Abstract
Ethylene regulates the photosynthetic efficiency of plants grown under challenging environments by the regulation of the antioxidant system and other biomolecules, such as osmolytes (proline). The role of ethylene in modulating proline biosynthesis and subsequent changes in antioxidant system to protect wheat (Triticum aestivum L. cv. WH-711) against heat stress was studied. The effects of exogenously sourced ethylene (as 200 µL L−1 ethephon: 2-chloroethylphosphonic acid) and proline (50 mM) were studied in the protection of photosynthetic performance and heat stress tolerance by studying mechanisms of proline biosynthesis, activity and gene expression of antioxidants, and ethylene evolution. The cultivars WH-711, RAJ-3765, PBW-373, HD-2967, PBW-550, DBW-17, PBW-343, and UP-2338 were screened for their proline accumulation capacity and tolerance to heat stress. Plants of the cultivar WH-711 with higher proline accumulation and heat tolerance capacity were subjected to a temperature of 40 °C for 6 h per day over 15 days and then allowed to recover at 28 °C. These plants showed increased H2O2 and TBARS (thiobarbituric acid reactive substances), proline accumulation, and ethylene evolution, activity of antioxidant enzymes, and reduced photosynthetic characteristics. Ethephon plus proline supplementation under heat stress upregulated the antioxidant defense system, reduced oxidative stress, and upregulated psbA and psbB expression and photosynthesis. The study’s outcome may be taken to improve photosynthetic performance and heat stress tolerance through ethylene-enhanced proline accumulation and antioxidant defense system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change has been a significant threat to the environment for decades. In the long run, temperature shifts have been a significant concern experienced globally (Marsicek et al. 2018). It is gradually rising due to the unorganized and unmethodical anthropogenic activities, which unavoidably affect plants. Plants are affected by the progressively increasing temperatures above the threshold at every stage of their life cycle and exhibit metabolically and physiologically irreversible alterations. High-temperature stress has been understood as an exposure to heat that is sufficiently higher than the threshold limit (usually 10–15 °C) for a specified period to induce irreversible alterations (Wahid et al. 2007). Heat stress impairs the growth and development of plants through disruption of the photosynthetic apparatus, which causes the functioning of pigment system II (PSII) to be compromised (Crafts-Brandner et al. 2000; Hasanuzzaman et al. 2013; Wang et al. 2018). Studies have shown that the photosynthetic attributes, stomatal conductance (Haworth et al. 2018; Zhou et al. 2018), intercellular CO2 levels (Wang et al. 2010), and the activity of ribulose 1,5-bisphosphate carboxylase (Rubisco) (Crafts-Brandner et al. 2000; Kumar et al. 2016; Perdomo et al. 2017) were adversely affected by heat stress resulting in significant yield suppression in cereals, oilseed and other cultivated crops (Zhang et al. 2012; Hussain et al. 2019). Recently, Hu et al. (2020) have reported that heat stress affected processes of photophosphorylation, thylakoid membrane fluidity, chlorophyll biosynthesis, and CO2 assimilation. The expression of heat stress-responsive nuclear genes for the plastid transcription machinery affected the transcript accumulation of plastid-encoded genes in A. thaliana, at least in part (Danilova et al. 2018). In wheat leaves, heat stress decreased the expression of the psbA, psbB, and psbC genes, which encode D1, CP47, and CP43 proteins, respectively (Fatma et al. 2021b).
When exposed to heat stress, plants respond in several ways to sustain their growth and production under stressful environments. The response of stress-exposed plant is affected by its developmental stage and the severity of the stress component (Brestic et al. 2012; Yamori et al. 2014). The uncontrolled generation of reactive oxygen species (ROS) under heat stress disrupts cellular functions, causes denaturation of DNA and protein and lipid peroxidation, and eventually cell death (Chaudhary et al. 2021). Medina et al. (2021) recently reviewed and summarized the physiological role and signaling of ROS produced in plants during heat stress. It has been shown that ROS help in heat stress signaling, upregulate the synthesis of heat shock proteins (HSPs) and coordinate several different signaling pathways and transcription factors for thermotolerance (Kotak et al. 2007; Liu et al. 2018; Argosubekti 2020).
The inorganic solutes act as osmoprotectants under stressful environments. Heat stress disturbs the osmotic homeostasis, and proline accumulation has been an adaptive strategy to counter the osmotic crisis (Wang et al. 2020). To reduce physiological and biochemical alterations due to heat stress, plants activate the signaling cascades and transcriptional factors (Hasanuzzaman et al. 2013). To minimize the adversity of heat shock, proline (osmolyte) production, and accumulation have been found crucially significant for the plants’ survival and sustenance. In heat-stressed sugarcane leaves, proline was responsible for the increased pressure potential (Wahid et al. 2007). Plants with increased proline content were less affected by stress conditions than wild-type plants with lower proline concentration. Proline synthesis upregulation under stressful conditions has proved as an adaptive criterion for stress tolerance and growth (Kishor et al. 2005). Proline accounts for maintaining cellular osmotic potential under stressful environments. It regulates photosynthesis by improving PSII electron transport and maintaining cellular redox potential, detoxifying ROS, and stabilizing proteins (Ashraf and Foolad 2007; Naliwajski and Sklodowska 2014; Iqbal et al. 2015).
Phytohormones behave as one of the significant compounds responsible for heat mitigation. Upregulation of several phytohormones has been observed in plant species under heat stress. Some plant hormones showed accelerated production, while others were comparatively suppressed in response to the stress stimuli. Plant hormones maintain cellular homeostasis for heat stress tolerance (Alonso-Ramírez et al. 2009; Nazar et al. 2011; Hasanuzzaman et al. 2012; Kwon et al. 2015; reviewed by Li et al. 2021). The proline and other compatible solutes have been shown to be regulated by phytohormones (Per et al. 2017; Iqbal et al. 2019). There is evidence of combined regulation of heat stress signaling by ethylene, salicylic acid, abscisic acid, and jasmonic acid (Larkindale et al. 2005; Muller and Munné-Bosch 2015). Ethylene regulates proline accumulation and osmotic adjustment (Per et al. 2017) and increases abiotic stress tolerance, such as heat (Khan et al. 2013; Gautam et al. 2022), salt stress (Jahan et al. 2021), and metal stress (Khan et al. 2020).
Ethylene, a gaseous signaling molecule, has been shown to be imperative for heat stress tolerance (Gautam et al. 2022). Ethylene-dependent responses in heat prone plants are dose and time determined; a low dose promotes plant defense signaling, while a higher dose inhibits (Chang et al. 2010; Khan et al. 2013; Riyazuddin et al. 2020). Under heat stress, plant cells tend to upregulate various cascades of ethylene signaling, among which the ethylene response factor (ERF) plays a significant role (Xu et al. 2019; Riyazuddin et al. 2020). Ethylene-regulated heat tolerance in plants is mediated by several mechanisms and procedures to acquire cellular integrity, protect photosynthetic setup, and reverse oxidative damage. Savada et al. (2017) have shown that the reproductive physiology of the plant was compromised in response to heat stress in Pisum sativum. The biosynthesis of ethylene occurred in a tissue-specific pattern that was influenced by the environment and developmental stage of the tissue. However, the information on ethylene and proline coordination and the mechanism as to how the interaction of these mitigates heat stress are less studied. Ethylene’s influence on proline production may affect heat stress tolerance. There could be a link between ethylene synthesis and the modulation of osmolyte (proline) accumulation to protect plants against heat stress. Iqbal et al. (2015) showed that ethylene has a role in regulating salinity stress by influencing the accumulation of osmoprotectants. Studies on ein2-5 and ein3-1 (ethylene insensitive) mutants validated the role of ethylene in osmolyte biosynthesis (Cui et al. 2015). The study was undertaken to understand the mechanisms induced by ethylene and proline individually or together to protect photosynthesis under heat stress.
Materials and Methods
Healthy seeds of wheat (Triticum aestivum L.) cultivars, WH-711, RAJ-3765, PBW-373, HD-2967, PBW-550, DBW-17, PBW-343, and UP-2338, were surface sterilized with 0.01% HgCl2 and repeatedly washed with deionized water before sowing in 15-cm-diameter earthen pots filled with acid-washed sand. Two plants per pot were maintained and were placed in the net house of the Department of Botany, Aligarh Muslim University, Aligarh (India), where day/night temperatures were 25/18 ± 3 °C, photoperiod (12 h, 680 µmol m−2 s−1), and relative humidity of 65 ± 5%. The plants were saturated with 300 ml of full-strength Hoagland’s nutrition solution on alternate days. In the experimentation, one set of plants was maintained at 25 °C (no stress). In contrast, the other set was subjected to 40 °C for 6 h daily for 15 days (heat stress) in the environmental growth chamber (Khera KI-261, New Delhi, India) and was allowed to recover at 25 °C and grown for the experimental period.
In the first experiment, screening of the cultivars was done to select heat-tolerant cultivar with high-proline accumulation capacity. The cultivar WH-711 and UP-2338 emerged as high and low proline accumulation with heat-tolerant and heat susceptible capacity. In the further experiment to study ethylene’s influence in the presence or absence of proline in mitigating heat stress, plants were treated with 200 µl L−1 ethephon (ETH, 2-chloroethylphosphonic acid) and/or 50 mM proline grown under normal and heat stress. A control set was also maintained. The ethephon concentration was based on our earlier experience (Gautam et al. 2022), while the concentration of proline was selected from a preliminary screening of the effect of proline (0, 50, and 100 mM) on photosynthesis and proline biosynthesis (unpublished). A surfactant teepol (0.5%) was added with the control and ethephon treatments. The treatments were arranged in a randomly blocked design. There were four replicates (n = 4) for each treatment. Plants were sampled 30 days after germination (DAG), and different parameters were recorded.
Determination of Photosynthetic and Growth Attributes
Net photosynthesis, stomatal conductance, and intercellular CO2 concentration were measured in fully expanded topmost intact leaves of plants in each treatment using Infrared Gas Analyzer (CID-340, photosynthesis system, Bioscience, Camas, WA, USA). The measurements were taken between 11 and 12 h at a light saturating intensity, a temperature of 22 °C and a relative humidity of about 60%. The content of chlorophyll was measured in the plants’ intact second top leaves with the help of SPAD Chlorophyll meter (502 DL PLUS, Spectrum Technologies, Plainfield, IL, USA).
To determine plant dry mass, plants were gently uprooted, cleaned under running tap water to eliminate dust, and dried in a hot air oven at 80 °C until constant weight. Leaf area was calculated using a leaf area meter (LA211, Systronic, New Delhi, India).
Measurement of Chlorophyll Fluorescence
Junior-PAM chlorophyll fluorometer (Heinz Walz, GmbH, Effeltrich, Germany) was used to measure chlorophyll fluorescence. Supplementary File S1 included details of the measurements.
Estimation of Proline and Glycine Betaine Content
The procedure described by Bates et al. (1973) was used to measure proline content in leaves. The details of the method are given in Supplementary File S1.
The method of Grieve and Grattan’s (1983) was followed to estimate glycine betaine (GB) content in leaves by monitoring the production of betaine-periodite complex. The details of the procedure are given in Syeed et al. (2021).
Contents of H2O2 and TBARS
The method of Okuda et al. (1991) was used to determine the content of H2O2 in leaves, and the content of thiobarbituric acid reactive substances (TBARS) as a measure of the status of lipid peroxidation in leaves was determined by the method given by Dhindsa et al. (1981). The details of the procedure are given in Supplementary File S1.
Assay of Antioxidants Enzyme Activities
Fresh leaves were homogenized in a chilled mortar and pestle with an extraction solution comprising 0.05% (v/v) Triton X-100 and 1% (w/v) PVP in potassium phosphate buffer (100 mM, pH 7.0). The supernatant obtained after centrifugation was used for the assay of superoxide dismutase (SOD, EC; 1.15.1.1) and glutathione reductase (GR, EC; 1.6.4.2) enzymes. For the assay of ascorbate peroxidase (APX, EC; 1.11.1.11), 2.0 mM ascorbate was supplemented with extraction buffer. The activity of SOD was measured using the methods of Beyer and Fridovich (1987) and Giannopolitis and Ries (1977). The activity of APX was assessed using the Nakano and Asada (1981) method, which involved recording the decrease in ascorbate absorbance at 290 nm. The activity of GR was determined using Foyer and Halliwell (1976) method, which involved measure of glutathione-dependent oxidation of NADPH at 340 nm. The details of the methods are given in Supplementary File S1.
Membrane Stability Index
Membrane stability index was determined by adopting the method of Das and Uprety (2006). Fresh leaf samples were cut into discs of small size. The samples (0.2 g) were collected in test tubes with 10 ml of double distilled water. The electrical conductivity (C1) of the samples was measured after 30 min of incubation at 40 °C in a water bath. The samples were transferred to other test tubes and incubated in a boiling water bath at 100 °C for 15 min, after which electrical conductivity (C2) was measured as described before, and the membrane stability index is computed and reported in percentage using the formula.
RNA Isolation, cDNA Synthesis, and Real-Time RT-PCR
TRIzol reagent (Ambion, Life Technologies, Austin, TX, USA) was used to extract total RNA from the leaves of treated and control plants according to the manufacturer’s instructions. The Nanodrop spectrophotometer was used to measure the amount of isolated RNA (Thermo Scientific, Waltham, MA, USA). Each sample was run on an agarose formaldehyde gel (Turano et al. 1997). Real-time PCR (RT-PCR) was performed in 96-well reaction plates (Roche, Mannheim, Germany) containing a 20-µl reaction mixture of 10X reaction buffer, 2 mM dNTPs, 1 mM MgCl2, 0.35 µM each of forward and reverse primers, 1 µl SYBR Green (10X), 10 µg cDNA template, and 5 U Taq polymerase on a thermal cycler (Lightcycler 480 II, Roche, Germany). Table S1 lists the primer pairs used in quantitative RT-PCR. The process is detailed out in Supplementary File S1.
Estimation of ACS Activity and Ethylene Evolution
Activity of 1-aminocyclopropanecarboxylic acid synthase (ACS; EC, 4.4.1.14) was determined by adopting the methods of Avni et al. (1994) and Woeste et al. (1999). Leaf tissue (5.0 g) was homogenized in a solution containing 100 mM HEPES (pH 8.0), 4 mM DTT, 2.5 mM pyridoxal phosphate, and 25% PVP. The homogenized material was centrifuged for 15 min at 12,000 × g. One milliliter of the supernatant was transferred to a 30 ml tube, along with 0.1 ml of 5 mM S-adenosyl methionine (AdoMet) and the mixture was incubated at 22 °C for 2 h. The amount of ACC produced was quantified by converting it to ethylene using 0.1 ml of 20 mM HgCl2 followed by addition of 0.1 ml of a 1:1 ratio of saturated NaOH/NaCl in an ice bath for 10 min. AdoMet was not included in the control group. A gas chromatograph was used to determine the amount of ethylene present. The details of the procedure are given earlier by Fatma et al. (2021a) and presented in Supplementary File S1.
Statistical Analysis
The data were statistically analyzed using analysis of variance (ANOVA) in SPSS 17.0 for Windows and reported as mean ± SE (n = 4). For the significant data, the least significant difference was calculated at p < 0.05. The data bars with the same letter were not significantly different by the least significant difference (LSD) test at p < 0.05.
Results
Screening of Cultivars for Heat Stress Tolerance: Plant Growth and Physiological Parameters
Plants grown under heat stress exhibited decrease in growth and photosynthetic characteristics as compared to control plant (Table 1). The cultivar WH-711 showed minimum reduction in leaf area and plant dry mass by 53.8% and 57%, chlorophyll content, net photosynthesis, and Fv/Fm by 57.2%, 61.7%, and 29.1%, respectively, and maximum proline accumulation of 93.8% compared to control plants under heat stress. Contrarily, the cultivar UP-2338 exhibited maximum decrease in the aforementioned parameters and less accumulation of proline compared to control plants under heat stress. On this basis, WH-711 was selected as the most heat-tolerant and UP-2338 as heat-sensitive cultivars. The screened cultivars showed heat stress tolerance in the order: WH-711 > RAJ-3765 > PBW-373 > HD-2967 > PBW-550 > DBW-17 > PBW-343 > UP-2338.
Ethephon in the Presence of Proline Increases Photosynthesis and Growth Under Heat Stress
Heat stress had a considerable impact on leaf gas exchange parameters and chlorophyll content (SPAD value). In particular, high-temperature stress decreased net photosynthesis, stomatal conductance, intercellular CO2 concentration, and SPAD value, Fv/Fm, and Rubisco activity by 47.5%, 28.5%, 47.2%, 58.5%, 19.4%, and 36.7%, respectively, in comparison to control. Under no stress condition, ethephon or proline application increased the above photosynthetic parameters; however, their combined application maximally increased these parameters in comparison to control. In addition, plants supplemented with ethephon under heat stress demonstrated increments in net photosynthesis, stomatal conductance, intercellular CO2 concentration, SPAD value, Fv/Fm, and Rubisco activity by 96.8%, 38.3%, 58.9%, 71.3%, 22.4%, and 63.0%, respectively, compared to the heat-stressed plants. Similarly, proline application to plants under heat stress showed increment in the above-mentioned parameters by 68.8%, 25.8%, 67.5%, 50.0%, 15.5%, and 32.6%, respectively, compared to the heat-stressed plants. Finally, the combined application of ethephon and proline maximally mitigated the negative effects of heat stress and significantly increased photosynthetic attributes by 156.2%, 62.5%, 193%, 106%, 53.4%, and 92.7%, respectively, compared to the plants exposed to heat stress (Table 2).
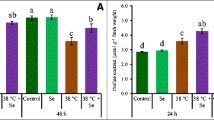
Notably, heat stress more severely hampered plant growth relative to control plants. Individual application of ethephon and proline resulted in increased leaf area and plant dry mass under both normal and heat stress conditions. In compared to heat-stressed plants, ethephon or proline treatment minimized the deleterious effects of heat stress and increased leaf area and plant dry mass. However, application of both ethephon and proline under heat stress maximally alleviated the negative effects caused by heat stress and showed significant increase in leaf area and plant dry mass by 76.1% and 177.0%, respectively, compared to heat-stressed plants (Fig. 1).
Leaf area (a) and plant dry mass (b) in wheat (Triticum aestivum L.) leaves at 30 days after sowing. Plants were treated with/without heat stress (40 °C for 6 h daily for 15 days) and 200 µl L−1 ETH and/or 50 mM proline. Data are presented as treatment mean ± SE (n = 4). Data followed by same letter are not significantly different by the LSD test at p < 0.05. ETH ethephon, HT heat stress, Pro proline
Effect of Ethephon and Proline on the Expression of Genes Encoding Core PSII Proteins
The expression of two photosynthetic genes, psbA and psbB, which encode D1 protein and CP47, respectively, was studied to know about the protective role of ethylene/proline under heat stress. Under normal condition, individual application of ethephon and proline equally raised psbA expression by 7.5 times and psbB expression by 9 times compared to control; however, heat stress decreased the expression by 0.5 times and 0.6 times. Under heat stress, plants supplemented with either ethephon or proline elevated the expression of psbA and psbB by 9 times and 13 times compared to control. Meanwhile, expression of psbA and psbB was maximally raised in plants treated with ethephon plus proline under heat stress by 11.2 times and 15 times compared to control (Fig. 2).
Relative expression of psbA (a) and psbB (b) in wheat (Triticum aestivum L.) leaves at 30 days after sowing. Plants were treated with/without heat stress (40 °C for 6 h daily for 15 days) and 200 µl L−1 ETH and/or 50 mM proline. Data are presented as treatment mean ± SE (n = 4). Data followed by same letter are not significantly different by the LSD test at p < 0.05. ETH ethephon, HT heat stress, Pro proline
Ethephon and Proline Reduce the Oxidative Stress and Maintain Membrane Stability Index Under Heat Stress
The extent of cellular damage caused by heat stress-induced oxidative stress was determined in terms of H2O2 content and membrane damage as TBARS content (Fig. 3). Relative to the control plants, heat stress significantly enhanced H2O2 and TBARS content by 138.8% and 169.1% in comparison to control plants. The individual application of ethephon and proline proved effective in mitigating the heat stress-induced oxidative stress and significantly reduced H2O2 content by 48.3% and 45% and TBARS content by 25.4% and 12.7%, respectively, in comparison to control plants. However, combined treatment of ethephon and proline maximally reduced heat stress-induced oxidative stress resulting in reduced H2O2 and TBARS content by 55.0% and 34.1%, respectively, relative to control plants.
Content of H2O2 (a), TBARS (b), and membrane stability index (C) in wheat (Triticum aestivum L.) leaves at 30 days after sowing. Plants were treated with/without heat stress (40 °C for 6 h daily for 15 days) and 200 µl L−1 ETH and/or 50 mM proline. Data are presented as treatment mean ± SE (n = 4). Data followed by same letter are not significantly different by the LSD test at p < 0.05. ETH ethephon, HT heat stress, Pro proline, TBARS thiobarbituric acid reactive substances
High-temperature stress significantly decreased membrane stability index by 29.4% in comparison to control plants. Under no stress, the individual application of ethephon and proline increased membrane stability index by 4.1% and 2.2%, respectively, compared to control plants, but the combined application of ethephon and proline increased it by 7.1%, compared to control plants. Supplementation of ethephon together with proline under heat stress showed maximum enhancement in membrane stability index by 9.1%, compared to control plants (Fig. 3).
Ethephon and Proline Accelerate Antioxidant Enzymes Activity Under Heat Stress
The activity of antioxidant enzymes increased in response to heat stress compared to control plants. Relative to the control plants, heat stress stimulated the activity of antioxidant enzymes, SOD, APX, and GR by 30.7%, 43.7%, and 62.1%, respectively. The individual application of ethephon and proline increased the activity of SOD by 62.2% and 57.1%, APX by 111.1% and 106.2%, and GR by 82.4% and 62.5%, respectively, compared to control plants. Under heat stress, supplementation of both ethephon and proline maximally increased activity of these antioxidant enzymes by 74.5%, 120.6%, and 68.5%, respectively, compared to heat-stressed plants (Fig. 4).
Activity of superoxide dismutase (SOD, a), ascorbate peroxidase (APX, b), and glutathione reductase (GR, c) in wheat (Triticum aestivum L.) leaves at 30 days after sowing. Plants were treated with/without heat stress (40 °C for 6 h daily for 15 days) and 200 µl L−1 ETH and/or 50 mM proline. Data are presented as treatment mean ± SE (n = 4). Data followed by same letter are not significantly different by the LSD test at p < 0.05. ETH ethephon, HT heat stress, Pro proline
Ethephon and Proline Increase Proline and Glycine Betaine Content Under Heat Stress
Figure 5 shows the content of proline and GB in plants grown under no stress or heat stress conditions and subjected to ethephon and proline treatments. Heat stress increased proline and GB content by 127.6% and 45.4%, respectively, compared to control. Ethephon and proline applied individually under no stress increased proline content by 145.2% and 124.2% and GB content by 81.8% and 54.6%, respectively, compared to the control. Plants supplemented with both ethephon and proline together under no stress condition exhibited more increase in proline and GB content by 172.5% and 110.1%, respectively, compared to control. Under heat stress, application of ethephon/proline manifested increase in proline and GB content, compared to the plants exposed to heat stress. The maximum increase in proline and GB content was observed with the combined application of ethephon and proline under heat stress by 34.1% and 56.2%, respectively, relative to the heat-stressed plants (Fig. 5).
Content of proline (a) and glycine betaine (b) in wheat (Triticum aestivum L.) leaves at 30 days after sowing. Plants were treated with/without heat stress (40 °C for 6 h daily for 15 days) and 200 µl L−1 ETH and/or 50 mM proline. Data are presented as treatment mean ± SE (n = 4). Data followed by same letter are not significantly different by the LSD test at p < 0.05. ETH ethephon, HT heat stress, Pro proline
Application of Ethephon and Proline on Gene Expression of Glutathione Reductase Under Heat Stress
Exogenous application of ethephon with proline increased the activity of antioxidant enzymes under heat stress, so we tested the changes in the expression level of GR genes by the application of ethephon with proline under heat stress (Fig. 6). Plants treated with combined application of ethephon and proline decreased GR expression by 70% and 97% compared to the control and heat-stressed plants, respectively.
Relative expression of GR in wheat (Triticum aestivum L.) leaves at 30 days after sowing. Plants were treated with/without heat stress (40 °C for 6 h daily for 15 days) and 200 µl L−1 ETH and/or 50 mM proline. Data are presented as treatment mean ± SE (n = 4). Data followed by same letter are not significantly different by the LSD test at p < 0.05. ETH ethephon, HT heat stress, Pro proline
Effect of Ethephon and Proline on ACS Activity and Ethylene Production Under Heat Stress
Plants exposed to heat stress showed higher ACS activity and ethylene production, and ethephon or proline application also increased these in comparison to control plants (Fig. 7). Under heat stress, ethephon/proline supplementation decreased ACS activity and ethylene production equally by about 62.0% and 46.7%, respectively, compared to control plants. Moreover, plants supplemented with ethephon and proline together under heat stress exhibited a maximum decrease in ACS activity and ethylene production by 77.3% and 67.7%, respectively, compared to plants exposed to heat stress.
ACS activity (a) and ethylene evolution (b) in wheat (Triticum aestivum L.) leaves at 30 days after sowing. Plants were treated with/without heat stress (40 °C for 6 h daily for 15 days) and 200 µl L−1 ETH and/or 50 mM proline. Data are presented as treatment mean ± SE (n = 4). Data followed by same letter are not significantly different by the LSD test at p < 0.05. ETH ethephon, HT heat stress, Pro proline
Discussion
A temperature rise beyond the threshold level for a long time is enough to harm agricultural plants and reduce global plant yield. On the other hand, the temperature threshold varies from species to species and between compartments within the cell (Hasanuzzaman et al. 2013; Asseng et al. 2015; Poór et al. 2021). High-temperature stress in plants is described as a temperature increase that exceeds a critical threshold for a sustained period, causing irreversible harm to plant growth and development processes (Xalxo et al. 2020; Gautam et al. 2021). The integrity of membranes, proteins, and cytoskeleton structure and the efficacy of cellular enzymatic activities are all disrupted by heat stress, impeding important physiological processes and producing metabolic imbalances (Suzuki et al. 2012). One of the principal impacts of high-temperature stress is the excessive generation of ROS, which leads to oxidative stress in the cells (Hemantaranjan et al. 2014; Gautam et al. 2021, 2022). Heat stress also causes loss of photosynthetic pigments and efficiency, disrupts thylakoid structure and electron transport chains in mitochondria and chloroplasts, reduces photoassimilate synthesis, and depletion of carbohydrate stores (Cortleven et al. 2019; Paul et al. 2020; Gautam et al. 2022). Plants enhance their inherent capacity to withstand heat stress (Song et al. 2012). The overproduction of ROS due to heat stress results in lipid peroxidation, DNA damage, protein oxidation, and cell death (Choudhury et al. 2017; Sarwar et al. 2018; Chaudhary et al. 2021). They increase the functions of the antioxidant system, as the ROS function as signaling molecule that confers plants to acclimate and adapt to abiotic stresses (Gautam et al. 2022). However, the effectiveness of these antioxidants is insufficient to minimize oxidative stress, necessitating more research into mechanisms that might enhance antioxidative metabolism. Also, osmolytes are another essential component that maintains the cell redox state by acting as an antioxidant and maintaining osmotic equilibrium. Phytohormones trigger antioxidant defense and osmolytes accumulation signals which might help to reduce ROS levels. In the present report, the screened cultivars had different potentials for heat stress tolerance. The cultivar WH-711 was most heat tolerant because of its higher capacity to accumulate proline and thus showed higher photosynthetic capacity under heat stress.
Ethylene, a gaseous hormone, is essential for plant growth and development as well as abiotic stress tolerance, such as high temperatures (Gautam et al. 2022). Indeed, ethylene is a fundamental regulator of abiotic stress responses in plants; that is, abiotic stress responses are connected to ethylene build-up at varied concentrations, affecting growth and development (Khan et al. 2014; Thao et al. 2015). Ethylene signaling also aids in the decrease of oxidative stress and the improvement of thermotolerance in plants (Wu and Yang 2019). In addition, the phytohormone and proline are important components that keep the cell redox status by acting as an antioxidant while also maintaining osmotic equilibrium. However, there is little information on how exogenous supplementation of ethephon and proline alters antioxidant metabolism and maintains photosynthesis of plants under heat stress. Both ethephon and proline alleviated heat stress impacts, and the effect of proline depended on ethylene. High-temperature stress increased oxidative stress as observed by increased H2O2 and TBARS levels; however, the same measurements were reduced in plants treated with ethephon and proline. Moreover, ethephon and proline increased the membrane stability index in plants, thus reducing oxidative stress. Abid et al. (2018) and Dwivedi et al. (2018) have also shown that supplementation of plant growth regulators positively affected membrane stability index and photosynthetic and growth attributes under water stress. However, there is no report available on ethylene- and proline-mediated regulation of membrane stability index. Further, plants’ build-up capacity of antioxidant enzymes upregulates under heat stress to reduce levels of H2O2 and TBARS. The present study demonstrated that the activity of antioxidant enzymes and GR gene expression was enhanced by ethephon and proline under heat stress and confirmed that the ethylene signaling system could modulate scavenger enzymes allowing plants to respond to heat-induced oxidative stress. Sharma et al. (2019) have reported that ethylene triggers an antioxidant defense system, reduces oxidative stress, and restores plant growth and photosynthetic efficiency. In addition to this, Zhang et al. (2011) showed that ERF95 known as ESE1 is a direct target of EIN3 and is involved in Arabidopsis’ salt stress response. Wu and Yang (2019) showed that ethylene signaling confers thermotolerance by decreasing MDA content and electrolyte leakage and regulating the heat shock factor’s transcript level in rice seedlings under heat stress. Huang et al. (2021) demonstrated that transcriptional cascade EIN3-ERF95/ERF97-HSFA2 might play a vital role in the heat stress response, indicating a link between ethylene and its downstream regulation in plant thermotolerance. The study of Wu and Yang (2019) emphasized that transcript levels of ethylene biosynthesis genes and signal transduction-related genes were upregulated under heat stress, resulting in an expansion of the ethylene signal response. They showed that the heat shock factors (Hsfs) activated the antioxidant system, lowering oxidative damage during heat stress. In the present study, ethephon and proline improved photosynthesis and growth under heat stress through the increased accumulation of osmolytes, such as proline and glycine betaine, and optimized ethylene levels under heat stress. Ethylene increases proline biosynthesis, which has a role in the regulation of abiotic stress tolerance (Iqbal et al. 2015). The increased proline metabolism was found to be related to ethylene levels in plants and salt tolerance (Alvarez et al. 2003; Szabados and Savoure 2010; Iqbal et al. 2015). Moreover, the inhibition of ethylene biosynthesis was shown to reduce proline accumulation under heat stress (Lv et al. 2011). In the present study, ethephon treatment under heat stress showed increased ACS activity and ethylene production. Notably, heat-stressed plants produced maximum ethylene; this was stress ethylene that hampered plant growth and the overall functioning. Previous research has described the importance of optimal ethylene levels and stress-induced ethylene generation (Fatma et al. 2021a; Sehar et al. 2021; Gautam et al. 2022). The physiological and metabolic alterations associated with high-temperature stress were altered by ethephon administration, which raised ethylene levels to an optimum and regulated antioxidant system and positively influenced the physiological and metabolic changes. The findings of Poór et al. (2021) also showed that supplementation of ethephon modulated osmoprotectants and the antioxidant defense system to control ROS and RNS metabolism and impart heat stress tolerance to plants. The ethylene and proline provided higher concentrations of cellular metabolites and antioxidant activity, increased quantum yield efficiency of PSII, and expression of psbA and psbB genes for improved photosynthetic performance under heat stress. The ethylene-stimulated transcription and activity of photosynthetic enzymes have been shown (Azoulay Shemer et al. 2008; Zhang et al. 2011). The balance of chlorophyll breakdown and biosynthesis and contribution of antioxidant activity are critical for the photosynthetic apparatus integrity under heat stress.
Conclusion
Conclusively, ethylene influences photosynthetic efficiency, proline, and antioxidant metabolism during heat stress. The simultaneous exogenous application of ethylene and proline considerably alleviates the harmful effects of heat stress by augmenting the antioxidant enzymes’ activity and glutathione reductase expression. It boosted photosynthesis by regulating gas exchange parameters and psbA and psbB expression. As a result, it may be said that ethylene-mediated proline accumulation and antioxidant system promoted photosynthesis under heat stress.
Change history
07 September 2023
Handling Editor has been updated with “Sudhir K. Sopory” instead of “Sarvajeet Singh Gill”
References
Abid M, Ali S, Qi LK, Zahoor R, Tian Z, Jiang D, Dai T (2018) Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci Rep 8:1–15
Alonso-Ramírez A, Rodríguez D, Reyes D, Jiménez JA, Nicolás G, López-Climent M, Nicolás C (2009) Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol 150:1335–2134
Alvarez I, Tomaro ML, Benavides MP (2003) Changes in polyamines, proline and ethylene in sunflower calluses treated with NaCl. Plant Cell Tissue Organ Cult 74:51–59
Argosubekti N (2020) A review of heat stress signaling in plants. In: IOP conference series: earth and environmental science. IOP Publishing, p 012041
Ashraf MFMR, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Asseng S, Ewert F, Martre P, Rötter RP, Lobell DB, Cammarano D, Zhu Y (2015) Rising temperatures reduce global wheat production. Nat Clim Change 5:143–147
Avni A, Bailey BA, Mattoo AK, Anderson JD (1994) Induction of ethylene biosynthesis in Nicotiana tabacum by a Trichoderma viride xylanase is correlated to the accumulation of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase transcripts. Plant Physiol 106:1049–1055
Azoulay Shemer T, Harpaz-Saad S, Belausov E, Lovat N, Krokhin O, Spicer V, Eyal Y (2008) Citrus chlorophyllase dynamics at ethylene-induced fruit color-break: a study of chlorophyllase expression, posttranslational processing kinetics, and in situ intracellular localization. Plant Physiol 148:108–118
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Brestic M, Zivcak M, Kalaji HM, Carpentier R, Allakhverdiev SI (2012) Photosystem II thermostability in situ: environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol Biochem 57:93–105
Chang C, Wang B, Shi L, Li Y, Duo L, Zhang W (2010) Alleviation of salt stress-induced inhibition of seed germination in cucumber (Cucumis sativus L.) by ethylene and glutamate. J Plant Physiol 167:1152–1156
Chaudhary C, Sharma N, Khurana P (2021) Decoding the wheat awn transcriptome and overexpressing TaRca1β in rice for heat stress tolerance. Plant Mol Biol 105:133–146
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Cortleven A, Leuendorf JE, Frank M, Pezzetta D, Bolt S, Schmülling T (2019) Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ 42:998–1018
Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci 97:13430–13435
Cui M, Lin ZuY, Efferth T, Li D, Tang Z (2015) Ethylene increases accumulation of compatible solutes and decreases oxidative stress to improve plant tolerance to water stress in Arabidopsis. J Plant Biol 58:193–201
Danilova MN, Kudryakova NV, Andreeva AA, Doroshenko AS, Pojidaeva ES, Kusnetsov VV (2018) Differential impact of heat stress on the expression of chloroplast-encoded genes. Plant Physiol Biochem 129:90–100
Das R, Uprety DC (2006) Interactive effect of moisture stress and elevated CO2 on the oxidative stress in Brassica species (No. RESEARCH)
Dhindsa RS, Plumb-Dhindsa PAMELA, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dwivedi SK, Arora A, Singh VP, Singh GP (2018) Induction of water deficit tolerance in wheat due to exogenous application of plant growth regulators: membrane stability, water relations and photosynthesis. Photosynthetica 56:478–486
Fatma M, Iqbal N, Gautam H, Sehar Z, Sofo A, D’Ippolito I, Khan NA (2021a) Ethylene and sulfur coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants 10:180
Fatma M, Iqbal N, Sehar Z, Alyemeni MN, Kaushik P, Khan NA, Ahmad P (2021b) Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 10:1216
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gautam H, Sehar Z, Rehman MT, Hussain A, AlAjmi MF, Khan NA (2021) Nitric oxide enhances photosynthetic nitrogen and sulfur-use efficiency and activity of ascorbate-glutathione cycle to reduce high temperature stress-induced oxidative stress in rice (Oryza sativa L.) plants. Biomolecules 11:305
Gautam H, Fatma M, Sehar Z, Iqbal N, Albaqami M, Khan NA (2022) Exogenously-sourced ethylene positively modulates photosynthesis, carbohydrate metabolism, and antioxidant defense to enhance heat tolerance in rice. Int J Mol Sci 23:1031
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2012) Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (’Triticum aestivum’ L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci 6:1314–1323
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Haworth M, Marino G, Brunetti C, Killi D, De Carlo A, Centritto M (2018) The impact of heat stress and water deficit on the photosynthetic and stomatal physiology of olive (Olea europaea L.)—a case study of the 2017 heat wave. Plants 7:776
Hemantaranjan A, Nishant Bhanu A, Singh MN, Yadav DK, Patel PK, Singh R, Katiyar D (2014) Heat stress responses and thermotolerance. Adv Plants Agric Res 1:00012
Hu S, Ding Y, Zhu C (2020) Sensitivity and responses of chloroplasts to heat stress in plants. Front Plant Sci 11:375
Huang J, Zhao X, Bürger M, Wang Y, Chory J (2021) Two interacting ethylene response factors regulate heat stress response. Plant Cell 33:338–357
Hussain HA, Men S, Hussain S, Chen Y, Ali S, Zhang S, Wang L (2019) Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci Rep 9:1–12
Iqbal N, Umar S, Khan NA (2015) Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Pysiol 178:84–91
Iqbal N, Fatma M, Gautam H, Umar S, Sofo A, D’ippolito I, Khan NA (2021) The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 10:1778
Iqbal N, Fatma M, Khan NA, Umar S (2019) Regulatory role of proline in heat stress tolerance: modulation by salicylic acid. In: Plant signaling molecules. Woodhead Publishing, pp 437–448
Jahan B, Iqbal N, Fatma M, Sehar Z, Masood A, Sofo A, Khan NA (2021) Ethylene supplementation combined with split application of nitrogen and sulfur protects salt-inhibited photosynthesis through optimization of proline metabolism and antioxidant system in mustard (Brassica juncea L.). Plants 10:1303
Khan MIR, Iqbal N, Masood A, Per TS, Khan NA (2013) Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav 8:e26374
Khan MIR, Asgher M, Khan NA (2014) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem 80:67–74
Khan A, Bilal S, Khan AL, Imran M, Shahzad R, Al-Harrasi A, Lee IJ (2020) Silicon and gibberellins: synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera L.). Plants 9:620
Kishor PK, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KS, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 5:424–438
Kotak S, Vierling E, Bäumlein H, Koskull-Döring PV (2007) A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. Plant Cell 19:182–195
Kumar RR, Goswami S, Singh K, Dubey K, Singh S, Sharma R, Praveen S (2016) Identification of putative RuBisCo Activase (TaRca1)—the catalytic chaperone regulating carbon assimilatory pathway in wheat (Triticum aestivum) under the heat stress. Front Plant Sci 7:986
Kwon CT, Kim SH, Kim D, Paek NC (2015) The rice floral repressor early flowering1 affects spikelet fertility by modulating gibberellin signaling. Rice 8:1–11
Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138:882–897
Li N, Euring D, Cha JY, Lin Z, Lu M, Huang LJ, Kim WY (2021) Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front Plant Sci 11:2318
Liu R, Zhang X, Ren A, Shi D, Shi L, Zhu J, Zhao MW (2018) Heat stress-induced reactive oxygen species participate in the regulation of HSP expression, hyphal branching and ganoderic acid biosynthesis in Ganoderma lucidum. Microbiol Res 209:43–54
Lv WT, Lin B, Zhang M, Hua XJ (2011) Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol 156:1921–1933
Marsicek J, Shuman BN, Bartlein PJ, Shafer SL, Brewer S (2018) Reconciling divergent trends and millennial variations in Holocene temperatures. Nature 554:92–96
Medina E, Kim SH, Yun M, Choi WG (2021) Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants 10:371
Müller M, Munné-Bosch S (2015) Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol 169:32–41
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Naliwajski MR, Skłodowska M (2014) Proline and its metabolism enzymes in cucumber cell cultures during acclimation to salinity. Protoplasma 251:201–209
Nazar R, Iqbal N, Syeed S, Khan NA (2011) Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol 168:807–815
Okuda T, Matsuda Y, Yamanaka A, Sagisaka S (1991) Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol 97:1265–1267
Paul P, Mesihovic A, Chaturvedi P, Ghatak A, Weckwerth W, Böhmer M, Schleiff E (2020) Structural and functional heat stress responses of chloroplasts of Arabidopsis thaliana. Genes 11:650
Per TS, Khan NA, Reddy PS, Masood A, Hasanuzzaman M, Khan MIR, Anjum NA (2017) Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem 115:126–140
Perdomo JA, Capó-Bauçà S, Carmo-Silva E, Galmés J (2017) Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front Plant Sci 8:490
Poór P, Nawaz K, Gupta R, Ashfaque F, Khan MIR (2021) Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep 41:1–24
Riyazuddin R, Verma R, Singh K, Nisha N, Keisham M, Bhati KK, Gupta R (2020) Ethylene: a master regulator of salinity stress tolerance in plants. Biomolecules 10:959
Sarwar M, Saleem MF, Ullah N, Rizwan M, Ali S, Shahid MR, Ahmad P (2018) Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci Rep 8:1–15
Savada RP, Ozga JA, Jayasinghege C, Waduthanthri KD, Reinecke DM (2017) Heat stress differentially modifies ethylene biosynthesis and signaling in pea floral and fruit tissues. Plant Mol Biol 95:313–331
Sehar Z, Iqbal N, Khan MIR, Masood A, Rehman M, Hussain A, Khan NA (2021) Ethylene reduces glucose sensitivity and reverses photosynthetic repression through optimization of glutathione production in salt-stressed wheat (Triticum aestivum L.). Sci Rep 11:1–12
Sharma A, Kumar V, Sidhu GPS, Kumar R, Kohli SK, Yadav P, Bhardwaj R (2019) Abiotic stress management in plants: role of ethylene. Mol Plant Abiotic Stress Biol Biotechnol 2019:185–208
Song L, Jiang Y, Zhao H, Hou M (2012) Acquired thermotolerance in plants. Plant Cell Tissue Organ Cult 111:265–276
Suzuki N, Koussevitzky SHAI, Mittler RON, Miller GAD (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Syeed S, Sehar Z, Masood A, Anjum NA, Khan NA (2021) Control of elevated ion accumulation, oxidative stress, and lipid peroxidation with salicylic acid-induced accumulation of glycine betaine in salinity-exposed Vigna radiata L. Appl Biochem Biotechnol 193:3301–3320
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trend Plant Sci 15:89–97
Thao NP, Khan MIR, Thu NBA, Hoang XLT, Asgher M, Khan NA, Tran LSP (2015) Role of ethylene and its cross talk with other signaling molecules in plant responses to heavy metal stress. Plant Physiol 169:73–84
Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Kawakami N (2008) High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol 146:1368–1385
Turano FJ, Thakkar SS, Fang T, Weisemann JM (1997) Characterization and expression of NAD (H)-dependent glutamate dehydrogenase genes in Arabidopsis. Plant Physiol 113:1329–1341
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang LJ, Fan L, Loescher W, Duan W, Liu GJ, Cheng JS, Li SH (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10:1–10
Wang QL, Chen JH, He NY, Guo FQ (2018) Metabolic reprogramming in chloroplasts under heat stress in plants. Int J Mol Sci 19:849
Wang Y, Xiong F, Nong S, Liao J, Xing A, Shen Q, Zhu X (2020) Effects of nitric oxide on the GABA, polyamines, and proline in tea (Camellia sinensis) roots under cold stress. Sci Rep 10:1–10
Woeste KE, Ye C, Kieber JJ (1999) Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol 119:521–530
Wu YS, Yang CY (2019) Ethylene-mediated signaling confers thermotolerance and regulates transcript levels of heat shock factors in rice seedlings under heat stress. Bot Stud 60:1–12
Xalxo R, Yadu B, Chandra J, Chandrakar V, Keshavkant S (2020) Alteration in carbohydrate metabolism modulates thermotolerance of plant under heat stress. Heat Stress Tolerance Plants Physiol Mol Genet Perspec 2020:77–115
Xu C, Xia Z, Huang Z, Xia C, Huang J, Zha M, Zhang C (2019) Understanding the physiological and transcriptional mechanism of reproductive stage soybean in response to heat stress. Crop Breed Genet Genom 2:1
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yamori W, Hikosaka K, Way DA (2014) Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth Res 119:101–117
Zhang L, Li Z, Quan R, Li G, Wang R, Huang R (2011) An AP2 domain-containing gene, ESE1, targeted by the ethylene signaling component EIN3 is important for the salt response in Arabidopsis. Plant Physiol 157:854–865
Zhang J, Li DM, Gao Y, Yu B, Xia CX, Bai JG (2012) Pretreatment with 5-aminolevulinic acid mitigates heat stress of cucumber leaves. Biol Plant 56:780–784
Zhou R, Wu Z, Wang X, Rosenqvist E, Wang Y, Zhao T, Ottosen CO (2018) Evaluation of temperature stress tolerance in cultivated and wild tomatoes using photosynthesis and chlorophyll fluorescence. Hortic Environ Biotechnol 59:499–509
Acknowledgements
Authors are thankful to the Council of Scientific & Industrial Research, New Delhi for support of financial assistance (Grant No. 38(1473)/19/EMR-II).
Author information
Authors and Affiliations
Contributions
ZS conducted the experiment, data collection and analysis, and writing and preparation of original draft, HG performed data analysis and manuscript editing, AM assisted in manuscript writing, NAK performed conceptualization, supervision, and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Handling Editor: Sudhir K. Sopory.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sehar, Z., Gautam, H., Masood, A. et al. Ethylene- and Proline-Dependent Regulation of Antioxidant Enzymes to Mitigate Heat Stress and Boost Photosynthetic Efficacy in Wheat Plants. J Plant Growth Regul 42, 2683–2697 (2023). https://doi.org/10.1007/s00344-022-10737-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10737-8