linked to thylakoid membranes, protect cells against oxidative stress by eliminating ROS, primarily by impeding the spread of lipid peroxidation via scavenging of lipid peroxyl radicals (Falk and Munné-Bosch 2010). In addition, phenolic compounds including anthocyanins and flavonols act as important antioxidant compounds in plants. Flavonols have been found to protect the flowers from the harmful exposure of UV-B rays, thus preventing ROS generation, while anthocyanins have been found to improve the longevity of flowers, like Petunia and Hibiscus (Kumar et al. 2008; Trivellini et al. 2007). During senescence, plant cells stimulate different ROS scavenging enzymes by activating specific signaling pathways, which include superoxide dismutase (SODs), catalase (CATs), and ascorbate peroxidase (APX) (Rogers 2012; Kou et al. 2014). The activity of SOD and CAT is important for stabilizing the lipid bilayer and averting the oxidation of unsaturated fatty acids (Saeed et al. 2014). In Iris versicolor, a decrease in antioxidant enzyme activity has been found to induce senescence (Ahmad and Tahir 2016a, b), while an increase in the activity of these antioxidant enzymes has been shown to improve the vase life of Consolida ajacis (Haq et al. 2022a, b). Thus, during the onset of aging process, the antioxidant defense system is upregulated. However, the production of ROS beyond the scavenging capacity of an antioxidant system leads to oxidative damage, which ends up in the death of petal tissue. All these events are summarized in Fig. 1.
Modulation of Ethylene Pathway for Extending the Longevity of Climacteric Flowers
The longevity of flowers is a crucial component in quality evaluation. Therefore, extending vase life is an important factor to convince customers to repurchase flowers (Vehniwal and Abbey 2019). Vase life is considered to be terminated with the appearance of senescence symptoms, like wilting, color fading, and abscission of petals (Olsen et al. 2015). In cut flowers particularly in ethylene-responsive systems, application of anti-ethylene treatments has been found to improve the vase life to a greater extent; however, a more viable approach to enhance the quality of cut flowers is the exploitation of alternative means ranging from conventional cross-pollination to advanced molecular methods, which involve direct modulation of the ethylene biosynthetic pathway (Table1). In Dianthus caryophyllus, repeated crossing doubled the flower longevity (Onozaki et al. 2011). The expression analysis revealed that the ethylene biosynthetic enzyme DcACS1 and DcACO2 exhibited low expression in these cultivars due to repeated crossing and selection, hence, improved longevity due to low ethylene production (Tanase et al. 2013). Moreover, hybridization through the interspecific crossing resulted in heterogeneous progeny with higher ethylene tolerance has been achieved in various Leptospermum species and Grevillea and Chamelaucium species (Bicknell 1995; Beal and Joyce 1999; Macnish et al. 2004). However, a significant drawback of this method is that it is not a practical strategy for improving existing cultivars, as substantial backcrossing with the parent plant may be required (Shibata 2008). Inducing mutations through irradiation is another viable approach to modulate ethylene output. Mutations induced in Lotus japonica by carbon ion beam significantly improved the flower longevity. These mutations altered the ethylene biosynthetic pathway and resulted in minimal ethylene production (Du et al. 2021). Besides irradiation, a more sophisticated way to introduce mutations in the desired genes is the CRISPR/Cas9 technology. This technology holds potential to edit the gene loci involved in ethylene biosynthesis as achieved in Petunia and ensures stable transmission of mutant alleles to the subsequent generations (Xu et al. 2020). Moreover, genetic engineering has significantly focused on targeting the ethylene pathway to enhance the lifespan of cut flowers. The transfer of mutated ethylene receptor gene etr1 from Arabidopsis thaliana in various ornamental plants, such as Campanula carpatica, Chrysanthemum, Kalanchoe blossfeldiana, and Pelargonium zonale, reduced their susceptibility to ethylene (Winkelmann et al. 2016; Gehl et al. 2018). In addition to receptors, the longevity of flowers has been improved by targeting specific elements in the ethylene signaling pathway, such as EIN2 and EIN3 (Shibuya et al. 2004; Shibuya and Clark 2006). Furthermore, inhibition of ethylene production in carnation by transforming with antisense ACC oxidase gene has proven to be effective (Savin et al. 1995). It is noteworthy that the ethylene sensitivity can also be altered by increasing cytokinin levels, through transformation with cytokinin biosynthesis gene PSAG12-IPT as achieved in petunia (Chang et al. 2003a, b). Thus, manipulation of the ethylene pathway presents a substantial avenue for enhancing postharvest quality in climacteric ornamental plants.
Conventional Ethylene Antagonists and Their Cost-Effective and Eco-Friendly Substitutes
Ethylene is a key hormone modulating senescence in ethylene-sensitive flowers. The ability of ethylene to induce flower senescence has developed naturally as a way to aid changes brought in by pollination (Chapin and Jones 2007a, b). Depending upon the mode of senescence, petal senescence can be ethylene regulated or ethylene independent (Dar et al. 2021). The response of climacteric flowers to ethylene varies among species and varieties (Wu et al. 2017). In ethylene-sensitive flower systems, an upsurge in endogenous ethylene triggers senescence and the exogenous application of the same speeds up the process. On the contrary, ethylene hardly influences senescence in ethylene-independent flowers. Non-climacteric flowers typically produce little ethylene and do not respond to exogenous treatment (Wang et al. 2020a, b). However, the interaction of ethylene with other hormones either promotes or hinders its production or action (Iqbal et al. 2017; Shibuya 2018). The ethylene biosynthesis pathway involves the following steps.
L-methionine → S-adenosyl-L-methionine (SAM) → 1-aminocyclopropane-1-carboxylate (ACC) → Ethylene (CH2 = CH2).
The enzymes accountable for ethylene biosynthesis are ACC synthase (ACS) and ACC oxidase (ACO). ACS converts SAM to ACC, while ACO converts ACC to ethylene. These genes have been identified in different flowers (Shibuya and Ichimura 2016). The mRNA transcripts of these genes are inversely related to the postharvest life of ornamental flowers (Tanase and Onozaki 2016). The ethylene signal is detected by the ETR1 receptor, a negative regulator of the ethylene response pathway. ETR1 then transmits the signal to downstream signaling components, such as CTR1, EIN2, EIN3, and ERF’S. These signaling components induce the expression of ethylene-responsive genes, such as proteases and lipoxygenases (Ceusters and Van de Poel 2018), which trigger the breakdown of proteins and membrane lipids, leading to the initiation of senescence (Hong et al. 2000; Jones et al. 2005). Ethylene burst also leads to respiratory upsurge which induces the degradation of carbohydrates and thus truncates vase life of flowers (Gonzalez-Candelas et al. 2010). However, exogenous sourcing of sugars such as glucose, trehalose, and mannitol accentuated the vase life of ethylene-responsive flowers, like Delphinium and Dianthus (Ichimura et al. 2000; Dar et al. 2014). Molecular studies revealed that sucrose treatments reduced ethylene production by curtailing ACO and ACS transcripts in climacteric flowers (Pun et al. 2016). Apart from sucrose, application of anti-ethylene substances like silver nanoparticles (Nag), aminooxyacetic acid (AOA), silver thiosulfate (STS), and 1-methylcyclopropene (1-MCP) treatments considerably diminished the deleterious effects of ethylene by inhibiting the accumulation of ACS and ACO transcripts in Dianthus and hybrids of Mokara Orchid (Naing et al. 2021; Wongjunta et al. 2021). Interestingly salicylic acid, a phenolic compound, has been suggested as a potential postharvest therapy to extend the lifespan of several types of cut flowers, including Rosa hybrida, Dianthus caryophyllus, Lillium pumilum, and chrysanthemum (Phi et al. 2021). Salicylic acid regulates petal aging in Nicotiana plumbaginifolia by enhancing the sugar and protein content of the petals. (Nisar et al. 2021a, b). In addition, molecular studies revealed that salicylic acid enhances the vase life by the diminishing activity of ACC oxidase besides, conferring membrane stability and improving water uptake (Kazemi et al. 2012). Convincingly, studies conducted on various cut flowers suggested a considerable role of Nitric oxide (NO) in mitigating postharvest senescence by multiple modes of action (Haq et al. 2021). Nitric oxide given as sodium nitroprusside (SNP) sustains optimal hydration of flower petals and inhibits the peroxidation of membrane lipids to minimize membrane effusion (Hassan et al. 2020). NO mitigates senescence in both ethylene-responsive as well as non-responsive systems. In ethylene-independent systems, NO ameliorates display life mainly by scavenging reactive oxygen species, while in ethylene-responsive systems, it accentuates the longevity of cut flowers by reducing the ethylene production (Liao et al. 2013; Naing et al. 2017; Lone et al. 2021a, b). Quite recently, role of novel postharvest treatments such as selenium and hydrogen nanobubble has been implicated in the regulation of postharvest senescence particularly in ethylene-sensitive systems. In climacteric flowers, endogenous ethylene production inhibits cell expansion via regulation of aquaporins involved in water transport through the cell membrane (Ma et al. 2008; Xue et al. 2020). Consequently, this results in a negative water balance, which limits the postharvest longevity of cut flowers (Van Meeteren and Aliniaeifard 2016). Moreover, ethylene-induced respiratory upsurge depletes primary respiratory substrates like carbohydrates and leads to oxidative stress via ROS production (John-karuppiah and Burns 2010; Bayanati et al. 2019). On the contrary, application of Selenium (Se) downregulated ethylene biosynthetic genes, which in turn inhibited the initiation of senescence-associated events, like respiratory upsurge, sugar depletion, ROS production, and petal wilting (Costa et al. 2020). Thus, Se can offer an economically viable and environmentally acceptable alternative to the existing conventional ethylene antagonists used for ethylene-sensitive cut flowers. Hydrogen-rich water (HRW) is another novel postharvest treatment reported to alleviate the deleterious effects of ethylene on cut flowers. Application of HRW reduces the accumulation of 1-aminocyclopropane-1-carboxylic acid (ACC) as well as inhibits the activity of ACC synthase (Wang et al. 2020a, b). HRW also prevents the degradation of proteins and nucleic acids by inhibiting proteases and nucleases, the key executioners of senescence (Li et al. 2021). HRW can accentuate the longevity of cut lilies by stimulating NO, which acts as a downstream signaling molecule (Huo et al. 2018). This suggests that HRW can not only reduce ethylene production but can serve as a strong inhibitor of nucleases and proteases besides, activating signaling molecules like NO which can ameliorate the display life of precious ornamentals. Boric acid is yet another cost-effective and eco-friendly substitute for conventional ethylene antagonists, like Silver thiosulfate (STS). Boric acid as a vase solution has been reported to preserve the quality of Tuberose and Jasmine cut flowers by delaying senescence symptoms (Manimaran et al. 2018; Baidya et al. 2020). Exogenous inclusion of Boric acid not only impedes ethylene biosynthesis but also improves other biochemical attributes, like proteins and sugars (Farooq et al. 2021a, b). Besides boric acid, ethanol is also used in the preservation of postharvest quality of horticultural produce owing to its safe and eco-friendly properties (Lin et al. 2020). Exogenous inclusion of ethanol has been found to improve the longevity of various cut flowers, effected by the ethylene and water stress (Pun et al. 2013; Sadeghi and Hashemabadi 2016). Ethanol being antimicrobial and anti-ethylene compound prevents microbial proliferation in vase solutions and impairs the activity of ACC synthase. Thus, ethanol reduces xylem occlusion and ethylene biosynthesis as reported in Alstroemeria and Rosa (Yaghoubi and Yadegri 2016; Manzoor et al. 2021; Nekouyar and Emani 2022). The aforementioned ethylene antagonists and their mechanism of action has been summarized in Table 2.
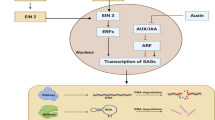
Synergistic or Antagonistic: Crosstalk of Ethylene with Other Growth Regulators During Petal Senescence
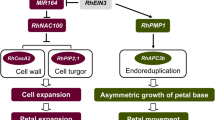
Petal senescence being a tightly regulated complex biological process is modulated by several phytohormones. There is a complex interplay between different growth regulators during petal senescence. Usually, cytokinins have an inhibitory effect on senescence, whereas ethylene and ABA show a stimulatory effect (Ma et al. 2018; Chen et al. 2018a, b). The role of auxins concerning petal senescence is somewhat contradictory and elusive. Treatment of carnation petals with auxin-induced senescence by stimulating ethylene production, while as auxins delayed senescence in Delphinium by reducing the ethylene sensitivity (Wulster et al.1982; Jones and Woodson 1999). Senescing carnation petals indicated a transient spike in the mRNA abundance of an AUX/IAA gene, whereas Aux/IAA genes were shown to be downregulated in Mirabilis jolapa during petal senescence (Xu et al. 2007; Price et al. 2008). The instigation of senescence in some flowers like Hemerocallis, Ipomoea, Ranunculus, and Lilium longiflorum has been reported to occur without any alterations in endogenous levels of auxin (Ahmad and Tahir 2016a, b). Gibberellic acid (GA) is known to act as an ethylene antagonist and thus delays senescence in some cut flowers, like roses and carnations (Chen et al. 2021). Gibberellic acid-mediated delay in senescence can be attributed to the decrease in climacteric ethylene production (Saks et al. 1992). GA treatment has been found to reduce the accumulation of ACC in carnation petals (Ma et al. 2018). Senescence in rose petals was expedited by silencing the GA20 oxidase gene RhGA20ox1 involved in GA biosynthetic pathway (Lü et al. 2014). Gibberellic acid delayed senescence in peony cut flowers via reduction in expression of MYB transcription factor gene PlMYB308, which otherwise leads to the accumulation of ABA and ethylene (Ji et al. 2022). The application of GA3 considerably improved the postharvest attributes and activity of antioxidant enzymes in non-climacteric gladiolus flowers by antagonizing ABA effects (Costa et al. 2016). However, GA treatment does not affect endogenous ACC levels or the longevity of ethylene-sensitive flower Grevillea (Irving et al. 2006). Thus, anti-aging effect of GA cannot be generalized but seems to be species specific.
Polyamines (PAs) have a vital role in plant juvenility by fostering cell proliferation, growth, and the postponement of senescence (Galston and Kaur-Sawhney 1995). The most prevalent polyamines in plants include putrescine, spermidine, and spermine. The interaction between ethylene and polyamines seems to regulate floral growth and senescence. More specifically, the balance between these two plant hormones determines whether the process of senescence will occur or not (Valero 2009). Polyamines are purported anti-senescent because of their ability to prevent senescence in climacteric flowers like Dianthus and Nicotiana by inhibiting ethylene biosynthesis (Ahmad and Tahir 2016a, b). Polyamines and ethylene compete for the same precursor S-adenosyl methionine (SAM). Therefore, an upsurge in polyamine biosynthesis limits the biosynthesis of ethylene, hence delaying the execution of senescence. Thus, owing to their common precursor SAM, ethylene and polyamines exhibit antagonistic roles in relation to petal senescence (Davarynejad et al. 2021). Application of polyamines prevents protein breakdown by downregulating protease activity and stabilizing proteins via binding of amine groups (Nisar et al. 2015). Polyamines also maintain the membrane integrity of petal tissue by attenuating lipoxygenase activity, hence preventing lipid peroxidation (Yousefi et al. 2019). Given the available data, polyamines are known to play a significant role in climacteric flowers, but their function in non-climacteric systems remains unclear. Abscisic acid (ABA) is believed to be the main regulator of aging in non-climacteric flowers (Da Costa et al. 2016). Investigations carried out on Narcissus pseudonarcissus indicated that the conversion of carotenoids to ABA leads to an increase in ABA concentration during flower senescence (Hunter et al. 2002). Treatment with ABA hastened the senescence process in Gladiolus by reducing water uptake and fresh weight and enhancing membrane seepage (Kumar et al. 2014). In climacteric flowers, ABA accelerates senescence by stimulating ethylene production or by increasing sensitivity to ethylene (Ronen and Mayak 1981; Muller et al. 2000). On the contrary, application of ABA in Hibiscus rosa-sinensis masked the expression of ethylene perception (HrsETR and HrsERS) and ethylene biosynthetic genes (HrACS and Hr ACO) (Trivellini et al. 2011). Silencing of PhHD-Zip in petunia decreased the expression of ethylene biosynthetic genes and curtailed ethylene output. Decrease in ethylene output impaired the expression of the ABA biosynthesis gene NCED (9-cis-epoxycarotenoid-dioxygenase). This indicates mediation of crosstalk by PhHD-Zip between ABA and ethylene (Chang et al. 2014). Cytokinins act as anti-senescent agents both in ethylene-responsive as well as ethylene-independent flower systems. In ethylene-independent flowers, cytokinins defer senescence by impeding ABA synthesis, while as in ethylene-responsive systems, cytokinins prevent senescence instigation by reducing ethylene output (Van Doorn and Woltering 2008; Trivellini et al. 2015). Application of cytokinin inhibits the initiation of senescence by maintaining sugar and protein content and reducing the membrane outflow of petal tissues (Iqbal et al. 2017; Chen et al. 2018a, b). In Nicotiana plumbaginifolia, exogenous inclusion of cytokinins retards the senescence execution by improving the activity of antioxidant enzymes (Tahir et al. 2018). Several studies have reported a negative correlation between senescence instigation and the cytokinins content (Hönig et al. 2018). On the contrary, increase in ethylene content has been positively correlated with cytokinin breakdown via O- glycosylation, thereby inducing senescence (Chang et al. 2003a, b). Isolated petunia petals treated with cytokinins exhibited decreased ethylene sensitivity and application of 6-methylpurine, a cytokinin oxidase inhibitor, dramatically reduced ethylene production and postponed flower senescence (Taverner et al. 2000). Transformation of miniature rose with cytokinins biosynthesis gene IPT (isopentenyl transferase) under the regulation of SAG12 promoter reduced ethylene sensitivity and retarded petal senescence (Zakizadeh et al. 2013). These results indicate antagonistic interaction between ethylene and cytokinins in the modulation of petal senescence.
Petal Senescence in Ranunculaceae
The family Ranunculaceae possesses a rich repository of beautiful ornamentals that could significantly transform the floriculture sector. Ranunculaceae is commonly known as the “buttercup family” or “crowfoot family.” The flowers of this family exhibit protogyny, which encourages cross-pollination or outbreeding. It comprises of 2500 species and 55 genera that exhibit great variation in floral morphology (Delpeuch et al. 2022). The key ornamentals of Ranunculaceae include Conslida sp., Anemone sp., Clematis sp., Helleborus sp., Ranunculus sp., Delphinium sp., nigella (Love-in-mist), and Aquilegia with considerable potential for use as a cut flower in the floriculture market; however, the family’s potential in the floricultural industry has not been fully explored from the perspective of cut flowers. Ranunculaceae is a highly ethylene-sensitive family with the level of sensitivity falling in the range of ‘3–4’ as per Van Doorn and woltering classification (1988). Higher ethylene sensitivity truncates the vase life of cut flowers by inducing petal abscission. Extensive postharvest studies have not been carried out on Ranunculaceae, resulting in a lack of precise regulatory mechanisms and standardized chemical formulations to enhance the postharvest longevity of these elegant flowers. Therefore, this article summarizes the preliminary postharvest studies conducted on some ornamentals of Ranunculaceae, which will provide a precise understanding of senescence mechanisms and identify the areas for further investigation.
Helleborus
Helleborus is a ravishing perennial ornamental that blooms in the winter or early spring. It has gained considerable commercial significance for usage as cut flowers and interior potted plants (Dhooghe et al. 2018). The widely grown ornamental species of Helleborus include Helleborus niger L. (Christmas rose) and Helleborus orientalis (Lenten Rose) (Rice and Strangman 1993). Helleborus niger flowers from mild winters (on Christmas) up to april, while Helleborus orientalis flowers from late winter to early spring (Salopek-Sondi and Magnus 2007; Shahri et al. 2011a, b). Helleborus flower consists of showy creamy white petaloid sepals. Whereas, petals are reduced to nectaries present at the base of flowers also called honey leaves (Dhooghe et al. 2018). It can act as a model system owing to its unique mode of senescence. Senescence studies conducted by shahri et al. (2011a, b) in Helleborus orientalis revealed that the flower senescence involves layer-by-layer stamen abscission surrounding the pistils, which is followed by the browning of nectaries at the base and finally the creamy white sepals harden and turn greenish. These sepals persist till the seed set is complete after which the complete flower abscises from the plant. Besides morphological changes, the various biochemical changes documented during the flower senescence include a decrease in sugars and proteins with an increase in protease activity. These persistent sepals become photosynthetically active during seed development. It is considered as important ecophysiological adaptation in Helleborus. Since Helleborus flowers in winter, therefore its leaves although green are covered by snow and debris. Moreover, they regularly deteriorate at their late stage with the formation of new leaves which are not photosynthetically active. Thus, green sepals are considered as the most reliable and direct source for providing assimilates to the developing seeds and fruits. The greening of sepals is induced by the stimulation of GA biosynthesis due to signals emanating from the developing fruits (Ayele et al. 2010). The protein extract of Helleborus orientalis analyzed through SDS-PAGE, demonstrated an increase in low-molecular weight proteins “so-called death proteins” and a decrease in high-molecular weight proteins during flower senescence. Thus in this flower system, senescence mechanisms and their post-transcriptional regulation can be better understood by analyzing the nature of these proteins (shahri et al. 2011a, b). Helleborus orientalis being an elegant cut flower has been least studied in terms of postharvest perspective. Recently, Abdulla and Çelikel (2018) reported that sucrose pulsing of Helleborus orientalis accentuated its postharvest longevity by via improving water uptake and fresh weight of flowers. Consequently, the positive response of Helleborus cut flowers to sucrose pulsing suggests a huge scope for postharvest quality improvement in Helleborus cut flowers using suitable postharvest treatments.
Anemone
Anemone is commonly known as “wind flower.” It occurs in various hues and colors, like red, blue, purple, and white. They are short-stemmed and spring flowering. Among the various species, Anemone coronaria is mostly used for cut flower production on large scale (Laura and Allavena 2007). They are usually harvested when buds are fully colored and half open. Anemones are highly sensitive to ethylene and exhibit ethylene-dependent mode of senescence (Reid 2004). The key senescence symptoms include petal in rolling and stem bending. The average lifespan of an individual flower in distilled water is about 5 days. Application of GA3 has been found to accentuate longevity and improve flower quality by preventing neck bending. Moreover, the inclusion of anti-ethylene treatments like AgNO3 in vase solutions improved the postharvest performance and flower quality of cut anemone flowers by reducing endogenous ethylene production (Sharifani, et al. 2004). Pulsing with ethylene action or synthesis inhibitors like STS (Silver thiosulfate) or MCP also reduced quality deterioration and improved their display life (Reid 2004). Moreover, Cyclodextrin nanosponges (CD-NS) synthesized by hydrolysis of starch are used for sustained release of 1-MCP (an ethylene biosynthesis inhibitor) (Manzoor et al. 2020). The use of CD-NS structure for sustained release of 1-MCP significantly improved the vase life of Anemone coronaria as compared to gaseous MCP (Seglie et al. 2013). The combination of treatments like BA (Benzyl adenine) and growth-retardant Paclobutrazol was also found to be effective in improving postharvest attributes, like preventing membrane leakage, improving anthocyanin content and inhibiting stem bending (Chernov et al. 2007). Thus anti-ethylene treatments and growth retardants can significantly mitigate the postharvest losses in Anemone cut flowers.
Clematis
Clematis is known for its profuse blooming and large elegant flowers measuring 20 cm in diameter (Rabiza-Świder et al. 2017). It is successfully cultivated as a cut flower in the U.S.A. on large scale (Greer and Dole 2009). Xylem occlusion leading to negative water balance is regarded as a key factor limiting the vaselife of clematis flowers. The first noticeable symptoms of senescence include loss of fresh weight and petal wilting (Rabiza-Świder et al. 2017). The root cause for the xylem occlusion was found to be tyloses and bacterial proliferation. Application of 8HQC (8-hydroxyquinoline citrate) and 2% sucrose reduced the bacterial proliferation and growth of xylem tyloses (Jedrzejuk et al. 2012). Moreover, the effect of the treatments including 8-hydroxyquinoline citrate (8HQC, 200 mgL−1) and a standard preservative (SP) solution comprising 200-mgL−1 8HQC and 20-gL−1 sucrose (S) on vase life was found to be cultivar specific. In cultivars like ‘General Sikorski,’ both treatments accentuated vase life by about 30% as compared to the control, however, in ‘Mazury’ cultivar, vase life was increased by about 40% as compared to the control, by the standard preservative solution only. On the contrary, in jalka and pillu cultivar, no effect was found on the vaselife by the application of these two solutions. These vase solutions also differentially affected the water uptake rates in different cultivars. The water uptake was significantly increased by both the vase solutions in ‘General Sikorski,’ while in ‘Piilu’ cultivar both the vase solutions reduced the water uptake as compared to control (Rabiza-Świder et al. 2017). Apart from 8HQC, the inclusion of other biocides like Al2(SO4)3, NaOCl and Ca(OCl)2, or citric acid as acidifier did not have any significant impact on the vase life of cut clematis flowers, thereby confirming the fact that the effect of biocides varies with different species and cultivars (Rabiza-Świder et al. 2017; Tekalign et al. 2011). Although clematis flowers do not produce measurable amounts of ethylene but they were highly responsive to exogenous ethylene treatment leading to shortening of vaselife. On the contrary, application of STS (Silver thiosulfate) and MCP significantly improved their vase life. MCP caused a maximum increase in vase life. The presence of silver ions in the STS complex hinders the ethylene effect by competing for the same active site (Rabiza-Świder et al. 2017). Moreover, the antimicrobial effect of STS improves water uptake and enhances flower longevity (Rezvanypour and Osfoori 2011). The cultivar-specific differences in terms of responding to different preservatives were also reflected in senescence mechanisms at the ultrastructural level. PCD studies conducted in two cultivars of clematis ‘Popieluszko and Andromeda’ revealed temporal differences in the degradation of different organelles during their cell death. In the long-lived cultivar, ‘Popieluszko’ symptoms of organelle degeneration were initiated at the open stage, while in the short-lived cultivar, ‘Andromeda’ symptoms of organelle degeneration were already initiated at the bud stage. This temporal difference in the initiation of senescence mechanisms may be the underlying reason for different longevity in different cultivars. Nuclear degradation being one of the earliest signs of PCD was well advanced in a short-lived cultivar at the bud stage while in long-lived cultivar, nuclei were found stable up to the last stage of development. The senescence process is usually intensified once the flowers are detached from the parent plant. However, application of preservatives has been found to retard the same (Lone et al. 2021a, b). Surprisingly, long-lived cultivars of clematis cut flowers held in preservative solutions had the same rate of chromatin degradation as that of control ones held in distilled water. In opposite to this, chromatin degradation was arrested in short-lived cultivars treated with preservatives. Mitochondria and plastids which are stable organelles were visible in wilted flowers of long-lived varieties as well as in short-lived cultivars treated with preservative (sucrose + 8HQC). The cell wall is one of the structures that degraded during the end phase of senescence. In long-lived cultivars, preservatives could not arrest cell wall degradation, whereas in short-lived cultivars, cell wall degradation was arrested by the application of preservatives (Rabiza-Świder et al. 2016). From the above studies, it can be concluded that although clematis is a beautiful cut flower with high market demand, but the selection of a suitable cultivar and optimization of appropriate postharvest treatments is the only means to commercialize it on large scale.
Delphinium
Delphinium is a fascinating ornamental with elegant spikes, mostly used as a cut flower (Ichimura et al. 2009). The spikes bear flowers of vibrant colors ranging from white to pink. Delphiniums are sensitive to ethylene but the sensitivity varies between the species and cultivars. In Delphinium hybrid cv. Bellamosum, ethylene sensitivity is age dependent, i.e., flowers become sensitive to ethylene toward the advanced stage of development (Ichimura et al. 2009). In Delphinium inter-organ signaling has been proposed to coordinate the senescence process within the flower similar to that of carnation (Shibuya et al. 2000). Interestingly, the climacteric upsurge of ethylene has not been observed in sepals and petals but in receptacle and gynoecium. The activity of ACC synthase and ACC oxidase was many folds higher in the gynoecium as compared to a receptacle, indicating production of ethylene in gynoecium signals the receptacle to produce ethylene and induce abscission of Delphinium sepals as they are directly connected to it rather than gynoecium (Ichimura et al. 2009). Moreover, wounding of the gynoecium and receptacle has also been found to induce sepal abscission as wounding leads to ethylene production in the receptacle (Yang and Hoffman 1984). The sepal abscission was however dramatically inhibited by the pulse treatment with STS (Silver thiosulfate) and AVG (Aminoethoxy vinyl glycine), but AVG inhibited the sepal growth of Delphinium flowers. Surprisingly, other ethylene antagonists like AOA (Aminooxyacetic acid) and 1-MCP (1-Methylcyclopropene) were found to be ineffective in inhibiting the abscission of sepals in cut Delphinium (Ichimura et al. 2009). This might be attributed to the accumulation of these inhibitors in insufficient concentration at the active site (Ichimura et al. 2002). Thus, STS is the only ethylene antagonist recommended to accentuate the quality of cut Delphinium flowers. Moreover, studies conducted in Delphinium grandiflorum revealed that the ethylene production was high in gynoecium and receptacle from day zero of harvest as compared to D. belladonna. During senescence, ethylene production exhibited a substantial increase in the receptacle as compared to gynoecium. Also, pollination induced the upsurge in ethylene production in gynoecium and receptacle, however, ACS and ACO transcripts were least abundant in gynoecium, while DgACO3 transcripts were highly abundant in the receptacle. This indicates the differential regulation of ACS and ACO in the gynoecium and receptacle (Okamoto et al. 2022). Mannitol is a key soluble carbohydrate in Delphinium. In addition, mannitol is also a major soluble carbohydrate of Scrophulariaceae, Oleaceae, Rubiaceae, and Umbellifereae (Bieleski 1982). The application of exogenous mannitol exhibited multifaceted effects on Delphinium, manifesting not only in the retardation of sepal abscission but also in the mitigation of ethylene responsiveness and the attenuation of the climacteric surge in ethylene synthesis. Mannitol treatment induced augmented concentrations of glucose and fructose within the sepal tissues, concomitant with the rise in mannitol levels in the sepals (Ichimura et al. 2000). These results indicate that mannitol is metabolized into readily usable forms of carbohydrates like glucose and fructose by mannitol dehydrogenase, which in turn reduce the climacteric upsurge in ethylene production and thus prevent the sepal abscission (Kikuchi et al. 1999). A combination of STS pulse and 4% sucrose treatment significantly accentuated the longevity of Delphinium elatum flowers, besides enriching the anthocyanin content of sepal tissues (Kuroshima et al. 2017). Likewise, in Delphinium malabaricum, treatment of sucrose along with AgNO3 significantly extended the vase life of Delphinium flowers. Sucrose not only acts as an osmoticum and respiratory substrate but also reduces ethylene sensitivity. Also, AgNO3, a broad-spectrum bactericide, markedly inhibited bacterial proliferation and maintained the solution flow through the xylem vessels and hence improved the postharvest attributes (Kolar et al. 2017).
Ranunculus
Ranunculus is commonly known as “buttercup.” It is a highly demanding crop in the global cut flower industry. Ranunculus cultivation is suitable for small-scale growers as it requires minimal space with huge economic returns as one bunch consisting of 10 Ranunculus stems costs $12.50 to $26.00 in the international market (Rauter et al. 2022). Ranunculus is an excellent model system for studying flower colorations because of its wide variety of petal colorations, including white, yellow, red, green, black, and brown (Liu et al. 2019). Senescence studies conducted on Ranunculus asiaticus revealed that the membrane integrity decreases with the progression of flower development. Moreover, the other biochemical attributes like sugars, proteins, and phenols also witnessed a sharp decrease as the flower development proceeded from the open to the senescent stage (Shahri and Tahir 2011a, b). The visible symptoms of senescence symptoms in Ranunculus asiaticus are wilting and then abscission of petals toward the end phase of senescence. The color of the petals also changes from dark red to brick red with the loss in luster and turgidity. The flowers usually last for five days from the day of blooming. (Shahri et al. 2010). Ranunculus asiaticus exhibits ethylene-independent mode of senescence. Although the flowers produce a climacteric rise in ethylene production but they are not sensitive to exogenous ethylene. Moreover, the ACC level was found to be constant with age (Kenza et al. 2000). Application of anti-ethylene treatments were ineffective in improving the quality of cut flowers, thereby, indicating that flowers need not be shielded from ethylene exposure (Shahri et al. 2011a, b). The anti-senescent postharvest treatments like GA3 significantly improved the vase life by maintaining higher carbohydrate content in petal tissues. Likewise, the application of salicylic acid and combination treatment of (sucrose + HQS + Citric acid) improved the postharvest performance of Ranunculus cut flowers by improving various biochemical attributes, like carbohydrate content, anthocyanin content, and water balance of petal tissues (Al-Hasnawi et al. 2018). Notably, individual sucrose treatments were found to be ineffective in delaying the senescence in cut flowers of Ranunculus asiaticus (Shahri et al. 2010). HQS being antibacterial prevents vascular occlusion by inhibiting bacterial growth, while citric acid maintains the pH of the preservative solution to facilitate absorption of all its constituents via xylem vessels (Al-Hasnawi et al. 2018). Moreover, salicylic acid and sucrose induce anthocyanin biosynthesis, thereby improving the display quality of these precious cut flowers (Hatamzadeh et al. 2012; Kazemi et al. 2011). Besides growth regulators, treatment of Ranunculus cut flowers with protein synthesis inhibitor ‘Cyclohexamide’ (CHI) has been reported to delay the execution of senescence by improving various postharvest attributes like membrane integrity and the content of soluble proteins in petal tissues through attenuation in protease activity. Moreover, carbohydrate content was also found to be higher in CHI-treated petal tissues, which may be attributed to a decrease in respiratory activity in petal tissues by application of CHI (Shahri and Tahir 2010a, b), as it reduces respiratory activity in higher plants. (Ellis and Macdonald 1970).
Consolida
Consolida ajacis, also known as “Doubtful Knight’s spur or Rocket larkspur,” blooms from May to July. It is a magnificent ornamental with promising potential as a cut flower because of its fascinating spikes of various hues and colors, adding charm to gardens and interior spaces (Haq et al. 2022b). The characteristic feature of Consolida ajacis flowers are hood and spur formed by the upper petaloid sepals. Flower senescence in Consolida ajacis is characterized by the withering of anthers and projection of the pistil out of the protective hood formed by upper petals, followed by wilting and abscission of tepals (Shahri and Tahir 2011a, b). The senescence process in Consolida ajacis involves a decrease in sugar and protein content of tepal tissues and increase in membrane seepage and protease activity. Moreover, SDS-PAGE analysis of the protein extract of Consolida ajacis revealed increase in low-molecular weight proteins and decrease in high-molecular weight proteins during flower senescence. Consequently, elucidating the nature of these polypeptides can provide vital insights about the post-transcriptional regulation of senescence mechanisms within this flower system. (Shahri and Tahir 2011a, b). Consolida ajacis is a highly ethylene-sensitive flower which limits its postharvest longevity. Application of sucrose and ethylene antagonists like STS (silver thiosulfate) significantly alleviated senescence symptoms in Consolida ajacis spikes by reducing the ethylene action and preventing climacteric rise in ethylene production (Ichmura et al. 2000, Finger 1999; Shahri et al. 2010). In addition to anti-ethylene treatments, pulsing with protein synthesis inhibitor Cyclohexamide (CHI) has been found to be highly efficacious in mitigating postharvest senescence in cut spikes of Consolida ajacis. Pulsing of spikes with CHI maintained higher fresh mass and dry mass of flowers by reducing respiratory loss. Moreover, higher protein content was detected in flowers pulsed with CHI relative to untreated ones. This may be attributed to diminished protease activity by CHI pulsing and hence prevent protein degradation (Shahri and Tahir 2010a, b). Recent studies entrench the extensive role of growth regulators like salicylic acid, cytokinins, and polyamines and in accentuating the marketability of Consolida ajacis cut spikes by delaying the execution of senescence. Salicylic acid being a phenolic compound is highly effective in orchestrating postharvest senescence in cut spikes of Consolida ajacis (Haq et al. 2022a). In another study, the role of nitric oxide was also envisaged in improving display quality in cut spikes of Consolida ajacis (Haq et al. 2021). These studies demonstrated that lipid peroxidation and vascular occlusion are the primary factors responsible for senescence instigation in Consolida ajacis. Salicylic acid and nitric oxide treatments improved the solution uptake by decreasing bacterial proliferation and maintained membrane integrity of flower tissues by attenuating lipoxygenase activity. In addition, these postharvest treatments significantly accentuated the activity of antioxidant enzymes to counter ROS involved in senescence instigation (Haq et al. 2021, 2022a). Owing to their multifaceted role in senescence regulation, these treatments can be recommended as effective anti-senescent therapies for mitigating postharvest losses in cut flowers of Consolida ajacis. Moreover, the implication of polyamines on various biochemical aspects of flower senescence in Consolida ajacis cut spikes was also tested. The investigation revealed that polyamines orchestrated the flower senescence by augmenting the antioxidant response, essential for ROS scavenging. The study also unveiled that Polyamines prevented the breakdown of protein and sugars besides, warranting the membrane stability of the tepal tissues. Thus, the depletion of sugars and Proteins, besides attenuation of antioxidant system was found to be the primary factors leading to senescence initiation in Consolida ajacis cut spikes (Farooq et al. 2021a). Cytokinins including Benzylamino purine (BAP), Kinetin (KIN), and cytokinin-like substances thidiazuron were also assessed in delaying the onset of senescence Consolida ajacis cut spikes. Inclusion of these postharvest treatments considerably improved the quality of cut spikes by augmenting phenolic content, besides maintaining optimum protein and sugar content in tepal tissues (Haq et al. 2022b). The aforementioned postharvest treatments have been summarized in tabulated form as shown in Table 3.
Conclusion and Future Perspectives
Ranunculaceae is an ethylene-responsive family, therefore the postharvest investigations conducted on cut flowers of this family have mostly evaluated the efficacy of conventional anti-ethylene treatments in alleviating postharvest senescence. However, using novel eco-friendly and cost-effective anti-ethylene substitutes over conventional ethylene blockers could be highly beneficial in preventing the onset of senescence. Moreover, earlier studies on senescence have entrenched the extensive role of growth regulators in accentuating the lifespan of cut flowers. As a result, exogenous sourcing of growth regulators such as cytokinins, salicylic acid, and polyamines can offer substantial opportunity for postharvest quality improvement in Ranunculus cut flowers. Ethylene being crucial in regulating flower senescence in Ranunculaceae, consequently elucidating crosstalk between ethylene and other phytohormones could be a promising strategy for modulating senescence in climacteric flowers. Senescence studies conducted on ornamentals of Ranunculaceae have primarily concentrated on understanding the biochemical mechanisms regulating their longevity. However, in recent years, significant advancements have been achieved in tagging the genes instigating senescence in various flowers, like Dianthus, Petunia, and Gladiolus. This has been achieved through gene expression studies, transcriptome profiling, and microarray technology. Extending the use of these technologies can be of immense help in tagging the genes responsible for senescence regulation in the ornamentals of Ranunculaceae. In addition, the antisense-RNA technology can help us to combat senescence in climacteric flowers by suppressing the transcripts of ethylene forming genes as achieved in Dianthus. Furthermore, the recent CRISPR technology can have far better implications in regulating the senescence process as it ensures the desirable fine tuning of senescence-associated genes along with their stable transmission to subsequent generations. Moreover, gaining insights into the crosstalk between the signaling cascades and the hormones can immensely widen our future understanding relating to postharvest physiology of flowers and as such the family Ranunculaceae can serve as a model system in this regard. Achieving successful outcomes in employing these strategies necessitates meticulous adherence to appropriate experimental designs, such as employing randomized designs to assess the potency of ethylene antagonists and growth regulators. Additionally, unraveling hormonal crosstalk and gene expression patterns of senescence markers demands judicious application of factorial and time series experimental designs to furnish insightful results. However, the application of the aforementioned methodologies is not devoid of hurdles. Concerns pertaining to environmental implications and potential adverse effects arising from the use of growth regulators may impede progress in these endeavors. Furthermore, implementing CRISPR technology to regulate senescence-associated genes in Ranunculaceae confronts the formidable challenge of mitigating off-target effects and the unintended modification of non-target genes, thereby necessitating stringent validation and optimization of the CRISPR-Cas9 system.
References
Abdulla MF, Çelikel FG (2018) Postharvest quality and extending vase life of Helleborus orientalis flowers by sucrose pulsing. In: XXX International Horticultural Congress IHC2018: International Symposium on Ornamental Horticulture and XI International. Vol. 1263, pp. 449–454
Ahmad SS, Tahir I (2015) Storage protocol for improving the postharvest performance in cut scapes of Iris versicolor. Acta Hortic 1060:71–79
Ahmad SS, Tahir I (2016a) How and why of flower senescence: understanding from models to ornamentals. Ind J Plant Physiol 21(4):446–456
Ahmad SS, Tahir I (2016b) Increased oxidative stress, lipid peroxidation and protein degradation trigger senescence in Iris versicolor L. flowers. Physiol Mol Biol Plants 22:507–514
Al-Hasnawi HAK, Khaleelb TH, Husseinc JK (2018) Effect of growth regulators and preservative solutions on vase life and water relations for flowers of Ranunculus asiaticus after cutting. Plant Arch 18:1391–1400
Arora A, Singh VP (2004) Cysteine protease gene expression and proteolytic activity during floral development and senescence in ethylene-insensitive Gladiolus grandiflora. J Plant Biochem Biotechnol 13:123–126
Arora A, Singh VP (2006) Polyols regulate the flower senescence by delaying programmed cell death in Gladiolus. J Plant Biochem Biotechnol 15:139–142
Avila-Rostant O, Lennon AM, Umaharan P (2010) Spathe color variation in Anthurium andraeanum Hort. and its relationship to vacuolar pH. HortScience 45:1768–1772
Ayele BT, Magnus V, Mihaljević S, Prebeg T, Čož-Rakovac R, Ozga JA, Salopek-Sondi B (2010) Endogenous gibberellin profile during Christmas rose (Helleborus niger L.) flower and fruit development. Plant Growth Regul 29:194–209
Baidya BK, Chakrabarty S, Sethy P (2020) Extending shelf life of loose tuberose florets (Polianthes tuberosa Linn. cv. Prajwal) by quick dipping in boric acid and sodium benzoate followed by low temperature storage. Int J Chem Stud 8:2607–2612
Baker JE, Wang CY, Lieberman M, Hardenburg R (1977) Delay of senescence in carnations by a rhizobitoxine analog and sodium benzoate. Hortic Sci 12:38–39
Barsan C, Sanchez-Bel P, Rombaldi C, Egea I, Rossignol M, Kuntz M, Zouine M, Latché A, Bouzayen M, Pech JC (2010) Characteristics of the tomato chromoplast revealed by proteomic analysis. J Exp Bot 61:2413–2431
Barth C, Moeder W, Klessig DF, Conklin PL (2004) The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol 134:1784–1792
Bayanati M, Tehranifar A, Razavi K, Nemati SH, Lohrasebi T, Ahmadi N (2019) Expression patterns analysis of SOD genes in responses to ethylene-induced oxidative stress in rose (Rosa hybrida) during flower development. S Afr J Bot 127:265–270
Beal PR, Joyce A (1999) Cut flower characteristics of terminal flowering tropical Grevillea: a brief review. Aust J Exp Agric 39:781–794
Beers EP, Woffenden BJ, Zhao C (2000) Plant proteolytic enzymes: Possible roles during programmed cell death. Plant Mol Biol 44:399–415
Bicknell R (1995) Breeding cut flower cultivars of Leptospermum using interspecific hybridisation. New Zeal J Crop Hortic 23:412–421
Bieleski RL (1982) Sugar alcohols. In: Loewus FA, Tanner W (eds) Plant carbohydrates I. intracellular carbohydrates. Encyclopedia of plant physiology, new series, vol 13A. SpringerVerlag, Berlin, pp 158–192
Bolouri-Moghaddam MR, Le Roy K, Xiang L, Rolland F, Van den Ende W (2010) Sugar signalling and antioxidant network connections in plant cells. FEBS J 277:2022–2037
Borochov A, Spiegelstein H, Philosoph-Hadas S (1997) Ethylene and flower petal senescence: interrelationship with membrane lipid catabolism. Physiol Plant 100:606–612
Carlos G, Bartoli M, Montaldi SE, Puntarulo S (1996) Oxidative stress, antioxidant capacity and ethylene production during ageing of cut carnation (Dianthus caryophyllus) petals. J Exp Bot 47:595–601
Cavaiuolo M, Cocetta G, Ferrante A (2013) The antioxidants changes in ornamental flowers during development and senescence. Antioxidants 2:132–155
Ceusters J, Van de Poel B (2018) Ethylene exerts species-specific and age-dependent control of photosynthesis. Plant Physiol 176:2601–2612
Chang H, Jones ML, Banowetz GM, Clark DG (2003a) Overproduction of cytokinins in petunia flowers transformed with PSAG12-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol 132:2174–2183
Chang H, Jones ML, Banowetz GM, Clark DG (2003b) Overproduction of cytokinins in petunia flowers transformed with PSAG12-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol 132:2174–2183
Chang X, Donnelly L, Sun D, Rao J, Reid MS, Jiang CZ (2014) A petunia homeodomain-leucine zipper protein, PhHD-Zip, plays an important role in flower senescence. PLoS ONE 9:e88320
Chapin LJ, Jones M (2007a) Nutrient remobilization during pollinationinduced corolla senescence in Petunia. Acta Hortic 755:181–190
Chapin LJ, Jones ML (2007b) Nutrient remobilization during pollination induced corolla senescence in Petunia. Acta Hortic 755:181–190
Chen C, Zeng L, Ye Q (2018a) Proteomic and biochemical changes during senescence of Phalaenopsis ‘Red Dragon’petals. Int J Mol Sci 19:1317
Chen WH, Lee YI, Yang CH (2018b) Ectopic expression of two FOREVER YOUNG FLOWER Orthologues from Cattleya orchid suppresses ethylene signaling and DELLA results in delayed flower senescence/abscission and reduced flower organ elongation in Arabidopsis. Plant Mol Biol Rep 36:710–724
Chen HW, Jiang YZ, Hsu FH, Yang HC (2021) Silencing of FOREVER YOUNG FLOWER-Like genes from Phalaenopsis orchids promotes flower senescence and abscission. Plant Cell Physiol 62:111–124
Chernov Z, Philosoph-Hadas S, Meir S, Salim S (2007) Quality improvement of cut flowers and potted plants with postharvest treatments based on various cytokinins and auxins. In: International Conference on Quality Management in Supply Chains of Ornamentals, vol. 755, pp. 143–154
Costa LCD, Araujo FFD, Lima PC, Pereira AM, Finger FL (2016) Action of abscisic and gibberellic acids on senescence of cut gladiolus flowers. Bragantia 75:377–385
Costa LC, Luz LM, Nascimento VL, Araujo FF, Santos MN, Franca CDF et al (2020) Selenium-ethylene interplay in postharvest life of cut flowers. Front Plant Sci 11:2055
Dar RA, Tahir I (2018) Effect of ethylene antagonist silver thiosulphate on the flower longevity of Clarkia pulchella Pursh. Hortic Res 26:5–12
Dar RA, Tahir I, Ahmad SS (2014) Sugars and sugar alcohols have their say in the regulation of flower senescence in Dianthus chinensis L. Sci Hortic 174:24–28
Dar RA, Nisar S, Tahir I (2021) Ethylene: a key player in ethylene sensitive flower senescence: a review. Sci Hortic 290:110491
Davarynejad GH, Nurzadehnamaghi M, Momen A (2021) Evaluation of the effect of exogenous application of polyamines on growth, nut traits and yield of ‘akbari’pistachio trees (pistacia vera l.). J Hortic Sci 34:547–561
De Vetten NC, Huber DJ (1990) Cell wall changes during the expansion and senescence of carnation (Dianthus caryophyllus) petals. Physiol Plant 78:447–454
Delpeuch P, Jabbour F, Damerval C, Schönenberger J, Pamperl S, Rome M, Nadot S (2022) A flat petal as ancestral state for Ranunculaceae. Front Plant Sci 5:35. https://doi.org/10.3389/fpls.2022.961906
Dhooghe E, Sparke J, Oenings P, Van Paemel T, Van Labeke MC, Winkelmann T (2018) Helleborus. Ornamental crops. Springer, Cham, pp 439–452
Doganlar ZB, Demir K, Basak H, Gul I (2010) Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. Afr J Agric Res 15(5):5–12
Du Y, Luo S, Zhao J, Feng Z, Chen X, Ren W, Zhou L (2021) Genome and transcriptome-based characterization of high energy carbon-ion beam irradiation induced delayed flower senescence mutant in Lotus japonicus. BMC Plant Biol 21(1):1–19
Eason JR (2006) Molecular and genetic aspect of flower senescence. Stewart Postharvest Rev 2:1–7
Ellis RJ, MacDonald IR (1970) Specificity of cycloheximide in higher plant systems. Plant Physiol 46:227–232
Falk J, Munné-Bosch S (2010) Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot 61:1549–1566
Farooq S, Lone ML, Parveen S, Altaf F, Tahir I (2021a) Polyamines accentuate vase life by augmenting antioxidant system in cut spikes of Consolida ajacis (L.) Schur. Ornam Hortic 27:495–504
Farooq S, Lone ML, Altaf F, Parveen S, Tahir I (2021b) Boric acid as a potential substitute for conventional ethylene antagonists in mitigating postharvest flower senescence of Digitalis purpurea. Ornamental Horticulture 27:516–525
Finger FL (1999) Pulsing with sucrose and silver thiosulfate extended the vase life of Consolida ajacis. In: VII International Symposium on Postharvest Physiology of Ornamental Plants, vol 543, pp. 63–67
Friedman WE, Moore RC, Purugganan MD (2004) The evolution of plant development. Am J Bot 91(10):1726–1741
Galston AW, Kaur-Sawhney R (1995) Polyamines as endogenous growth regulators. Plant hormones: physiology, biochemistry and molecular biology. Springer, Dordrecht, pp 158–178
Gehl C, Wamhoff D, Schaarschmidt F, Serek M (2018) Improved leaf and flower longevity by expressing the etr1-1 allele in Pelargonium zonale under control of FBP1 and SAG12 promoters. Plant Growth Regul 86:351–363
Gonzalez A (2009) Pigment loss in response to the environment: a new role for the WD/bHLH/MYB anthocyanin regulatory complex. New Phytol 182:1–3
Gonzalez-Candelas L, Alamar S, Sanchez-Torres P, Zacarias L, Marcos JF (2010) A transcriptomic approach highlights induction of secondary metabolism in citrus fruit in response to Penicillium digitatum infection. BMC Plant Biol 10:194
Greer L, Dole JM (2009) Woody cut stems for growers and florists. Timber Press, Portland
Halevy AH, Mayak S (1979) Senescence and postharvest of cut flowers. Hort Rev 1:204–236
Haq AU, Lone ML, Farooq S, Parveen S, Altaf F, Tahir I et al (2021) Nitric oxide effectively orchestrates postharvest flower senescence: a case study of Consolida ajacis. Funct Plant Biol 50(2):570–581
Haq AU, Farooq S, Lone ML, Altaf F, Parveen S, Tahir I et al (2022a) Assessing the efficacy of Thidiazuron, 6-Benzylamino purine and kinetin in modulating flower senescence in cut spikes of Consolida ajacis (L.) Schur. J King Saud Univ Sci. 34(8):10235
Haq AU, Lone ML, Farooq S, Parveen S, Altaf F, Tahir I, El-Serehy HA (2022b) Efficacy of salicylic acid in modulating physiological andbiochemical mechanisms to improve postharvest longevity in cut spikes of Consolida ajacis (L.) Schur. Saudi J Biol Sci 29(2):713–720
Hassan F, Ali E, Mazrou R (2020) Involvement of ethylene synthetic inhibitors in regulating the senescence of cut carnations through membrane integrity maintenance. Hortic Res 28:39–48
Hatamzadeh A, Hatami M, Ghasemnezhad M (2012) Efficiency of salicylic acid delay petal senescence and extended quality of cut spikes of Gladiolus grandiflora cv wings sensation. Afr J Agric Res 7(4):540–545
Hirota T, Izumi M, Wada S, Makino A, Ishida H (2018) Vacuolar protein degradation via autophagy provides substrates to amino acid catabolic pathways as an adaptive response to sugar starvation in Arabidopsis thaliana. Plant Cell Physiol 59(7):1363–1376
Hönig M, Plíhalová L, Husičková A, Nisler J, Doležal K (2018) Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int J Mol Sci 19(12):4045
Hossain Z, Mandal AKA, Datta SK, Biswas AK (2006) Decline in ascorbate peroxidase activity-a prerequisite factor for tepal senescence in gladiolus. J Plant Physiol 163:186–194
Hunter DA, Steele BC, Reid MS (2002) Identification of genes associated with perianth senescence in Daffodil (Narcissus pseudonarcissus L. “Dutch Master”). Plant Sci 163:13–21
Huo J, Huang D, Zhang J, Fang H, Wang B, Wang C, Ma Z, Liao W (2018) Comparative proteomic analysis during the involvement of nitric oxide in hydrogen gas-improved postharvest freshness in cut lilies. Int J Mol Sci 19:3955
Ichimura K, Kohata K, Goto R (2000) Soluble carbohydrates in Delphinium and their influence on sepal abscission in cut flowers. Physiol Plant 108:307–313
Ichimura K, Shimizu H, Hiraya T, Hisamatsu T (2002) Effect of 1- methylcyclopropene (1-MCP) on the vase life of cut carnation Delphinium and sweet pea flowers. Bull Natl Inst Flor Sci 2:1–8
Ichimura K, Shimizu-Yumoto H, Goto R (2009) Ethylene production by gynoecium and receptacle is associated with sepal abscission in cut Delphinium flowers. Postharvest Biol Technol 52(3):267–272
Irving DE, Joyce DC, Simons DH (2006) Vase treatments containing gibberellic acid do not increase longevity of cut Grevillea ‘Sylvia’inflorescences. Aust J Exp Agric 46(11):1535–1539
Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan MIR (2017) Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front Plant Sci 8:475
Jedrzejuk A, Rochala J, Zakrzewski J, Rabiza-Świder J (2012) Identification of xylem occlusions occurring in cut clematis (Clematis L., fam. Ranunculaceae Juss.) stems during their vase life. Sci World J 2012:35–48
Jeong IS, Lee S, Bonkhofer F, Tolley J, Fukudome A, Nagashima Y, Koiwa H (2018) Purification and characterization of Arabidopsis thaliana oligosaccharyltransferase complexes from the native host: a protein super-expression system for structural studies. Plant J 94(1):131–145
Ji X, Wang M, Xu Z, Wang K, Sun D, Niu L (2022) PlMYB308 regulates flower senescence by modulating ethylene biosynthesis in Herbaceous Peony. Front Plant Sci. https://doi.org/10.3389/fpls.2022.872442
John-Karuppiah K, Burns JK (2010) Degreening behavior in ‘Fallglo’ and ‘Lee×Orlando’ is correlated with differential expression of ethylene signaling and biosynthesis genes. Postharvest Biol Technol 58:185–193
Jones ML (2013) Mineral nutrient remobilization during corolla senescence in ethylene-sensitive and -insensitive flowers. AoB Plants 5:plt023
Jones ML, Chaffin GS, Eason JR, Clark DG (2005) Ethylene-sensitivity regulates proteolytic activity and cysteine protease gene expression in Petunia corollas. J Exp Bot 56(420):2733–2744
Jones ML, Larsen PB, Woodson WR (1995) Ethylene-regulated expression of a carnation cysteine protease during flower petal senescence. Plant Mol Biol 28:505–512
Jones ML, Woodson WR (1999) Differential expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in carnation. Plant Physiol 119(2):755–764
Kamdee C, Kirasak K, Ketsa S, van Doorn WG (2015) Vesicles between plasma membrane and cell wall prior to visible senescence of Iris and Dendrobium flowers. J Plant Physiol 188:37–43
Kato Y, Yamamoto Y, Murakami S, Sato F (2005) Post-translational regulation of CND41 protease activity in senescent tobacco leaves. Planta 222:643–651
Kazemi M, Zamani S, Aran M (2011) Effect of some treatment chemicals on keeping quality and vase-life of gebrera cut flowers. Am J Plant Physiol 6:99–105
Kazemi M, Hadavi E, Hekmati J (2012) Effect of salicylic acid, malic acid, citric acid and sucrose on antioxidant activity, membrane stability and ACC-oxidase activity in relation to vase life of carnation cut flowers. J Adv Agric Technol 8(6):2053–2063
Kenza M, Umiel N, Borochov A (2000) The involvement of ethylene in the senescence of ranunculus cut flowers. Postharvest Biol Technol 19(3):287–290
Khunmuang S, Kanlayanarat S, Wongs-Aree C, Meir S, Philosoph-Hadas S, Oren-Shamir M, Ovadia R, Buanong M (2019) Ethylene induces a rapid degradation of petal anthocyanins in cut Vanda ‘Sansai Blue’orchid flowers. Front Plant Sci 10:1004
Kikuchi K, Kanahama K, Kanayama Y (1999) Changes in sugar metabolic enzymes with senescence of cut Delphinium flowers. J Jpn Soc Hortic Sci 68:338
Kolar F, Pai S, Dixit G (2017) Delphinium malabaricum (huth) munz: a potential ornamental crop from Western Ghats. J Hortic Sci 1(1):16–21
Kou L, Yang T, Luo Y, Liu X, Huang L, Codling E (2014) Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biol Technol 87:70–78
Kumar N, Bhandari P, Singh B, Gupta AP, Kaul VK (2008) Reversed phase-HPLC for rapid determination of polyphenols in flowers of rose species. J Sep Sci 31:262–267
Kumar M, Singh VP, Arora A, Singh N (2014) The role of abscisic acid (ABA) in ethylene insensitive Gladiolus (Gladiolus grandiflora Hort) flower senescence. Acta Physiol Plant 36(1):151–159
Kuroshima M, Ichimura K, Suzuki R, Ubukata M (2017) Effects of sucrose in combination with STS on the quality and vase life of cut Delphinium elatum flowers. Hortic Res 16(2):197–202
Laura M, Allavena A (2007) Anemone coronaria breeding: current status and perspectives. Eur J Hortic Sci 72(6):241
Lesham YY (1992) Membrane-associated phospholytic and lipolytic enzymes. In: Lesham YY (ed) Plant membranes: a biophysical approach to structure, development and senescence. Kluwer Academic Publishers, Dordrecht, pp 174–191
Li L, Yin Q, Zhang T, Cheng P, Xu S, Shen W (2021) Hydrogen nanobubble water delays petal senescence and prolongs the vase life of cut carnation (Dianthus caryophyllus L.) flowers. Plants 10(8):1662
Liao WB, Zhang ML, Yu JH (2013) Role of nitric oxide in delaying senescence of cut rose flowers and its interaction with ethylene. Sci Hortic 155:30–38
Lin X, Wang L, Hou Y, Zheng Y, Jin P (2020) A combination of melatonin and ethanol treatment improves postharvest quality in bitter melon fruit. Foods 9(10):1376
Liu Y, Lin T, Du L, Wang J, Yang X, Zhang J, Yang X (2019) Sampling for DUS Test of flower colors of Ranunculus asiaticus L. in view of spatial and temporal changes of flower colorations, anthocyanin contents, and gene expression levels. Molecules 24(3):615
Lone ML, Ul Haq A, Farooq S, Altaf F, Tahir I (2021a) Nitric oxide effectively curtails neck bending and mitigates senescence in isolated flowers of Calendula officinalis L. Physiol Mol Biol Plants 27:835–845
Lone ML, Farooq S, Parveen S, Tahir I (2021b) 6-Benzylamino purine outperforms Kinetin and Thidiazuron in ameliorating flower longevity in Calendula officinalis L. by orchestrating physiological and biochemical responses. Ornam Hortic 27:183–195
Lü P, Zhang C, Liu J, Liu X, Jiang G, Jiang X, Gao J (2014) Rh HB 1 mediates the antagonism of gibberellins to ABA and ethylene during rose (Rosa hybrida) petal senescence. Plant J 78(4):578–590
Ma N, Xue J, Li Y, Liu X, Dai F, Jia et al (2008) Rh-PIP2;1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol 148:894–907
Ma N, Ma C, Liu Y, Shahid MO, Wang C, Gao J (2018) Petal senescence: a hormone view. J Exp Bot 69(4):719–732
Macnish AJ, Irving DE, Joyce DC, Vithanage V, Wearing AH et al (2004) Variation in ethylene-induced postharvest flower abscission responses among Chamelaucium Desf. (Myrtaceae) genotypes. Sci Hortic 102:415–432
Maillard A, Diquélou S, Billard V, Laîné P, Garnica M, Prudent M, Garcia-Mina JM, Yvin JC, Ourry A (2015) Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front Plant Sci 6:317
Manimaran P (2018) Standardization of postharvest management techniques for Jasminum nitidum flowers. Chem Sci Rev Lett 7(26):652–658
Manzoor A, Bashir MA, Hashmi MM (2020) Nanoparticles as a preservative solution can enhance postharvest attributes of cut flowers. Italus Hortus 27:1–14
Manzoor A, Ahmad R, Naveed MS (2021) Impact of different biocidal compounds for improvement of vase life and quality of cut flowers: a review. Integr Plant Biol 1(1):1–14
Marty F (1999) Plant vacuoles. Plant Cell 11:587–600
Mayak S, Legge RI, Thompson JE (1983) Superoxide radical production by microsomal membranes from senescing carnation flowers: an effect on membrane fluidity. Phytochem 22:1375–1380
Meir S, Philosoph-Hadas S, Riov J, Tucker ML, Patterson SE, Roberts JA (2019) Re-evaluation of the ethylene-dependent and-independent pathways in the regulation of floral and organ abscission. J Exp Bot 70(5):1461–1467
Mibus H, Sriskandarajah S, Serek M (2009) Genetically modified flowering potted plants with reduced ethylene sensitivity. Acta Hortic 847:75–80
Morkunas I, Borek S, Formela M, Ratajczak L (2012) Plant responses to sugar starvation. Carbohydrates-comprehensive studies on glycobiology and glycotechnology. InTech, London, pp 409–438
Mulisch M, Krupinska K (2013) Ultrastructural analyses of senescence associated dismantling of chloroplasts revisited. In: Biswal B, Krupinska K, Biswal UC (eds) Plastid development in leaves during growth and senescence. Advances in photosynthesis and respiration, vol 36. Springer, New York, pp 307–335
Muller R, Stummann BM, Serek M (2000) Characterization of an ethylene receptor family with differential expression in rose (Rosa hybrida L.) flowers. Plant Cell Rep 19:1232–1239
Naing AH, Lee K, Arun M, Lim KB, Kim CK (2017) Characterization of the role of sodium nitroprusside (SNP) involved in long vase life of different carnation cultivars. BMC Plant Biol 17(1):1–12
Naing AH, Soe MT, Kyu SY, Kim CK (2021) Nano-silver controls transcriptional regulation of ethylene-and senescence-associated genes during senescence in cut carnations. Sci Hortic 287:110280
Nekouyar N, Emani MH (2022) The interactive effect of sodium benzoate and ethanol on the vase life of cut roses cv.‘Avalanche.’ J Ornam Plants 12(3):203–212
Nisar S, Dar RA, Bhat AA, Farooq Z, Tahir I (2021a) Some important biochemical changes orchestrating flower development and senescence in Nicotiana plumbaginifolia Viv. and Petunia hybrida Vilm. flowers. J Hortic Sci Biotechnol 96(6):759–769
Nisar S, Dar RA, Tahir I (2021b) Salicylic acid retards senescence and makes flowers last longer in Nicotiana plumbaginifolia (Viv). Plant Physiol Reports 26(1):128–136
Okamoto M, Niki T, Azuma M, Shibuya K, Ichimura K (2022) Expression of ethylene biosynthesis genes in the gynoecium and receptacle associated with sepal abscission during senescence in Delphinium grandiflorum. Plant Growth Regul. https://doi.org/10.1007/s10725-022-00822-z
Olsen A, Lütken H, Hegelund JN, Müller R (2015) Ethylene resistance in flowering ornamental plants. Improvements and Future Perspectives Hortic Res 2:15038
Onozaki T, Yagi M, Tanase K, Shibata M (2011) Crossings and selections for six generations based on flower vase life to create lines with ethylene resistance or ultra-long vase life in carnations (Dianthus caryophyllus L.). J Jpn Soc Hortic Sci 80:486–498
Pak C, van Doorn W (2005) Delay of Iris flower senescence by protease inhibitors. New Phytol 165(2):473–480
Peary JS, Prince TA (1990) Floral lipoxygenase: activity during senescence and inhibition by phenidone. J Am Soc Hortic Sci 115:455–457
Phi C, Van N, Thi L, Thi P, Xuan V, Thi C (2021) Influence of salicylic acid on some physiological responses of chrysanthemum “Mai Vang.” Asian J Plant Sci 20:44–44
Phillips HL, Kende H (1980) Structural changes in flowers ofIpomoea tricolor during flower opening and closing. Protoplasma 102(3):199–215
Price AM, Aros Orellana DF, Salleh FM, Stevens R, Acock R, Buchanan-Wollaston V, Stead AD, Rogers HJ (2008) A comparison of leaf and petal senescence in wallflower reveals common and distinct patterns of gene expression and physiology. Plant Physiol 147(4):1898–1912
Promyou S, Ketsa S, van Doorn WG (2012) Salicylic acid alleviates chilling injury in anthurium (Anthurium andraeanum L.) flowers. Postharvest Biol Technol 64(1):104–110
Pun UK, Niki T, Ichimura K (2013) Ethanol reduces sensitivity to ethylene and delays petal senescence in cut Tweedia caerulea flowers. Plant Growth Regul 69(2):125–130
Pun UK, Yamada T, Azuma M, Tanase K, Yoshioka S, Shimizu-Yumoto H, Satoh S, Ichimura K (2016) Effect of sucrose on sensitivity to ethylene and enzyme activities and gene expression involved in ethylene biosynthesis in cut carnations. Postharvest Biol Technol 121:151–158
Rabiza-Świder J, Rochala J, Jędrzejuk A, Skutnik E, Łukaszewska A (2016) Symptoms of programmed cell death in intact and cut flowers of clematis and the effect of a standard preservative on petal senescence in two cultivars differing in flower longevity. Postharvest Biol Technol 118:183–192
Rabiza-Świder J, Skutnik E, Jędrzejuk A (2017) The effect of preservatives on water balance in cut clematis flowers. J Hortic Sci Biotechnol 92(3):270–278
Rauter S, Stock M, Black B, Drost D, Dai X, Ward R (2022) Overwintering improves ranunculus cut flower production in the US Intermountain West. Horticulturae 8(12):1128
Reid MS (2004) Cut flowers and greens. Agriculture Handbook, Davis, p 66
Rezvanypour S, Osfoori M (2011) Effect of chemical treatments and sucrose on vase life of three cut rose cultivars. J Agric Res 7:133–139
Rice G, Strangman E (1993) The gardener’s guide to growing hellebores. David & Charles, Devon, p 160
Rogers HJ (2012) Is there an important role for reactive oxygen species and redox regulation during floral senescence? Plant Cell Environ 35:217–233
Rogers HJ (2013) From models to ornamentals: how is flower senescence regulated? Plant Mol Biol 82:563–574
Ronen M, Mayak S (1981) Interrelationship between abscisic acid and ethylene in the control of senescence processes in carnation flowers. J Exp Bot 32:759–765
Rubinstein B (2000) Regulation of cell death in flower petals. Plant Mol Biol 44:303–318
Sadeghi HN, Hashemabadi D (2016) Improvement postharvest quality of cut alstroemeria (Alstroemeria hybrida) by stem-end splitting and ethanol. J Ornam Plants 6:49–58
Saeed T, Hassan I, Abbasi NA, Jilani G (2014) Effect of gibberellic acid on the vase life and oxidative activities in senescing cut gladiolus flowers. Plant Growth Regul 72:89–95
Sairam RAJK, Vasanthan B, Arora A (2012) Calcium regulates gladiolus flower senescence by influencing senescence associated genes. Indian J Plant Physiol 17:28–36
Saks Y, van Staden J, Smith MT (1992) Effect of gibberellic acid on carnation flower senescence evidence that the delay of carnation flower senescence by gibberellic acid depends on the stage of flower development. Plant Growth Regul 12:105–110
Salopek-Sondi B, Magnus V (2007) Developmental studies in the Christmas rose (Helleborus niger L.). Int J Plant Dev Biol 1:151–159
Sanikhani M, Mibus H, Stummann BM, Serek M (2008) Kalanchoe blossfeldiana plants expressing the Arabidopsis etr1-1 allele show reduced ethylene sensitivity. Plant Cell Rep 27:729–737
Savin KW, Baudinette SC, Graham MW, Michael MZ, Nugent GD et al (1995) Antisense ACC Oxidase RNA delays carnation petal senescence. J Hortic Sci 30:970–972
Savin KW, Baudinette SC, Graham MW et al (2019) Antisense ACC oxidase rna delays carnation petal senescence. HortScience 30:970–972
Seglie L, Devecchi M, Trotta F, Scariot V (2013) β-Cyclodextrin-based nanosponges improve 1-MCP efficacy in extending the postharvest quality of cut flowers. Sci Hortic 159:162–165
Shahri W, Tahir I (2010a) Effect of cycloheximide on postharvest performance in cut spikes of Consolida ajacis cv violet blue. J Appl Hortic 13(2):134–138
Shahri W, Tahir I (2010b) Effect of cycloheximide on senescence and post-harvest performance of Ranunculus asiaticus L. flowers. Pak J Bot 42(5):3577–3585
Shahri W, Tahir I (2011a) Flower development and senescence in Ranunculus asiaticus L. J Fruit Ornam Plant Res 19(2):123–131
Shahri W, Tahir I (2011b) Physiological and biochemical changes associated with flower development and senescence in Consolida ajacis Nieuwl cv. violet blue. Front Agric China 5(2):201
Shahri W, Tahir I, Islam ST, Ahmad M (2010) Response of some ornamental flowers of family Ranunculaceae to sucrose feeding. Afr J Plant Sci 4(9):346–352
Shahri W, Tahir I, Islam ST, Bhat MA (2011a) Physiological and biochemical changes associated with flower development and senescence in so far unexplored Helleborus orientalis Lam. cv olympicus. Physiol Mol Biol Plants 17:33–39
Shahri W, Tahir I, Islam ST, Bhat MA (2011b) Effect of ethylene antagonists (STS and AOA) on postharvest senescence of Ranunculus asiaticus L. flowers. Res J Bot 6(2):95–99
Sharifani M, Parizadeh M, Mashayekhi K (2004) The effects of pre-storage treatments on postharvest quality of cut anemone (Anemone coronaria) flowers. In: V International Postharvest Symposium. Vol. 682, pp. 701–708
Shan C, Zhao X (2015) Lanthanum delays the senescence of Lilium longiflorum cut flowers by improving antioxidant defense system and water retaining capacity. Sci Hortic 197:516–520
Shibata M (2008) Importance of genetic transformation in ornamental plant breeding. Plant Biotech J 25:3–8
Shibuya K (2018) Molecular aspects of flower senescence and strategies to improve flower longevity. Breed Sci 68:99–108
Shibuya K, Clark DG (2006) Ethylene: current status and future directions of using transgenic techniques to improve flower longevity of ornamental crops. J Crop Improv 18:391–412
Shibuya K, Ichimura K (2016) Physiology and molecular biology of flower senescence. In: Pareek S (ed) Postharvest ripening physiology of crops. CRC Press, Boca Raton, pp 109–129
Shibuya K, Barry KG, Ciardi JA, Loucas HM, Underwood BA, Nourizadeh S, Ecker JR, Klee HJ, Clark DG (2004) The central role of PhEIN2 in ethylene responses throughout plant development in petunia. Plant Physiol 136:2900–2912
Shibuya K, Yamada T, Ichimura K (2016) Morphological changes in senescing petal cells and the regulatory mechanism of petal senescence. J Exp Bot 67(20):5909–5918
Smith MT, Saks Y, Staden JV (1992) Ultrastructural changes in the petals of senescing flowers of Dianthus caryophyllus L. Ann Bot 69(3):277–285
Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A (1999) The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11:431–443
Stead AD, Van Doorn WG (1994) Strategies of flower senescence: a review. Molecular and cellular aspects of plant reproduction. Cambridge University Press, Cambridge, pp 215–238
Sumitomo K, Narumi T, Satoh S, Hisamatsu T (2008) Involvement of the ethylene response pathway in dormancy induction in chrysanthemum. J Exp Bot 59:4075–4082
Tahir I, Nisar S, Dar RA (2018) Gibberellin and cytokinins modulate flower senescence and longevity in Nicotiana plumbaginifolia. In: XXX International Horticultural Congress IHC2018: International Symposium on Ornamental Horticulture and XI International, vol 1263. pp 469–476
Tanase K, Onozaki T (2016) Regulation of ethylene-and senescence-related genes in pot carnation flowers during flower senescence. Hort J 85(3):254–263
Tanase K, Otsu S, Satoh S, Onozaki T (2013) Expression and regulation of senescence-related genes in carnation flowers with low ethylene production during senescence. J Jpn Soc Hortic Sci 82:179–187
Taverner EA, Letham DS, Wang J, Cornish E (2000) Inhibition of carnation petal inrolling by growth retardants and cytokinins. Funct Plant Biol 27(4):357–362
Tekalign T, Tilahun S, Humphries G (2011) Influence of pulsing biocides and preservative solution treatment on the vase life of cut rose (Rosa hybrida L) varieties. Ethiop J Appl Sci Technol 2(2):1–18
Thompson JE, Froese CD, Madey E, Smith MD, Hong YW (1998) Lipid metabolism during plant senescence. Prog Lipid Res 37:119–141
Tripathi SK, Tuteja N (2007) Integrated signaling in flower senescence: an overview. Plant Signal Behav 2(6):437–445
Trivellini A, Vernieri P, Ferrante A, Serra G (2007) Physiological characterization of flower senescence in long life and ephemeral hibiscus (Hibiscus rosa-sinensis L). Acta Hortic 755:457–464
Trivellini A, Ferrante A, Vernieri P, Serra G (2011) Effects of abscisic acid on ethylene biosynthesis and perception in Hibiscus rosa-sinensis L. flower development. J Exp Bot 62(15):5437–5452
Trivellini A, Cocetta G, Vernieri P, Mensuali-Sodi A, Ferrante A (2015) Effect of cytokinins on delaying petunia flower senescence: a transcriptome study approach. Plant Mol Biol 87(1):169–180
Valero D (2009) The role of polyamines on fruit ripening and quality during storage: what is new. XI Int Symp Plant Bioregulators in Fruit Prod 884:199–205
Van Doorn WG (2001) Categories of petal senescence and abscission: a re-evaluation. Ann Bot 87(4):447–456
Van Doorn WG (2004) Is petal senescence due to sugar starvation? Plant Physiol 134:35–42
Van Doorn WG, Woltering EJ (2008) Physiology and molecular biology of petal senescence. J Exp Bot 59(3):453–480
Van Meeteren U, Aliniaeifard S (2016) Stomata and postharvest physiology. In: Pareek S (ed) Postharvest ripening physiology of crops. CRC Press, Boca Raton, pp 157–216
Van Doorn WG, Balk PA, van Houwelingen AM, Hoeberichts FA, Hall RD, Vorst O, van der Schoot C, van Wordragen MF (2003) Gene expression during anthesis and senescence in Iris flowers. Plant Mol Biol 53:845–863
Van Doorn WG, Kirasak K, Sonong A, Srihiran Y, van Lent J, Ketsa S (2011) Do plastids in Dendrobium cv Lucky Duan petals function similar to autophagosomes and autolysosomes? ATG. 7(6):584–597
Vehniwal SS, Abbey L (2019) Cut flower vase life-influential factors, metabolism and organic formulation. Hort Int J 3(6):275–281
Wagstaff C, Leverentz MK, Griffiths G, Thomas B, Chanasut U, Stead AD, Rogers HJ (2002) Cysteine protease gene expression and proteolytic activity during senescence of Alstroemeria petals. J Exp Bot 53:233–240
Wagstaff C, Malcolm P, Rafiq A, Leverentz M, Griffiths G, Thomas B, Rogers H (2003) Programmed cell death (PCD) processes begin extremely early in Alstroemeria petal senescence. New Phytol 160:49–59
Wang C, Fang H, Gong T, Zhang J, Niu L, Huang D, Huo J, Liao W (2020a) Hydrogen gas alleviates postharvest senescence of cut rose ‘Movie star’ by antagonizing ethylene. Plant Mol Biol 102:271–285
Wang Y, Zhao H, Liu C, Cui G, Qu L, Bao M, Wang Y (2020b) Integrating physiological and metabolites analysis to identify ethylene involvement in petal senescence in Tulipa gesneriana. Plant Physiol Biochem 149:121–131
Wiemken-Gehrig V, Wiemken A, Matile P (1974) Mobilisation von Zellwandstoffen in der welkenden Blüte von Ipomoea tricolor Cav. Planta 115(4):297–307
Wilhelmova N, Domingues PMDN, Srbova M, Fuksova H, Wilhelm J (2006) Changes in non-polar aldehydes in bean cotyledons during aging. Biol Plant 50:559–564
Winkelmann T, Warwas M, Raffeiner B, Serek M, Mibus H (2016) J Plant Growth Regul 35:390–400
Woltering EJ, van Doorn WG (1988) Role of ethylene in senescence of petals—morphological and taxonomical relationships. J Exp Bot 39:1605–1616
Wongjunta M, Wongs-Aree C, Salim S, Meir S, Philosoph-Hadas S, Buanong M (2021) Involvement of ethylene in physiological processes determining the vase life of various hybrids of Mokara orchid cut flowers. J Agron 11(1):160
Wu F, Zhang C, Wang X, Guo J, Dong L (2017) Ethylene-influenced development of tree peony cut flowers and characterization of genes involved in ethylene biosynthesis and perception. Postharvest Biol Technol 125:150–160
Wulster G, Sacalis J, Hanes H (1982) The effect of inhibitors of protein synthesis on ethylene-induced senescence in isolated carnation petals. J Am Soc Hortic 107:112–115
Xu Y, Hanson MR (2000) Programmed cell death during pollination induced petal senescence in petunia. Plant Physiol 122:1323–1333
Xu X, Gookin T, Jiang, C, Reid MS (2007) Genes associated with opening and senescence of Mirabilis jalapa flowers. J Exp Bot 58:2193–2201
Xu X, Jiang C, Reid MS (2008) Functional analysis of a putative ubiquitin ligase that is highly expressed during flower senescence. J Exp Bot 58:3623–3630
Xu J, Kang BC, Naing AH, Bae SJ, Kim JS, Kim H, Kim CK (2020) CRISPR/Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 enhances Petunia flower longevity. Plant Biotechnol J 18(1):287–297
Xue J, Huang Z, Wang S, Xue Y, Ren X, Zeng X et al (2020) Dry storage improves the vase quality of cut peony by increasing water uptake efficiency through aquaporins regulation. Plant Physiol Biochem 148:63–69
Yaghoubi KD, Yadegari M (2016) The effect of ethanol and cycloheximide on the vase life of cut flowers Alstroemeria (Alstroemeria hybrida). J Ornam Plants 6:73–84
Yamada T, Ichimura K (2007) Relationship between petal abscission and programmed cell death in Prunus yedoensis and Delphinium belladonna. Planta 226:1195–1205
Yamada T, Ichimura K, van Doorn WG (2006) DNA degradation and nuclear degeneration during programmed cell death in petals of Antirrhinum, Argyranthemum, and Petunia. J Exp Bot 57(14):3543–3552
Yamada T, Ichimura K, Kanekatsu M, van Doorn WG (2009) Homologs of genes associated with programmed cell death in animal cells are differentially expressed during senescence of Ipomoea nil petals. Plant Cell Physiol 50:610–625
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Ann Rev Plant Physiol 35:155–189
Yoshida K, Kondo T, Okazaki Y, Katou K (1995) Cause of blue petal colour. Nature 373:291
Yousefi F, Jabbarzadeh Z, Amiri J, Rasouli-sadaghiani MH (2019) Response of roses (Rosa hybrida l. ‘herbert stevens’) to foliar application of polyamines on root development, flowering, photosynthetic pigments, antioxidant enzymes activity and npk. Sci Rep 9:1–11
Zakizadeh H, Lütken H, Sriskandarajah S, Serek M, Müller R (2013) Transformation of miniature potted rose (Rosa hybrida cv Linda) with PSAG12-ipt gene delays leaf senescence and enhances resistance to exogenous ethylene. Plant Cell Rep 32(2):195–205
Acknowledgements
The authors thank Professor Zahoor Ahmad Kaloo for providing the necessary suggestions.
Author information
Authors and Affiliations
Contributions
AUH contributed to literature survey and drafting of manuscript. SF, MLL, SP, and FA contributed to literature survey and reviewing. IT contributed to editing and reviewing.
Corresponding author
Ethics declarations
Conflict of interest
The authors affirm that they have no conflicts of interest about this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haq, A.u., Farooq, S., Lone, M.L. et al. Flower Senescence Coordinated by Ethylene: An Update and Future Scope on Postharvest Biology in the “Buttercup” Family. J Plant Growth Regul 43, 402–422 (2024). https://doi.org/10.1007/s00344-023-11122-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11122-9