Abstract
Proline plays adaptive roles in plant tolerance to cadmium (Cd)-induced stress, but many gaps remain to be elucidated as the responses triggered by exogenously supplied proline or endogenously overproduction are not well known. Thus, we assayed the nutritional status, metabolite profiling, and antioxidative responses in wild type and transgenic tobacco (Nicotiana tabacum L.) containing the P5CSF129A gene under control of the cauliflower mosaic virus (CaMV35S) or stress inducible rd29A promoters. The plants were exposed or unexposed to Cd (0 and 50 μmol L−1 CdCl2·H2O) for 24 and 72 h. The wild type plants were also treated with or without exogenous proline (1 mmol L−1). Plants supplied with exogenous proline exhibited lower Cd translocation from roots to leaves than plants overproducing proline, avoiding oxidative damages in the leaves of these plants. Meanwhile, tobacco overproducing proline was less susceptible to Cd-induced nutritional changes than wild type plants and presented better metabolic adjustment under Cd exposure compared to plants supplied with exogenous proline. Plants overproducing proline increased the synthesis of sugars and organic acids under Cd exposure, which contributed to absence of oxidative stress, since both superoxide dismutase and catalase were not active against Cd-induced oxidative stress in these genotypes. Plants overproducing proline under the control of rd29A presented higher proline concentration in comparison to the CaMV35S promoter. With exception of rd29A plants that presented high proline and reduced glutathione (GSH) concentrations, the other plants presented an inverse correlation between proline and GSH synthesis after 72 h of Cd exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) can induce several damages to plants, such as nutritional imbalance, photosynthesis impairment, and oxidative stress (Gallego et al. 2012; Lavres et al. 2019; Carvalho et al. 2020). Oxidative stress has been discussed as a primary effect of Cd exposure (Clemens 2006). Once formed, reactive oxygen species (ROS) must be detoxified as efficiently as possible by enzymatic and non-enzymatic antioxidants to minimize eventual damages in plants (Soares et al. 2019). Superoxide dismutase (SOD, EC 1.15.1.1) which dismutates superoxide (O2·−) into hydrogen peroxide (H2O2) and H2O, and catalase (CAT, EC 1.11.1.6) that reduces H2O2 into H2O are between the most important enzymes involved in antioxidative defense (Gratão et al. 2005). However, Iannone et al. (2015) suggested that CAT did not play a crucial role in protection against Cd toxicity in tobacco (Nicotiana tabacum L.) plants, since this species is able to activate alternative defense mechanisms such as ameliorated synthesis of proline and glutathione.
The importance of proline for tobacco tolerance to Cd was displayed by Islam et al. (2009), who described that exogenous proline supply decreased lipid peroxidation in tobacco cells exposed to Cd. Exogenous proline supply also contributed to adjusting the nutritional status of olive (Olea europaea L.) under Cd exposure (Zouari et al. 2016). Nevertheless, the role of proline in modulating these responses is unknown. Also endogenous proline accumulation is believed to play adaptive roles in plant tolerance against Cd-induced toxicity (Islam et al. 2009; Zouari et al. 2016). Under Cd exposure, proline is synthesized mainly from glutamate that is converted to proline by two successive reductions catalyzed by ∆1-pyrroline-5-carboxylate synthetase (P5CS, EC 2.7.2.11) and pyrroline-5-carboxylate reductase (P5CR, EC 1.5.1.2), respectively (Verbruggen and Hermans 2008; Repkina et al. 2019). However, proline synthesis can be limited by P5CS activity that is subject to feedback inhibition by the product proline (Hong et al. 2000). Thus, the use of transgenic plants containing the mutated enzyme P5CSF129A (EC 2.7.2.11/1.2.1.41) that presents twice more proline accumulation than wild type plants containing the enzyme P5CS can be an alternative to understand the role of proline in tolerance mechanisms against abiotic stress (Hong et al. 2000).
Siripornadulsil et al. (2002) pointed out that microalga Chlamydomonas reinhardtii P. A. Dang mutated to express free proline was more tolerant to Cd than the wild type. However, this increased tolerance was attributed to a higher proline-induced glutathione synthesis. Glutathione (GSH, γ-Glu-Cys-Gly) can be oxidized to GSSG during ROS scavenging, which contributes to preventing the oxidative damages in cells (Yadav 2010). Once oxidized, glutathione reductase (GR, EC 1.6.4.2) catalyzes the reduction of GSSG into GSH (Gratão et al. 2005). Clemens (2006) stated that in plants exposed to Cd, symptoms of oxidative stress such as lipid peroxidation often are a consequence of GSH depletion due to the binding of Cd2+ to GSH and/or its use as substrate for the synthesis of phytochelatins [PCs, (γ-Glu-Cys)n-Gly, with n = 2–11]. Phytochelatins are involved in Cd chelation and its transport from the cytosol to the vacuole (Yadav 2010). It is known that GSH and PCs synthesis are strongly induced by Cd, but there is no available information about the synthesis of these thiol compounds under Cd exposure in plants overproducing proline. Therefore, we do not know if there could be competition between proline and GSH, since both need glutamate to be synthesized (Verbruggen and Hermans 2008; Yadav 2010).
The action of enzymatic and non-enzymatic antioxidants in ROS scavenging depends on the plant’s ability to reconfigure its metabolic network to allow both the maintenance of metabolic homeostasis and the production of compounds that ameliorate the stress (Obata and Fernie 2012). Sun et al. (2010) reported that the concentrations of proline, serine, sucrose, and other metabolites with compatible properties to these increased in Arabdopsis thaliana L. exposed to 50 µmol L−1 Cd compared to control, attenuating the Cd-induced stress. Like proline and GSH, organic acids, amino acids, sugars, and other related metabolites also can attenuate the Cd-induced oxidative stress by acting as chelators, antioxidants, and osmoprotectants (Sharma and Dietz 2006). However, there is no information on metabolic adjustment under Cd-induced stress in plants overexpressing proline or plants supplied with exogenous proline. Thus, our aim with this study was to better understand how exogenous proline supply or proline overproduction could affect the nutritional status, metabolite profile, activity of antioxidant enzymes, concentrations of proline and glutathione, and transcript levels of genes related to the metabolism of proline and glutathione and contribute to attenuate the Cd-induced stress in tobacco (notably in the leaves).
Materials and Methods
Plant Material and Experimental Design
The study was conducted in a greenhouse with controlled conditions: 12 h/22 °C and 12 h/18 °C, 12/12 h light/dark, photosynthetic active radiation of 170 μmol m−2 s−1 at the leaf level delivered by a combination of blue and red Philips® Green-Power LED modules, and 65% relative humidity. Seeds of wild type (Petite Havana SR1) and transgenic (T3) tobacco (N. tabacum L.) containing the P5CSF129A mutated gene under control of the cauliflower mosaic virus (CaMV35S; (called 35S from this point)) or stress inducible rd29A promoters were sown in trays containing vermiculite as substrate, with a daily supply of deionized water. Eleven days after sowing, the nutrient solution of Hoagland and Arnon (1950) at 15% of the ionic strength was supplied, for 14 days. Then, the seedlings were placed in a styrofoam support and transferred to hydroponics composed of plastic trays containing 12 L of nutrient solution of Hoagland and Arnon (1950) at 30% of the ionic strength. From this point, the ionic strength of the solution was gradually increased until it reached 100% at 43 days after sowing. The undiluted nutrient solution was composed of 6 mmol L−1 Ca(NO3)2·4H2O, 6 mmol L−1 KNO3, 2.5 mmol L−1 MgSO4·7H2O, 1 mmol L−1 KH2PO4, 100 μmol L−1 H3BO3, 100 μmol L−1 MnSO4·4H2O, 30 μmol L−1 ZnSO4·7H2O, 0.1 μmol L−1 CuSO4·5H2O, 1 μmol L−1 Na2MoO4·4H2O, and 75 μmol L−1 FeNa-EDTA. Solutions were replaced weekly and remained constantly aerated throughout the entire experiment through plastic tubes connected to an air compressor.
Wild type (WT) and transgenic tobacco plants (35S or rd29A) were either unexposed or exposed to Cd (50 μmol L−1 CdCl2·H2O), for 24 and 72 h. In addition, we evaluated the effect of exogenous proline supply (1 mmol L−1) on WT plants either unexposed or exposed to 50 μmol L−1 CdCl2·H2O, for 24 and 72 h. The exposure times were chosen considering the fact that processes like gene expression involved on plant adjustment to stress Cd-induced tends to occur more often in short-term (Hendrix et al. 2020b; Zdunek-Zastocka et al. 2021). On the 44th day after sowing proline was added to the nutrient solution of WT plants, and on the 45th day after sowing Cd was added to the nutrient solution of all genotypes assayed. Moreover, WT, WT receiving exogenous proline (WT + Pro), and transgenic tobacco (35S and rd29A) collected before Cd supply (time 0 h) were used as control treatments. The trays used to grow the tobacco plants were distributed in completely randomized design with four replicates per condition, and ten plants by replicate. At the moment of the harvest, plants were separated into shoot and roots. The plant material collected to determine the biomass production, nutrients and Cd concentrations was dried in a forced ventilation oven at 60 °C for 72 h, whereas the plant material collected to perform the other analyses was snap frozen in liquid N and stored at – 80 °C until the analyses were carried out.
Determination of Nutrients and Cd Concentrations in Roots and Leaves
After drying in an oven at 60 °C for 72 h, the plant material was ground in a Wiley type mill (Model 4, Thomas Scientific, Swedesboro, USA) and digested with 70–71% HNO3 in a heat block (Cuypers et al. 2002). The concentrations of P, K, Ca, Mg, S, Cu, Fe, Mn, Zn, and Cd were determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent Technologies, 700 Series, Belgium). Blank reagent samples were used in the digestion for quality control. Standard reference material (NIST 1570a—spinach) was also used to assure the accuracy and precision of the analytical methods. From Cd concentrations, we calculated the Cd translocation factor by dividing the Cd concentration in the leaves by the Cd concentration in the roots.
Determination of Metabolite Profiling in the Leaves
Metabolites were extracted from 100 mg of frozen leaves tissue in 0.5 mL of cold extraction solution [isopropanol/acetonitrile/water (3:2:2, v/v/v)] containing succinic acid as internal standard (1 mg mL−1), as described by Zhao et al. (2015, 2016). Tungsten magnetic beads were added to the mixture, and then, the samples were subjected to agitation in a Vibration Mill (Retsch GmbH & Co., KG, Haan, Germany) for 30 s and 20 Hz. Beads were removed and samples centrifuged for 16,000×g for 10 min at 4 °C. The supernatant was filtered (Millex 0.22 µM filter, Millipore) and stored at − 80 °C.
For derivatization of the samples, 30 μL of methoxyamine (15 μg μL−1 in pyridine) were added to 100 µL of the filtered extract previously lyophilized. The samples were vortex-mixed for 1 min and incubated at room temperature in dark for 16 h. After this step, 30 μL of N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) with 1% of trimethylchlorosilane (TMCS) were added and the samples were incubated in the dark for 1 h. Then, 30 μL of heptane were added to the samples, vortex-mixed, and injected in a gas chromatography mass spectrometer (GC–MS) (Pegasus 4D GCxGC-TOFMS, Leco Corporation, St. Joseph, USA). At this stage, control samples (blanks) and a series of alkanes (C12–C40) were used, which it made possible to calculate the retention indices (Schauer et al. 2005). After derivatization, the samples were injected into the 7890A gas chromatograph (Agilent Technologies, Santa Clara, USA) coupled to a Comb-xt automatic processor (Leap Technologies, Carrboro, USA).
The temperature of sample injection was 280 °C and the septum purge flow was 20 mL min−1 for 60 s. Helium gas flow was constant through the column with a flow rate of 1 mL min−1. The column temperature was maintained at 80 °C for 2 min and then increased by 15 °C every minute until reaching 305 °C for 10 min. The column effluent was inserted into GCxGC-TOFMS ionization source (Pegasus 4D, Leco Corp.) equipped with two fused silica columns: the first-dimension column (Agilent DB-5) with 20 m length (0.18 mm inner diameter × 0.18 μm film) and the second-dimension column of 0.96 m (RXT-170.10 mm inner diameter × 0.10 μm film). The transfer line and ion source temperatures were 280 and 250 °C, respectively. The ions were generated by an electron beam of 70 eV in an ionization flow of 2.0 mA, and 10 spectra s−1 in a mass range of m/z 45–800. ChromaTOF v. 4.51 software (Leco Corp.) was used to perform baseline correction and export all MS files in NetCDF. Peak detection, retention time alignment, and library matching were carried out using the TargetSearch package (Cuadros-Inostroza et al. 2009). For the identification of the metabolites, retention indices, spectra with similarity (score) > 600, and metabolites with at least 3 fragments (mass count) were compared with data stored in the database Golm-Metabolome—GMD—(available at http://gmd.mpimp-golm.mpg.de/) (Kopka et al. 2005). The intensity of each metabolite was normalized by the fresh weight (mg) of the corresponding sample and the total ion current (TIC) of each sample.
Determination of H2O2 Concentration and Lipid Peroxidation in the Leaves
Concentrations of H2O2 were determined as described by Alexieva et al. (2001), with modifications. Firstly, 0.2 g of frozen samples were macerated in 2 mL of 0.1% (w/v) trichloroacetic acid (TCA) in the presence of 20% (w/w) of polyvinyl polypyrrolidone (PVPP). After complete homogenization, 2 mL of extract was centrifuged at 10,000×g for 10 min at 4 °C. An aliquot of 0.2 mL was taken from the supernatant and then an aliquot of 0.2 mL of 100 mmol L−1 potassium phosphate buffer (pH 7.0) and 0.8 mL of 1 mol L−1 potassium iodide was added to the mixture. The solution was left for 1 h on ice in darkness to stabilize the reaction. The readings were made in a spectrophotometer at 390 nm (Genesys 10S UV–VIS, Thermo Fisher Scientific, Waltham, USA).
The lipid peroxidation was determined estimating the malondialdehyde (MDA) concentration from 2-thiobarbituric acid (TBA) reactive compounds (Heath and Packer 1968). The initial procedures for MDA measurements were the same as described for H2O2. Following centrifugation, 0.25 mL of sample supernatant was added to 1 mL of 20% (w/v) TCA containing 0.5% TBA. The samples were incubated for 60 min at 95 °C, and then, cooled in an ice bath for 1 min to stop the reaction. Subsequently, the samples were centrifuged at 10,000×g for 10 min to separate the residues formed during heating and to clarify the samples. The absorbance was measured at 535 and 600 nm by using a spectrophotometer (Genesys 10S UV–VIS).
Protein Extraction and Enzymatic Activities Determination in the Leaves
Proteins were extracted from 250 mg of leaves samples that were homogenized with a mortar and pestle in 100 mmol L−1 potassium phosphate buffer (pH 7.5) containing 1 mmol L−1 ethylenediaminetetraacetic acid (EDTA), 3 mmol L−1 dithiothreitol (DTT), and 4% (w/v) PVPP (Azevedo et al. 1998). The resulting homogenate was centrifuged at 10,000×g for 30 min at 4 °C, and the supernatant was stored at − 80 °C for determination of the activities of antioxidative enzymes. Total soluble protein concentrations were determined by the method of Bradford (1976), using bovine serum albumin—BSA (Protein Standard, Sigma-Aldrich) as standard.
Total SOD activity was determined using a spectrophotometer (Giannopolitis and Ries 1977). The assays contained 1.79 mL of 50 mmol L−1 sodium phosphate (pH 7.8), 225 µL of 1 mmol L−1 ρ-nitro blue tetrazolium chloride (NBT), 780 µL of 50 mmol L−1 methionine, 30 µL of 10 mmol L−1 EDTA, 150 µL of 0.1 mmol L−1 riboflavin, and 25 µL of protein extract. The reaction mixture was exposed to light for 5 min and measured at 560 nm.
Total CAT activity was determined using a spectrophotometer following the method described by Kraus et al. (1995), with modifications by Azevedo et al. (1998). The reaction medium was composed of 1 mL of 100 mmol L−1 potassium phosphate buffer (pH 7.5) and 25 μL of 0.25% H2O2 solution. The reaction started after adding 25 μL of plant extract, and CAT activity was determined following the decomposition of H2O2 at 10 s intervals for 1 min at 240 nm.
Total GR activity was determined following the method described by Smith et al. (1988), with modifications by Azevedo et al. (1998). The reaction medium (1 mL) was composed of 100 mmol L−1 potassium phosphate buffer (pH 7.5), 100 µL of 1 mmol L−1 GSSG, 100 µL of 0.1 mmol L−1 NADPH, 500 µL of 1 mmol L−1 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), and 35 µL of protein extract. The activity was estimated by GSSG reduction accompanied by monitoring the change in absorbance at 412 nm for 1 min.
Determination of Proline Concentration in the Leaves
The determination of proline concentration was performed by using 100 mg of leaf tissue homogenized in 3% sulfosalicylic acid. After centrifugation, the supernatant was taken for determination of the proline concentration at 520 nm, according to Bates et al. (1973).
Determination of GSH and GSSG Concentrations in the Leaves
The concentrations of GSH and GSSG were determined using spectrophotometry as described by Anderson (1985), with modifications by Borges et al. (2018). Fresh leaves tissue (200 mg) was homogenized in 1 mL of 50 mmol L−1 sulfosalicylic acid and centrifuged at 10,000×g for 20 min at 4 °C. Then, 0.2 mL of supernatant were added to 1.8 mL of 100 mmol L−1 potassium phosphate buffer (pH 7.0) containing 0.5 mmol L−1 EDTA and 100 μL of DTNB. The mixture was kept in the dark for 5 min and then taken to spectrophotometer (Genesys 10S UV–VIS) at 412 nm to measure GSH concentration. Then, 100 μL of 0.4 mmol L−1 NADPH and 2 μL of GR (1 U/reaction) were added to the mixture, which was kept in the dark for 20 min and read again in the spectrophotometer at 412 nm to measure the concentrations of GSH + GSSG. The concentrations of GSSG were obtained by the difference between the concentrations of GSH + GSSG and GSH. The redox state of glutathione was also calculated as GSH/GSSG (Jozefczak et al. 2015).
Gene Expression Analysis in the Leaves
The expression of genes related to the synthesis and degradation of proline (P5CSF129A, P5CS-1, P5CS-2, P5CR, PDH1, and P5CDH), glutathione (GSH, GSH2, and GR1), phytochelatins (PCS1), and metal transporters (MRP3 and PDR8) plants was measured in the leaves of tobacco by real-time reverse transcription PCR (RT-qPCR), as described by Keunen et al. (2015). Total RNA was extracted using the RNAqueous® Total RNA Isolation Kit (Ambion, Life Technologies, Merelbeke, Belgium), followed by a DNAse treatment (DNAse I Kit, Invitrogen, Thermo Fisher Scientific). Then, extracted RNA was quantified in RNAse-free PCR tubes using the QuantiFluor RNA System (Promega Corp., Madison, USA) and 1 μL of RNA samples using a portable fluorometer (Quantus Fluorometer, Promega Corp., Madison, USA). Random primers and SuperScript III RT Kit (Invitrogen) were used to convert RNA (1 μg) into cDNA according to the manufacturer. After this step, cDNA was diluted 10 times in 1/10 diluted Tris–EDTA (TE) buffer (Tris–HCl 1 mmol L−1, Na2-EDTA 0.1 mmol L−1, pH 8.0) and subsequently stored at 20 °C.
Real-time PCR quantification was performed in 96-well optical plates using the 7500 Fast Real-Time PCR System (Applied Biosystems, Life Technologies, Gent, Belgium) and the Fast SYBR Green Master Mix (Applied Biosystems). Amplification occurred under universal cycling conditions (20 s at 95 °C, 40 cycles of 3 s at 95 °C, and 30 s at 60 °C), followed by the generation of a dissociation curve to verify amplification specificity. Forward and reverse primers (300 nmol L−1) were manually designed using tobacco sequences deposited in GenBank (Table 1), and quality verified using NetPrimer (http://www.premierbiosoft.com/netprimer/) software. Gene expression was calculated using the 2−ΔCq method in relation to WT expression at the time 0 h (control). All data were normalized by using the expression of two stable reference genes: EF1-a (Elongation Factor 1-alpha) (Ye et al. 2016) and NtEFa (Elongation Factor a) (Fässler et al. 2011). Primer efficiencies were determined using a standard curve of a two-fold dilution series generated from a pooled sample.
Statistical Analysis
To perform statistical analysis, each combination of genotype (WT, WT + Pro, 35S, and rd29A) and Cd rate (0 and 50 μmol L−1 CdCl2·H2O) was considered as a treatment, totalling eight treatments. For the longitudinal analysis, the times 0 (control treatments), 24 and 72 h have been considered as factors (except for metabolite profiling that was only determined at the 24 h and analyzed as described below). Thus, for each treatment, a normal regression model was built to test a possible difference in the mean of the response variable between the different Cd exposure times within the same treatment. To compare the means between genotypes within each Cd rate in a fixed time and to compare the means between Cd rate within each genotype in a fixed time, an analysis of variance (ANOVA) was performed. When the F test was significant in the ANOVA, we applied the post hoc tests (Tukey test) to provide specific information on which means differed from each other. For all tests mentioned above, the p-value was fixed in 5%. All statistical analyses were performed using R software v. 3.0.2 (R Core Team 2019). Graphs were created and plotted with SigmaPlot v. 10.0 (SIGMAPLOT 2006), and the results are expressed as means ± standard error of the mean.
Multivariate (Partial least square discriminant analysis—PLS-DA) and univariate (ANOVA) analyses were performed on the entire metabolomics data set using the MetaboAnalyst 4.0 (Chong et al. 2018). Data were normalized by the median, log-transformed, and scaled by Auto scaling prior to data analysis. Differentially abundant metabolites were identified based on the variable importance in projection (VIP ≥ 1), followed by one-way ANOVA (FDR, adjusted p ≤ 0.05).
Results
Proline Overproduction Allowed Continuous Root Biomass Production Even Under Cd Exposure and Exogenous Proline Supply Decreased Root-to-Shoot Cd Translocation in WT Plants
The biomass production of tobacco supplied with exogenous proline or overproducing proline (35S and rd29A) did not differ from that of WT, regardless of Cd rate (Figs. 1A, B). However, all tobacco plants after 72 h exposed to Cd exhibited lower biomass compared to unexposed plants. Analyzing the data over the time, we observed that shoot biomass of all plants continued increasing after 24 or 72 h in both unexposed and Cd exposed conditions (Fig. 1A). However, only the roots biomass of 35S and rd29A plants continued increasing after 24 or 72 h of Cd exposure (Fig. 1B).
Biomass production (A, B), Cd concentrations (C, D) in the leaves (A, C) and roots (B, D), and Cd translocation factor (Cd TF, E) in wild type (WT), WT supplied with 1 mmol L−1 of exogenous proline 24 h before Cd exposure (WT + Pro), and transgenic tobacco (Nicotiana tabacum L.) plants containing the mutated P5CSF129A gene under control of the cauliflower mosaic virus CaMV35S (35S) or stress inducible rd29A (rd29A) promoters, exposed to Cd (0 and 50 μmol L−1 CdCl2·H2O) for 0 (control), 24 and 72 h. Distinct upper case letters on the bars indicate difference between Cd rate (0 vs 50 μmol L−1 CdCl2·H2O) within each genotype (WT, WT + Pro, 35S, and rd29A) over the time, and distinct lower case letters indicate difference between genotypes within each Cd rate for each exposure time (Tukey test, n = 4, p ≤ 0.05). p-values in bold in the tables within each figure indicate difference between exposure times within each genotype and Cd rate (p ≤ 0.05). ND non-detected
Cadmium concentration in the leaves of WT did not differ from that of tobacco supplied with exogenous proline or overproducing proline, regardless of Cd exposure time (Fig. 1C). Meanwhile, Cd concentration in the roots of 35S and WT supplied with exogenous proline was higher compared to the other plants after 72 h of Cd exposure (Fig. 1D). Cadmium concentrations in the leaves and roots of all plants increased over time due to Cd exposure (Figs. 1C, D). Interestingly, Cd translocation from roots to shoots in WT supplied with exogenous proline was lower compared to WT grown without exogenous proline supply and to 35S and rd29A after 24 or 72 h of Cd exposure (Fig. 1E). Cadmium translocation presented a tend to increase over the time in all genotypes (Fig. 1E).
Cadmium Exposure and Exogenous Proline Supply or Proline Overproduction Induced Changes in Nutrients Concentrations in the Leaves and Roots of Tobacco Plants
The concentrations of P, K, Mg, S, Cu, Fe, Mn, and Zn in the leaves of plants supplied with exogenous proline or overproducing proline did not differ from WT collected before Cd supply (control) (Tables 2 and 3). Meanwhile, both plants overproducing proline tended to present higher K concentrations in their roots in relation to WT (Table 2), while WT supplied with exogenous proline tended to present lower Mn and Zn concentrations in their roots compared to other plants (Table 3). 24 h after the beginning of the study, rd29A contained the highest P and Cu concentrations in the leaves under Cd exposure. There was no difference in nutrient concentrations in the leaves and roots between the genotypes after 72 h, regardless of Cd rate (Tables 2 and 3).
Cadmium exposure after 24 h increased P concentration in the leaves of rd29A (Table 2) and decreased Mn and Zn concentrations in the leaves of WT grown without or with exogenous proline supply (Table 3). In general, there was no effect of Cd exposure on nutrient concentrations in the leaves of plants after 72 h, as well as there was no effect of Cd exposure after 24 or 72 h on macronutrient concentrations in the roots of plants (Table 2), regardless of exogenous proline supply or proline overproduction. Nevertheless, 24 h of Cd exposure decreased Fe concentrations in the roots of 35S, whereas 72 h of Cd exposure decreased Mn concentrations in the roots of genotypes overproducing proline (Table 3). Iron concentrations increased after 72 h of Cd exposure in the roots of WT supplied with exogenous proline (Table 3).
Analyzing the nutrient concentrations over the time, we observed that Mg concentration in the leaves of plants tended to decrease, especially under Cd exposure. For the other nutrients there was no pattern as a result of exogenous proline supply, proline overproduction, or Cd exposure (Tables 2 and 3). Nutrient concentrations in the roots of plants were less affected over time than nutrient concentrations in the leaves, with exception of Fe concentration that decreased over time, especially in plants unexposed to Cd (Tables 2 and 3). There was no significant effect of genotypes, Cd rate or exposure time on Ca concentrations (results not shown), which ranged from 19.35 to 29.61 g kg−1 DW in the leaves and from 13.12 to 22.68 g kg−1 DW in the roots.
The Metabolite Profiling of Plants Overproducing Proline Was Much More Differentiated Than in WT Plants Receiving Exogenous Proline Supply in Order to Adjust to Cd-Induced Stress
To assay the response of plants to Cd exposure we compared WT with WT that received exogenous proline supply and with plants overproducing proline, in the absence and presence of Cd. In total, 163 metabolites with known structures were identified by GC-TOFMS and classified as sugars and sugar acids (21.5%), amino acids and their analogues (12.9%), lipids (12.3%), organic acids (12.3%), steroids (6.7%), alkanes (4.3%), alkaloids (3%), nucleic acids (2.4%), benzenoids (1.8%), and others (19.1%) (Supplementary Table l). To reduce the data dimensionality and visualize the response of tobacco to Cd, we performed a supervised PLS-DA obtaining score plots (Fig. 2A–C). In the models obtained for WT supplied with exogenous proline and for rd29A (Fig. 2A and C), the samples coming from plants exposed to Cd were not grouped with the correspondent ones unexposed to Cd, which indicates that the metabolites’ response under Cd exposure can be distinguished from the other ones. Based on the parameter VIP (≥ 1) and ANOVA (FDR, adjusted p ≤ 0.05), 3, 18 , and 34 responsive metabolites were identified in WT supplied with exogenous proline and in 35S and rd29A, respectively. Responsive metabolites were composed mainly of amino acids and derivatives, organic acids, and sugars (Supplementary Table 2). The differentially abundant metabolites for each comparison were plotted as heatmaps with hierarchical clustering (Fig. 2D–F).
Partial least squares discriminant analysis (PLS-DA) of the metabolites identified by GC-TOFMS (A–C) and hierarchical clustering with heatmap showing the relative abundance of the metabolites (D, F) in the leaves of wild type (WT), WT supplied with 1 mmol L−1 of exogenous proline 24 h before Cd exposure (WT + Pro), and transgenic tobacco (Nicotiana tabacum L.) plants containing the mutated P5CSF129A gene under control of the cauliflower mosaic virus CaMV35S (35S) or stress inducible rd29A (rd29A) promoters, exposed to Cd (0 and 50 μmol L−1 CdCl2·H2O) after 24 h. Score plot model (A) and hierarchical clustering (D) for WT + Pro − Cd, WT + Pro + Cd, WT − Cd, and WT + Cd; score plot model (B) and hierarchical clustering (E) for 35S − Cd, 35S + Cd, WT − Cd, and WT + Cd; score plot model (C) and hierarchical clustering (F) for rd29A − Cd, rd29A + Cd, WT − Cd, and WT + Cd. Outliers (WT + Pro + Cd) were removed from the models (A). PLS-DA cross validation parameters: R2 (correlation analysis: 0.99, 0.99, 0.99), and Q2 (predictive capability: 0.84, 0.83, 0.84) for the three first principal components from the models A, B and C, respectively. The relative metabolite level is depicted by the color scale (D–F): red indicates up-regulation and blue indicates down-regulation (Color figure online)
From the heatmaps we observed that each genotype adjusted to Cd exposure using different metabolic strategies. Only 3 metabolites were significantly abundant in WT supplied with exogenous proline (Fig. 2D). Ornithine was up-regulated in WT, while erythronic acid was up-regulated in WT supplied with exogenous proline and threonine was up-regulated after Cd exposure in WT supplied with exogenous proline (Fig. 2D). Hierarchical clustering distinguished 5 and 6 groups of metabolites for the comparisons related to 35S and rd29A, respectively (Figs. 2E, F). Metabolites such as galactose-6-phosphate, N-tetradecanoyl-homoserine lactone, and erythronic acid were down-regulated in 35S compared to WT unexposed to Cd, but other metabolites such as lactulose and hydroquinone were up-regulated in 35S compared to WT exposed to Cd (Fig. 2E). Cadmium exposure led to accumulation of N-tetradecanoyl-homoserine lactone and N-(3-oxohexanoyl)-homoserine lactone in WT, and lactulose, hydroquinone, and glyceric acid in 35S (Fig. 2E). Comparing WT and rd29A we observed that metabolites such as sucrose and galactose-6-phosphate were more accumulated in rd29A compared to WT unexposed and exposed to Cd (Fig. 2F). Cadmium exposure induced the accumulation of several metabolites in WT (e.g., raffinose and mannitol) and rd29A (e.g., sucrose and cysteine) (Fig. 2F).
There Was No Lipid Peroxidation and Reduction on Protein Concentration Cd-Induced in the Leaves of Tobacco Plants, Despite Decreased SOD Activity Over Time in WT Plants Exposed to Cd
The H2O2 concentration in the leaves of tobacco supplied with exogenous proline or tobacco overproducing proline did not differ from WT, regardless of Cd rate (Fig. 3A). Only the 35S presented higher H2O2 concentration in their leaves after Cd exposure for 24 h compared to unexposed plants. From the analysis of the data over time, we can see that there were only small variations on H2O2 concentrations in the leaves of plants, regardless of genotypes or Cd rate (Fig. 3A).
Concentrations of hydrogen peroxide (H2O2, A), malondialdehyde (B) and soluble protein (C) and activities of superoxide dismutase (SOD, D) and catalase (CAT, E) in the leaves of wild type (WT), WT supplied with 1 mmol L−1 of exogenous proline 24 h before Cd exposure (WT + Pro), and transgenic tobacco (Nicotiana tabacum L.) plants containing the mutated P5CSF129A gene under control of the cauliflower mosaic virus CaMV35S (35S) or stress inducible rd29A (rd29A) promoters, exposed to Cd (0 and 50 μmol L−1 CdCl2·H2O) for 0 (control), 24 and 72 h. Distinct upper case letters on the bars indicate difference between Cd rate (0 vs 50 μmol L−1 CdCl2·H2O) within each genotype (WT, WT + Pro, 35S, and rd29A) over the time, and distinct lower case letters indicate difference between genotypes within each Cd rate for each exposure time (Tukey test, n = 3, p ≤ 0.05). p-values in bold in the tables inside each figure indicate difference between exposure times within each genotype and Cd rate (p ≤ 0.05)
In general, there was no relation between the concentrations of H2O2, MDA, and soluble protein (Fig. 3A–C). The MDA concentrations in the leaves were very similar to each other in the control treatments and after 24 h, but after 72 h, plants unexposed to Cd that received exogenous proline supply presented higher MDA concentrations compared to the other plants. Cadmium exposure did not increase MDA concentrations in the leaves, regardless of the genotypes or exposure time. There was also no effect of genotypes nor Cd rate on soluble protein concentrations in the leaves of plants (Fig. 3C). However, we can see that Cd exposure decreased the soluble protein concentration in the leaves of WT over time, differently from which occurred with plants supplied with exogenous proline or overproducing proline after 72 h of Cd exposure (Fig. 3C).
The activities of the enzymes SOD and CAT of plants supplied with exogenous proline or overproducing proline did not differ from WT, regardless of Cd rate (Fig. 3D, E). There was also no effect of Cd exposure on SOD and CAT activities, regardless of genotypes. Over the time, we can observe that tobacco grown without exogenous proline supply or proline overproduction tended to present lower SOD activity due to Cd exposure for 72 h (Fig. 3D). Meanwhile, WT supplied or not with exogenous proline presented an increase on CAT activities in their leaves after 72 h compared to WT of the control, regardless of Cd rate (0 vs 72 h; Fig. 3E).
Plants Overproducing Proline Under the Control of the rd29A Promoter Presented Higher Proline Accumulation, Which Correlated with Genes Controlling the Metabolism of Proline
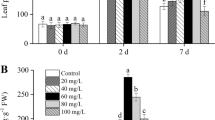
In the absence of Cd, rd29A presented higher proline concentrations compared to other plants at the times 0 and 24 h (Fig. 4). Differently from the results observed in Cd absence, both transgenic tobacco lines presented similar proline concentrations to WT after 24 h of Cd exposure, regardless of exogenous proline supply. 72 h after, rd29A presented higher proline concentration in relation to other plants, regardless of Cd rate. Proline concentration was not affected by Cd exposure in the leaves of WT and 35S. On the other hand, proline concentrations in the leaves of rd29A exposed to Cd was 90% lower compared to plants unexposed to Cd after 24 h, but after 72 h, proline concentrations in the leaves of rd29A exposed to Cd was 97% higher compared to rd29A unexposed to Cd. The analysis of the data over the time showed that proline concentrations in the leaves of rd29A exposed to Cd after 72 h were 63% higher in relation to control (Fig. 4).
Proline concentration in the leaves of wild type (WT), WT supplied with 1 mmol L−1 of exogenous proline 24 h before Cd exposure (WT + Pro), and transgenic tobacco (Nicotiana tabacum L.) plants containing the mutated P5CSF129A gene under control of the cauliflower mosaic virus CaMV35S (35S) or stress inducible rd29A (rd29A) promoters, exposed to Cd (0 and 50 μmol L−1 CdCl2·H2O) for 0 (control), 24 and 72 h. Distinct upper case letters on the bars indicate difference between Cd rate (0 vs 50 μmol L−1 CdCl2·H2O) within each genotype (WT, WT + Pro, 35S, and rd29A) over the time, and distinct lower case letters indicate difference between genotypes within each Cd rate for each exposure time (Tukey test, n = 3, p ≤ 0.05). p-values in bold in the tables inside each figure indicate difference between exposure times within each genotype and Cd rate (p ≤ 0.05)
The higher proline concentration observed in rd29A collected at the times 0 and 24 h, in the absence of Cd (Fig. 4), probably occurred due to a higher P5CSF129A expression (Fig. 5A), since there was no significant difference in the expression of P5CS-1, P5CS-2, and P5CR (Fig. 5B–D) as compared to the other plants. Although unexposed rd29A presented the highest expression of PDH1 compared to the other plants (Fig. 5E), proline was highly accumulated in these conditions (Fig. 4). There was no variation between genotypes unexposed to Cd concerning the expression of P5CDH (Fig. 5F). On the other hand, 24 h after Cd exposure plants overproducing proline presented similar proline concentrations to WT, regardless of exogenous proline supply (Fig. 4), even the transgenic plants presenting lower P5CDH expression compared to WT (Fig. 5F). This result probably is related to the low expression of P5CSF129A and P5CR under Cd exposure after 24 h (Fig. 5A and D).
Relative expression (fold change) of the genes P5CSF129A (A), P5CS-1 (B), P5CS-2 (C), P5CR (D), PDH1 (E), P5CDH (F), GSH1 (G), GSH2 (H), GR1 (I), PCS1 (J), MRP3 (K), and PDR8 (L) in the leaves of wild type (WT), WT supplied with 1 mmol L−1 of exogenous proline 24 h before Cd exposure (WT + Pro), and transgenic tobacco (Nicotiana tabacum L.) plants containing the mutated P5CSF129A gene under control of the cauliflower mosaic virus CaMV35S (35S) or stress inducible rd29A (rd29A) promoters, exposed to Cd (0 and 50 μmol L−1 CdCl2·H2O) for 0 (control), 24 and 72 h. Distinct upper case letters on the bars indicate difference between Cd rate (0 vs 50 μmol L−1 CdCl2·H2O) within each genotype (WT, WT + Pro, 35S, and rd29A) after 24 h, and distinct lower case letters indicate difference between genotypes within each Cd rate for each exposure time (Tukey test, n = 3, p ≤ 0.05). Asterisks (*) over the letters on the bars inside each figure indicate difference between exposure times within each genotype and Cd rate (p ≤ 0.05). **Relative expression levels to transgenic tobacco overexpressing proline under control of constitutive promoter CaMV35S (35S) unexposed to Cd at the time 0 h (control)
The Synthesis of GSH and the Capacity to Keep Glutathione in Its Reduced Form Was Limited in Tobacco Plants Overproducing Proline Under Control of 35S Promoter
The concentrations of GSH and GSH + GSSG, and the redox state of GSH in tobacco supplied with exogenous proline or overproducing proline did not differ from that of WT collected immediately before Cd supply, differently from the concentration of GSSG that was lower in 35S (Fig. 6A–D). 24 and 72 h after the beginning of the study, there was no difference in the concentrations of GSH, GSSG, and GSH + GSSG (Fig. 6A–C), and in the redox state of GSH (Fig. 6D) between the genotypes unexposed to Cd. On the other hand, when the tobacco lines were exposed to Cd for 72 h, 35S presented lower concentrations of GSH and GSH + GSSG and a lower redox state of GSH (Fig. 6A and C, D). Cadmium exposure induced higher GSH accumulation in WT (regardless of exogenous proline supply) and rd29A compared to the same plants unexposed to Cd after 72 h (Fig. 6A). The concentration of GSH + GSSG was higher in WT exposed to Cd compared to WT unexposed to Cd after 72 h, regardless of exogenous proline supply (Fig. 6C). The redox state of GSH in WT and rd29A exposed to Cd was higher than in the same plants unexposed to Cd after 72 h (Fig. 6D). Analyzing these results over the time, 35S have low capacity to keep glutathione in its reduced form (GSH) under Cd exposure (Fig. 6A–D), even when presenting similar GR activities as in other plants after 24 and 72 h of Cd exposure (Fig. 6E).
Concentrations of reduced glutathione (GSH, A), oxidized glutathione (GSSG, B) and total glutathione (GSH + GSSG, C), redox state of glutathione (GSH/GSSG, D) and activity of glutathione reductase (GR, E) in the leaves of wild type (WT), WT supplied with 1 mmol L−1 of exogenous proline 24 h before Cd exposure (WT + Pro), and transgenic tobacco (Nicotiana tabacum L.) plants containing the mutated P5CSF129A gene under control of the cauliflower mosaic virus CaMV35S (35S) or stress inducible rd29A (rd29A) promoters, exposed to Cd (0 and 50 μmol L−1 CdCl2·H2O) for 0 (control), 24 and 72 h. Distinct upper case letters on the bars indicate difference between Cd rate (0 vs 50 μmol L−1 CdCl2·H2O) within each genotype (WT, WT + Pro, 35S, and rd29A) over the time, and distinct lower case letters indicate difference between genotypes within each Cd rate for each exposure time (Tukey test, n = 3, p ≤ 0.05). p-values in bold in the tables inside each figure indicate difference between exposure times within each genotype and Cd rate (p ≤ 0.05)
There was no difference in the expression of the genes GSH1 and GSH2 in the leaves of unexposed plants (Fig. 5G, H), but after 24 h of Cd exposure the expression of GSH1 and GSH2 in rd29A was lower than WT. The low expression of GSH1 and GSH2 did not limit the synthesis of GSH in rd29A in relation to other plants exposed to Cd for 24 h (Fig. 6A). Although the expression of GR1 (encoding for GR) was lower in the leaves of 35S compared to other plants after 24 h of Cd exposure (Fig. 5I), this genotype presented similar GR activity as the other plants (Fig. 6E).
There was no difference in PCS1 expression between the plants after 24 h, regardless of Cd rate. Taking together the results of PCS1 expression (Fig. 5J) and GSH concentration (Fig. 6A) we can conclude that PCs concentrations in the genotypes assayed should be similar to each other after 24 h. Nevertheless, the higher expression of the gene MRP3 that encodes for the ABCC3 transporters (vacuolar membrane-localized protein involved in the vacuolar transport of PC–Cd complexes) (Brunetti et al. 2015) in the leaves of WT supplied with exogenous proline compared to 35S and rd29A after 24 h of Cd exposure (Fig. 5K) suggests that PCs synthesis was possibly higher when there was exogenous proline supply. There was no effect of genotypes nor Cd rate on the expression of PDR8 (pleiotropic drug resistance) that is involved in Cd cellular efflux (Kim et al. 2007). However, PDR8 expression in the leaves of WT and rd29A exposed to Cd after 24 h decreased over the time (Fig. 5L).
Discussion
Although it is believed that proline accumulation plays an adaptive role in plant tolerance against Cd-induced toxicity (Islam et al. 2009; Zouari et al. 2016; Repkina et al. 2019), this process is not completely known. In this study we assayed the effect of exogenous proline supply and proline overproduction on tolerance mechanisms of tobacco plants exposed to Cd in order to better understand the role of proline in mitigating Cd-induced toxicity. It is known that Cd exposure can change a series of physiological, biochemical, and molecular events in plants that leads to inhibition of biomass production (Clemens 2006; Gallego et al. 2012), as occurred with all tobacco plants after 72 h of Cd exposure (Fig. 1A, B). The biomass production of plants supplied with exogenous proline or overproducing proline under control of 35S and rd29A promoters did not differ from that of the WT, under Cd exposure (Fig. 1A, B). Differently from our results, Zouari et al. (2016) described that olive plants exposed to Cd produced more biomass when it received exogenous proline supply, and attributed this result to a differentiated proline-induced distribution of Cd between roots and leaves. In our study, only WT plants grown with exogenous proline supply exhibited Cd concentration in their leaves below 100 mg kg−1 DW after 72 h of Cd exposure (Fig. 1C), which can be attributed to a higher Cd accumulation in the roots (Fig. 1D) and a lower root-to-shoot Cd translocation (Fig. 1E) in this treatment. It is known that Cd accumulation in the roots is strongly related to PCs synthesis in this tissue (Clemens 2006). Therefore, it is possible that exogenously applied proline allowed high proline accumulation in the roots of tobacco exposed to Cd helping to reduce oxidative damage and contributing to a more reducing cellular environment, which may then have increased GSH concentration, allowing higher PCs synthesis (Siripornadulsil et al. 2002).
Some studies have also pointed out beneficial effects of proline on maintaining the nutritional plant status (Islam et al. 2009; Zouari et al. 2016), which is desirable since the modulation of nutritional status is involved in plant tolerance against Cd-induced stress (Carvalho et al. 2020). Indeed, in general tobacco lines overproducing proline were less susceptible to Cd-induced nutritional changes compared to WT (Tables 2 and 3). However, Cd exposure increased P concentrations in the leaves of rd29A and decreased Mn and Zn concentrations in the leaves of WT plants, regardless of exogenous proline supply, 24 h after the beginning of the study (Tables 2 and 3). Interestingly, when P concentration in the leaves of rd29A increased due Cd to exposure (Table 2), there was a decrease in proline concentration (Fig. 4). Aleksza et al. (2017) mentioned that phosphate starvation led to gradual increase in proline concentration in A. thaliana as well as in transcriptional activation of P5CS-1. In fact, the expression of P5CS-1 in our study was higher in tobacco plants which presented lower P concentrations after 24 h of Cd exposure (Fig. 5A; Table 2). However, there is no relationship between P concentration and P5CSF129A expression (Table 2; Fig. 5A). Bertoli et al. (2012) evaluated the effect of Cd on uptake and translocation of nutrients in tomato (Solanum lycopersicum L.) and also found lower Mn and Zn concentrations in the leaves of plants exposed to Cd, which was attributed to the antagonistic competition between Cd and Mn/Zn. In our study, Cd exposure also decreased S concentration in the leaves of 35S plants after 72 h (Table 2), which possibly contributed to the lower concentrations of GSH and GSH + GSSG in 35S compared to other plants after 72 h (Fig. 6A and C), since S is a component of cysteine that is used for GSH synthesis (Yadav 2010).
Tobacco 35S also presented lower Fe concentrations in their roots after 24 h of Cd exposure, which was different from WT plants supplied with exogenous proline that presented higher Fe concentration after 72 h of Cd exposure (Table 3). Sharmila et al. (2017) suggested that proline accumulation in leaves of Indian mustard exposed to Cd was coupled to Fe depletion. If we consider that the same could occurs in the roots, our results make sense since 35S plants should synthesize more proline and WT plants do not need to synthesize proline due to exogenous supply. Furthermore, both transgenic plants overproducing proline presented decreased Mn concentrations in their roots after 72 h of Cd exposure (Table 3). The lower concentrations of cationic micronutrients, such as Mn, in the roots of plants have been often attributed to competition of Cd2+ for the same nutrient transporters (Bertoli et al. 2012), but lower Mn concentrations in the roots can indicate protective mechanisms to counteract the entrance of positive charges originating from Cd accumulation in roots (Carvalho et al. 2020). Changes on nutrient concentrations are often coupled to negative outcomes on plant development under Cd exposure, but the degree of plant tolerance to short Cd exposure is related to its capacity to adjust the nutritional status in order to improve its performance under Cd-induced stress (Carvalho et al. 2020). Manganese deficiency can increase root endodermal suberization (Chen et al. 2019), which is an important mechanism to immobilize Cd in cell walls (Gallego et al. 2012). Dicotyledonous species such as tobacco present several proline- or hydroxyproline-rich glycoprotein constituents in the cell wall (Stiefel et al. 1988). Thereby, it is probable that there is some link between proline, Mn status, and root suberization, but this assumption needs to be carefully investigated. Cd-induced changes on nutrient homeostasis can also affect the metabolic network of plants that must be reconfigured under stress conditions to allow the maintenance of metabolic homeostasis and the production of compounds that ameliorate the stress (Obata and Fernie 2012). Thus, the ameliorated capacity of tobacco plants overproducing proline to cope with nutritional alterations (Tables 2 and 3) could facilitate metabolic adjustments under Cd-induced stress (Fig. 2).
Only 3 metabolites were significantly different in WT plants grown with exogenous proline supply (Fig. 2A and D), but 18 and 34 responsive metabolites were identified in plants overproducing proline under control of 35S and rd29A promoters, respectively (Fig. 2E, F), indicating us that proline is involved in metabolic network adjustments, especially under stress conditions. In 35S plants, Cd exposure induced the accumulation of sugars (lactulose and similar to glycerolaldopyranosid) and organic acids (glyceric and galactaric acids) (Fig. 2E). Karalija and Selović (2018) related proline seed priming with an increased sugar content in maize (Zea mays L.), indicating an interaction of increased proline and soluble sugars on antioxidant plant protection. Sugars are involved in direct ROS quenching in different organelles and act in an integrated cellular redox network (for a comprehensive review see Keunen et al. 2013). Like sugars, organic acids are important metabolites to decrease the Cd-induced stress in plants (Sun et al. 2010). Sun et al. (2006) pointed out that organic acids are related to Cd hyperaccumulation in the leaves of Solanum nigrum L. due to their roles in Cd complexation, transportation and storage, mainly in the vacuoles. Similarly to what was observed in 35S plants, proline overproduction under control of rd29A promoter allowed accumulation of sugars (sucrose, galactose-6-phosphate, and mannitol), organic acids (erythronic acid), and amino acids (cysteine and glutamic acid) after 24 h of Cd exposure (Fig. 2F). The interaction between proline and sugars (Karalija and Selović 2018), such as sucrose that acts on hydroxyl (°OH) scavenging (Van den Ende and Valluru 2009), is important to reduce oxidative damage and contribute to a more reducing cellular environment (Siripornadulsil et al. 2002). Thus, as speculated by Siripornadulsil et al. (2002), a more reducing cellular environment probably contributed for the increased cysteine concentration in rd29A plants after Cd exposure (Fig. 2F), which is essential for both GSH and PCs synthesis (Yadav 2010). The synthesis of antioxidants like sugars and GSH is indispensable to avoid the Cd-induced oxidative damages.
Cd-induced oxidative damage leads to a vast number of responses in plants depending on both, the Cd concentration and the exposure time (Gallego et al. 2012). These triggered responses are particularly important to be understood in leaves, since ROS production can impair photosynthesis that in turn is one of the main causes of Cd-induced growth inhibition (Zouari et al. 2016). Whereas oxidative stress has often been discussed as a primary effect of Cd2+ exposure (Clemens 2006), only the plants overproducing proline under the control of the 35S promoter presented higher H2O2 concentrations in their leaves after 24 h of Cd exposure compared to unexposed plants (Fig. 3A). However, Cd exposure did not induce lipid peroxidation (Fig. 3B) in all tobacco genotypes assayed, regardless of exposure time. Our results are similar to those described by Repkina et al. (2019) who also did not observe any symptom of Cd-induced oxidative stress in wheat leaves in the first 48 h of Cd exposure and attributed this result to an efficient action of non-enzymatic antioxidants and Cd-chelators (e.g., sugars, GSH, and PCs). In our study, the activities of SOD and CAT also did not increase due to Cd exposure, regardless of exogenous proline supply or proline overproduction (Fig. 3D, E). Plants not supplied with exogenous proline or overproducing proline tended to present lower SOD activity over time after Cd exposure (Fig. 3D). Perhaps SOD and CAT are not the main line of defense against Cd-induced oxidative stress in tobacco plants, as previously speculated in other studies. Martins et al. (2014) pointed out that SOD was not heavily involved in antioxidative responses of tobacco exposed to Cd. Iannone et al. (2015) stated that CAT did not play a crucial role in tobacco protection against Cd toxicity, since this species is able to activate alternative defense mechanisms such as ameliorated synthesis of proline and GSH.
Proline accumulation is a common physiological response in many plant species under biotic and abiotic stresses (Hong et al. 2000; Sharma and Dietz 2006), such as Cd exposure (Islam et al. 2009; Sun et al. 2010; Iannone et al. 2015; Zouari et al. 2016; Repkina et al. 2019), since this amino acid has protective functions such as osmotic adjustment, stabilization of cellular structures, and ROS scavenging (Verbruggen and Hermans 2008; Borgo et al. 2015). However, in our study Cd exposure only led to proline accumulation in tobacco plants overproducing proline under the control of the rd29A promoter, after 72 h (Fig. 4). This result is probably related to the expression of the genes linked to proline synthesis, since the results measured in the first 24 h of study indicate that the expression of P5CSF129A in transgenic tobacco was higher in rd29A than 35S plants (Fig. 5A). The first glutamate reduction in tobacco overproducing proline is regulated by P5CSF129A. Kumar et al. (2010) reported that proline accumulation in salt-stressed rice (Oryza sativa L.) was closely correlated with the expression of the P5CSF129A gene. Moreover, the 35S promoter is presumed to be a constitutive promoter, but it contains several domains and subdomains that can confer different developmental and tissue-specific expression patterns in different species (Borgo et al. 2015). These same authors reported that tobacco overproducing proline under control of 35S promoter presented higher proline concentrations in the roots than in leaves. Despite the fact that tobacco overproducing proline under control of the 35S promoter, they did not present higher proline concentrations in their leaves after Cd exposure (Fig. 4). Nevertheless, the concentration of this amino acid was two-fold higher compared to WT plants after 24 and 72 h of Cd exposure, which is in agreement with the results described by Hong et al. (2000) for this genotype.
In non-transgenic plants, the levels of both P5CS and P5CR transcripts were correlated with proline concentration in A. thaliana leaves (Verbruggen and Hermans 2008). In the case of WT plants supplied with exogenous proline that presented Cd-induced expression of P5CS-1 (Fig. 5B) and low proline concentration (Fig. 4) after 24 h, it is possible that post-transcriptional changes took place, which limited proline synthesis. The low proline concentration in the leaves of WT that received exogenous proline supply (Fig. 4) can also be associated to low proline exportation via xylem from roots to leaves due to the local proline storage in root cells, in the absence and presence of Cd. This assumption makes sense considering that PDH1 expression is strongly induced by exogenous proline addition (Verbruggen and Hermans 2008), but PDH1 expression in the leaves of WT plants grown with or without exogenous proline addition was similar to each other (Fig. 5E). Gagneul et al. (2007) reported that excessive proline was sequestered into the vacuoles in the roots of non-stressed Limonium latifolium L., whereas high proline concentrations were detected in the cytosol of plants under salt-stress. Therefore, proline coming from exogenous supply could be stored in the roots and used when necessary in plants under Cd-induced stress. Zouari et al. (2016) determined proline concentrations in the roots and leaves of olive plants exposed to Cd in response to exogenous proline supply and observed higher proline concentrations in the roots (low exportation to leaves), which was the tissue more damaged by the Cd-induced oxidative stress. Although proline can also accumulate due to a low degradation rate in reactions catalyzed by proline dehydrogenase (PDH, EC 1.5.99.8) and pyrroline-5-carboxylate dehydrogenase (P5CDH, EC 1.2.1.88) (Verbruggen and Hermans 2008), there was no clear correlation between proline concentration and the expression of the PDH1 and P5CDH genes in rd29A plants (Figs. 4 and 5E, F). With the exception of rd29A that presented high proline concentration, there was no clear action of proline on the absence of Cd-induced oxidative stress in the leaves of the other plants, which can be associated with changes in Cd translocation from roots to shoots and with the synthesis of non-enzymatic antioxidants, such as sugars, organic acids, and GSH.
Glutathione is the most important non-enzymatic antioxidant in plants exposed to Cd (Gratão et al. 2005; Yadav 2010; Hendrix et al. 2020a). However, GSH1 and GSH2 genes that encode for enzymes controlling GSH synthesis (γ-glutamylcysteine synthetase—GSH1, EC 6.3.2.2; and glutathione synthetase—GSH2, EC 6.3.2.3) were not induced upon Cd exposure in the first 24 h (Fig. 5G, H), as well as the GSH concentration in the leaves remained the same in all genotypes (Fig. 6A). Vögeli-Lange and Wagner (1996) proposed that leaf cells of tobacco have a responsive ‘sensing system’ to keep GSH concentration at a fixed level even under stress conditions. On the other hand, some studies have shown that GSH induction by Cd in the leaves of plants occurs more often under prolonged Cd exposure and depends on signaling and substrates for GSH synthesis, PCs synthesis from GSH, action of other antioxidants over ROS, and Cd concentrations in this tissue (Clemens 2006; Mendoza-Cózatl and Moreno-Sánchez 2006). Thus, it is probable that the higher Cd concentration detected in the leaves of the plants after 72 h of Cd exposure compared to 24 h of Cd exposure (Fig. 1C) contributed to the higher GSH concentration observed in the leaves of WT, regardless of exogenous proline supply, and rd29A plants at the end of the study (Fig. 6A). Hendrix et al. (2020a) reported that GSH concentrations in the leaves of A. thaliana exposed to Cd also did not differ from the control in the first 24 h, but its concentration increased in relation to control plants after 72 h of Cd exposure. Mendoza-Cózatl and Moreno-Sánchez (2006) suggested that plants exposed to Cd rates up to 50 µmol L−1 can present an increase in GSH concentration, but high Cd concentrations often lead to GSH depletion due its use as a substrate for PCs synthesis. In our study, the higher Cd-induced PCS1 expression (Fig. 5J) and the higher MRP3 expression in the leaves of WT that received exogenous proline supply compared to the other tobacco plants after 24 h of Cd exposure (Fig. 5K) suggest a possible higher PCs synthesis in this treatment. PCS1 encodes for phytochelatin synthase (PCS, EC 2.3.2.15) (Wojas et al. 2008). So, the lower concentrations of GSH and GSH + GSSG observed in the leaves of 35S plant in comparison to the other plants after 72 h of Cd exposure (Fig. 6A and C) possibly is related to lower Cd-induced S concentrations (Table 2) or some Cd-induced limitation on GSH synthesis or both. Wojas et al. (2008) reported that Cd-induced γ-glutamylcysteine (γ-EC) accumulation in tobacco leaves that resulted in lower GSH concentrations in this tissue. As GSH2 expression (GSH2 ligates a glycine residue with γ-EC to form GSH) in 35S did not decrease after 24 h of Cd exposure (Fig. 5H), it is more likely that γ-EC has been used to bind Cd, as related by Wojas et al. (2008) in tobacco overexpressing PCS under control of a 35S promoter.
The transgenic tobacco 35S also presented a lower redox state of GSH after 72 h of Cd exposure (Fig. 6D), and a lower capacity to keep glutathione in its reduced form (GSH) over time under Cd exposure (Fig. 6A–D), even when presenting similar GR activities as the other plants (Fig. 6E). These results could indicate a relationship between proline and GSH, but in our study there was no significative correlation between proline and GSH concentrations for all genotypes assessed (data not shown). Thus, besides the possible limitation in GSH synthesis due to the use of γ-EC to bind Cd, the lower redox state of GSH in 35S plants after 72 h of Cd exposure can also be associated to the presence of glutathione in its oxidized form due to an inefficient action of CAT on H2O2 scavenging in tobacco (Iannone et al. 2015). Queval et al. (2011) observed that under limited CAT activity in the leaves of A. thaliana plants in response to increased H2O2 availability, glutathione metabolism was changed and there was predominance of GSSG. This fact helps to explain the higher GSSG concentration in relation to GSH in all plants, even those unexposed to Cd (Fig. 6A, B). Noctor et al. (2012) pointed out that there is a close relationship between H2O2 concentration and GSH status, and at moderate rates of endogenous H2O2 production a decrease in the leaf GSH/GSSG ratio is common. Although the low redox state of GSH can limit the action of ascorbate–glutathione cycle on ROS scavenging (Gratão et al. 2005), Jozefczak et al. (2015) described that in GSH-deficient mutants of A. thaliana exposed to Cd, the more oxidized environment contributed to the activation of alternative pathways using both O2·− and H2O2 scavengers. Thus, it is possible that the Cd-induced increase in sugars such as lactulose and organic acids such as glyceric and galactaric acids in the leaves of 35S plants after 24 h of Cd exposure (Fig. 2E) may also have occurred after 72 h of Cd exposure, contributing for ROS scavenging. As mentioned before, both sugars and organic acids play important roles on Cd-tolerance (Sharma and Dietz 2006; Sun et al. 2006, 2010; Keunen et al. 2013; Soares et al. 2019).
Conclusion
Exogenous proline supply induced different responses compared to endogenous proline overproduction in tobacco exposed to Cd. Plants supplied with exogenous proline presented lower Cd translocation from roots to leaves compared to plants overproducing proline, which certainly contributed to attenuate oxidative damages in the leaves of plants. On the other hand, tobacco overproducing proline was less susceptible to Cd-induced nutritional changes as compared to wild type and showed better metabolic adjustment under stress conditions than plants grown with exogenous proline supply. In this sense, the absence of Cd-induced oxidative stress in the leaves of plants overproducing proline is associated with an enhanced proline-induced synthesis of metabolites such as sugars and organic acids, since both SOD and CAT were not the main line of defense against Cd-induced oxidative stress in tobacco. Moreover, the absence of Cd-induced oxidative stress in tobacco overproducing proline under control of the stress inducible rd29A promoter can be attributed to a higher proline and GSH concentrations. Transgenic tobacco under the control of the stress inducible rd29A promoter exhibited higher proline concentrations than plants overproducing proline under the control of the constitutive 35S promoter. With exception of rd29A plants that presented high proline and GSH concentrations, the others presented an inverse correlation between proline and GSH synthesis after 72 h of Cd exposure, suggesting a signaling network between proline and GSH. However, new studies are necessary to elucidate this assumption and to assay the action of exogenous proline supply and proline overproduction under prolonged Cd exposure, in roots and leaves.
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Aleksza D, Horváth GV, Sándor HG, Szabados L (2017) Proline accumulation is regulated by transcription factors associated with phosphate starvation. Plant Physiol 175:555–567
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:548–555
Azevedo RA, Alas RM, Smith RJ, Lea PJ (1998) Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol Plant 104:280–292
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bertoli AC, Cannata MG, Carvalho R, Bastos ARR, Freitas MP, Augusto AS (2012) Lycopersicon esculentum submitted to Cd-stressful conditions in nutrition solution: nutrient contents and translocation. Ecotoxicol Environ Saf 86:176–181
Borges KLR, Salvato F, Alcântara BK, Nalin RS, Piotto FA, Azevedo RA (2018) Temporal dynamic responses of roots in contrasting tomato genotypes to cadmium tolerance. Ecotoxicology 27:245–258
Borgo L, Marur CJ, Vieira LGE (2015) Effects of high proline accumulation on chloroplast and mitochondrial ultrastructure and on osmotic adjustment in tobacco plants. Acta Sci Agron 37:191–199
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing principle of protein-dye-binding. Anal Biochem 72:248–254
Brunetti P, Zanella L, De Paolis A, Di Litta D, Cecchetti V, Falasca G, Barbieri M, Altamura MM, Costantino P, Cardarelli M (2015) Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J Exp Bot 66:3815–3829
Carvalho MEA, Castro PRC, Kozak M, Azevedo RA (2020) The sweet side of misbalanced nutrients in cadmium-stressed plants. Ann Appl Biol 176:275–284
Chen A, Husted S, Salt DE, Schjoerring JK, Persson DP (2019) The intensity of manganese deficiency strongly affects root endodermal suberization and ion homeostasis. Plant Physiol 181:729–742
Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46:W486-494
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Cuadros-Inostroza A, Caldana C, Redestig H, Kusano M, Lisec J, Pena-Cortes H, Willmitzer L, Hannah MA (2009) TargetSearch—a bioconductor package for the efficient preprocessing of GC-MS metabolite profiling data. BMC Bioinform 10:428
Cuypers A, Vangronsveld J, Clijsters H (2002) Peroxidases in roots and primary leaves of Phaseolus vulgaris Copper and zinc phytotoxicity: a comparison. J Plant Physiol 159:869–876
Dobrá J, Vanková R, Havlová M, Burman AJ, Libus J, Štorchová H (2011) Tobacco leaves and roots differ in the expression of proline metabolism-related genes in the course of drought stress and subsequent recovery. J Plant Physiol 168:1588–1597
Fässler E, Plaza S, Pairraud A, Gupta SK, Robinson B, Schulin R (2011) Expression of selected genes involved in cadmium detoxification in tobacco plants grown on a sulphur-amended metal-contaminated field. Environ Exp Bot 70:158–165
Gagneul D, Aïnouche A, Duhazé C, Lugan R, Larher FR, Bouchereau A (2007) A reassessment of the function of the so-called compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiol 144:1598–1611
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. Plant Physiol 59:309–314
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hendrix S, Jozefczak M, Wójcik M, Deckers J, Vangronsveld J, Cuypers A (2020a) Glutathione: A key player in metal chelation, nutrient homeostasis, cell cycle regulation and the DNA damage response in cadmium-exposed Arabidopsis thaliana. Plant Physiol Biochem 154:498–507
Hendrix S, Iven V, Eekhout T, Huybrechts M, Pecqueur I, Horemans N, Keunen E, De Veylder L, Vangronsveld J, Cuypers A (2020b) Suppressor of Gamma Response 1 modulates the DNA damage response and oxidative stress response in leaves of cadmium-exposed Arabidopsis thaliana. Front Plant Sci 11:366
Hoagland D, Arnon DI (1950) The water culture method for growing plants without soil. California Agriculture Experimental Station, Berkeley
Hong Z, Lakkineni K, Zhang Z, Verma DPS (2000) Removal of feedback inhibition of ∆1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Iannone MF, Groppa MD, Benavides MP (2015) Cadmium induces different biochemical responses in wild type and catalase-deficient tobacco plants. Environ Exp Bot 109:201–211
Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, Murata Y (2009) Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol 166:1587–1597
Jozefczak M, Bohler S, Schat H, Horemans N, Guisez Y, Remans T, Vangronsveld J, Cuypers A (2015) Both the concentration and redox state of glutathione and ascorbate influence the sensitivity of arabidopsis to cadmium. Ann Bot 116:601–612
Karalija E, Selović A (2018) The effect of hydro and proline seed priming on growth, proline and sugar content, and antioxidant activity of maize under cadmium stress. Environ Sci Pollut Res 25:33370–33380
Keunen E, Peshev D, Vangrosveld J, Van den Ende W, Cuypers A (2013) Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ 36:1242–1255
Keunen E, Schellingen K, Straeten DVD, Remans T, Colpaert J, Vangronsveld J, Cuypers A (2015) ALTERNATIVE OXIDASE1a modulates the oxidative challenge during moderate Cd exposure in Arabidopsis thaliana leaves. J Exp Bot 66:2967–2977
Kim D-Y, Bovet L, Maeshima M, Martinoia E, Lee Y (2007) The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J 50:207–218
Kopka J, Schauer N, Krueger S, Birkemeyer C, Usadel B, Bergmuller E, Dormann P, Weckwerth W, Gibon Y, Stitt M, Willmitzer L, Fernie AR, Steinhauser D (2005) GMD@CSB.DB: the Golm metabolome database. Bioinformatics 21:1635–1638
Kraus TE, McKersie BD, Fletcher RA (1995) Paclobutrazol-induced tolerance of wheat leaves to paraquat may involve increased antioxidant enzyme activity. J Plant Physiol 145:570–576
Kumar V, Shriram V, Kishor PBK, Jawali N, Shitole MG (2010) Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotechnol Rep 4:37–48
Lavres J, Rabêlo FHS, Capaldi FR, Reis AR, Rossi ML, Franco MR, Azevedo RA, Abreu-Junior CH, Nogueira NL (2019) Investigation into the relationship among Cd bioaccumulation, nutrient composition, ultrastructural changes and antioxidative metabolism in lettuce genotypes under Cd stress. Ecotoxicol Environ Saf 170:578–589
Martins LL, Mourato MP, Baptista S, Reis R, Carvalheiro F, Almeida AM, Fevereiro P, Cuypers A (2014) Response to oxidative stress induced by cadmium and copper in tobacco plants (Nicotiana tabacum) engineered with the trehalose-6-phosphate synthase gene (AtTPS1). Acta Physiol Plant 36:755–765
Mendoza-Cózatl DG, Moreno-Sánchez R (2006) Control of glutathione and phytochelatin synthesis under cadmium stress. Pathway modeling for plants. J Theor Biol 238:919–936
Noctor G, Mhamdi A, Chaouch S, Han Y, Neuker-Mans J, Marquez-Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Obata T, Fernie AR (2012) The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol Life Sci 69:3225–3243
Queval G, Jaillard D, Zechmann B, Noctor G (2011) Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ 34:21–32
R Core Team (2019) R: a language and environment for statistical computing. R foundation for statistical computing. Vienna-Austria. https://cran.r-project.org/bin/windows/base/old/3.0.2/
Repkina N, Talanova V, Ignatenko A, Titov A (2019) Involvement of proline and non-protein thiols in response to low temperature and cadmium stresses in wheat. Biol Plant 63:70–77
Schauer N, Steinhauser D, Strelkov S, Schomburg D, Allison G, Moritz T, Lundgren K, Roessner-Tunali U, Forbes MG, Willmitzer L, Fernie AR, Kopka J (2005) GC-MS libraries for the rapid identification of metabolites in complex biological samples. FEBS Lett 579:1332–1337
Sharma SS, Dietz K (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Sharmila P, Kumari PK, Singh K, Prasad NVSRK, Pardha-Saradhi P (2017) Cadmium toxicity-induced proline accumulation is coupled to iron depletion. Protoplasma 254:763–770
SIGMAPLOT (2006) SigmaPlot for Windows Version 10.0. Systat Software. Berkshire-UK. http://www.sigmaplot.co.uk/products/sigmaplot/produpdates/prod-updates3.php
Siripornadulsil S, Train S, Verma DPS, Sayre RT (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14:2837–2847
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5’-dithilbis (2-nitrobenzoic acid). Anal Biochem 175:408–413
Soares C, Carvalho MEA, Azevedo RA, Fidalgo F (2019) Plants facing oxidative challenges—a little help from the antioxidant networks. Environ Exp Bot 161:4–25
Stiefel V, Pérez-Grau L, Albericio F, Giralt E, Ruiz-Avila L, Ludevid MD, Puigdomènech P (1988) Molecular cloning of cDNAs encoding a putative cell wall protein from Zea mays and immunological identification of related polypeptides. Plant Mol Biol 11:483–493
Sun R, Zhou Q, Jin C (2006) Cadmium accumulation in relation to organic acids in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. Plant Soil 285:125–134
Sun X, Zhang J, Zhang H, Ni Y, Zhang Q, Chen J, Guan Y (2010) The responses of Arabidopsis thaliana to cadmium exposure explored via metabolite profiling. Chemosphere 78:840–845
Van den Ende W, Valluru R (2009) Sucrose, sucrosyl oligosaccharides, and oxidative stress: scavenging and salvaging? J Exp Bot 60:9–18
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Vögeli-Lange R, Wagner GJ (1996) Relationship between cadmium, glutathione and cadmium-binding peptides (phytochelatins) in leaves of intact tobacco seedlings. Plant Sci 114:11–18
Wojas S, Clemens S, Hennig J, Skłodowska A, Kopera E, Schat H, Bal W, Antosiewicz DM (2008) Overexpression of phytochelatin synthase in tobacco: distinctive effects of AtPCS1 and CePCS genes on plant response to cadmium. J Exp Bot 59:2205–2219
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Ye X, Ling T, Xue Y, Xu C, Zhou W, Hu L, Chen J, Shi Z (2016) Thymol mitigates cadmium stress by regulating glutathione levels and reactive oxygen species homeostasis in tobacco seedlings. Molecules 21:1339–1353
Zdunek-Zastocka E, Grabowska A, Michniewska B, Orzechowski S (2021) Proline concentration and its metabolism are regulated in a leaf age dependent manner but not by abscisic acid in pea plants exposed to cadmium stress. Cells 10:946
Zhao Y, Zhao J, Zhao C, Zhou H, Li Y, Zhang J, Li L, Hu C, Li W, Peng X, Lu X, Lin F, Xu G (2015) A metabolomics study delineating geographical location-associated primary metabolic changes in the leaves of growing tobacco plants by GC-MS and CE-MS. Sci Rep 9:16346
Zhao J, Zhao Y, Hu C, Zhao C, Zhang J, Li L, Zeng J, Peng X, Lu X, Xu G (2016) Metabolic profiling with gas chromatography–mass spectrometry and capillary electrophoresis–mass spectrometry reveals the carbon–nitrogen status of tobacco leaves across different planting areas. J Proteome Res 15:468–476
Zouari M, Ahmed CB, Elloumi N, Bellassoued K, Delmail D, Labrousse P, Abdallah FB, Rouina BB (2016) Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol Environ Saf 128:195–205
Funding
This research was supported by the National Council for Scientific and Technological Development—CNPq, Brazil [Grant Number #150260/2017-2]; and the Research Foundation Flanders—FWO, Belgium [Grant Number #BOF16KV06].
Author information
Authors and Affiliations
Contributions
LB conducted the research and wrote the manuscript. FHSR helped to write the manuscript. IGFB, TRC, and CAL performed the analyzes concerning metabolite profile. TGR performed the statistical analysis. PDCS, AFR, and JL helped to analyze the results and review the manuscript. AN and RAA concepted the study and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling Editor: Mikihisa Umehara.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borgo, L., Rabêlo, F.H.S., Budzinski, I.G.F. et al. Proline Exogenously Supplied or Endogenously Overproduced Induces Different Nutritional, Metabolic, and Antioxidative Responses in Transgenic Tobacco Exposed to Cadmium. J Plant Growth Regul 41, 2846–2868 (2022). https://doi.org/10.1007/s00344-021-10480-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10480-6