Abstract
Acquired thermotolerance in plants refers to the ability to cope with lethal high temperatures following acclimatization at sublethal high temperatures. Acquired thermotolerance reflects an actual thermotolerance mechanism naturally occurring in plants and has been extensively used in thermotolerant line identification. In recent years, great progress has been achieved in the elucidation of biochemical, physiological, and molecular mechanisms of thermotolerance acquisition by using genomic approaches, including microarray analysis and mutation, knockout, and overexpression of related genes. Heat shock proteins (HSPs), such as Hsp101, BOBBER1, and Hsa32, have been shown to be important for inducement and maintenance of acquired thermotolerance. Downstream target genes and upstream regulation factors of HsfA2, including Hsa32, Apx2, small ubiquitin-like modifier proteins, FK506-binding proteins ROF1 (FKBP62) and ROF2 (FKBP65), and heat shock transcription factor binding protein, have been revealed to be involved in thermotolerance acquisition regulation. Moreover, the role of abscisic acid, ethylene, hydrogen peroxide, and salicylic acid in acquired thermotolerance has been demonstrated by molecular evidence from Arabidopsis mutants and transgenic lines. Most importantly, different molecular mechanisms of thermotolerance acquisition have been shown to underlie various acclimatization methods. Establishment of an experimental system similar to natural conditions is important for further exploration of natural thermotolerance mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermotolerance refers to the ability of an organism to cope with excessively high temperatures. Like other organisms, plants have both an inherent ability to survive high temperatures (basal thermotolerance) and the ability to acquire thermotolerance. Acquired thermotolerance may be induced by either exposure to short but sublethal high temperatures (de Klerk and Pumisutapon 2008; Pumisutapon et al. 2012) or by a gradual temperature increase to lethally high levels, as would be experienced under natural conditions (Larkindale and Vierling 2008). In natural environments, in fact, plants not only experience regular and gradual daily temperature range fluctuations, but also become acclimated by gradual increases to high temperatures that would otherwise be lethal. Acquired thermotolerance may therefore reflect the natural mechanism that contributes to thermotolerance in plants. In accord with this idea, plants grown under laboratory conditions only showed differences in thermotolerance after acclimatization treatments, with the diversity revealed by the acclimatization treatment mirroring actual field performance under high temperature conditions (Howarth et al. 1997; Srikanthbabu et al.2002). The acquired thermotolerance assay has been used to identify thermosensitive and thermotolerant genotypes of groundnut, pea, wheat, sunflower, pearl millet, sorghum, and sunflower (Srikanthbabu et al.2002; Howarth et al. 1997; Kumar et al. 1999). In addition, the acquired thermotolerance assay has been employed to evaluate the roles of certain stress genes in thermotolerance (Li et al. 2003). For instance, a transgenic plant overexpressing HsfA1b exhibited a higher basal thermotolerance than the non-acclimated wild type (Li et al. 2003). Because this superiority was lost following heat acclimatization, however, the higher basal tolerance to high temperature possessed by the transgenic plant does not necessarily provide conclusive evidence for the role of HsfA1b in thermotolerance under natural conditions.
In recent years, genomic approaches, such as mutation, knockout, and overexpression of target genes, have facilitated research into the molecular mechanisms of acquired thermotolerance (Dafny-Yelin et al. 2008; Perez et al. 2009; Liu et al. 2009b; Meiri et al. 2010). This review aims to summarize the progress in acquired thermotolerance research in plants in the past decades. It begins with a discussion of the influence of plant materials and acclimatization methods on thermotolerance acquisition, and then focuses on physiological responses and molecular mechanisms involved in thermotolerance acquisition in plants.
Factors involved in acquired thermotolerance development
Effect of growth stage of plant materials on acquired thermotolerance development
Thermotolerance phenotypes vary depending on the growth stage at which plants are assayed (Larkindale et al. 2005; Clarke et al. 2004; Hong et al. 2003; Abernethy et al. 1989; Harrington and Alm 1988). A study on Arabidopsis mutants suggested that the UV-sensitive mutants uvh6 and uvh3 showed defects in acquired thermotolerance during seed germination or after 2.5-day growth in the dark. ABA biosynthesis and ABA signaling mutants, such as aba1, aba2, aba3, abi1, and abi2, showed defects in acquired thermotolerance only after 4 days or more of growth (Larkindale et al. 2005). In four Arabidopsis mutants (hot1-1, hot2-1, hot3-1, and hot4-1) defective for acquired thermotolerance, the hot1-1 mutants exhibited defects after 2.5, 7, or 10 days of growth, while the acquired thermotolerance defects in the hot2-1 mutants were apparent after 2.5 or 10 days of growth. In contrast, 2.5-day-old hot3-1 and hot4-1 showed thermotolerance equivalent to approximately 30 or 60 % of wild type, respectively, whereas 10-day-old hot3-1 and hot4-1 behaved like the wild type, indicating that different genes contribute to thermotolerance acquisition at different stages of the plant life cycle (Hong et al. 2003). Interestingly, a study on thermotolerance acquisition during early germination of wheat seed showed that the protective effect of acclimatization prior to heat stress did not occur without initial application of 9–12 h of imbibition. Following 9 or more hours of imbibition, thermotolerance was acquired after subsequent 38 and 40 °C pretreatments, suggesting that imbibition time is important even though the mechanism is presently unknown (Abernethy et al. 1989). Further investigation is required to determine the relationship between acquired thermotolerance and growth period.
Effect of acclimatization method on acquired thermotolerance development
Different acclimatization temperatures, acclimatization duration, or recovery periods between acclimatization and heat stress have been found to result in varied thermotolerance development (Larkindale and Vierling 2008; Howarth et al. 1997; Kumar et al. 1999; Harrington and Alm 1988; Senthil-Kumar et al. 2003) (Table 1). Howarth et al. (1997) reported that acclimatization at 43 or 45 °C induced more thermotolerance in sorghum and pearl millet seedlings than at 40 °C (Howarth et al. 1997). Acclimatization at 43 °C for 1 h induced maximum thermotolerance in pearl millet. When acclimatization time exceeded 4 h, acquired thermotolerance gradually decreased. After acclimatization at 43 °C for 12 h, thermotolerance of pearl millet was similar to that of the non-acclimated control, demonstrating the dependence of acquired thermotolerance on acclimatization duration (Howarth et al. 1997). Tobacco cells and soybean seedlings that were allowed recovery time between acclimatization treatment and heat stress showed greater thermotolerance than those subjected to immediate heat stress following acclimatization. The longer the recovery period, the greater the acquired thermotolerance, suggesting that special physiological changes related to thermotolerance induction might occur during the recovery period (Harrington and Alm 1988; Lin et al. 1984) Indeed, a large proportion of heat shock proteins (HSPs) have been found to be synthesized during the recovery period, which may impart thermoprotection to plants to subsequent heat stress (Harrington and Alm 1988; Lin et al. 1984).
Recently, Larkindale and Vierling (2008) compared the effect of two different heat acclimatization treatments on thermotolerance development in Arabidopsis, i.e., a gradual increase to 45 °C (G acclimatization, 22–45 °C over 6 h) and a stepped heat pretreatment (S acclimatization, 90 min at 38 °C plus 120 min at 22 °C before 45 °C). They found that G and S acclimatization treatments allowed much longer plant survival upon exposure to 45 °C than no acclimatization (D, direct treatment without acclimatization). G acclimatization induced greater thermotolerance than S acclimatization. Compared with S or D treatments, plants subjected to G acclimatization showed larger number of altered transcripts as well as more transcripts with greater fold-changes and higher absolute expression levels, which may have contributed to the increased heat tolerance in G-acclimated plants (Table 1). These results are the first evidence for different molecular mechanisms underlying different acclimatization methods. Further study on transcripts important for acquired thermotolerance may contribute to the elucidation of thermotolerance acquisition mechanisms.
Crosstalk between acquired thermotolerance and other stress tolerance
In nature, plants frequently encounter several environmental stresses, such as salt, drought, high temperature, and cold, simultaneously. Cross-tolerance has been observed in plants, whereby a response to one stress also helps to protect the plant from another coincident or subsequent environmental stress (Sabehat et al. 1998). Wen et al. (2005) reported that salt adaptation induced thermotolerance in the halophyte Artemisia anethifolia L. by increasing thermotolerance of the PSII apparatus, including PSII reaction centers, oxygen-evolving complexes, and the light-harvesting complex. Such cross-tolerance has been also observed in moss, where acquired thermotolerance was induced by gradual dehydration (Meyer and Santarius 1998). This is not always the case, however. For example, water stress was not observed to induce thermotolerance in cotton seedlings (Burke and O’Mahony 2001). The mechanisms of crosstalk between acquired thermotolerance and other stress tolerance require further research.
Physiological responses involved in acquired thermotolerance
High temperature has been found to induce accumulation of reactive oxygen species (ROS), including 1O2, H2O2, O2 −, and ·OH (Vallelian-Bindschedler et al. 1998), which causes oxidative stress in plants and consequent protein denaturation, condensation (Salvucci et al. 2001), enzyme inactivation, membrane damage, lipid peroxidation (Liu and Huang 2000), and inhibition of photosynthesis, respiration, and plant growth (Jiang and Huang 2001). Acclimatization treatments might alleviate heat stress damage by reducing ROS accumulation (Larkindale et al. 2005) and electrolyte leakage (Howarth et al. 1997) and by increasing antioxidant enzyme activity (Yuan et al. 2011), protein synthesis (Kumar et al. 1999), chlorophyll stability (Burke 1998), and cell viability (Senthil-Kumar et al. 2003).
Analysis of Arabidopsis mutants defective in acquired thermotolerance provided direct genetic evidence that protection of membrane integrity and recovery of protein activity/synthesis are required for acquisition of thermotolerance (Hong et al. 2003). Unlike wild-type plants, hot2-1 mutants with higher electrolyte leakage levels under control conditions were not protected by acclimatization treatment, indicating the importance of membrane properties in acquired thermotolerance (Hong et al. 2003). Luc (firefly luciferase) is a very thermolabile protein, the reactivation of which reflects ability to reactivate damaged proteins in plants (Lee and Vierling 2000). The phenotypes of Arabidopsis wild type and hot1-1 and hot3-1 mutants correlated with Luc activity recovery levels, proving that recovery of protein activity/synthesis was required for thermotolerance acquisition (Hong et al. 2003).
Over the past decade, several thermotolerance line identification methods have been established, including a temperature induction response technique (TIR) based on survival percentage evaluation (Clarke et al.2004; Senthil-Kumar et al. 2003), a hypocotyl elongation assay (Kaplan et al. 2004; Nishizawa et al. 2006), a cell viability assay based on 2,3,5-triphenyl tetrazolium chloride reduction (Song et al. 2012; Yildiz and Terzi 2008), a chlorophyll accumulation assay (Camejo et al. 2005; Dash and Mohanty 2001), a membrane permeability assay (Song et al. 2008), a malondialdehyde content assay (Song et al. 2006), and a chlorophyll fluorescence analysis based on chlorophyll fluorescence parameters such as the maximum quantum yield and photochemical quenching (qN) (Ducruet et al. 2007; Tsai and Hsu 2009).
Molecular responses involved in acquired thermotolerance
Transcript changes involved in acquired thermotolerance
Microarray and cluster analyses have revealed groups of genes that are upregulated or downregulated during thermotolerance acquisition. Upregulated genes include those encoding HSPs (Hsp101, organelle Hsp100/ClpB proteins, Hsp70 s, and small HSPs), regulatory proteins that control signal transduction and gene expression (including protein kinase, protein phosphatase, and transcription factors HsfA3, HsfA7a, NF-X1 DREB2A, DREB2B, DREB2C, and DREB2H), various stress-related proteins (such as late embryogenesis abundant proteins and cold-regulated protein COR6.6), and genes associated with oxidative stress, photosynthesis, and programmed cell death. Eight upregulated genes important in acquired thermotolerance were identified by phenotypic analysis of T-DNA insertion mutants. These genes encode cytosolic ascorbate peroxidase (APX2, At3g09640), transcription factors HsfA7a (At3g51910), NF-X1 (At1g10170), ProOx, SGT1a (At4g23570), Hsp110 (At1g79920), choline kinase (At1g74320), and thaumatin (At4g36010) (Larkindale and Vierling 2008; Lim et al. 2006; Epple et al. 2003; Beere 2004). Genes downregulated exclusively in acclimated plants include those encoding cytochrome P450 s, genes associated with disease resistance (including genes encoding classical pathogenesis response proteins PR1 and PR5) and cell detoxification (mainly genes encoding glutathione S-transferases), and auxin-regulated genes (Larkindale and Vierling 2008; Lim et al. 2006). There were more transcripts that decreased in abundance than increased, suggesting that suppression of transcription is critical for thermotolerance (Larkindale and Vierling 2008).
Genome analysis has shown that different acclimatization methods give rise to different transcript profiles and degrees of thermotolerance. G acclimatization induced the greatest heat tolerance as well as the largest number of altered transcripts in comparison with S acclimatization and direct heating with no acclimatization (Larkindale and Vierling 2008). Enhanced survival of G-acclimated plants can be attributed to four general factors: greater expression of heat stress-induced transcripts (including many of the classical HSP/ molecular chaperone genes), higher expression of transcripts unique to thermotolerant plants, increases in transcripts unique to the specific heat treatment (gradual heating), and more effective repression of many transcripts potentially damaging or presumably not needed during stress (Larkindale and Vierling 2008).
Translationally controlled tumor protein (TCTP) is an important component of the target of rapamycin signaling pathway, a major regulator of cell growth in animals and fungi. Knowledge of the molecular function of TCTP in plants is still limited, however. Recently, BoTCTP from cabbage was found to be induced by high temperature. Silencing of BoTCTP by RNA interference resulted in reduced vegetative growth rate and impaired tolerance to high temperature, suggesting the involvement of the BoTCTP gene in response to heat stress (Cao et al. 2010). Phospholipase D (PLD) is a key enzyme involved in membrane phospholipid catabolism during plant growth, development, and stress responses. Recently, a novel PLD gene, CbPLD, was cloned and characterized from Chorispora bungeana (Yang et al. 2012). The transcripts of CbPLD were induced and greatly increased in abundance under heat stress, indicating that CbPLD may play an important role in response to high temperature in C. bungeana. The functions and mechanisms of TCTP and CbPLD in plant thermotolerance acquisition remain to be investigated.
Heat shock proteins involved in acquired thermotolerance
Heat-shock proteins (HSPs) are a group of evolutionarily conserved polypeptides, which are induced in all organisms in response to environmental stresses and during various developmental processes (Dafny-Yelin et al. 2008). HSPs produced in plants in response to temperatures above optimum function as molecular chaperones to prevent aggregation of denatured proteins, to assist in folding of nascent polypeptides, to aid in refolding of denatured proteins, or to resolubilize aggregated denatured proteins (Wang et al. 2004; Parsell et al. 1994). In plants, HSPs can be classified into five groups on the basis of molecular mass: the small HSP (sHsp) family (Wang et al. 2004), the chaperonins (GroEL and Hsp60), the Hsp70 (DnaK) family, the Hsp90 family, and the Hsp100 (Clp) family.
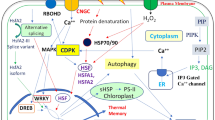
Hsp100 proteins (also known as Clp [caseinolytic protease proteins]), which are divided into class 1, including ClpA and ClpB, and class 2, such as ClpX and ClpY (HslU), are essential for acquired thermotolerance in plants. Deletion of Hsp104 led to loss of acquired thermotolerance (Sanchez and Lindquist 1990). Antisense inhibition of Lehsp100/ClpB and HSP101 resulted in decreased thermotolerance acquisition (Yang et al. 2006; Queitsch et al. 2000). Loss-of-function mutation of Hsp101 (Hong and Vierling 2000; Nieto-Sotelo et al. 1999) eliminated thermotolerance acquisition at several different growth stages, proving that Hsp101 expression is essential for acquired thermotolerance (Fig. 1).
Overview of signaling pathways and factors involved in acquired thermotolerance. This figure shows signaling components involved in heat stress response and protective factors leading to acquired thermotolerance that are described in the text. Black arrows indicate connections with experimental evidence, and hollow arrows with swallow tails indicate negative regulation relationships. Hollow arrows with even tails and question marks represent as-yet-unidentified factors in corresponding signal transduction pathways
In contrast, Arabidopsis mutants defective in abscisic acid, salicylic acid, ethylene, and oxidative burst signaling all accumulated wild-type levels of Hsp101 and class I and II sHsps in spite of thermotolerance acquisition defects (Larkindale et al. 2005). Exogenous application of these signaling agents enhanced plant thermotolerance without an accompanying accumulation of HSPs (Larkindale and Knight 2002), implying that thermotolerance acquisition does not correlate with HSP accumulation. Consistent with these observations, accumulation of Hsp58, Hsp60, Hsp46, Hsp40, and Hsp14 induced by mild heat pretreatments during the initial hours of imbibition conferred no acquired thermotolerance in wheat seed (Abernethy et al. 1989). Similarly, Hsp101 and Hsp17.6 induced by water stress did not contribute to thermotolerance in cotton seedling cotyledons (Burke and O’Mahony 2001). Flower tissues with accumulated sHSPs appeared to be susceptible to heat stress (Dafny-Yelin et al. 2005). HSPs are developmentally as well as environmentally regulated (Dafny-Yelin et al. 2008). The developmentally regulated HSPs might be not metabolically available to assist in enhancing thermotolerance (Burke and O’Mahony 2001), possibly explaining the diverse functions of HSPs. The underlying expression, regulation, and action mechanism of HSPs at different developmental and physiological state requires further investigation.
Hsp90 is the most abundant constitutively expressed HSP in eukaryotic cells, and is involved in developmental processes related to maturation and folding of several protein kinases and nuclear steroid hormone receptors (Ludwig-Müller et al. 2000). In the Arabidopsis TU8 mutant, reduced levels of Hsp90 were found to be responsible for deficiency in thermotolerance acquisition following acclimatization treatment (Ludwig-Müller et al. 2000) (Fig. 1). Conversely, application of monocillin I (MON), an Hsp90 inhibitor derived from the rhizosphere fungus Paraphaeosphaeria quadriseptata, was found to enhance expression of Hsp101 and Hsp70 and promote heat tolerance in Arabidopsis seedlings (McLellan et al. 2007). Co-cultivation of P. quadriseptata with Arabidopsis consistently leads to enhanced heat tolerance in Arabidopsis, suggesting that Hsp90 inhibition contributes to thermotolerance induction (McLellan et al. 2007). Additional work is necessary to verify the function of Hsp90 in heat stress response and to explain the contradictory results.
Hsp70 gene families comprise four major subgroups, localized respectively to cytosol, endoplasmic reticulum, plastids, and mitochondria. The cytosolic Hsp70 gene has been shown to contribute to thermotolerance acquisition (Lee and Schoffl 1996) (Fig. 1). Plastid stroma Hsp70 s may play a role in photosystem thermotolerance acquisition (Su and Li 2008). A T-DNA insertion knockout mutation of Hsp70 led to reduced chlorophyll accumulation, suggesting that plastid stroma Hsp70 s may play a role in photosystem protection during heat shock and/or recovery (Su and Li 2008).
sHSPs are divided into six different classes, which are located in cytoplasm, nucleus, endoplasmic reticulum, mitochondria, or plastids, respectively (Siddique et al. 2008). sHSPs are produced in response to heat, cold, drought, or salinity (Burke and O’Mahony 2001; Dafny-Yelin et al. 2008) and during various developmental processes, such as embryogenesis, germination, and fruit development (Medina-Escobar et al. 1998; Dafny-Yelin et al. 2008). sHSPs might function as molecular chaperones in vitro and in vivo, probably preventing irreversible protein aggregation and maintaining denatured proteins in a folding-competent state under heat-stress conditions (Basha et al. 2006; Ahrman et al. 2007). The sHSP genes hsp18.1, hsp17.4, and hsp17.6A have been demonstrated to be involved in thermotolerance acquisition (Dafny-Yelin et al. 2008) (Fig. 1). BOBBER1, an sHSP expressed in most tissues throughout plant development and required for partitioning and patterning of the apical domain of embryo, leaves, flowers, and inflorescence meristems (Schmid et al. 2005), is involved in both basal and acquired thermotolerance (Perez et al. 2009) (Fig. 1).
Rarely explored is the question of how long a previously acquired thermotolerance may be sustained, which is important for immobile plants that frequently face unpredictable temperature fluctuations and other stresses. Recently, Hsa32, a highly conserved 32-kDa Hsp present in land plants but absent in most other organisms, has been found to be essential for sustained acquired thermotolerance although it does not affect growth and development under normal conditions (Charng et al. 2006). Hsa32 is essential for tolerance against severe heat challenges after acclimatization treatment followed by a long recovery, which is apparent from the fast decay of thermotolerance observed in the absence of this protein (Charng et al. 2006). Hsa32 might be required not for induction but rather for maintenance of acquired thermotolerance (Charng et al. 2006; Kaplan et al. 2004) (Fig. 1). Interestingly, decreased thermotolerance after long recovery in the Hsa32 mutant hsa32-1 is reversible. A second acclimatization treatment after recovery but before severe heat stress protected hsa32-1 plants from being killed, suggesting that the subsequent thermotolerance acquisition does not require Hsa32 and seems to overcome the thermotolerance defect of the mutant. It is possible that Hsa32 is not essential for thermotolerance when other HSPs are also present at a sufficient level to compensate for its absence (Charng et al. 2006). Detailed and systematic study of Hsa32 transcriptomic, proteomic, and metabolomic profiles may provide insights into the action of this protein.
Thermotolerance acquisition depends not only upon synthesis of HSPs but also upon their selective cellular localization (Lin et al. 1984). In soybean seedlings, acclimatization at 40 °C for 3 h followed by a chase at 28 °C led to HSP synthesis, which then accumulated and provided thermal protection during a subsequent 45 °C treatment. At the same time, some HSPs were observed to selectively localize in cellular organelles during 40 °C acclimatization and to relocalize rapidly (completed within 15 min) during a second 45 °C heat stress after a 28 °C incubation, suggesting that selective localization of HSPs during HS (heat shock) is important for thermotolerance development (Lin et al. 1984).
Heat shock transcription factors involved in acquired thermotolerance
Heat stress transcription factors (HSFs) are the central regulators responsible for the expression of heat-responsive genes, sHSPs, and additional molecular chaperones (Kotak et al. 2004, 2007). Most current information on plant HSF function is derived from tomato and Arabidopsis. In tomato, HsfA1a, HsfA2, and HsfB1 form a regulatory network that is responsible for the expression of HS responsive genes (Perez et al. 2009). Arabidopsis thaliana contains 21 Hsf homologs that can be sorted into classes A, B, and C (Kotak et al. 2004, 2007; Charng et al. 2007). In this model plant, 21 Hsfs form a complex HSF network in which AtHsfA2 plays a dominant role in acquired thermotolerance regulation through transcriptional regulation of certain heat-induced genes during recovery after acclimatization (Charng et al. 2006, 2007; Schramm et al. 2006; Nishizawa et al. 2006; Ogawa et al. 2007) (Fig. 1).
HsfA2 knockout mutants displayed reduced acquired thermotolerance while HsfA2-overexpressing transgenic plants exhibited increased acquired thermotolerance, indicating that HsfA2 is required for acquired thermotolerance (Li et al. 2005) (Fig. 1). Disruption of HsfA2 lowered transcript and protein expression levels of sHSP genes during recovery, including Hsp18.1-CI, Hsp25.3-P, and Hsa32, which are essential for maintenance of acquired thermotolerance during recovery periods (Charng et al. 2007). In addition, Apx2, which encodes ascorbate peroxidase, a cytosolic hydrogen peroxide (H2O2) scavenging enzyme, was significantly induced by overexpression of HsfA2 and greatly suppressed by T-DNA insertion mutation of HsfA2 during recovery (Li et al. 2005; Charng et al. 2007). These results provide direct genetic evidence for the function of HsfA2 in acquired thermotolerance by sHSP and Apx2 expression regulation.
Recently, several protein regulators, such as small ubiquitin-like modifier (SUMO), ROF1 (FKBP62), ROF2 (FKBP65), and AtHSBP, have been found to be involved in the regulation of HsfA2 in acquired thermotolerance (Miura and Hasegawa 2010; Meiri et al. 2010; Hsu et al. 2010) (Fig. 1).
SUMO proteins are expressed throughout the eukaryotic kingdom. In plants, SUMOylation is involved in stress responses, pathogen defense, abscisic acid signaling, floral induction, and gene regulation (Miura et al. 2007, 2009; Jin et al. 2008; Miura and Hasegawa 2010). A recent study showed that SUMO1 overexpression led to a decrease in HsfA2 transcriptional activation. With regard to phenotype, seedlings overexpressing AtSUMO1 resembled AtHsfA2 knockout seedlings, suggesting negative modification of AtHsfA2 by AtSUMO1 (Miura and Hasegawa 2010). AtHsfA2 is a major transcription factor of sHSPs (Schramm et al. 2006), which play an important role in thermotolerance acquisition (Charng et al. 2006; Larkindale and Huang 2004; von Koskull-Doring et al. 2007). Seedlings overexpressing AtSUMO1 exhibited lower levels of sHSP compared with wild-type plants, demonstrating that AtSUMO1 interferes with sHSP expression. Although the mechanism by which AtSUMO1 regulates AtHsfA2 is not clear, this interference is possibly due, at least in part, to modification of AtHsfA2. SUMOylation of AtHsfA2 might be involved in thermotolerance acquisition by sHSP expression regulation (Cohen-Peer et al. 2010) (Fig. 1).
FK506-binding proteins (FKBPs) belong to the large peptidyl-prolyl cis–trans isomerase (PPIase) family characterized by its enzymatic activity, namely, the peptidyl–prolyl cis–trans isomerization of polypeptide bonds (Galat 2003). Two Arabidopsis FKBPs, ROF1 (FKBP62) and ROF2 (FKBP65), share 85 % sequence identity and similar domain structures (Aviezer-Hagai et al. 2007). ROF1 (FKBP62) was recently shown to be involved in long term acquired thermotolerance through its interaction with Hsp90.1 and modulation of the heat shock transcription factor HsfA2 (Meiri and Breiman 2009). ROF2 participates in long term acquired thermotolerance in a different manner than ROF1, i.e., ROF1 contributes to HsfA2 transcription activity (Meiri and Breiman 2009), while ROF2, in the presence of ROF1, completely suppresses this activity. To explain this phenomenon, Meiri et al. (2010) have proposed a model in which ROF2 is transcribed by HsfA2 and participates in acquired thermotolerance via its interaction with ROF1 and negative regulation of HsfA2 by a feedback mechanism. Under normal growth conditions, ROF1 interacts with Hsp90.1 in the cytoplasm. During heat stress, various proteins are synthesized, including sHSPs, HsfA2, and ROF2. HsfA2 interacts with Hsp90.1 and is responsible for translocation of the ROF1-Hsp90.1-HsfA2 complex to the nucleus (Meiri et al. 2010; Yokotani et al. 2008). During the recovery period, this complex maintains sHSP expression levels and ROF2 concentrations. After a recovery period of about 6 h, ROF2 interacts with ROF1 in the nucleus and this heterodimer, together with Hsp90.1, abrogates the transcriptional activity of HsfA2 (Meiri et al. 2010; Yokotani et al. 2008). Therefore, despite the antagonistic effect between ROF1 and ROF2, these proteins may cooperate to modulate HsfA2 transcriptional activity and further affect sHSP accumulation during the recovery period, thus ultimately regulating acquired thermotolerance development (Fig. 1).
AtHSBP, an Arabidopsis HSF binding protein, is heat inducible and ubiquitously expressed in all tissues. Under normal conditions, it is primarily expressed in the cytoplasm, but translocates to the nucleus in response to thermal stress (Liu et al. 2006a). Recent studies indicate that AtHSBP participates in acquired thermotolerance as a negative regulator (Fig. 1). More specifically, AtHSBP knockout mutation, overexpression, and protoplast two-hybrid assays showed that AtHSBP might interact with HSFs, such as AtHsfA1a, AtHsfA1b, and AtHsfA2, and negatively affect AtHSF DNA-binding capacity to eventually decrease AtHsp101, AtHsp70, AtsHsp18.2, and AtsHsp17.4 expression during HS recovery (Nishizawa et al. 2006; Hsu et al. 2010).
These results provide a summary of recent progress on HSF regulatory mechanisms, focusing on downstream target genes and upstream regulation factors, in thermotolerance acquisition. Interestingly, HSF-interacting proteins mentioned above, such as SUMO, ROF1 (FKBP62), ROF2 (FKBP65), and AtHSBP, all act as negative regulators for HsfA2 transcriptional activity. Further investigation of positive regulators of HSFs will aid in integral clarification of HSF modulation mechanisms in acquired thermotolerance.
While class A HSFs are associated directly with heat stress responses, class B HSFs seem to have diverse roles in plants. CphsfB1, an HSF cloned from papaya, was found to be induced by heat stress in leaves while showing constitutive expression in radicles, suggesting that CphsfB1 plays different roles, i.e., a heat-stress-dependent response and a non-dependent response, in the two different plant tissues. In papaya, CphsfB1 may act together with class-A HSFs to regulate heat stress response (Tarora et al. 2010).
Growth inhibition is generally observed when plants are stressed. Several HSFs, including AtHsfA3, AtHsfA2, and OsHsfA2e, have been reported to confer a dwarf phenotype (Ogawa et al. 2007; Yokotani et al. 2008). However, the underlying mechanisms are still far from elucidation. Recently, a study on BhHsf1, an HSF gene cloned from the resurrection plant Boea hygrometrica, showed that overexpression of BhHsf1 induced thermotolerance in plants by upregulation of stress-related genes, including AtAPX2, AtGolS1, AtMKP6.25, and various Hsps, such as Hsp22.0-ER, Hsp18.1-CI, Hsp17.6B-CI, Hsp17.6C-CI, Hsp25.3-P, Hsp26.5-P, Hsp70, and Hsp70T-2. Simultaneously, overexpression of BhHsf1 downregulated expression of genes related to DNA replication and mitotic cell cycle, which led to cell proliferation, cell expansion, and eventually growth retardation (Zhu et al. 2009). These results imply that HSFs may help integrate the processes of growth retardation and stress tolerance.
Signal transduction involved in acquired thermotolerance
A wide range of signaling molecules, such as abscisic acid (ABA), ethylene, H2O2, and salicylic acid (SA), are involved in high temperature sensing and signaling. Evidence for their involvement includes the protective effects of exogenous SA, ethylene precursor 1-aminocyclopropane-1-carboxylic acid, H2O2, and ABA application (Larkindale and Knight 2002; Rai et al. 2011) and increases in endogenous SA, ethylene, H2O2, and ABA concentration during heating or recovery periods (Ludwig-Müller et al. 2000; Larkindale and Huang 2005). An investigation of 45 Arabidopsis mutant phenotypes provided molecular evidence for the importance of such signaling pathways in acquired thermotolerance. ABA was most likely to be involved in processes associated with acquired thermotolerance, whereas SA and active oxygen species were critical for events during both basal and acquired thermotolerance (Fig. 1). Ethylene and genes related to antioxidant metabolism were more likely to be critical in basal heat tolerance, with less of a role in processes required for acquired thermotolerance (Larkindale et al. 2005). The role of calcium in thermotolerance acquisition during or after recovery has been confirmed (Larkindale and Knight 2002) (Fig. 1).
Phosphatidylinositol 4,5-bisphosphate phospholipase C (PIP2-PLC) is a lipid-associated enzyme that employs PIP2 to produce inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. These two messenger substances play crucial roles in amplifying extracellular stimuli and mediating various physiological processes caused by different abiotic stresses (Zhao et al. 2004). Results obtained in an earlier study indicated that free SA and PIP2-PLC were involved in thermotolerance acquisition (Fig. 1). PIP2-PLC stimulation was preceded by an increase in free SA content and followed by an increase in IP3 production, indicating that PIP2-PLC was involved in the SA-mediated signal pathway that leads to thermotolerance acquisition (Liu et al. 2006a) (Fig. 1). Another study on the roles of free SA, ABA, and PIP2-PLC in thermotolerance development confirmed the role of free SA as an upstream element in the stimulation of PIP2-PLC in heat acclimatization-induced thermo tolerance (Liu et al. 2006b). This study also demonstrated that the response of PIP2-PLC to heat acclimatization was preceded by a peak in ABA, implying that PIP2-PLC mediated free SA and ABA induced thermotolerance. Moreover, ABA elevation corresponding to heat acclimatization preceded a free SA peak (Liu et al. 2006b) (Fig. 1).
Work with pea leaves has revealed the role of conjugated salicylic acid (SA 2-O-ß-d-glucose, or SAG) in acquired thermotolerance. Liu et al. (2006b) demonstrated that free SA involved in acquired thermotolerance might be derived from the conversion of SAG to free SA. Additionally, direct application of SAG induced thermotolerance in plants, indicating a possible role for SAG in thermotolerance acquisition. Further analysis with isotope labeling and western blotting by the same research group confirmed that SAG indeed participates in thermotolerance acquisition regulation and might act as a signal molecule in the same manner as free SA (Liu et al. 2006b, 2009b). The translation between free SA and SAG and its function in thermotolerance acquisition regulation, however, require further research.
Plasma membrane H+-ATPase (PM H+-ATPase), an important functional protein located on the plasma membrane (PM), establishes a proton electrochemical gradient across the PM and the tonoplast and is also involved in stomatal opening, cell elongation, and intracellular pH regulation (Kuhlbrandt 2004). Studies with pea leaves suggest that PM H+-ATPase might be involved in the development of SA-induced thermotolerance. Heat acclimatization might first induce a peak in endogenous SA, and then free SA may function as an upstream element to trigger peaks in PM H+-ATPase transcript levels and enzyme protein amounts (Liu et al. 2009a). The stimulation of PM H+-ATPase can then induce the amplification of signals, such as Ca2+ (Kinoshita et al. 1995) and HSP (Fan et al. 2000; Liu et al. 2001), to maintain PM integrity and finally induce thermotolerance (Fig. 1). Further investigation on signal cross-talk should accelerate our understanding of signal transduction networks involved in thermotolerance acquisition.
Conclusion
Acquired thermotolerance reflects an actual thermotolerance mechanism naturally occurring in plants. A growing number of researchers are beginning to focus on acquired thermotolerance instead of basal thermotolerance (Clarke et al. 2004; Larkindale and Vierling 2008; Dafny-Yelin et al. 2008; Perez et al. 2009). Evidence exists that different heat acclimatization methods lead to different thermotolerance development and transcript alteration, implying divergent molecular mechanisms underlying various acclimatization methods (Larkindale and Vierling 2008). Establishment of an experimental system similar to natural conditions is thus important for further exploration into thermotolerance acquisition.
Although great progress has been achieved in the elucidation of physiological and molecular mechanisms underling thermotolerance acquisition, there are still many important topics to be addressed by future research, including the identity of HSP cellular targets and the reason why thermosensitive Arabidopsis mutants accumulate wild-type levels of Hsp101 and small HSPs. Several HSF-interacting proteins that negatively regulate HSF transcriptional activity have recently been revealed. The question still remains as to whether there are active regulation factors involved in HSF regulation. In addition, an understanding of the signal pathways in thermotolerance acquisition is currently lacking, and how the signals interact with each other remains unclear. Finally, elucidation of the establishment of signal network involved in acquired thermotolerance remains a subject for further exploration. A better understanding of all these processes is essential for an overall and detailed understanding of natural thermotolerance acquisition mechanisms at the whole plant level.
Abbreviations
- HS:
-
Heat shock
- HSP:
-
Heat shock protein
- HSF:
-
Heat shock transcription factor
- SUMO:
-
Small ubiquitin-like modifier protein
- ROF1 (FKBP62):
-
FK506-binding proteins
- ROF2 (FKBP65):
-
FK506-binding proteins
- HSBP:
-
HSF binding protein
- IP3:
-
Inositol 1,4,5-trisphosphate
References
Abernethy RH, Thiel DS, Petersen NS, Helm K (1989) Thermotolerance is developmentally dependent in germinating wheat seed. Plant Physiol 89(2):569–576
Ahrman E, Lambert W, Aquilina JA, Robinson CV, Emanuelsson CS (2007) Chemical cross-linking of the chloroplast localized small heat-shock protein, Hsp21, and the model substrate citrate synthase. Protein Sci 16:1464–1478
Aviezer-Hagai K, Skovorodnikova J, Galigniana M, Farchi-Pisanty O, Maayan E, Bocovza S, Efrat Y, von Koskull-Doring P, Ohad N, Breiman A (2007) Arabidopsis immunophilins ROF1 (At-FKBP62) and ROF2 (AtFKBP65) exhibit tissue specificity, are heat-stress induced, and bind HSP90. Plant Mol Biol 63:237–255
Basha E, Friedrich KL, Vierling E (2006) The N-terminal arm of small heat shock proteins is important for both chaperone activity and substrate specificity. J Biol Chem 281(52):39943–39952
Beere H (2004) ‘The stress of dying’: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651
Burke JJ (1998) Characterization of acquired thermotolerance in soybean seedlings. Plant Physiol Biochem 36(8):601–607
Burke JJ, O’Mahony PJ (2001) Protective role in acquired thermotolerance of developmentally regulated heat shock proteins in cotton seeds. J Cotton Sci 5:174–183
Camejo D, Rodriguez P, Morales MA, Dell’Amico JM, Torrecillas A, Alarcon JJ (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol 162:281–289
Cao BH, Lu YQ, Chen GJ, Lei JJ (2010) Functional characterization of the translationally controlled tumor protein (TCTP) gene associated with growth and defense response in cabbage. Plant Cell Tiss Organ Cult 103:217–226
Charng YY, Liu HC, Liu NY, Hsu FC, Ko SS (2006) Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol 140:1297–1305
Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT (2007) A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol 143:251–262
Clarke SM, Mur LA, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38:432–447
Cohen-Peer R, Schuster S, Meiri D, Breiman A, Avni A (2010) Sumoylation of Arabidopsis heat shock factor A2 (HsfA2) modifies its activity during acquired thermotholerance. Plant Mol Biol 74:33–45
Dafny-Yelin M, Guterman I, Menda N, Ovadis M, Shalit M, Pichersky E, Zamir D, Lewinsohn E, Adam Z, Weiss D, Vainstein A (2005) Flower proteome: changes in protein spectrum during the advanced stages of rose petal development. Planta 222:37–46
Dafny-Yelin M, Tzfira T, Vainstein A, Adam Z (2008) Non-redundant functions of sHSP-CIs in acquired thermotolerance and their role in early seed development in Arabidopsis. Plant Mol Biol 67:363–373
Dash S, Mohanty N (2001) Evaluation of assay for the analysis of thermo-tolerance and recovery potentials of seedlings of wheat (Triticum aestivum L.) cultivars. J Plant Physiol 158:1153–1165
de Klerk GJ, Pumisutapon P (2008) Protection of in-vitro grown Arabidopsis seedlings against abiotic stresses. Plant Cell Tiss Organ Cult 95:149–154
Ducruet JM, Peeva V, Havaux M (2007) Chlorophyll thermofluorescence and thermoluminescence as complementary tools for the study of temperature stress in plants. Photosynth Res 93:159–171
Epple P, Mack AA, Morris VR, Dangl JL (2003) Antagonistic control of oxidative stress-induced cell death in Arabidopsis by two related, plant specific zinc finger proteins. Proc Natl Acad Sci 100:6831–6836
Fan ZH, Zhou RG, Li XZ, Bai J (2000) Calcium-calmodulin and the inducement of heat shock proteins in wheat seedling. Acta Phytophysiol Sin 26(4):331–336
Galat A (2003) Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity-targets-functions. Curr Top Med Chem 3(12):1315–1347
Harrington HM, Alm DM (1988) Interaction of heat and salt shock in cultured tobacco cells. Plant Physiol 88:618–625
Hong SW, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97:4392–4397
Hong SW, Lee U, Vierling E (2003) Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiol 132:757–767
Howarth CJ, Pollock CJ, Peacock JM (1997) Development of laboratory-based methods for assessing seedling thermotolerance in pearl millet. New Phytol 137:129–139
Hsu SF, Lai HC, Jinn TL (2010) Cytosol-localized heat shock factor-binding protein, AtHSBP, functions as a negative regulator of heat shock response by translocation to the nucleus and is required for seed development in Arabidopsis. Plant Physiol 153:773–784
Jiang YW, Huang BR (2001) Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci 41(2):436–442
Jin JB, Jin YH, Lee J, Miura K, Yoo CY, Kim WY, Van Oosten M, Hyun Y, Somers DE, Lee I, Yun DJ, Bressan RA, Hasegawa PM (2008) The SUMO E3 ligase, AtSIZ1, regulates flowering by controlling a salicylic acid-mediated floral promotion pathway and through affects on FLC chromatin structure. Plant J 53:530–540
Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL (2004) Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol 136:4159–4168
Kinoshita T, Nishimura M, Shimazaki K (1995) Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell 7:1333–1342
Kotak S, Port M, Ganguli A, Bicker F, von Koskull-Doring P (2004) Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature combination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular localization. Plant J 39:98–112
Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316
Kuhlbrandt W (2004) Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol 5:282–295
Kumar G, Krishnaprasad BT, Savitha M, Gopalakrishna R, Mukhopadhyay K, Ramamohan G, Udayakumar M (1999) Enhanced expression of heat-shock proteins in thermotolerant lines of sunflower and their progenies selected on the basis of temperature-induction response (TIR). Theor Appl Genet 99:359–367
Larkindale J, Huang B (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol 161:405–413
Larkindale J, Huang B (2005) Effect of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regul 47(1):17–28
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128:682–695
Larkindale J, Vierling E (2008) Core genome responses involved in acclimation to high temperature. Plant Physiol 146:748–761
Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138:882–897
Lee JH, Schoffl F (1996) An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol Gen Genet 252:11–19
Lee GJ, Vierling E (2000) A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol 122:189–197
Li HY, Chang CS, Lu LS, Liu CA, Chan MT, Charng YY (2003) Overexpression of Arabidopsis thaliana heat shock factor gene (AtHsf1b) enhances chilling tolerance in transgenic tomato. Bot Bull Acad Sin 44:129–140
Li CG, Chen QJ, Gao XQ, Qi BS, Chen NZ, Xu SM, Chen J, Wang XC (2005) AtHsfA2 modulates expression of stress responsive genes and enhanced tolerance to heat and oxidative stress in Arabidopsis. Sci China C Life Sci 48(6):540–550
Lim CJ, Yang K, Hong JK, Choi JS, Yun DJ, Hong JC, Chung WS, Lee SY, Cho MJ, Lim CO (2006) Gene expression profiles during heat acclimation in Arabidopsis thaliana suspension-culture cells. J Plant Res 119:373–383
Lin CY, Roberts JK, Key JL (1984) Acquisition of thermotolerance in soybean seedlings. Plant Physiol 74:152–160
Liu XZ, Huang BR (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40:503–510
Liu HT, Zhao H, Li B, Sun DY, Zhou RG (2001) Involvement of calcium-calmodulin in the expression of HSP26 gene in wheat. Acta Bot Sin 43(7):766–768
Liu HT, Huang WD, Pan QH, Weng FH, Zhan JC, Liu Y, Wan SB, Liu YY (2006a) Contributions of PIP2-specific-phospholipase C and free salicylic acid to heat acclimation-induced thermotolerance in pea leaves. J Plant Physiol 163:405–416
Liu HT, Liu YY, Pan QH, Yang HR, Zhan JC, Huang WD (2006b) Novel interrelationship between salicylic acid, abscisic acid and PIP2-specific-phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J Exp Bot 57:3337–3347
Liu YY, Liu HT, Pan QH, Yang HR, Zhan JH, Huang WD (2009a) The plasma membrane H+-ATPase is related to the development of salicylic acid-induced thermotolerance in pea leaves. Planta 229:1087–1098
Liu HT, Yang HR, Huang WD, Hou ZX, Tang K (2009b) Salicylic acid 2-O-β-d-glucose: a possible signal substance involved thermotolerance induced by heat acclimation. Chin Bull Bot 44(2):211–215
Ludwig-Müller J, Krishna P, Forreiter C (2000) A glucosinolate mutant of Arabidopsis is thermosensitive and defective in cytosolic Hsp90 expression after heat stress. Plant Physiol 123:949–958
McLellan CA, Turbyville TJ, Wijeratne EMK, Kerschen A, Vierling E, Whitesell L, Queitsch C, Gunatilaka AAL (2007) A rhizosphere fungus enhances Arabidopsis thermotolerance through production of an HSP90 inhibitor. Plant Physiol 145:174–182
Medina-Escobar N, Cardenas J, Munoz-Blanco J, Caballero JL (1998) Cloning and molecular characterization of a strawberry fruit ripening-related cDNA corresponding to a mRNA for a low-molecular-weight heat-shock protein. Plant Mol Biol 36:33–42
Meiri D, Breiman A (2009) Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90.1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J 59:387–399
Meiri D, Tazat K, Cohen-Peer R, Farchi-Pisanty O, Aviezer-Hagai K, Avni A, Breiman A (2010) Involvement of Arabidopsis ROF2 (FKBP65) in thermo tolerance. Plant Mol Biol 72:191–203
Meyer H, Santarius KA (1998) Short-term thermal acclimation and heat tolerance of gametophytes of mosses. Oecologia 115:1–8
Miura K, Hasegawa PM (2010) Sumoylation and other ubiquitin-like post-translational modifications in plants. Trends Cell Biol 20:223–232
Miura K, Jin JB, Hasegawa PM (2007) Sumoylation, a post-translational regulatory process in plants. Curr Opin Plant Biol 10:495–502
Miura K, Lee J, Jin JB, Yoo CY, Miura T, Hasegawa PM (2009) Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proc Natl Acad Sci 106:5418–5423
Nieto-Sotelo J, Kannan KB, Martinez LM, Segal C (1999) Characterization of a maize heat-shock protein 101 gene, HSP101, encoding a ClpB/ Hsp100 protein homologue. Gene 230:187–195
Nishizawa A, Yabuta Y, Yoshida E, Maruta T, Yoshimura K, Shigeoka S (2006) Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J 48:535–547
Ogawa D, Yamaguchi K, Nishiuchi T (2007) High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J Exp Bot 58:3373–3383
Parsell DA, Kowal A, Singer MA, Lindquist S (1994) Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372:475–478
Perez DE, SteenHoyer J, Johnson AI, Moody ZR, Lopez J, Kaplinsky NJ (2009) BOBBER1 Is a noncanonical Arabidopsis small heat shock protein required for both development and thermo tolerance. Plant Physiol 151:241–252
Pumisutapon P, Visser RGF, de Klerk GJ (2012) Moderate abiotic stresses increase rhizome growth and outgrowth of axillary buds in Alstroemeria cultured in vitro. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-012-0160-7
Queitsch C, Hong S-W, Vierling E, Lindquist SL (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12:479–492
Rai MK, Shekhawat NS, Harish Gupta AK, Phulwaria M, Ram K, Jaiswal U (2011) The role of abscisic acid in plant tissue culture: a review of recent progress. Plant Cell Tiss Organ Cult 106:179–190
Sabehat A, Weiss D, Lurie S (1998) Heat-shock proteins and cross tolerance in plants. Physiol Plant 103:437–441
Salvucci ME, Osteryoung KW, Crafts-Brandner SJ, Vierling E (2001) Exceptional sensitivity of rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiol 127:1053–1064
Sanchez Y, Lindquist SL (1990) HSP104 required for induced thermotolerance. Science 248:1112–1115
Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37:501–506
Schramm F, Ganguli A, Kiehlmann E, Englich G, Walch D, von Koskull-Doring P (2006) The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Mol Biol 60:759–772
Senthil-Kumar M, Srikanthbabu V, Mohanraju B, Kumar G, Shivaprakash N, Udayakumar M (2003) Screening of inbred lines to develop a thermotolerant sunflower hybrid using the temperature induction response (TIR) technique: a novel approach by exploiting residual variability. J Exp Bot 54:2569–2578
Siddique M, Gernhard S, von Koskull-Doring P, Vierling E, Scharf KD (2008) The plant sHSP superfamily: five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperones 13:183–197
Song LL, Ding W, Zhao MG, Sun BT, Zhang LX (2006) Nitric oxide protects against oxidative stress under heat stress in the calluses from two ecotypes of reed. Plant Sci 171:449–458
Song LL, Ding W, Shen J, Zhang ZG, Bi YR, Zhang LX (2008) Nitric oxide mediates abscisic acid induced thermotolerance in the calluses from two ecotypes of reed under heat stress. Plant Sci 175(6):826–832
Song LL, Jiang YL, Zhao HQ, Zhang ZG (2012) Comparative study on calli from two reed ecotypes under heat stress. Russ J Plant Physiol 59(3):381–388
Srikanthbabu V, Kumar G, Krishnaprasad BT, Gopalakrishna R, Savitha M, Udayakumar M (2002) Identification of pea genotypes with enhanced thermotolerance using temperature induction response (TIR) technique. J Plant Physiol 159:535–545
Su PH, Li HM (2008) Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol 146:1231–1241
Tarora K, Tamaki M, Shudo A, Urasaki N, Matsumura H, Adaniya S (2010) Cloning of a heat stress transcription factor, CphsfB1, that is constitutively expressed in radicles and is heat-inducible in the leaves of Carica papaya. Plant Cell Tiss Organ Cult 102:69–77
Tsai CM, Hsu BD (2009) Thermotolerance of the photosynthetic light reactions in two Phaseolus species: a comparative study. Photosynthetica 47:255–262
Vallelian-Bindschedler L, Schweizer P, Mosinger E, Metraux JP (1998) Heat induced resistance in barley to powdery mildew (Blumeria graminis f. sp. hordei) is associated with a bust of AOS. Physiol Mol Plant Pathol 52:185–199
von Koskull-Doring P, Scharf KD, Nover L (2007) The diversity of plant heat stress transcription factors. Trends Plant Sci 12:452–457
Wang WX, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9(5):244–252
Wen XG, Qiu NW, Lu QT, Lu CM (2005) Enhanced thermotolerance of photosystem II in salt-adapted plants of the halophyte Artemisia anethifolia. Planta 220:486–497
Yang JY, Sun Y, Sun AQ, Yi SY, Qin J, Li MH, Liu J (2006) The involvement of chloroplast HSP100/ClpB in the acquired thermotolerance in tomato. Plant Mol Biol 62:385–395
Yang N, Yue XL, Chen XL, Wu GF, Zhang TG, An LZ (2012) Molecular cloning and partial characterization of a novel phospholipase D gene from Chorispora bungeana. Plant Cell Tiss Organ Cult 108:201–212
Yildiz M, Terzi H (2008) Evaluation of acquired thermotolerance in wheat (Triticum Aestivum and T Durum) cultivars grown in Turkey. Pak J Bot 40(1):317–327
Yokotani N, Ichikawa T, Kondou Y, Matsui M, Hirochika H, Iwabuchi M, Oda K (2008) Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta 227:957–967
Yuan Y, Qian HM, Yu YD, Lian FQ, Tang DQ (2011) Thermotolerance and antioxidant response induced by heat acclimation in Freesia seedlings. Acta Physiol Plant 33(3):1001–1009
Zhao J, Guo YQ, Kosaihira A, Sakai K (2004) Rapid accumulation and metabolism of polyphosphoinositol and its possible role in phytoalexin biosynthesis in yeast elicitor-treated Cupressus lusitanica cell cultures. Planta 219:121–131
Zhu Y, Wang Z, Jing Y, Wang L, Liu X, Liu Y, Deng X (2009) Ectopic over-expression of BhHsf1, a heat shock factor from the resurrection plant Boea hygrometrica, leads to increased thermotolerance and retarded growth in transgenic Arabidopsis and tobacco. Plant Mol Biol 71:451–467
Acknowledgments
This work was supported by the Scientific Research and Innovation Program of the Shanghai Municipal Education Commission (Tissue Culture and High-yield Cultivation Techniques of Taxus chinensis var. mairei [11CXY60]) and the Key Course Construction Program of the Shanghai Municipal Education Commission (Plant Physiology).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, L., Jiang, Y., Zhao, H. et al. Acquired thermotolerance in plants. Plant Cell Tiss Organ Cult 111, 265–276 (2012). https://doi.org/10.1007/s11240-012-0198-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0198-6