Abstract
Nitric oxide (NO) is a free-radical gasotransmitter signaling molecule associated with a varied spectrum of signal transduction pathways linked to inducing cross-adaptation against abiotic stresses. It has crucial roles from seed germination to plant maturity, depending upon its cellular concentration. The functional cross-talk of NO among different stress signaling cascades leads to alteration in the expression of developmental genes that regulate biosynthesis and function of plant growth regulators (PGRs). NO-PGRs and secondary signaling compounds cross-talk trigger reprogramming of stress-responsive gene expressions, transcriptional gene modulations, redox regulating machinery, oxidative metabolisms, and multiple regulatory pathways under plant abiotic stress. Recent findings suggest NO as critical components of numerous plant signaling network that interplays with auxin, gibberellins (GA), abscisic acid (ABA), ethylene (ET), jasmonic acid (JA), brassinosteroids (BRs), H2O2, melatonin, hydrogen sulfide (H2S), salicylic acid (SA), and other PGRs to modulate growth and development under multiple stresses. Considering the importance of NO signaling crosstalk under stress adaptation, in this review, we point out the biosynthesis and metabolism of NO and its crosstalk with numerous other signaling compounds. Further, recent cellular and molecular advances in NO signaling cross-talk under abiotic stress adaptations also have been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitric oxide (NO) is an essential gasotransmitter, which acts as a signaling molecule during plant stress. NO cross-talk with other signaling molecules to transduce stress signals between the cells. These signaling molecules include reactive oxygen species (ROS), phytohormones [Auxin (Aux), gibberellin (GA), cytokinin (CK), ethylene (ET), and abscisic acid (ABA), jasmonic acid (JA)], plant growth regulators’ melatonin (MT), and other signaling molecules. This suggests that two or more biosynthesis pathways share some common path to regulate signals in better ways, also known as crosstalk. Several endogenous growth regulators such as ABA and GA are reported previously for breaking of seed dormancy/inducing seed germination. In recent years, nitrate, nitrite, hydroxylamine, azide, NO, and sodium nitroprusside (SNP) compounds were also identified to regulate seed dormancy and germination processes through phytohormonal cross-talk (Krasuska et al. 2017).
For instance, ET and NO crosstalk with ABA during seed germination and dormancy period counteract the action of ABA (Arc et al. 2013). Similarly, in canola and maize, exogenous application of NO enhances seed germination in a dose-dependent manner (Fan et al. 2013a, b). Nevertheless, different mechanisms stimulate seed germination by light and NO reported (Beligni et al. 2000; Poor et al. 2019). It remains unclear whether the GA- and NO-promoting germination mechanism acts synergistically or antagonistically. CK and NO crosstalk were also reported to regulate the photo-morphogenesis process observed in Arabidopsis, parsley, or tobacco cell (Tun et al. 2001). Exogenous application of NO and CKs inhibit hypocotyl elongation in Arabidopsis and lettuce dark-grown seedlings (Beligni et al. 2000). Recently, Wu et al. (2016b) reported that hydrogen peroxide, NO, and UVR 8 interact with each other and are subjected to anthocyanin accumulation in reddish sprouts. Likewise, NO plays a crucial role in inhibiting primary root growth in Arabidopsis by regulating PHYTOCHROME INTERACTING FACTOR 3 (PIF 3) under light conditions (Bai et al. 2014). There is evidence that IAA and NO regulate the same responses in plants due to sharing some common steps during the signal transduction pathway. For example, growth of maize root segment influenced by NO in a dose-dependent manner similar to indole acetic acid (IAA) (Gouvea et al. 1997).
Studies suggested that NO plays a crucial role in stomatal movement, together with H2O2, abscisic acid (ABA) under water stress (Garcia-Mata et al. 2002; Desikan et al. 2002; Garcia-Mata et al. 2003; Desikan et al. 2004). NO regulate stomatal closure through Ca2 þ–dependent stomatal closure mechanism (Desikan et al. 2001). Synergistic effects of ABA and NO on stomatal closure were observed in Pisum sativum and Vicia faba plants (Neill et al. 2003). Some research also confirmed NO in guard cells (Garcia-Mata et al. 2002), leading to stomata closure through NR activity. Recently, it is suggested that UVR8, H2O2, and NO interact with each other under UV light and close the stomata by regulating the UVR8 pathway (Tossi et al. 2014). NO also increases the chlorophyll content in potato, lettuce, and Arabidopsis (Beligni et al. 2000). NO preserves and increases chlorophyll content similarly to CKs “chlorophyll retention effect” in pea and potato (Leshem and Wills 1998).
Rapid synthesis of NO and a parallel accumulation of ROS are typically observed under biotic and abiotic stresses. Consequently, these adverse responses activate the senescence process, ultimately leading to the death of plant cells. Earlier studies suggest that both NO and ROS play important roles in regulating programmed cell death (PCD) either independently or synergistically (Wang et al. 2013). Therefore, NO plays crucial functions in nutrient homeostasis, ion transport, plastid development, and alleviation of antioxidant genes during normal and unfavorable conditions as signaling compounds. Some of the pivotal roles of NO in plant growth and development are highlighted in Fig. 1.
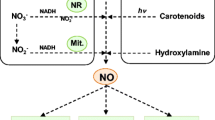
Schematic illustration of nitric oxide (NO) pools in the cells triggered under stress and their subsequent metabolism. The possible different pathways can lead to the generation of NO pool in the cytosol (enzymatic and non-enzymatic pathways) and apoplast (non-enzymatic pathway). Cytoplasmic organelles such as peroxisome, mitochondria, chloroplast, and acidic vacuoles are the prime compartments for NO biosynthesis. Various effectors regulating (up- or down-regulation) NO pools are phytohormones (ABA, cytokinin, GA, auxin, brassinosteroids), ambient light, transition metals, phenolics, abiotic stressors, and feedback inhibition (NO pool-mediated reduction of NO2− to NO). Solid lines ( ) and dotted lines (
) and dotted lines ( ) are used to avoid the overcrowding and clarity of the figure; (
) are used to avoid the overcrowding and clarity of the figure; ( ) depicts inhibition/inhibitory effect
) depicts inhibition/inhibitory effect
Abiotic (drought, salinity, heavy metals, extreme temperature, etc.) stresses are a significant concern for low agricultural production worldwide. They are steadily increasing due to uninvited anthropogenic activities in the natural environment (Asgher et al. 2017). These stresses adversely affect plant growth and development (Khan et al. 2015a; Fancy et al. 2017) by producing ROS (singlet oxygen, hydrogen peroxide, hydroxyl radicals, superoxide radicals, etc.). These are needed for the proper functioning of cells under normal conditions but adversely affect the cell programming system under stressful environments (Gupta et al. 2016; Asgher et al. 2017). The multiple stresses induce modulation of phytohormonal regulation, metabolism, and signaling in plants, which affects the plant defense system through metabolic adjustment, stomatal regulation, and behavioral changes in plant growth and development (Zhang et al. 2006a). The NO has been considered either a protective mediator or stress-inducing agent and plays a crucial role in intracellular redox signaling, ion homeostasis, and activation of antioxidant defense mechanisms (Asgher et al. 2017). Several studies suggested NOs’ role in maintaining pigment composition, stomatal movements, root growth and development, water relations, membrane stability, hormonal balance, osmotic adjustments, and ion channels’ activities in plants under different circumstances through cross-talk with other signaling compounds (Li et al. 2015; Shan et al. 2015; Kaya et al. 2020a, b; Wu et al. 2020; Santos et al. 2020).
On recognizing the importance of NO crosstalk in plants under multiple abiotic stresses, in this review, we have explored biosynthesis and metabolism pathways of NO in different cellular sites and their regulating factors. Then, we have discussed the NO cross-talk with other signaling compounds, their regulatory roles, and crucial molecular mechanisms of NO crosstalk under multiple abiotic stresses. This information will help us understand the role of NO crosstalk as a central hub in regulating plant processes under different environmental stresses.
NO Biosynthesis and Metabolism
Nitric Oxide (NO) has multifaceted physiological role in plants as a bioactive gasotransmitter. Eight different enzymatic and non-enzymatic processes that can produce NO in plants have been identified to date. Nitrite (NO2−) or more reduced compounds (L-arginine or hydroxylamine) are produced due to NO generation through oxidation (Mur et al. 2013). Cytoplasm, mitochondria, chloroplast, peroxisome, and apoplast are the major cellular sites for NO2−reduction (Roszer 2012a,b). Reduced NO can be generated through nitrate reductase activity (NR; EC 1.6.6.1 to EC 171) via mitochondrial electron transport chain (mETC) or heme-containing proteins. The oxidative NO can be synthesized through L-arginine and other compounds. In the acidic compartments of plant tissues, non-enzymatic reduction of NO2−/NO can also happen (Roszer 2012a, b; León and Costa‐Broseta 2020). Mechanism of production or synthesis of oxidized and reduced, enzymatic, and non-enzymatic NO are discussed in this section and highlighted in Fig. 2.
Mechanisms of Reductive Synthesis
By Nitrate Reductase (NR)
Nitrate reductase can reduce NO2− to NO with low efficacy through primary nitrate (NO3−) oxidoreductase activities (Rockel et al. 2002). In cyanobacterium (Anabaena doliolum), green algae, and vascular plants, NR catalytic reductions from NO2− to NO have a crucial role during stress response (Mur et al. 2013; Floryszak-Wieczorek et al. 2016). It indicates one of the oldest forms of NO production mechanisms in plants (Astier et al. 2018). Cytoplasm and chloroplast association are the main pool of NR activity (Kolbert et al. 2019). However, using a reduced cytochrome c as an electron donor, NO2−/NO-reductase (NI-NOR) reduces NO2− to NO. NO NI-NOR generation is similar to NO3−-reduced root-specific NR activity, but NO-NOR may act as a separate protein and needs to be regarded as NR-generated NO (Mohn et al. 2019).
By Mitochondrial ETC (Electron Transport Chain)
Mitochondria can use NO2− as an alternate electron acceptor for ATP synthesis; reduction of NO2− to NO takes place inside complex III (cytochrome bc1) and IV (cytochrome-c oxidase, CCO) (Gupta and Igamberdiev 2011a; Kolbert et al. 2019). The mechanism creates hypoxia in plant cells which results in mitochondrial NO generation. Hypoxia increases NR transcription activities, which converts NO3− to NO2− and results in a cytoplasmic accumulation of NO2−. NO2− reduction is limited in the hypoxic cell, and a continued supply of NO2− for reduced NO synthesis is permitted (Roszer 2012a, b). Therefore, a specific system of O2 transport in plants reduces NO synthesis/mitochondrial NO2− or NO. The NO generated within the mitochondria inhibits the germination of CCO (Gniazdowska et al. 2010a, b), which enhances the energy status of O2-limited cells (Gupta and Igamberdiev 2011b). The reduced mitochondrial NO generation inhibits the photo-respiratory cycle and fermentative metabolism (Oliveira et al. 2013). NO released from mitochondria into the cytosol is oxidized by plant hemoglobin (NO3−) due to hypoxia (Igamberdiev and Hill 2004). This leads to NO/NO2− exchange of mitochondria in the cytoplasm, maintaining a continuous supply of NO2 for ATP synthesis under hypoxia (Gupta and Igamberdiev 2011b). The cytoplasmic conversion NO/ NO3−/NO2 ensures that the low redox level helps adapt to the hypoxic NADH/NADP+ and NADPH/NADP+ ratios (Igamberdiev et al. 2010).
By Heme-Containing Proteins
Plant peroxisomes can produce NO under hypoxic or anoxic conditions by reducing NO2− (Igamberdiev et al. 2010). NO2−/NO may reduce the capacity of deoxygenated heme-containing proteins in the peroxisome matrix, which is the primary production mechanism (Igamberdiev et al. 2010; Sturms et al. 2011). The plant plasma membrane, cytosol, and endoplasmic reticulum have shown a similar reductional NO generation (Igamberdiev et al. 2010). Cyanobacteria (Sturms et al. 2011) and mammalian tissues have also been affected by a reduction in the use of heme-proteins from NO2− to NO (e.g., hemoglobin’s) (Tiso et al. 2011).
Mechanisms of Oxidative NO Synthesis
NOS (EC 1.14.23.29) proteins and NOS encoding genes (Roszer 2010) have been identified in prokaryotes, unicellular eukaryotes, invertebrates, non-mammalian vertebrates, and mammals. However, higher plants lack homologous sequences for known NOS encoding genes (Mur et al. 2013). Oxidative L-arginine synthesis is also present in plants' cells, but the responsible enzyme NO synthase (NOS) has not yet been found. Some of the pathways.
From L-arginine
The chloroplasts and leaf peroxisomes of the vascular plants and the green algae have been identified as a site for enzymatic oxidation of L-arginine to NO and L-citruline (Roszer 2012b). The chloroplastic oxidation of L-arginine to NO requires NADPH and in the absence of Ca2+ (Jasid et al. 2006). In the peroxisomes of leaves, Ca2+, calmodulin, FAD, FMN, and NADPH are required for L-arginine/L-citrulline conversion (del Rio et al. 2003; del Río 2011). It has been recently found that L-arginine-oxylated NO synthesis requires both Ca2+ and NADPH, with tetrahydrobiopterin (BH4) in Ostreococcus green algae species (Foresi et al. 2010). Plant mitochondria also oxidize L-arginine to NO with the help of enzymes available in the matrix or intermembrane space (Guo and Crawford 2005). It is debatable whether plant mitochondria contain a specific NO oxidative synthesis enzyme (Barroso et al. 1999).
Other Forms of Oxidative NO Synthesis
Polyamines and hydroxylamine have recently been shown to increase the synthesis of oxidative NO in plant cells (Wimalasekera et al. 2011). The exact mechanism of polyamines in increasing NO synthesis remains uncertain (Fröhlich and Durner 2011). However, NO cannot mediate the effect of polyamines on plants. Pathways possibly responsible include an interaction between polyamines and NR-catalyzed NO (Rosales et al. 2012) and the indirect impact of polyamine synthesis on L-arginine metabolism (Zhang et al. 2011). Hydroxylamine is an intermediate in the nitrification process and can be oxidized to NO in tobacco cell cultures (Rumer et al. 2009). This mechanism could be a substitute for oxidative NO synthesis via L-arginine. However, the underlying molecular mechanism is still unknown for hydroxylamine's role in NO synthesis (Rumer et al. 2009). The possible contribution to NO plant synthesis for other enzymes needs to be explored.
Non-enzymatic NO Generation
Non-enzymatic NO generation includes release from nitrous acid (HNO2) after protonation. Acidic environments such as apoplast of germinating and hypoxic seeds favor this type of chemical NO release (Yamasaki 2000; Bethke et al. 2004). Consequently, in the aleuronic layer of barley, the NO release from NO2− has been shown (Bethke et al. 2004). Phenolic compounds found in aleuron apoplast and on seed coat increase this non-enzymatic NO release. The NO release in germinating seeds may protect them from soil microorganisms (Bethke et al. 2004). In addition, seed dormancy is interrupted by NO, which suggests that proper germination requires NO2− together with an enzymatic NO synthesis (Roszer 2012b). Together, NO release can synergize with the reduction of the enzyme NO2−/NO to invoke germination NO burst. During germination, NO-mediated programmed cell death occurs when aleuron cells are removed (Lombardi et al. 2010). However, the release of NO from S-nitrosoglutathione (GSNO) (del Río 2011) is another possible, unexplored mechanism for non-enzymatic NO generation. This compound is formed in the oxidative environment of peroxisomes, which allows both GSNO and GSNO to react with glutathione (Barroso et al. 2006). The GSNO is a compound NO-donor and could carry reserve NO distributed in the plant's tissues. GSNO genesis is facilitated by environmentally friendly light and metal transition (Floryszak-Wieczorek et al. 2006). Hydroxylamine is another possible non-synthesis substrate (Rumer et al. 2009), but GSNOR would not support hydroxylamine production.
NO Metabolism
NO metabolism includes a redox range, which displays distinctive properties and reactivity such as nitrosonium (NO+), NO radical (NO*), and nitroxyl anion (NO−) (Gisone et al. 2004). Nitrosation in aqueous phases in organic molecules in –S, –N, –O, and –C centers results in NO+. The biological relevance of NO+ was disputed under slightly acidic or physiologic conditions, but a variety of nitroso-compounds forming effectively under neutral physiological conditions could be interpreted as NO+ reactions (Stamler et al. 1992). These compounds include metal-nitrosyl-complexes, thionitrites (RS-NO), nitrosamines (RNH–NO), alkyl- and aryl-nitrites (RO–NO), and tri- and tetra-oxides (N2O3 and N2O4) of dinitrogen. Numerous nuclear centers in biological systems whose potential nitrosative vulnerability were demonstrated in in vitro studies (Stamler et al. 1992). Dimerization and dehydration quickly convert NO− to the N2O (Basylinski and Hollocher 1985) and reacts with Fe (III) heme (Goretski and Hollocher 1988).
NO* is also reversible in sulfhydryl oxidation, leading to low molecular weight and protein-associated thiols. The transmission of electrons and collisions is standard and generally results in NO radical (NO*) as the main product. S-nitrosothiols are thought to be a (minor) product of the NO disulfide reaction (Stamler et al. 1992). The significant NO reactions are those with O2 and its different redox and transition ions in biological terms. When discussing the chemical and physiological effects, NO is a highly diffused secondary messenger that may generate relative effects far from its production site in plants. Hence, the concentration and the source of NO are the main determinants of its biological effects (Wink and Mitchell 1998). The direct effects of NO are the result of the interaction between NO and metal complexes. NO form complexes, including those found regularly in metalloproteins, of transition metal ions. Heme-containing protein reactions have been studied extensively for NO-complexes.
NO also forms non-heme transition metal complexes, and biochemical focus was given to its responses to the Fe–S center of the proteins, including several mitochondrial electron transportations and enzyme proteins (Henry et al. 1991). NO's reaction to heme-containing proteins includes cytochrome P450 interactions with more considerable physiological consequences (Wink et al. 1993). Tyrosine nitration is also a directly established effect of NO on proteins. Tyrosine nitration is selective and reversible and ONOO− dependent. In vivo nitration pathways were shown to be ONOO− independent (Davis et al. 2001). NO can also stop lipid peroxidation (Rubbo et al. 1995). Nitrosating oxidation or nitration is the indirect effect of NO, generated by the interaction of the NO and O2− (Wink et al. 1993). None of these substances can undergo autoxidation (i.e., reactions to O2) to produce N2O3 in aquatic solutions (Ford et al. 1993). Since NO and O2 are 6–20 times more soluble in lipid layers, the auto-oxidation rate in a lipid phase (Ford et al. 1993) dramatically increases. The primary N2O3 reactions are thought to occur in the membrane fraction.
In its response to O2, NO generates ONOO at a rate near diffusion, which acts as a nitrating agent as well as a powerful oxidant to modify the proteins (nitrotyrosine formation), lipids (lipid oxidation, lipid nitration), and nucleic acids (DNA oxidation and DNA nitration). In short, there are numerous potential reactions of NO depending on the cell milieu facilitating biochemical modifications. The production site, source, and NO concentration collectively determine its effects. In addition, a relative equilibrium exists between oxidative and nitrosating stress. The mechanism of NO biosynthesis and its metabolism are highlighted in Fig. 1.
NO: Plant Signaling Component Hub
Perceiving the cues within cells and outside the environment is vital for the plant life cycle. This perception is accomplished by plant signaling. Plant signaling involves an exchange of information between plant cells from receptors to effector through signaling molecules. Discoveries of molecular components related to signaling provided evidence about the signal response as a cumulative effect of cross-talk between different signaling pathways (Taylor et al. 2004). This cross-talk generally results from pathway integration with the unique signal response as a combination. Such cross-talk involved in physiological processes ranged from development to stress responses (Peck and Mittler 2020). Some of the critical signaling compounds are ROS, PGRs, and signaling peptides discussed in this section in NO as a central signaling molecule. NO orchestrate a plethora of signaling responses in plants. These responses act at inter- and intra-cellular levels to modulate plant growth and development. NO-mediated transcriptional changes or secondary messenger activation regulates these processes (Falak et al. 2021). These processes include photosynthesis, organelles motility, hypersensitive response, programmed cell death, seed germination, cell wall lignification, flowering, pollen tube growth, fruit ripening as well as legume–rhizobium symbiosis, and biotic and abiotic stress (Turkan 2017; Sami et al. 2018; Inmaculada Sánchez-Vicente et al. 2019).
NO signaling operates at various levels, specifically with ROS of the anti-oxidant system (Ma et al. 2016) and affects seed dormancy, plant reproduction mechanisms (Jiménez-Quesada et al. 2016), plant–rhizobia interaction (Damiani et al. 2016), and plant–pathogen interactions (Thalineau et al. 2016). Moreover, higher NO/ROS content correlates with the compromised antioxidant system in plants (Gaupels et al. 2016). This interplay of NO/ROS homeostasis is also vital for N nutrition and plant immunity. These processes are mainly governed by NR activity, which is an essential part of NO signaling after stress induction. Hormonal control on NO/ROS homeostasis is a crucial factor in plant development and stress response, as reported by Sivakumaran et al. (2016). Also, mitochondria play a vital role in the modulation of NO and ROS signaling by changing hypoxic or anoxic conditions (Gupta and Igamberdiev 2016). Other than the direct involvement of NO in ROS production, post-translational modification of NO enzymes is essential for NO/ROS homeostasis, emphasizing the ascorbate–glutathione cycle (Begara-Morales et al. 2016). NO self-regulation also affects ROS levels (Romero-Puertas and Sandalio 2016). This regulation indicates the fine-tuning of NO and ROS as signaling components.
Another facet of NO signaling operates as a secondary messenger in conjugation with other signaling molecules as cytosolic Ca2+ levels, cyclic guanosine 5′-monophosphate (cGMP), cyclic adenosine diphosphate ribose (cADPR), phosphatidic acid, H2O2, JA and SA, and Mitogen-Associated Protein kinases (Santner and Estelle 2009; Foyer and Noctor 2015; Duszyn et al. 2019; Yang et al. 2019). NO- cGMP-dependent pathway in plants opens avenues of NO crosstalk with cGMP signaling (Gross and Durner 2016). While NO-mediated cGMP signaling is well known in mammals, this system is not well defined in plants. However, the identification of enzymes of the cGMP pathway in higher plants supports this hypothesis. This crosstalk provides the molecular basis of physiological and developmental responses generated through NO signaling.
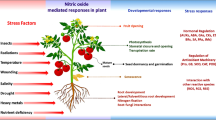
Further, downstream target protein studies give cues about the indirect effect of NO signaling (Simon and Dresselhaus 2015). Other than cross-talk in conventional pathway, NO directly interacts with other molecules to affect the biological processes in the plant, for example, NO–sulfur (Fatma et al. 2016), NO–inositol (Lytvyn et al. 2016), NO–heme oxidase 1 (Wu et al. 2016a), and NO-H2O2 interactions (Molassiotis et al. 2016). Understanding this cross-talk in light of NO response and signaling will provide insights into its mechanism. The NO crosstalk with other crucial signaling compounds is highlighted in Fig. 3 and discussed in this section.
Model highlights the NO crosstalk with PGRs and other signaling compounds in plant growth regulation and stress conditions. NO crosstalk is very complex in nature; it crosstalk with numerous signaling compounds such as H2S, H2O2, Ca, melatonin, ethylene, abscisic acid, and salicylic acid to regulate various homeostasis processes under normal and stress conditions
Molecular Understanding of NO Crosstalk with Crucial Signaling Compounds
In the signaling cascade, phytohormones are instrumental for orchestrating plant growth, development, and stress responses (Santner and Estelle 2009). NO is an essential cue in signaling cascade interactions with all major hormones and other endogenous molecules (Freschi 2013). Here, NO acts as a secondary messenger for plant hormones involved in stress responses (Saito et al. 2009; Liu et al. 2010). The subsequent section will discuss the NO accumulation in specific tissues to perform particular functions in routes with hormonal regulation.
NO–ABA Crosstalk
Abscisic acid (ABA) is referred to as stress hormone cross-talk with NO during various environmental challenges and activates the antioxidant system (Hancock et al., 2011; Freschi 2013). The ABA-induced response was reduced after the decrease in NO synthesis, which suggests that it is acting downstream of ABA under stress treatments (Tossi et al. 2012; Zhang et al. 2009). On contrary to this, NO counteracts the ABA (Lozano-Juste and Leon 2010a, 2010b). This mechanism operates at cell, tissue, and organ level and indicates the specificity of NO–ABA signaling under specific physiological events. The role of NO–ABA crosstalk was reported in different physiological processes, for example, during germination (Liu et al. 2009) as transcriptional inducer and in the maintenance of seed dormancy (Bethke et al. 2006). Under stress conditions, ROS generation induces the ABA–NO crosstalk by activating antioxidants and transcription factors (Lu et al. 2009; Zhang et al. 2007a). Other signaling molecules such as cGMP MAPK and type 2C protein phosphatases act downstream of NO–ABA interplay and antioxidant system to modulate plant stress response (Desikan et al. 2002; Dubovskaya et al. 2011; Mioto et al. 2013). Mutant studies suggested the role of this cross-talk for salinity stress (Lu et al. 2009; Kong et al. 2016), heat and drought stress (Zandalinas et al. 2016), and thermotolerance of plant calluses (Song et al. 2008).
NO–GA Cross-Talk
Gibberellic acid is a crucial phytohormone associated with seed germination and plant growth. In the signaling cascade, NO promotes the biosynthesis of GA by transcriptional regulation of GA biosynthesis genes (Bethke et al. 2007). NO acts as a balance center for ABA-induced dormancy and GA-stimulated germination. The molecular basis of this balance lies in the activation of the anti-oxidant system along with post-translational modification of other enzymes involved in ethylene synthesis (Gniazdowska et al. 2010a, b; Hebelstrup et al. 2012). GAs have been reported to control hypocotyl growth in coordination with DELLA protein degradation (de Lucas et al. 2008). Interestingly enough, higher NO levels antagonize hypocotyl growth (Beligni and Lamattina 2000). Moreover, NO was also reported in the repression of PIF genes and augmenting DELLA protein content (Lozano-Juste and León 2011). That led to the possibility of NO–GAs–light interplay in the regulation of seed germination events. NO–GAs module also operates at various stress conditions, for example, aluminum toxicity in wheat (He et al. 2012), cadmium toxicity in Arabidopsis (Zhu et al. 2012), and deprived phosphorous condition (Asgher et al. 2017).
NO–Auxins Crosstalk
Auxin is an essential phytohormone associated with cell elongation. NO as a signaling molecule in NO–Auxin crosstalk modulates auxin degradation enzyme activity (Xu et al. 2010), interferes with auxin transport through PIN1 efflux carrier (Fernández-Marcos et al. 2011), and activates auxin signaling by S-nitrosylation of the auxin receptor protein (Terrile et al. 2012). The role of auxin in plant root architecture, lateral root growth, and root hairs is well documented (Overvoorde et al. 2010). Interestingly, most root architecture phenotypes are also influenced by NO as signal molecules (Fernández-Marcos et al. 2011). In vitro cultures suggest that auxin application does not affect NO release (Tun et al. 2001). This advises downstream action of NO in auxin signaling response (Chen et al. 2010). NO–Auxin crosstalk operates from synthesis to perception in response to environmental and developmental cues. This crosstalk was also reported in plant stress responses, for example, iron deficiency (Chen et al. 2010), drought and water stress conditions (Pagnussat et al. 2002; Liao et al. 2012) due to extensive involvement with root architecture regulation, and cadmium toxicity (Yuan et al. 2016; Xu et al. 2010).
NO–Melatonin Crosstalk
Melatonin is the novel amine-derivative hormone class involved in plant growth, development, aging, and stress response. Interaction of NO with melatonin regulates the melatonin synthesis genes and changes the phytohormone level (Zhu et al. 2019). Further, downstream action of NO activates MAPK-associated defense responses. Exogenous application of melatonin induces glycerol, sugar production, ultimately increasing NO and salicylic acid levels. NO–melatonin crosstalk affects several physiological processes like root growth, aging, and iron deficiency alleviation (Zhu et al. 2019; Kaya et al. 2020a).
NO–JA Crosstalk
Jasmonic acid is a fatty acid-derivative phytohormone mainly associated with herbivory and pathogen response. Abiotic stress, such as drought stress, affects the JA-associated signaling genes (Huang et al. 2008). NO treatment induces JA-biosynthesis genes that indicate interplay of NO–JA module (Palmieri et al. 2008). CDPKs are induced by JA, starting the ABA-induced stomatal closure (Munemasa et al. 2007). External treatment with MeJA and ABA increases NO and ROS content in guard cells (Munemasa et al. 2007). Evidence suggests calcium signaling acting downstream of NO–ROS crosstalk. Apart from that, JA associated with NO synthesis increases ROS scavenging enzyme as reported for chilling stress tolerance in Cucumis sativus (Liu et al. 2016).
NO–CK Crosstalk
Cytokinins are a class of phytohormones associated with plant cell division in plant shoot and root. CK–NO module of signaling affects the biosynthesis of nitric oxide; however, peroxynitrite (NO-derived) binds with zeatin to reduce its activity (Liu et al. 2013). Type-A response regulators are a crucial component of CK signaling regulated by NO-mediated S-nitrosylation (Feng et al. 2013). NO–CK crosstalk also operates in different stress responses, such as water stress conditions (Shao et al. 2010) and salt stress conditions. Antagonistic relation of CK on NO levels was also reported in Vicia faba seedlings grown under dark (Song et al. 2011) and leaf development in aging leaves. The molecular basis of this regulation is supposed to be the limitation of phosphorelay activity caused due to S-nitrosylation (Fan et al. 2013a, b).
NO–ET Crosstalk
Ethylene also known as ripening/senescence/stress hormone is important for plant growth regulation. Heavy metal stress often increases the activity of the 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) enzyme that is associated with ET (Khan et al. 2015b). Understanding the NO–ET crosstalk provides the operating mechanism of plant stress adaptation mechanism under these stresses. ET–NO crosstalk leads to activation of MAPK cascades and polyamine synthesis during cadmium stress in soybean and pea seedlings (Chmielowska-Bąk et al. 2013; Rodríguez-Serrano et al. 2006). Similarly, treatment of Cd and spermine leads to NO generation in roots in Triticum aestivum seedlings which ultimately inhibits the root growth (Groppa et al. 2008). Fe-deficiency signaling is affected by NO–ET crosstalk with the induction of several genes associated with the iron accumulation and transport (Garcia et al. 2010). Other than heavy metals, NO–ET module works profoundly in salinity stress (Liu et al. 2015).
NO–SA Crosstalk
Salicylic acid is an important plant hormone essential for plant growth, development, and pathological processes. NO–SA interplay regulates plant stress responses; for example, combination of NO and SA prevents nickel toxicity by proline accumulation, reduced lipid peroxidation, and chlorophyll content enhancement in Brassica napus (Kazemi et al. 2010). On contrary to this, NO–SA combination increases the Cd concentration in the cell wall of Arachis hypogaea to prevent organelles from toxic effects (Xu et al. 2015). In addition, ROS also participates with NO in SA-induced closure of stomata (Khokon et al. 2011). Here, SA activates peroxidase enzyme that promotes ROS accumulation, leading to NO generation in guard cells and ultimately stomata closure. Similarly, the combination of NO–SA acts synergistically in alleviating salt stress by improving divalent cations absorption (Dong et al. 2015). Again, pretreatment of SA in Spinacia oleracea modulates the NR activity for improvement in chilling tolerance (Aydin and Nalbantoğlu 2011). This implicates SA interplay in the NO generation pathway that can be used for the future generation of climate-smart crops.
NO–Sulfur Crosstalk
Sulfur (S) is a vital part of essential molecules, such as the thioredoxin system, reduced glutathione (GSH), methionine, and coenzyme A. Under salt stress conditions, NO–S crosstalk changes the ET and ABA levels in guard cells to affect the photosynthetic and stomatal response. NO interacts with GSH and forms S-nitrosoglutathione (GSNO) to impart better stress tolerance (Wang et al. 2015b). Further, NO–sulfur crosstalk is essential for S-assimilation, as shown for Cys synthesis modulation by ET production (Fatma et al. 2016). Interactions of nitro and sulfhydryl groups are crucial during nitration (Leterrier et al. 2011). NO also interacts with H2S to provide salinity stress tolerance by upregulation of salinity stress-induced genes like HvSOS1 and HvHA1 (Chen et al. 2015). This process is mainly governed by transcriptional activation of vacuolar transport and compartmentalization genes where NO acts as a signaling molecule.
NO–BRs Crosstalk
Brassinosteroids (BRs) are the novel class of plant hormones implicated in plant growth, development, and immunity. Recently, reports have suggested NO–BRs interplay in plant root architecture as well as in root development (Tossi et al. 2013). In addition, alleviation of Copper toxicity was mediated by NO–BRs crosstalk in conjunction with ABA in Raphanus sativus seedlings (Choudhary et al. 2012).
These reports suggest precise NO interaction with hormones and other signaling components for fine-tuning the plant growth, development, and stress response. Further experiments on targeted NO homeostasis in controlled induced conditions (Temporal and spatial) will shed light on components of these cross-talks. Direct target identification of NO signaling in biosynthesis, perception, and signal transduction will be important to decipher the underlying regulatory mechanisms.
Molecular Understanding of NO Crosstalk During Plant Stress
Nitric oxide is an essential gasotransmitter with a regulatory role during plant growth and development. These regulatory roles are amplified when NO crosstalk with other signaling molecules or PGRs. The NO crosstalk with other compounds regulate various biosynthetic pathways, signaling processes, and metabolism and ultimately maintains plant growth and development under multiple stresses. Therefore, the mechanism of NO crosstalk under numerous abiotic stress tolerance is highlighted in Fig. 4 and discussed in this section. The NO crosstalk with PGRs and other signaling compounds under multiple stresses and their improved traits for stress tolerance are presented in Table 1.
Drought Stress
It has been well established that NO is required for ABA-induced stomatal closure and provides tolerance to plants under drought stress (Garcia-Mata and Lamattina 2002). Further, stomatal closure is regulated by ABA-induced NO production in Arabidopsis guard cells. Although, Desikan et al. (2002) revealed no stomatal closer in response to ABA in double-mutant nia1 nia2, which are associated with reduced NO production. This suggests the role of other intermediaries in NO–ABA crosstalk. Plants accumulate more ABA in drought stress, leading to activation of NADPH oxidase enzymes such as RBOHF and RBOHD (respiratory burst oxidase homolog F and D), resulting in more superoxide accumulation. This phenomenon is needed for stomatal closure through NO production via NR and activates MAPK signaling cascade (Desikan et al. 2002; Bright et al. 2006; Fency et al. 2017). Several studies showed that the exogenous application of NO could promote the accumulation of ABA in plants under drought stress, which can be reversed by the application of NO scavenger (Zhao et al. 2001; Fency et al. 2017). Thus, there is ambiguity in NO's function in the increased or decreased ABA signaling under water deficit. The NO-mediated S-nitrosylation could be crucial for drought tolerance, as reported in several studies. The central component of ABA signaling is OST1/SnRK2.6 (open stomata 1/sucrose non-fermenting 1-related protein kinase 2.6) induced by the S-nitrosylation process in plants. The protein kinase activity of OST1/SnRK2.6 is inhibited by S-nitrosylation at Cys 137 position. This ABA-induced S-nitrosylation of SnRK2.6 acts as a negative feedback regulator of ABA signaling in plants (Wang et al. 2015a).
There are reports which emphasize the role of transcription factors from MYB family to regulate tolerance mechanism in plants under abiotic stresses. Transcription factor, AtMYB2, is associated with salt, and drought stress tends to inhibit its DNA binding activity after the S-nitrosylation process (Serpa et al. 2007). Another transcription factor, AtMYBB30, has been found to lose its DNA binding activity after S-nitrosylation (Tavares et al. 2014; Fency et al. 2017). Thus, protein kinases and transcriptions factors play a vital role in mitigating plant stress under water deficit. Several recent studies suggested that NO crosstalk is a central player of drought stress tolerance. Wang et al. 2020 reported that crosstalk between NO and H2S mediates priming-induced drought tolerance via accumulation of osmolytes (proline and glycine betaine). Sami et al. (2018) found that NO crosstalk with phytohormones mediates the alteration in plant metabolism, and post-translational modification such as S-nitrosylation confers multiple stress tolerance including drought. Likewise, Shan et al. (2015) reported that NO induced by exogenous application of JA upregulated the AsA–GSH cycle activity and reduced drought stress in wheat crops. Moreover, recent studies suggested that NO crosstalk with other signaling compounds and phytohormones mitigate the drought stress by improving the relative water contents, photosynthetic capacity, antioxidant defense, ionic balance, and other plant growth attributes (Shan et al. 2015; Khan et al. 2017; Kaya et al. 2019). However, the exact mechanism of NO crosstalk under drought tolerance at the molecular level needed to be explored. These studies point out that NO crosstalk plays a crucial role during drought stress tolerance by antioxidant and osmolytes regulation.
Temperature Stress
Plant growth and development are severely affected by low temperature (cold and freezing) and high-temperature stress. Plants have evolved mechanisms during evolution to combat temperature stresses. NO cross-talk plays an essential role in a plant's battle against temperature fluctuations (Majlath et al. 2012; Parankusam et al. 2017; Kolbert et al. 2019). For example, exogenous application of NO induces the expression of MfSAMS1 and thereby increased S-adenosylmethionine (SAM), polyamines (PAs) concentration, and PA oxidation under cold stress in alfalfa (Medicago sativa) (Guo et al. 2014). In this context, SAMs are acting by up-regulating PA oxidation and H2O2-induced antioxidant defense (Guo et al. 2014). There is an antagonistic relationship between NO and ET during fruit ripening in cold stress. For example, Zaharah et al. (2011) studied the different NO levels for fumigation on mango fruits and observed a significant reduction in ET production during fruit ripening. They also found reduced chilling injury, softening, ripening, and delayed fruit color development in mango fruits under cold storage conditions. Thus, cross-talk between NO and ET delays fruit senescence and thereby fruit quality during cold fruit storage.
The crop productivity is adversely affected by heat stress due to adverse effects on photosynthesis, respiration, membrane stability, membrane permeability, and water relations (Kolbert et al. 2019). Heat stress affects cytoskeleton structure, cell metabolism, and membrane fluidity by increasing the accumulation of proteins that affect ROS, NO, and other phytohormones (Wahid et al. 2007). It has been suggested that NO acts via reduction of ROS level through activating antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and expression of heat shock factor during heat stress in plants (Neill et al. 2002; Song et al. 2006; Wang et al. 2014; Fency et al. 2017). Exogenous application of NO (pre-treatment) increased the survival rate of maize (Zea mays) seedlings and wheat (Triticum aestivum) leaves and reduced heat stress-induced loss in rice (Oryza sativa) seedlings (Lamattina et al. 2001; Uchida et al. 2002). Similarly, crosstalk between NO and H2S regulates the H2O2-induced thermotolerance in maize seedlings. It also affects the Ca and calmodulin levels in tobacco seedlings (Li et al. 2015). These reports suggest that NO crosstalk needed to be further explored for its role during thermotolerance in plants.
Salinity
Soil salinity is one of the main factors for reduced crop production in major food and fodder crops and, by large, emerged due to extensive use of groundwater for irrigation across the world (Slinger et al. 2005). The role of NO to address plant salt tolerance has been extensively studied in various plant species (Zhang et al. 2007b; Hasanuzzaman et al. 2018). For example, artificial application of sodium nitroprusside (SNP, act as NO donor) protects plants against salt stress by altering growth habit and protects from oxidative damage by maintaining plant ion homeostasis (Zhang et al. 2006b). Moreau et al. (2008) studied the effect of NO using Atnoa1 plants (defective in GTPase activity) and concluded a role of NO under salt stress. The S-nitrosylated proteins play an essential role under NaCl stress and negatively affect salt concentration (Tanou et al. 2009). However, exogenous application of NO increased (pre-treatment) the concentration of NaCl-induced S-nitrosylated protein that played a protective role under stress conditions (Tanou et al. 2009). Arora et al. (2016) stated that NO can interact with different metal proteins such as zinc–sulfur clusters, heme–iron, copper, and iron–sulfur clusters and form a stable metal nitrosyl complex that can modify the protein structure as well as function. They also observed the binding of thiols to NO and their role in transporting it to the site of action. Camejo et al. (2013) observed decreased S-nitrosylation of proteins during short-term and long-term salt concentrations. A recent report suggested that pretreatments with CaCl2, H2O2, and SNP improve β-amylase activity, which influences starch breakdown and improved seedling establishments in Chenopodium (Hajihashemi et al. 2020).
Similarly, Singh and Bhatla (2018) reported that NO bind with ACC oxidase and form a ternary complex (ACC–ACC oxidase—NO), which lead to a reduction of ethylene biosynthesis and induce LR formation in sunflower under salt stress conditions. Likewise, Arora and Bhatla (2017) reported that melatonin and NO crosstalk maintain redox homeostasis and differential modulations of SOD isoform in sunflower under salt stress. Moreover, several recent updates on NO crosstalk with other signaling compounds alleviate salinity stress (Fatma et al. 2016; Shi et al. 2017; Kaya et al. 2019). However, there was a significant reduction in S-nitrosylation under long-term salt treatment. Thus, there were inconsistencies between different studies due to differences in plant genotypes/species, tissue-examined, variable NaCl concentration, and duration of time. Further, NO, S-nitrosylation, and associated enzyme GSNOR play an essential role in mitigating salt stress in plants. However, there is a need to focus more on proteomic approaches to identify salt stress signaling components directly and indirectly regulated by redox enzymes and GSNOR.
Heavy Metal Stress
Heavy metals (HMs) such as mercury (Hg), cadmium (Cd), arsenic (As), chromium (Cr), thallium (Tl), and lead (Pb) have an unknown biological function and are very harmful for plants in higher concentrations. They tend to bio-accumulate (accumulation in plant cell with the time) and non-biodegradable. Plants taking up these HMs through roots from the soil and hyper-accumulation of these HMs bring rapid cellular homeostasis changes (Ghori et al. 2019). Nitric oxide (NO) has a broad spectrum of regulation functions with widespread inter- and intra-cellular messenger activities (Wei et al. 2020). Many enzymatic reactions accelerated through NO, including nitrate reductase and L-Ar-dependent nitric oxide synthase-related reactions, an essential component for HMs tolerance (Wei et al. 2020). Like other stresses, NO also plays a vital role in enhancing antioxidant enzyme activities and alleviates the toxicity of HMs. Rodriguez-Serrano et al. (2009) studied the cadmium (Cd) toxicity effect on nitric oxide (NO) metabolism in pea (Pisum sativum), and results implicated that Cd toxicity inactivated the NO synthase-dependent NO production. Consequently, it leads to calcium (Ca) deficiency in leaves. This suggests that the Cd toxicity effect can be counteracted by calcium (Ca). Exogenous SNP application acts as NO donor to the rice leaves and reduces the Cu and NH4+ accumulation (Mazid et al. 2011). Moreover, Wang et al. (2010) report that NO actions reduce Cu toxicity through antioxidant enzymes, which accelerates the metallothionein and metallothionein. There was an increase in total chlorophyll content and fresh or dry weight of leaves against Cu toxicity in tomato. Also, reports suggested the cross-protection role of putrescine and NO toward Cd toxicity in mung bean seedlings (Nahar et al. 2016). Singh et al. (2008) also found the detoxification and anti-oxidative properties of NO for Cd and Cu toxicity in wheat. Exogenous application of SNP accelerated the ROS scavenging enzymes, which reduced the accumulation of H2O2 and diminished the toxic effect of Cu in tomato (Cui et al. 2009). Similar results were observed in rice against Cd toxicity. The exogenous application of NO ameliorates the tolerance against Cd toxicity by increasing the pectin and hemicelluloses content in the root cell wall (Xiong et al. 2009). In soybean seedlings, the short-term treatment with Cd accelerated the geneS expression of encoding the protein of NO synthesis and ET (Chmielowska-Bak et al. 2013; Kolbert et al. 2019). Likewise, recent studies on the role of NO crosstalk on HMs stress tolerance suggest that it acts via regulating the root growth (biomass, formation, and length), photosynthetic activity, antioxidant defense, accumulation of osmoprotectants, and inhibition of HMs transport to grain and above plant parts (Khan et al. 2020; Kaya et al. 2020a, b; Singh et al. 2020).
Other Stresses
During the stress condition, NO is generated from L-arg-dependent NO synthase. This NO can react with superoxide (O2−) to form ONOO, a powerful oxidant that can lead to tyrosine nitration of proteins. Tyrosine nitration is an indicator of nitrosative stress in plants which acts as the defense system for the plants during stress (Nabi et al. 2019). Recent reports have explained that a wide range of abiotic stresses is leading to NO synthesis and signaling. It is gaining more attention mainly due to its properties like small size, no charge, free radicals, and highly diffusible nature across the cell membranes and many plant physiological functions like growth, development, maturation, and senescence. It is believed that NO signaling is involved in the respiratory electron transport system in mitochondria, where it confers the modulation of ROS and accelerates the antioxidant signaling defense system in the plant, which is exposed to several abiotic factors (Mazid et al. 2011; Santisree et al. 2020). The regulatory function of NO crosstalk is not only limited to drought, cold, heat, cold, and HMs stress but also has a regulatory role during combined stress, nutrient deficiency, and high and low light stress. For example, some studies suggested the NO crosstalk role during the N, P, Mg, and Fe deficient soil and suggested that it regulates the nutrient deficiency by improving root attributes, better translocation of ions, and regulating phytohormones concentration (Yang et al. 2016; Su et al. 2016; Zhu et al. 2017).
Conclusion and Perspectives
NO has gained attention during the last few decades due to its substantial role as a gasotransmitter and defense molecule during numerous environmental stresses. Most of the NO crosstalk functions are associated with redox, oxidative, ion, and hormonal homeostasis through the modulations of downstream genes in the signaling pathway. A large body of research has addressed the elementary mechanism of NO crosstalk regarding plant development and its role as a central hub under abiotic stress tolerance. Broadly, these studies indicate how NO crosstalk with other signaling compounds regulates the cell machinery in optimum ways. Although the mode of NO crosstalk with other signaling compounds is not always synergistic, sometimes antagonist responses also benefit plants under stressful situations. Moreover, the NO crosstalk response under similar stress could vary plant by plant due to the complex nature of signaling compounds and their interacting signals. Components of this crosstalk include genes, transcription factors, and enzymes associated with the NO synthesis and expression during different environmental signals, which need to be more elaborate to understand the exact mechanism of NO crosstalk. However, most studies have shown that the NO crosstalk regulates stress responses via the synthesis and expression of SOD, CAT, APX, MDA, GR, POX, DHAR, and other antioxidant defense enzymes and genes. These factors help in the maintenance of oxidative stress situations at the cell level. Likewise, stress proteins (HSP), phytochelatins, signaling cascades (MAPK, CDPK, GMP), osmoprotectants (sugar, proline), and ion proteins (H+-ATPase) are linked with NO crosstalk. However, the molecular mechanism of NO crosstalk is still unclear and needs to explore more for deep understanding and development of multiple stress tolerance varieties. Most studies focused on single stress conditions, and the mechanism of NO crosstalk under combined and multiple stress still needs to be deciphered. These studies are limited to the germination and vegetative stage. However, the responses of NO crosstalk under the reproductive phase and yield attributing traits are still unclear, which need to be investigated to develop higher yield lines under stress situations. In recent years, integrating omics approaches (integrating genomics, proteomics, metabolomics, and transcriptomics) has further clues on understanding gene–gene, gene–protein, gene–environment interactions and can be a potential approach to understanding the complex NO signaling mechanisms. Further, the integration of omics approaches to next-generation techniques explores the signaling mechanism at molecular levels and insights into full understanding of regulatory pathways and crosstalk mechanism to develop climate-resilient crops. Moreover, the engineering of NO biosynthesis and crosstalk pathways will be crucial for providing novel insights into the crop stress improvements program.
Data Availability
The present paper covered the concluded remarks on No Nitric oxide cross-talking covered in various findings studied by researchers.
References
Ahmad P, Abass Ahanger M, Nasser Alyemeni M, Wijaya L, Alam P, Ashraf M (2018) Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J Plant Interact 13:64–72
Alamri S, Ali HM, Khan MIR, Singh VP, Siddiqui MH (2020) Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol Biochem 155:20–34
Amooaghaie R, Enteshari S (2017) Role of two-sided crosstalk between NO and H2S on improvement of mineral homeostasis and antioxidative defense in Sesamum indicum under lead stress. Ecotoxicol Environ Saf 139:210–218
Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Kubiś J (2009) Interaction between polyamine and nitric oxide signaling in adaptive responses to drought in cucumber. J Plant Growth Regul 28:177–186
Arc E, Sechet J, Corbineau F, Rajjou L, Marion-Poll A (2013) ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front Plant Sci 4:63
Arora D, Bhatla SC (2017) Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic Biol Med 106:315–328
Arora D, Jain P, Singh N, Kaur H, Bhatla SC (2016) Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free Radic Res 50:291–303
Asgher M, Per TS, Masood A, Fatma M, Freschi L, Corpas FJ, Khan NA (2017) Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ Sci Pol Res 24:2273–2285
Astier J, Gross I, Durner J (2018) Nitric oxide production in plants: an update. J Exp Bot 69:3401–3411
Aydın B, Nalbantoğlu B (2011) Effects of cold and salicylic acid treatments on nitrate reductase activity in spinach leaves. Turk J Biol 35:443–448
Bai S, Yao T, Li M, Guo X, Zhang Y, Zhu S, He Y (2014) PIF3 is involved in the primary root growth inhibition of Arabidopsis induced by nitric oxide in the light. Mol Plant 7:616–625
Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma J, Lupiáñez JA, del Río LA (1999) Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem 274:36729–36733
Barroso JB, Corpas FJ, Carreras A, Rodríguez-Serrano M, Esteban FJ, Fernández-Ocana A, Chaki M, Romero-Puertas MC, Valderrama R, Sandalio LM, del Río LA (2006) Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. J Exp Bot 57:1785–1793
Basylinski DA, Hollocher TC (1985) Evidence from the reaction between trioxodinitrate (II) and 15NO that trioxidinitrate (II) decomposes into nitrosyl hydride and nitrite in neutral aqueous solution. Inorg Chem 24:4285–4288
Begara-Morales JC, Sánchez-Calvo B, Chaki M, Valderrama R, Mata-Pérez C, Padilla MN, Corpas FJ, Barroso JB (2016) Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs). Front Plant Sci 7:152. https://doi.org/10.3389/fpls.2016.00152
Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210:215–221
Bethke PC, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16:332–341
Bethke PC, Libourel IGL, Jones RL (2006) Nitric oxide reduces seed dormancy in Arabidopsis. J Exp Bot 57:517–526
Bethke PC, Libourel IGL, Aoyama N, Chung YY, Still DW, Jones RL (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143:1173–1188
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Camejo D, del Carmen R-P, Rodríguez-Serrano M, Sandalio LM, Lázaro JJ, Jiménez A, Sevilla F (2013) Salinity-induced changes in S-nitrosylation of pea mitochondrial proteins. J Proteom 79:87–99
Campos FV, Oliveira JA, Pereira MG, Farnese FS (2019) Nitric oxide and phytohormone interactions in the response of Lactuca sativa to salinity stress. Planta 250:1475–1489
Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ (2010) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154:810–819
Chen YS, Lo SF, Sun PK, Lu CA, Ho TH, Yu SM (2015) A late embryogenesis abundant protein HVA1 regulated by an inducible promoter enhances root growth and abiotic stress tolerance in rice without yield penalty. Plant Biotech J 13:105–116
Chmielowska-Bąk J, Lefèvre I, Lutts S, Deckert J (2013) Short term signaling responses in roots of young soybean seedlings exposed to cadmium stress. J Plant Physiol 170:1585–1594
Choudhary SP, Kanwar M, Bhardwaj R, Yu JQ, Tran LS (2012) Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 7:e33210
Cui X, Zhang Y, Chen X, Jin H, Wu X (2009) Effects of exogenous nitric oxide protects tomato plants under copper stress. In: 2009 3rd International Conference on Bioinformatics and Biomedical Engineering. 1–7
Damiani I, Pauly N, Puppo A, Brouquisse R, Boscari A (2016) Reactive oxygen species and nitric oxide control early steps of the legume—rhizobium symbiotic interaction. Front Plant Sci 7:454. https://doi.org/10.3389/fpls.2016.00454
Davis KL, Martin E, Turko IV, Murad F (2001) Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol 41:203–236
de Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias Pedraz JM, Lorrain S, Fankhauser C, Blázquez MA, Titarenko E, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451:480–484
Del Río LA, Corpas FJ, Sandalio LM, Palma JM, Barroso JB (2003) Plant peroxisomes, reactive oxygen metabolism and nitric oxide. IUBMB Life 55:71–81
del Río LA (2011) Peroxisomes as a cellular source of reactive nitrogen species signal molecules. Arch Biochem Biophys 506:1–11
Desikan R, Soheila AH, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127:159–172
Desikan R, Griffiths R, Hancock J, Neill S (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA 99:16314–16318
Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 55:205–212
Diao Q, Song Y, Shi D, Qi H (2017) Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) seedlings. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00203
Dong F, Simon J, Rienks M, Lindermayr C, Rennenberg H (2015) Effects of rhizopheric nitric oxide (NO) on N uptake in Fagus sylvatica seedlings depend on soil CO2 concentration, soil N availability and N source. Tree Physiol 35:910–920
Dubovskaya LV, Bakakina YS, Kolesneva EV, Sodel DL, McAinsh MR, Hetherington AM, Volotovski ID (2011) cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutant abi1-1. New Phytol 191:57–69
Duszyn M, Świeżawska B, Szmidt-Jaworska A, Jaworski K (2019) Cyclic nucleotide gated channels (CNGCs) in plant signallingCurrent knowledge and perspectives. J Plant Physiol 241:153035
Falak N, Imran QM, Hussain A, Yun BW (2021) Transcription factors as the “Blitzkrieg” of plant defense: A pragmatic view of nitric oxide’s role in gene regulation. Int J Mol Sci 22:522. https://doi.org/10.3390/ijms22020522
Fan HF, Du CX, Ding L, Xu YL (2013a) Effects of nitric oxide on the germination of cucumber seeds and antioxidant enzymes under salinity stress. Acta Physiol Plant 35:2707–2719
Fan HF, Du CX, Guo SR (2013b) Nitric oxide enhances salt tolerance in cucumber seedlings by regulating free polyamine content. Environ Exp Bot 86:52–59
Fancy NN, Bahlmann AK, Loake GJ (2017) Nitric oxide function in plant abiotic stress. Plant Cell Environ 40:462–472
Fatma M, Masood A, Per TS, Khan NA (2016) Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front Plant Sci 7:521. https://doi.org/10.3389/fpls.2016.00521
Feng J, Wang C, Chen Q, Chen H, Ren B, Li X, Zuo J (2013) S-nitrosylation of phosphotransfer proteins represses cytokinin signaling. Nat Commun 4:1529
Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Nat Acad Sci USA 108:18506–18511
Floryszak-Wieczorek J, Milczarek G, Arasimowicz M, Ciszewski A (2006) Do nitric oxide donors mimic endogenous NO-related response in plants? Planta 224:1363–1372
Floryszak-Wieczorek J, Arasimowicz-Jelonek M, Izbiańska K (2016) The combined nitrate reductase and nitrite-dependent route of NO synthesis in potato immunity to Phytophthora infestans. Plant Physiol Biochem 108:468–477
Ford PC, Wink DA, Stanbury DM (1993) Autoxidation kinetics of aqueous nitric oxide. FEBS Lett 326:1–3
Foresi N, Correa-Aragunde N, Parisi G, Caló G, Salerno G, Lamattina L (2010) Characterization of a nitric oxide synthase from the plant kingdom: NO generation from the green alga Ostreococcus tauri is light irradiance and growth phase dependent. Plant Cell 22:3816–3830
Foyer CH, Noctor G (2015) Defning robust redox signalling within the context of the plant cell. Plant Cell Environ 38:239
Freschi L (2013) Nitric oxide and phytohormone interactions: Current status and perspectives. Front Plant Sci 4:1–22
Fröhlich A, Durner J (2011) The hunt for plant nitric oxide synthase (NOS): is one really needed? Plant Sci 181:401–404
García MJ, Lucena C, Romera FJ, Alcántara E, Pérez-Vicente R (2010) Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J Exp Bot 61:3885–3899
Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl-channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100:11116–11121
Garcıa-Mata C, Lamattina L (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128:790–792
Gaupels F, Furch AC, Zimmermann MR, Chen F, Kaever V, Buhtz A, Kehr J, Sarioglu H, Kogel KH, Durner J (2016) Systemic induction of NO-, redox-, and cGMP signaling in the pumpkin extra fascicular phloem upon local leaf wounding. Front Plant Sci 7:154. https://doi.org/10.3389/fpls.2016.00154
Ghori NH, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ SciTech 16:1807–1828
Gisone P, Dubner D, PÉREZ MD, Michelin S, Puntarulo S (2004) The role of nitric oxide in the radiation-induced effects in the developing brain. In Vivo 18:281–292
Gniazdowska A, Krasuska U, Bogatek R (2010a) Dormancy removal in apple embryos by nitric oxide or cyanide involves modifications in ethylene biosynthetic pathway. Planta 232:1397–1407
Gniazdowska A, Krasuska U, Dębska K, Andryka P, Bogatek R (2010b) The beneficial effect of small toxic molecules on dormancy alleviation and germination of apple embryos is due to NO formation. Planta 232:999–1005
González A, de Los Ángeles Cabrera M, Henríquez MJ, Contreras RA, Morales B, Moenne A (2012) Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol 158:1451–1462
Goretski J, Hollocher TC (1988) Trapping of nitric oxide produced during denitrification by extracellular hemoglobin. J Biol Chem 263:2316–2323
Gouvea CMCP, Souza JF, Magalhaes CAN, Martins IS (1997) NO·–releasing substances that induce growth elongation in maize root segments. Plant Growth Regul 21:183–187
Groppa MD, Rosales EP, Iannone MF, Benavides MP (2008) Nitric oxide, polyamines and Cd-induced phytotoxicity in wheat roots. Phytochem 69:2609–2615
Gross I, Durner J (2016) In search of enzymes with a role in 3’, 5’-cyclic guanosine monophosphate metabolism in plants. Front Plant Sci 7:576. https://doi.org/10.3389/fpls.2016.00576
Guillas I, Zachowski A, Baudouin E (2011) A matter of fat: interaction between nitric oxide and sphingolipid signaling in plant cold response. Plant Signal Behav 6:140–142
Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17:3436–3450
Guo Z, Tan J, Zhuo C, Wang C, Xiang B, Wang Z (2014) Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosylmethionine synthetase in Medicago sativa subsp. falcata that confers cold tolerance through up-regulating polyamine oxidation. Plant Biotech J 12:601–612
Gupta KJ, Igamberdiev AU (2011) The anoxic plant mitochondrion as a nitrite: NO reductase. Mitochondrion 11:537–543
Gupta KJ, Igamberdiev AU (2016) Reactive nitrogen species in mitochondria and their implications in plant energy status and hypoxic stress tolerance. Front Plant Sci 7:369. https://doi.org/10.3389/fpls.2016.00369
Gupta KJ, Igamberdiev AU, Manjunatha G et al (2011) The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci 181:520–526
Gupta K, Sengupta A, Chakraborty M, Gupta B (2016) Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01343
Gupta P, Srivastava S, Seth CS (2017) 24-Epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 411:483–498
Hajihashemi S, Skalicky M, Brestic M, Pavla V (2020) Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol Biochem 154:657–664
Hancock JT, Neill SJ, Wilson ID (2011) Nitric oxide and ABA in the control of plant function. Plant Sci 181:555–559
Hasanuzzaman M, Oku H, Nahar K, Bhuyan MB, Al Mahmud J, Baluska F, Fujita M (2018) Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotech Rep 12:77–92
He HY, He LF, Gu MH, Li XF (2012) Nitric oxide improves aluminum tolerance by regulating hormonal equilibrium in the root apices of rye and wheat. Plant Sci 183:123–130
Hebelstrup KH, Van Zanten M, Mandon J, Voesenek LA, Harren FJ, Cristescu SM, Møller IM, Mur LA (2012) Haemoglobin modulates NO emission and hyponasty under hypoxia-related stress in Arabidopsis thaliana. J Exp Bot 63:5581–5591
Henry Y, Ducrocq C, Drapier JC, Servent D, Pellat C, Guissani A (1991) Nitric oxide, a biological effector. Electron paramagnetic resonance detection of nitrosyl-iron-protein complexes in whole cells. Eur Biophys J 20:1–15
Huang D, Wu W, Abrams SR, Cutler AJ (2008) The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J Exp Bot 59:2991–3007
Igamberdiev AU, Hill RD (2004) Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot 55:2473–2482
Igamberdiev AU, Bykova NV, Shah JK, Hill RD (2010) Anoxic nitric oxide cycling in plants: participating reactions and possible mechanisms. Physiol Plant 138:393–404
Jasid S, Simontacchi M, Bartoli CG, Puntarulo S (2006) Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiol 142:1246–1255
Jiménez-Quesada MJ, Traverso JÁ, Alché J (2016) NADPH oxidase-dependent superoxide production in plant reproductive tissues. Front Plant Sci 7:359. https://doi.org/10.3389/fpls.2016.00359
Karpets YV, Kolupaev YE, Yastreb TO, Oboznyi AI (2016) Induction of heat resistance in wheat seedlings by exogenous calcium, hydrogen peroxide, and nitric oxide donor: functional interaction of signal mediators. Russ J Plant Physiol 63:490–498
Kaya C, Ashraf M, Wijaya L, Ahmad P (2019) The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol Biochem 143:119–128
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020a) Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plant 168:345–360
Kaya C, Higgs D, Ashraf M, Alyemeni MN, Ahmad P (2020b) Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol Plant 168:256–277
Kazemi N, Khavari-Nejad RA, Fahimi H, Saadatmand S, Nejad-Sattari T (2010) Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci Hort 126:402–407
Khan MI, Nazir F, Asgher M, Per TS, Khan NA (2015a) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015b) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:642
Khan MN, Mobin M, Abbas ZK, Siddiqui MH (2017) Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 68:91–102
Khan MN, Siddiqui MH, AlSolami MA, Alamri S, Hu Y, Ali HM, Al-Amri AA, Alsubaie QD, Al-Munqedhi BM, Al-Ghamdi A (2020) Crosstalk of hydrogen sulfide and nitric oxide requires calcium to mitigate impaired photosynthesis under cadmium stress by activating defense mechanisms in Vigna radiata. Plant Physiol Biochem 156:278–290
Khokon MD, Okuma EI, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2011) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ 34:434–443
Kolbert ZS, Barroso JB, Brouquisse R, Corpas FJ, Gupta KJ, Lindermayr C, Loake GJ, Palma JM, Petřivalský M, Wendehenne D, Hancock JT (2019) A forty year journey: the generation and roles of NO in plants. Nitric Oxide 93:53–70
Kong X, Wang T, Li W, Tang W, Zhang D, Dong H (2016) Exogenous nitric oxide delays salt induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol Plant 38:1–9
Krasuska U, Ciacka K, Gniazdowska A (2017) Nitric oxide-polyamines cross-talk during dormancy release and germination of apple embryos. Nitric Oxide 68:38–50
Kushwaha BK, Singh S, Tripathi DK, Sharma S, Prasad SM, Chauhan DK, Kumar V, Singh VP (2019) New adventitious root formation and primary root biomass accumulation are regulated by nitric oxide and reactive oxygen species in rice seedlings under arsenate stress. J Hazard Mat 361:134–140
Lamattina L, Beligni MV, Garcia-Mata C, Laxalt AM (2001) Method of enhancing the metabolic function and the growing conditions of plants and seeds. US Patent 6, 242
León J, Costa-Broseta Á (2020) Present knowledge and controversies, deficiencies, and misconceptions on nitric oxide synthesis, sensing, and signaling in plants. Plant Cell Environ 43:1–15
Leshem YY, Wills RBH (1998) Harnessing senescence delaying gases nitric oxide and nitrous oxide: a novel approach to postharvest control of fresh horticultural produce. Biol Plant 41:1–10
Leterrier M, Chaki M, Airaki M, Valderrama R, Palma JM, Barroso JB, Corpas FJ (2011) Function of S-nitrosoglutathione reductase (GSNOR) in plant development and under biotic/abiotic stress. Plant Signal Behav 6:789–793
Li X, Gong B, Xu K (2014) Interaction of nitric oxide and polyamines involves antioxidants and physiological strategies against chilling-induced oxidative damage in Zingiber officinale Roscoe. Sci Hort 170:237–248
Li ZG, Luo LJ, Sun YF (2015) Signal crosstalk between nitric oxide and hydrogen sulfide may be involved in hydrogen peroxide-induced thermotolerance in maize seedlings. Russ J Plant Physiol 62:507–514
Liao WB, Huang GB, Yu JH, Zhang ML (2012) Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol Biochem 58:6–15
Liu YG, Shi L, Ye NH, Liu R, Jia WS, Zhang JH (2009) Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol 183:1030–1042
Liu Y, Ye N, Liu R, Chen M, Zhang J (2010) H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J Exp Bot 61:2979–2990
Liu WZ, Kong DD, Gu XX, Gao HB, Wang JZ, Xia M, Gao Q, Tian LL, Xu ZH, Bao F, Hu Y (2013) Cytokinins can act as suppressors of nitric oxide in Arabidopsis. Proc Nat Acad Sci USA 110:1548–1553
Liu W, Li RJ, Han TT, Cai W, Fu ZW, Lu YT (2015) Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol 168:343–356
Liu Y, Yang X, Zhu S, Wang Y (2016) Postharvest application of MeJA and NO reduced chilling injury in cucumber (Cucumis sativus) through inhibition of H2O2 accumulation. Postharvest Biol Tech 119:77–83
Liu M, Zhang H, Fang X, Zhang Y, Jin C (2018) Auxin acts downstream of ethylene and nitric oxide to regulate magnesium deficiency-induced root hair development in Arabidopsis thaliana. Plant Cell Physiol 59:1452–1465
Liu X, Yin L, Deng X, Gong D, Du S, Wang S, Zhang Z (2020) Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J Hazard Mat 395:122679
Lombardi L, Ceccarelli N, Picciarelli P, Sorce C, Lorenzi R (2010) Nitric oxide and hydrogen peroxide involvement during programmed cell death of Sechium edule nucellus. Physiol Plant 140:89–102
Lozano-Juste J, Leon J (2010) Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR- and AtNOA1-dependent nitric oxide biosynthesis in Arabidopsis. Plant Physiol 152:891–903
Lozano-Juste J, León J (2010) Nitric oxide modulates sensitivity to ABA. Plant Signal Behav 5:314–316
Lozano-Juste J, León J (2011) Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis. Plant Physiol 156:1410–1423
Lu G, Gao C, Zheng X, Han B (2009) Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 229:605–615
Lv X, Ge S, Jalal Ahammed G, Xiang X, Guo Z, Yu J, Zhou Y (2017) Crosstalk between nitric oxide and MPK1/2 mediates cold acclimation-induced chilling tolerance in tomato. Plant Cell Physiol 58:1963–1975
Lytvyn DI, Raynaud C, Yemets AI, Bergounioux C, Blume YB (2016) Involvement of inositol biosynthesis and nitric oxide in the mediation of UV-B induced oxidative stress. Front Plant Sci 7:430. https://doi.org/10.3389/fpls.2016.00430
Ma Z, Marsolais F, Bykova NV, Igamberdiev AU (2016) Nitric oxide and reactive oxygen species mediate metabolic changes in barley seed embryo during germination. Front Plant Sci 7:138. https://doi.org/10.3389/fpls.2016.00138
Majláth I, Szalai G, Soós V, Sebestyén E, Balázs E, Vanková R, Dobrev PI, Tari I, Tandori J, Janda T (2012) Effect of light on the gene expression and hormonal status of winter and spring wheat plants during cold hardening. Physiol Plant 145:296–314
Mazid M, Khan TA, Mohammad F (2011) Role of nitric oxide in regulation of H2O2 mediating tolerance of plants to abiotic stress: a synergistic signaling approach. J Stress Physiol Biochem 7(2):34
Mioto PT, Mercier H (2013) Abscisic acid and nitric oxide signaling in two different portions of detached leaves of Guzmania monostachia with CAM up-regulated by drought. J Plant Physiol 170:996–1002
Mohn MA, Thaqi B, Fischer-Schrader K (2019) Isoform-specific NO synthesis by Arabidopsis thaliana nitrate reductase. Plants 8:67
Molassiotis A, Job D, Ziogas V, Tanou G (2016) Citrus plants: A model system for unlocking the secrets of NO and ROS-inspired priming against salinity and drought. Front Plant Sci 7:229. https://doi.org/10.3389/fpls.2016.00229
Montilla-Bascón G, Rubiales D, Hebelstrup KH, Mandon J, Harren FJ, Cristescu SM, Mur LA, Prats E (2017) Reduced nitric oxide levels during drought stress promote drought tolerance in barley and is associated with elevated polyamine biosynthesis. Sci Rep 7:1–15
Moreau M, Lee GI, Wang Y, Crane BR, Klessig DF (2008) AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J Biol Chem 283:32957–32967
Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y (2007) The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol 143:1398–1407
Mur LA, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJ, Hebelstrup KH, Gupta KJ (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants 5:pls052. https://doi.org/10.1093/aobpla/pls052
Nabi RB, Tayade R, Hussain A, Kulkarni KP, Imran QM, Mun BG, Yun BW (2019) Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ Exp Bot 161:120–133
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf 126:245–255
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:11–35
Oliveira H, Salgado I, Sodek L (2013) Nitrite decreases ethanol production by intact soybean roots submitted to oxygen deficiency: a role for mitochondrial nitric oxide synthesis? Plant Signal Behav 8:e23578
Overvoorde P, Fukaki H, Beeckman T (2010) Auxin control of root development. Cold Spring Harb Perspect Biol 2:a001537
Ozfidan-Konakci C, Yildiztugay E, Elbasan F, Kucukoduk M, Turkan I (2020) Hydrogen sulfide (H2S) and nitric oxide (NO) alleviate cobalt toxicity in wheat (Triticum aestivum L.) by modulating photosynthesis, chloroplastic redox and antioxidant capacity. J Hazard Mat 388:122061
Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129:954–956
Palmieri MC, Sell S, Huang X, Scherf M, Werner T, Durner J, Lindermayr C (2008) Nitric oxide responsive genes and promoters in Arabidopsis thaliana: A bioinformatics approach. J Exp Bot 59:177–186
Parankusam S, Adimulam SS, Bhatnagar-Mathur P, Sharma KK (2017) Nitric oxide (NO) in plant heat stress tolerance: current knowledge and perspectives. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01582
Peck S, Mittler R (2020) Plant signaling in biotic and abiotic stress. J Exp Bot 71:1649–1651. https://doi.org/10.1093/jxb/eraa051
Poór P, Czékus Z, Ördög A (2019) Role of nitric oxide in physiological and stress responses of plants under darkness. In: Hasanuzzaman M, Fotopoulos V, Nahar K, Fujita M (eds) Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. Wiley, New York, pp 515–531
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110
Rodríguez-serrano MA, Romero-puertas MC, Zabalza AN, Corpas FJ, Gómez M, Del Rio LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Rodríguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueño MC, Luis A, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243
Romera FJ, García MJ, Alcántara E, Pérez-Vicente R (2011) Latest findings about the interplay of auxin, ethylene and nitric oxide in the regulation of Fe deficiency responses by strategy I plants. Plant Signal Behav 6:167–170
Romero-Puertas MC, Sandalio LM (2016) Nitric oxide level is self-regulating and also regulates its ROS partners. Front Plant Sci 7:316. https://doi.org/10.3389/fpls.2016.00316
Rosales EP, Iannone MF, Groppa MD, Benavides MP (2012) Polyamines modulate nitrate reductase activity in wheat leaves: involvement of nitric oxide. Amino Acids 42:857–865
Roszer T (2012a) Nitric oxide synthesis in leaf peroxisomes and in plant-type mitochondria. In: Roszer T (ed) The biology of subcellular nitric oxide. Springer, New York, pp 67–80
Roszer T (2012b) Nitric oxide synthesis in the chloroplast. In: Roszer T (ed) The biology of subcellular nitric oxide. Springer, New York, pp 49–66
Roszer T, Kiss-Tóth E, Rózsa D et al (2010) Hypothermia translocates nitric oxide synthase from cytosol to membrane in snail neurons. Cell Tissue Res 342:191–203
Ruan HH, Shen WB, Xu LL (2004) Nitric oxide involved in the abscisic acid induced proline accumulation in wheat seedling leaves under salt stress. Acta Bot Sin 46:1307–1315
Rubbo H, Parthasarathy S, Barnes S, Kirk M, Kalyanaraman B, Freeman BA (1995) Nitric oxide inhibition of lipoxygenasedependent liposome and low density lipoprotein oxidation: termination of radical chain propagation reactions and formation of nitrogen containing oxidized lipid derivatives. Arch Biochem Biophys 324:15–25
Rumer S, Kapuganti JG, Kaiser WM (2009) Oxidation of hydroxylamines to NO by plant cells. Plant Signal Behav 4:853–855
Saito N, Yoshimasa N, Mori IC, Murata Y (2009) Nitric oxide functions in both methyl jasmonate signaling and abscisic acid signaling in Arabidopsis guard cells. Plant Signal Behav 4:119–120
Sami F, Faizan M, Faraz A, Siddiqui H, Yusuf M, Hayat S (2018) Nitric oxide-mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO-mediated post translational modifications in modulating diverse plant stress. Nitric Oxide 73:22–38
Sánchez-Vicente I, María G, Fernández-Espinosa OL (2019) Nitric oxide molecular targets: reprogramming plant development upon stress. J Exp Bot 70:4441–4460
Santisree P, Sanivarapu H, Gundavarapu S, Sharma KK, Bhatnagar-Mathur P (2020) Nitric oxide as a signal in inducing secondary metabolites during plant stress. In: Mérillon JM, Ramawat KG (eds) Co-evolution of secondary metabolites. Springer, Cham, pp 593–621
Santner A, Estelle M (2009) Recent advances and emerging trends in plant hormone signalling. Nat 459:1071–1078
Santos MP, Zandonadi DB, de Sá AFL, Costa EP, de Oliveira CJL, Perez LE, Façanha AR, Bressan-Smith R (2020) Abscisic acid-nitric oxide and auxin interaction modulates salt stress response in tomato roots. Theor Exp Plant Physiol 32(4):301–313
Serpa V, Vernal J, Lamattina L, Grotewold E, Cassia R, Terenzi H (2007) Inhibition of AtMYB2 DNA-binding by nitric oxide involves cysteine S-nitrosylation. Biochem Biophy Res Commun 361:1048–1053
Shan C, Zhou Y, Liu M (2015) Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 252:1397–1405
Shao R, Wang K, Shangguan Z (2010) Cytokinin-induced photosynthetic adaptability of Zea mays L. to drought stress associated with nitric oxide signal: probed by ESR spectroscopy and fast OJIP fluorescence rise. J Plant Physiol 167:472–479
Shi H, Liu W, Wei Y, Ye T (2017) Integration of auxin/indole-3-acetic acid 17 and RGA-LIKE3 confers salt stress resistance through stabilization by nitric oxide in Arabidopsis. J Exp Bot 68:1239–1249
Si T, Wang X, Wu L, Zhao C, Zhang L, Huang M, Cai J, Zhou Q, Dai T, Zhu JK, Jiang D (2017) Nitric oxide and hydrogen peroxide mediate wounding-induced freezing tolerance through modifications in photosystem and antioxidant system in wheat. Front Plant Sci 8:1284
Siddiqui M, Alamri SA, Mutahhar YY, Al-Khaishany MA, Al-Qutami HM, Nasir Khan MA (2017) Nitric Oxide and calcium induced physiobiochemical changes in tomato (Solanum Lycopersicum) plant under heat stress. Fresen Environ Bull 26:1663–1672
Simaei M, Khavari-Nejad RA, Bernard F (2012) Exogenous application of salicylic acid and nitric oxide on the ionic contents and enzymatic activities in NaCl-stressed soybean plants. Am J Plant Sci 3(10):1495–1503
Simon R, Dresselhaus T (2015) Peptides take centre stage in plant signalling. J Exp Bot 66:5135–5138
Singh N, Bhatla SC (2018) Nitric oxide regulates lateral root formation through modulation of ACC oxidase activity in sunflower seedlings under salt stress. Plant Signal Behav 13:e1473683
Singh HP, Batish DR, Kaur G, Arora K, Kohli RK (2008) Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot 63:158–167
Singh S, Husain T, Kushwaha BK, Suhel M, Fatima A, Mishra V, Singh SK, Tripathi DK, Rai M, Prasad SM, Dubey NK (2020) Regulation of ascorbate-glutathione cycle by exogenous nitric oxide and hydrogen peroxide in soybean roots under arsenate stress. J Haz Mat. https://doi.org/10.1016/j.jhazmat.2020.123686
Sivakumaran A, Akinyemi A, Mandon J, Cristescu SM, Hall MA, Harren FJ, Mur LA (2016) ABA suppresses Botrytis cinerea elicited NO production in tomato to influence H2O2 generation and increase host susceptibility. Front Plant Sci 7:709. https://doi.org/10.3389/fpls.2016.00709
Slinger D, Tenison K (2005) Salinity glove box guide: NSW Murray & Murrumbidgee catchments. An initiative of the Southern salt action team. NSW Department of Primary Industries, Newington
Song L, Ding W, Zhao M, Sun B, Zhang L (2006) Nitric oxide protects against oxidative stress under heat stress in the calluses from two ecotypes of reed. Plant Sci 171:449–458
Song L, Ding W, Shen J, Zhang Z, Bi Y, Zhang L (2008) Nitric oxide mediates abscisic acid induced thermotolerance in the calluses from two ecotypes of reed under heat stress. Plant Sci 175:826–832
Song XG, She XP, Wang J, Sun YC (2011) Ethylene inhibits darkness-induced stomatal closure by scavenging nitric oxide in guard cells of Vicia faba. Funct Plant Biol 38:767–777
Stamler JS, Singel DJ, Loscalzo J (1992) Biochemistry of nitric oxide and its redox-activated forms. Science 258:1898–1902
Sturms R, Dispirito AA, Hargrove MS (2011) Plant and cyanobacterial hemoglobins reduce nitrite to nitric oxide under anoxic conditions. Biochem 50:3873–3878
Sun H, Bi Y, Tao J, Huang S, Hou M, Xue R, Liang Z, Gu P, Yoneyama K, Xie X, Shen Q (2016) Strigolactones are required for nitric oxide to induce root elongation in response to nitrogen and phosphate deficiencies in rice. Plant Cell Env 39:1473–1484
Tanou G, Job C, Rajjou L, Arc E, Belghazi M, Diamantidis G, Molassiotis A, Job D (2009) Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J 60:795–804
Tavares CP, Vernal J, Delena RA, Lamattina L, Cassia R, Terenzi H (2014) S-nitrosylation influences the structure and DNA binding activity of AtMYB30 transcription factor from Arabidopsis thaliana. Biochim Biophys Acta 1844:810–817
Taylor JE, McAinsh MR (2004) Signalling crosstalk in plants: emerging issues. J Exp Bot 55:147–149
Terrile MC, París R, Calderón-Villalobos LI, Iglesias MJ, Lamattina L, Estelle M, Casalongué C (2012) Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J 70:492–500
Thalineau E, Truong HN, Berger A, Fournier C, Boscari A, Wendehenne D, Jeandroz S (2016) Cross-regulation between N metabolism and nitric oxide (NO) signaling during plant immunity. Front Plant Sci 7:472. https://doi.org/10.3389/fpls.2016.00472
Tian X, He M, Wang Z, Zhang J, Song Y, He Z, Dong Y (2015) Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul 77:343–356
Tiso M, Tejero J, Basu S, Azarov I, Wang X, Simplaceanu V, Frizzell S, Jayaraman T, Geary L, Shapiro C, Ho C (2011) Human neuroglobin functions as a redox-regulated nitrite reductase. J Biol Chem 286:18277–18289
Tossi V, Cassia R, Bruzzone S, Zocchi E, Lamattina L (2012) ABA says NO to UV-B: a universalresponse? Trends Plant Sci 17:510–517
Tossi V, Lamattina L, Cassia R (2013) Pharmacological and genetical evidence supporting nitric oxide requirement for 2, 4-epibrassinolide regulation of root architecture in Arabidopsis thaliana. Plant Signal Behav 8:e24712
Tossi VE, Lamattina L, Jenkins G, Cassia R (2014) UV-B-induced stomatal closure in Arabidopsis is regulated by the UVR8 photoreceptor in an NO-dependent mechanism. Plant Physiol 164:2220–2230
Tun NN, Holk A, Scherer GF (2001) Rapid increase of NO release in plant cell cultures induced by cytokinin. FEBS Lett 509:174–176
Turkan I (2017) Emerging roles for ROS and RNS—versatile molecules in plants. J Exp Bot 68:4413–4416. https://doi.org/10.1093/jxb/erx236
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163:515–523
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang L, Yang L, Yang F, Li X, Song Y, Wang X, Hu X (2010) Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to copper toxicity. J Plant Physiol 167:1298–1306
Wang Y, Li L, Cui W, Xu S, Shen W, Wang R (2012) Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351:107–119
Wang Y, Loake GJ, Chu C (2013) Cross-talk of nitric oxide and reactive oxygen species in plant programed cell death. Front Plant Sci. https://doi.org/10.3389/fpls.2013.00314
Wang L, Guo Y, Jia L, Chu H, Zhou S, Chen K, Wu D, Zhao L (2014) Hydrogen peroxide acts upstream of nitric oxide in the heat shock pathway in Arabidopsis seedlings. Plant Physiol 164:2184–2196
Wang D, Liu Y, Tan X, Liu H, Zeng G, Hu X, Jian H, Gu Y (2015a) Effect of exogenous nitric oxide on antioxidative system and S-nitrosylation in leaves of Boehmeria nivea (L.) Gaud under cadmium stress. Environ Sci Pol Res 22:3489–3497
Wang P, Du Y, Hou YJ, Zhao Y, Hsu CC, Yuan F, Zhu X, Tao WA, Song CP, Zhu JK (2015b) Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc Natl Acad Sci USA 112:613–618
Wang H, Ji F, Zhang Y, Hou J, Liu W, Huang J, Liang W (2019) Interactions between hydrogen sulphide and nitric oxide regulate two soybean citrate transporters during the alleviation of aluminium toxicity. Plant Cell Environ 42:2340–2356
Wei L, Zhang M, Wei S, Zhang J, Wang C, Liao W (2020) Roles of nitric oxide in heavy metal stress in plants: cross-talk with phytohormones and protein S-nitrosylation. Environ Pol. https://doi.org/10.1016/j.envpol.2020.113943
Wimalasekera R, Tebartz F, Scherer GF (2011) Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci 181:593–603
Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25:434–456
Wink DA, Osawa Y, Darbyshire JF, Jones CR, Eshenaur SC, Nims RW (1993) Inhibition of cytochrome P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys 300:115–123
Wu H, Zheng Y, Liu J, Zhang H, Chen H (2016a) Heme oxygenase-1 delays gibberellin-induced programmed cell death of rice aleurone layers subjected to drought stress by interacting with nitric oxide. Front Plant Sci 6:1267. https://doi.org/10.3389/fpls.2015.01267
Wu Q, Su N, Zhang X, Liu Y, Cui J, Liang Y (2016b) Hydrogen peroxide, nitric oxide and UV RESISTANCE LOCUS8 interact to mediate UV-B-induced anthocyanin biosynthesis in radish sprouts. Sci Rep 6:1–12
Wu P, Xiao C, Cui J, Hao B, Zhang W, Yang Z, Ahammed GJ, Liu H, Cui H (2020) Nitric oxide and its interaction with hydrogen peroxide enhance plant tolerance to low temperatures by improving the efficiency of the calvin cycle and the ascorbate-glutathione cycle in cucumber seedlings. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10242-w
Xiong J, An L, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Xu J, Wang W, Yin H, Liu X, Sun H, Mi Q (2010) Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326:321–330
Xu LL, Fan ZY, Dong YJ, Kong J, Bai XY (2015) Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of two peanut cultivars under cadmium stress. Biol Planta 59:171–182
Xu YF, Chu XT, Fu JJ, Yang LY, Hu TM (2016) Crosstalk of nitric oxide with calcium induced tolerance of tall fescue leaves to high irradiance. Biol Plant 60:376–384
Yamasaki H (2000) Nitrite-dependent nitric oxide production pathway: implications for involvement of active nitrogen species in photoinhibition in vivo. Philos Trans R Soc Lond B Biol Sci 355:1477–1488
Yang L, Ji J, Wang H, Harris-Shultz KR, Abd Allah EF, Luo Y, Guan Y, Hu X (2016) Carbon monoxide interacts with auxin and nitric oxide to cope with iron deficiency in Arabidopsis. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00112
Yang J, Deng X, Wang X, Wang J, Du S, Li Y (2019) The calcium sensor OsCBL1 modulates nitrate signaling to regulate seedling growth in rice. PLoS ONE 14:e0224962
Yuan HM, Huang X (2016) Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signalling in Arabidopsis. Plant Cell Environ 39:120–135
Zaharah SS, Singh Z (2011) Postharvest nitric oxide fumigation alleviates chilling injury, delays fruit ripening and maintains quality in cold-stored ‘Kensington Pride’mango. PostharvestBiol Tech 60:202–210
Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A, Inupakutika MA, Mittler R (2016) ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot 67:5381–5390
Zhang J, Jia W, Yang J, Ismail AM (2006a) Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res 97:111–119
Zhang Y, Wang L, Liu Y, Zhang Q, Wei Q, Zhang W (2006b) Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 224:545–555
Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, Tan M (2007a) Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol 175:36–50
Zhang F, Wang Y, Yang Y, Wu HAO, Wang DI, Liu J (2007b) Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Environ 30:775–785
Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60:1579–1588
Zhang X, Shen L, Li F, Meng D, Sheng J (2011) Methyl salicylate-induced arginine catabolism is associated with up-regulation of polyamine and nitric oxide levels and improves chilling tolerance in cherry tomato fruit. J Agric Food Chem 59:9351–9357
Zhang X, Liu Y, Liu Q, Zong B, Yuan X, Sun H, Wang J, Zang L, Ma Z, Liu H, He S (2018) Nitric oxide is involved in abscisic acid-induced photosynthesis and antioxidant system of tall fescue seedlings response to low-light stress. Environ Exp Bot 155:226–238
Zhao Z, Chen G, Zhang C (2001) Interaction between reactive oxygen species and nitric oxide in drought-induced abscisic acid synthesis in root tips of wheat seedlings. Funct Plant Biol 28:1055–1061
Zhu XF, Jiang T, Wang ZW, Lei GJ, Shi YZ, Li GX, Zheng SJ (2012) Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J Hazard Mat 239:302–307
Zhu XF, Zhu CQ, Wang C, Dong XY, Shen RF (2017) Nitric oxide acts upstream of ethylene in cell wall phosphorus reutilization in phosphorus-deficient rice. J Exp Bot 68:753–760
Zhu Y, Gao H, Lu M, Hao C, Pu Z, Guo M, Hou D, Chen LY, Huang X (2019) Melatonin-nitric oxide crosstalk and their roles in the redox network in plants. Int J Mol Sci. https://doi.org/10.3390/ijms20246200
Acknowledgements
Authors are very thankful to the researchers whose excellent work has been cited in the presented study, which helps us to prepare an up to date review.
Author information
Authors and Affiliations
Contributions
Author contribution statement: RKS and HSJ conceived the idea. All authors contributed equally in writing and figure and table preparation. All authors have read and approved the final version of review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they do not have any conflict of interest.
Ethical Approval
All the authors have been agreed to submit it.
Consent to Participate
Before the submission of paper, all the author have given the consent to publish.
Consent to Publish
All the authors have given the consent to publish.
Additional information
Handling Editor: M. Naeem.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singhal, R.K., Jatav, H.S., Aftab, T. et al. Roles of Nitric Oxide in Conferring Multiple Abiotic Stress Tolerance in Plants and Crosstalk with Other Plant Growth Regulators. J Plant Growth Regul 40, 2303–2328 (2021). https://doi.org/10.1007/s00344-021-10446-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10446-8