Abstract
The branching trait is influenced by various environmental signals, including the Red light:Far Red light (R:FR), an indicator of competition and an inhibitor of axillary bud growth. Branch development is influenced by an array of hormones, including auxin which indirectly suppresses bud growth as a consequence of auxin transport and signaling in the main stem. The suppressive effect of auxin sourced from the shoot apex and transported basipetally is a major mechanism contributing to apical dominance, a form of correlative inhibition where superior growing points restrict the growth of those lower on the plant. The current study shows that increased apical dominance is a mechanism that suppresses the branching of plants grown in a low R:FR. The elevated apical dominance was not due to increased levels of the natural auxin IAA, but was associated with enhanced expression of auxin-induced genes both in the presence and absence of exogenous auxin. A direct test of bud sensitivity to auxin confirmed that a low R:FR promoted auxin responsiveness leading to reduced bud growth. Thus, the low R:FR enhancement of auxin sensitivity is a mechanism that contributes to the reduced branching phenotype of plants growing in competitive environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants display enormous diversity in above-ground architectural traits and variation in axillary branching patterns effectively generates a spectrum of plant forms. Axillary meristems formed in leaf axils develop into buds, and the buds may grow out to form a branch/tiller or remain dormant/semi-dormant. Branching is an important trait that was often selected for during domestication of major crops and has been modified by modern breeding programs in both annuals and perennials (Dong et al. 2019). The manipulation of branching by pruning off terminal shoots is a recurring operation to improve productivity in orchards (Lauri et al. 2009). Shoot branching is thought to optimize resource allocation in response to biotic and abiotic stresses in natural populations occupying diverse ecological landscapes (Baker et al. 2012; Remington et al. 2015). The plasticity in bud outgrowth offers a wide range of possible plant forms from prolific branching to a complete absence of branches and all possibilities in between.
Genetic factors specify the underlying architecture of plants by regulating axillary meristem formation and subsequent development into a bud, but the plasticity in bud growth to form a branch or remain dormant/semi-dormant is determined by the integration of endogenous hormonal and developmental signals, as well as environmental cues (Domagalska and Leyser 2011). Recent discoveries integrating physiology, genetics, and molecular tools indicate that bud outgrowth is, to a large extent, controlled by multiple phytohormones through their biosynthesis, transport, and signaling, in coordination with diverse environmental signals (reviewed in Barbier et al. 2019).
Auxin has long been known to play a central role in the control of branching, although the precise mechanisms remain unclear. The term “apical dominance” describes the inhibition of axillary branching by the main shoot apex, a phenomenon that results in part from the inhibitory effects of basipetally transported, apex-derived auxin on bud growth (Thimann and Skoog 1933). Removing auxin sources by decapitating the shoot stimulates bud outgrowth and application of exogenous auxin at the decapitated stump restores apical dominance (Thimann and Skoog 1933; Cline 1996). It is noteworthy that this transported auxin indirectly suppresses bud growth without entering the bud (Hall and Hillman 1975; Booker et al. 2003). Exactly how auxin inhibits bud growth is not completely understood, although two hypotheses, the canalization hypothesis, and the second messenger hypothesis, enjoy strong support.
The auxin canalization hypothesis proposes that auxin establishes its own conduit of transport through the formation of narrow canals of vascular connection enabling auxin transport from source to sink (Sachs 1981). The bud (source) at a higher auxin concentration must move auxin into the polar auxin transport stream (PATS) at a lower concentration in the stem (sink) to establish vascular connections, which eventually sustain growth (Li and Bangerth 1999; Balla et al. 2011). Decapitation removes the apical auxin source and is therefore expected to create an ample gradient for auxin export out of buds. While many lines of research support these basic principles (Bennett et al. 2006; Prusinkiewicz et al. 2009; van Rongen et al. 2019), bud growth in response to decapitation has been detected before stem auxin levels declined (Morris et al. 2005) and bud growth independent of polar auxin transport has also been reported (Brewer et al. 2015).
An alternative theory invokes the action of a second messenger regulating bud growth responses to auxin in the PATS. Two hormones, cytokinins and a strigolactone-derivative have been advanced as second messengers controlling the bud growth. Cytokinins have a promotive effect by acting at the node or within the bud, whereas strigolactone also operates within or near the bud to inhibit branching (Bennett et al. 2006; Waldie et al. 2010). Auxin suppresses the transcription of a group of Adenylate Isopentenyltransferase (IPT) genes encoding cytokinin biosynthetic enzymes (Tanaka et al. 2006). In contrast, auxin promotes the biosynthesis of bud inhibitory strigolactone by elevating the expression of More Axillary Growth 3 (MAX3) and MAX4 that encode enzymes involved in strigolactone biosynthesis (Bennett et al. 2006; Brewer et al. 2009). Some evidence indicates that strigolactones may inhibit branching through their effects on auxin transport (Bennett et al. 2016), while other research suggests that their effects on shoot branching may be independent of auxin transport (Brewer et al. 2015). The physiological models associating auxin suppression of bud growth with cytokinins, and strigolactones remain inconclusive.
A number of lines of investigations have also associated the action of abscisic acid (ABA) with the suppression of bud growth (Arney and Mitchell 1969; Tucker and Mansfield 1972; Tucker 1977; Gocal et al. 1991; Cline and Oh 2006). Recent studies demonstrated that ABA functions downstream of auxin and strigolactones to suppress bud growth (Reddy et al. 2013; González-Grandío et al. 2017). Sugar signaling is also associated with apical dominance, as rapid decapitation-induced bud growth was coincident with enhanced mobilization of sugars into axillary buds and sucrose itself can stimulate bud growth (Mason et al. 2014; reviewed in Kebrom 2017).
Plants have highly developed light-sensing capabilities and mechanisms to respond when challenged by competition for light. Reduced R:FR is an indicator of neighboring competition and is perceived largely by the phytochrome B (phyB) photoreceptor (Casal 2012). Low R:FR, or loss of phyB function, stimulates a suite of shade avoidance responses, including a reduction in branching (Kebrom et al. 2006; Finlayson et al. 2010; González-Grandío et al. 2013; Reddy et al. 2013). Auxin plays a predominant role in the generation of shade avoidance phenotypes (Halliday et al. 2009). Early responses to shade or a low R:FR in Arabidopsis seedlings are contingent on biosynthesis of the natural auxin indole-3-acetic acid (IAA) occurring via Tryptophan Aminotransferase of Arabidopsis 1 (TAA1) (Tao et al. 2008) and polar auxin transport by PINOID3 (PIN3) (Keuskamp et al. 2010). Phytochrome Interacting Factor (PIF) transcription factors acting downstream of phyB elevate the transcription of YUCCA genes to promote auxin biosynthesis and also promote the expression of the auxin signaling genes Indole-3-Acetic Acid Inducible 19 (IAA19), and IAA29 in response to shade (Hornitschek et al. 2012; Li et al. 2012; Lorrain et al. 2008). Overall, the low R:FR-mediated inactivation of phyB or non-functional phyB alters auxin biosynthesis, transport, and signaling.
Ongoing work has shed light on the role of auxin in axillary bud inhibition in phyB mutants or in response to changes in the R:FR (Reddy and Finlayson 2014; Yao and Finlayson 2015; Holalu and Finlayson 2017). A large part of the suppression of branching by phyB deficiency could be attributed to elevated auxin-dependent apical dominance (Reddy and Finlayson 2014). However, this increased apical dominance was not the result of elevated IAA levels, but was due to enhanced auxin responsiveness of the phyB mutant. Although it has been established that phyB represses branching in part by promoting auxin signaling, the function of low R:FR has not been previously demonstrated. It was hypothesized that low R:FR, like phyB deficiency, would promote apical dominance by elevating auxin signaling independent of auxin abundance.
Materials and Methods
Plant Growth and Light Treatments
Wild type Arabidopsis thaliana (Col-0, ABRC CS60000) was used throughout. Plants were grown in six cell inserts (one plant per cell) filled with MetroMix LC1, and provided optimal water and nutrients (Hoagland’s solution). The plants were grown in a growth chamber modified with an overhead array of FR light emitting diodes (735 nm). The chamber was split into two equal parts with a light-impermeable baffle. Photosynthetically active radiation was provided by fluorescent lamps at 180 μmoles m−2 s−1 photosynthetic photon flux density (PPFD). Plants were initially exposed to high R:FR (4.41) for 4 days, and then given supplemental FR to reduce the R:FR to 0.075. Spectra of the light sources are provided in Fig. 1. Plants received a photoperiod of 16/8 h light/dark and temperatures of 24/18 °C day/night.
Decapitation and Architectural and Branch Elongation Analyses
Plants were decapitated below the lowest cauline branch at one day before the predicted occurrence of anthesis. Architectural characteristics and branch elongation were measured at 10 days post anthesis as described in Finlayson et al. (2010) except that the correlative inhibition index was calculated for each record individually. The correlative inhibition index is given as the slope of the top three branch lengths plotted against their positions, multiplied by − 1 for ease of presentation. The “rosette branches response” was calculated by dividing the number of rosette branches for each record by the average number of rosette branches for the un-decapitated plants within each light treatment. The “rosette branch decapitation response” was similarly calculated by dividing the rosette branch length for each record by the average rosette branch length of the un-decapitated plants within each light treatment and branch position.
Analysis of Hormone Abundance
IAA abundances were determined in whole seedling shoots (10 shoots per replicate) at 14 days after sowing, and in basal 15 mm segments of the main inflorescence stem (8 to 10 segments per replicate) at anthesis. IAA was extracted and quantified using isotope dilution selected ion monitoring gas chromatography-mass spectroscopy as described in Reddy et al. (2013). Four biological replicates were measured for each genotype.
Analysis of Gene Expression
For seedlings, 14 day old plants were sprayed until lightly wetted with 50 μM napthaleneacetic acid (NAA) in a solution containing 1% ethanol and 0.03% Silwet, or with a control solution lacking the NAA. Whole shoots were harvested 45 min after the treatment was applied, with ten shoots comprising one replicate. Mature plants were treated in a similar manner at the time of anthesis. Basal 1.5 cm segments of the main inflorescence stem adjacent to the rosette were harvested 45 min after treatment, with 8 to 10 segments comprising one replicate. Total RNA was extracted and gene expression was measured by QPCR using the methods of Su et al. (2011). Average gene expression responses were based on the normalized expression patterns of all the tested genes. For each gene target, each gene expression value was normalized by dividing by the average value for all the samples/replicates. The average of all normalized gene expression values was then used to estimate the average response for each treatment/tissue. Primers for IAA11, IAA19 and GH3.5 were taken from Effendi et al. (2011). Primers for IAA29 are described in Reddy and Finlayson (2014). Four biological replicates were measured for each genotype/treatment combination.
Split Tip Assay of Bud Growth
A split tip assay of axillary bud growth response to auxin was conducted based on modifications to the split plate method of Chatfield et al. (2000). Plants were grown in inserts as described above, under the different light regimens. Stem sections spanning buds were excised from the main inflorescence when the buds were less than 3 mm long, rinsed in 20% bleach followed by sterile water, and inserted into the split tip system. Split tips were comprised of 1 mL (nominal) pipettor tips filled with 0.8% agar with 0.4X MS salts and 0.2% sucrose (Fig. 2). The tips were notched with a blade to permit insertion of the stem section and to provide discontinuity between the agar in contact with the apical stem portion and the agar in contact with the basal stem portion. The upper agar portion of split tips providing auxin contained 80 nM NAA. Once assembled the split tips were placed in clear disposable plastic culture tubes (17 × 100 mm) containing 250 μL of water (to maintain humidity) and closed with vented caps. The split tips were replaced in their respective light regimens and bud lengths were measured at four days after transfer. Nineteen to 20 biological replicates were measured for each treatment combination.
Statistics
Comparisons between means were made using a two-tailed t-test or ANOVA followed by Tukey’s HSD implemented in R.
Results
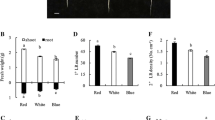
Shoot branching phenotypes generated by the different R:FR treatments were evaluated by assessing the number of branches, the correlative inhibition index and branch elongation/normalized branch elongation by position (Fig. 3). The correlative inhibition index quantifies branching using the lengths of the top three branches (Finlayson et al. 2010). More negative slopes obtained by regressing the top three branch lengths by their ordinal positions (“n”, “n-1”, “n-2”) indicate greater correlative inhibition, or weaker branching, as the lower branches are increasingly inhibited by more apical growing points supplying inhibitory signals. Branch “n” is the topmost rosette branch (in the axil of the last rosette leaf formed), with sequentially lower branches assigned labels “n-1”, “n-2”, etc. Since the correlative inhibition index is typically derived from elongated branches, it integrates not only the buds’ initial growth, but also the subsequent rate of elongation at sequential positions. Plants grown under a low R:FR had fewer rosette branches and a higher correlative inhibition index than those grown under a high R:FR (Fig. 3a, c). Decapitation was conducted to eliminate the source of apical signals, including apically-sourced auxin, which is known to inhibit branching. Decapitated plants grown under either a high or a low R:FR produced more branches than their intact counterparts, but the response to decapitation was significantly stronger in plants grown under a low R:FR (Fig. 3b). Decapitation also sharply reduced correlative inhibition in low R:FR grown plants, but it had no effect on those grown in a high R:FR (Fig. 3c). Branch growth following decapitation was also evaluated on a positional basis (Fig. 3d, e). Decapitation promoted the growth of lower branches more than that of upper branches, and the response was significantly greater in lower branches of plants grown under a low R:FR, compared to a high R:FR. In fact, the branches of decapitated plants grown under a low R:FR grew to greater lengths than those grown under a high R:FR. Overall, the branching of intact plants grown under a low R:FR was weaker than in plants grown under a high R:FR, but they also showed a stronger branching response to decapitation than their high R:FR grown counterparts.
Number of rosette branches (a), number of rosette branches response (b), correlative inhibition index (c), branch lengths (d) and branch lengths response (e) of intact and decapitated Col-0 grown under high and low R:FR at 10 days after anthesis. Inset in (e) expands Y-axis for first two positions. Data are means ± SE with n = 15–23. Bars with different letters are significantly different at α = 0.01. Asterisks in (e) indicate a significant difference in response to decapitation at each branch position between plants grown in high and low R:FR at α = 0.01
Since plants grown under a low R:FR showed weaker branching, but greater responsiveness to decapitation than those grown under a high R:FR it was possible that the low R:FR branching suppression might result from elevated auxin abundance. IAA was therefore quantified in young shoots and in mature basal stem segments of plants grown under a high and a low R:FR. There was no difference in IAA levels in young shoots (Fig. 4a), but IAA levels were significantly lower in basal stems of mature plants grown under a low R:FR compared to those grown under a high R:FR, both on a FW basis and on a shoot segment basis (Fig. 4b, c). The results did not support the hypothesis that a low R:FR inhibits branching by increasing IAA levels since IAA abundance was not positively correlated with the suppression of branching under a low R:FR.
IAA abundance in high and low R:FR grown shoots of 14 day old Col-0 seedlings (a) and basal stem segments of mature plants (b and c) expressed on a per weight basis (b) and per 15 mm stem segment (c). Data are means ± SE with n = 4. Asterisks indicate a significant difference between high and low R:FR at α = 0.001
Previous work showed that the R:FR and phyB modulate auxin sensitivity in young seedlings and mature plants (Bou-Torrent et al. 2014; Hersch et al. 2014; Reddy and Finlayson 2014; de Wit et al. 2015; Pucciariello et al. 2018). The expression of auxin-inducible genes was therefore assessed under the different light regimens, with and without exogenous auxin application, to determine if the R:FR altered auxin responsiveness in a manner consistent with the observed branching phenotypes. The gene targets included IAA11, IAA19, and IAA29 that encode auxin-induced transcription factors involved in auxin signal transduction, and GH3.5 which encodes an auxin-inducible IAA conjugating enzyme. In the absence of exogenous auxin the expression of IAA19 and IAA29 was elevated in young shoots grown under a low R:FR compared to a high R:FR, the expression of IAA11 was suppressed and the expression of GH3.5 was unaltered (Fig. 5a–d). Exogenous application of auxin significantly elevated the expression of IAA19, IAA29 and GH3.5 in low R:FR grown plants compared to those grown in a high R:FR. Overall, the average response of auxin-induced genes was equivalent in seedlings grown under both a high and a low R:FR, but the gene expression of plants grown under a low R:FR was more responsive to the addition of exogenous auxin (Fig. 5e). IAA11, IAA19 and IAA29 expression was significantly elevated in untreated basal stem sections of mature plants grown under a low R:FR compared to a high R:FR (Fig. 5F-I), and expression of all 4 marker genes was elevated in basal stem segments of low R:FR plants following auxin application. Both the baseline and inducible average responses of auxin-induced genes were elevated in basal stem sections of mature plants grown under a low R:FR (Fig. 5j). The results indicate that auxin responsive gene expression may be promoted by growth under a low R:FR even though IAA levels are reduced.
Expression of auxin-responsive genes in shoots of 14 day old Col-0 seedlings (a-d) and in basal stem segments of mature plants (f–i) grown under high and low R:FR with and without auxin (NAA) treatment. Data are means ± SE with n = 4. Overall average response for seedlings, as reported by the normalized expression of the genes presented in (a–d), is shown in (e). Overall average response for basal stem segments of mature plants, as reported by the normalized expression of the genes presented in (f–i), is shown in (j). Data in (e) and (j) are means ± SE with n = 16. Bars with different letters are significantly different at α = 0.05
To further probe how the contrasting R:FR regimens impacted the plants’ ability to perceive auxin, a split tip assay was used as a direct read-out of auxin responsiveness. In this assay (modified from Chatfield et al. 2000) a section of stem including a bud is suspended between discontinuous agar media masses. Auxin provided in the apical agar mass is transported through the stem where it indirectly inhibits the growth of the associated bud (Fig. 2). Bud growth was greater in isolated buds grown under a low R:FR compared to a high R:FR (Fig. 6a). Apically supplied auxin inhibited the growth of buds from plants grown under both a high and a low R:FR (Fig. 6a), however the inhibitory effect was significantly greater in sections from plants grown under a low R:FR compared to a high R:FR (Fig. 6b). The assay demonstrated that a low R:FR promoted the inhibitory response of bud growth to auxin transported in the PATS.
Discussion
The suppression of shoot branching by a low R:FR was shown to result, at least in part, from increased auxin responsiveness that was independent of auxin abundance in the stem. A previous study demonstrated that phyB normally acts to attenuate auxin signaling, which promotes branch growth (Reddy and Finlayson 2014). Therefore, it may now be concluded that phyB transduces some of the effects of the R:FR on branching by modulating auxin responsiveness. A high R:FR results in activated phyB which attenuates auxin responsiveness to stimulate branching, while a low R:FR inactivates phyB which promotes auxin responsiveness to inhibit branching. The signaling pathway downstream of phyB that modulates auxin signaling to regulate branching likely involves Phytochrome Interacting Factors (PIFs). Under a high R:FR phyBfr moves into the nucleus and phosphorylates/inactivates/sequesters several PIFs, including PIFs 4, 5 and 7 to inhibit shade avoidance responses. Conversely, under a low R:FR phyBr does not enter the nucleus and the PIFs are able to bind to the promoters of their target genes to regulate their expression and elicit shade avoidance responses. PIF gene targets include auxin signaling genes that enhance auxin sensitivity and shade avoidance phenotypes (Hornitschek et al. 2012).
While it is apparent that branching responses to the R:FR result in part from phyB-modulation of auxin responsiveness, this is not the only mechanism involved. Previous research showed that low R:FR promotes bud ABA accumulation and signaling to repress bud growth (Reddy et al. 2013; González-Grandío et al. 2017). A time course analysis indicated that the R:FR impacts bud ABA homeostasis more rapidly than auxin responsiveness, but that the effects of the change in auxin responsiveness on branching are likely stronger than those of ABA (Holalu and Finlayson 2017). Additionally, the ABA and auxin pathways interact, with auxin signaling contributing to bud ABA accumulation, and bud ABA limiting bud IAA accumulation (Yao and Finlayson 2015).
Ongoing research has indicated that sugar supply and/or signaling may contribute to regulate bud growth (Kebrom et al. 2012; Mason et al. 2014; Barbier et al. 2015; Fichtner et al. 2017). In the case of pea, the rapid bud growth response to decapitation was associated with redirection of sugars to the developing buds (Mason et al. 2014). Plants grown under a low R:FR have fewer leaves than those grown under a high R:FR and therefore might be expected to produce less sugars, which could limit bud growth. However, in the present study the buds of low R:FR grown plants were actually more responsive to decapitation than those of high R:FR grown plants and grew to greater lengths which is not consistent with a presumed sugar deficiency under a low R:FR. Further research on the potential role of sugars in the regulation of branching by the R:FR is necessary to address this issue.
Decapitation was effective at promoting the growth of buds from lower rosette positions, and this effect was much stronger in plants grown under a low R:FR. The growth promotion response to decapitation increased exponentially at sequentially lower rosette positions, then abruptly declined, and in fact the very lowest buds rarely grew appreciably, even with decapitation. Thus, the lowest buds were virtually unresponsive to decapitation, indicating that other factors were involved in their arrest. It is possible that elevated ABA accumulation may contribute to this arrest, in view of the known role of low R:FR in promoting bud ABA accumulation (Reddy et al. 2013; Holalu and Finlayson 2017; González-Grandío et al. 2017). It is also possible that these lowest buds were insufficiently developed to respond to decapitation, and that given time growth might be initiated. Further research is necessary to explore these possibilities.
The data indicated that a low R:FR inhibited the frequency of branching and promoted correlative inhibition, but in some cases, it exerted positive effects on branch elongation. In the case of decapitation, all of the branches of plants grown under a low R:FR elongated more than those grown under a high R:FR, and even without decapitation the topmost branch of plants grown under a low R:FR outstripped their high R:FR counterparts. Additionally, isolated buds in the split-tip system elongated more rapidly when grown under a low R:FR than a high R:FR. Thus, the R:FR exerts contrasting effects on different aspects of branch development. It is possible that a low R:FR inhibits the initiation of bud growth by promoting systemic auxin signaling in the PATS, but enhances bud/branch elongation by promoting auxin signaling in the bud itself. Dissecting these potential antagonistic effects of auxin signaling warrants further study.
References
Arney SE, Mitchell DL (1969) The effect of abscisic acid on stem elongation and correlative inhibition. New Phytol 68:1001–1015
Baker RL, Hileman LC, Diggle PK (2012) Patterns of shoot architecture in locally adapted populations are linked to intraspecific differences in gene regulation. New Phytol 196:271–281
Balla J, Kalousek P, Reinohl V, Friml J, Prochazka S (2011) Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J 65:571–577
Barbier F, Péron T, Lecerf M, Perez-Garcia M-D, Barrière Q, Rolčík J, Boutet-Mercey S, Citerne S, Lemoine R, Porcheron B, Roman H, Leduc N, Le Gourrierec J, Bertheloot J, Sakr S (2015) Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot 66:2569–2582
Barbier FF, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA (2019) An update on the signals controlling shoot branching. Trends Plant Sci 24:220–236
Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16:553–563
Bennett T, Hines G, van Rongen M, Waldie T, Sawchuk MG, Scarpella E, Ljun K, Leyser O (2016) Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biol 14(4):e1002446. https://doi.org/10.1371/journal.pbio.1002446
Booker J, Chatfield S, Leyser O (2003) Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 15:495–507
Bou-Torrent J, Galstyan A, Gallemi M, Cifuentes-Esquivel N, Molina-Contreras MJ, Salla-Martret M, Jikumaru Y, Yamaguchi S, Kamiya Y, Martínez-García JF (2014) Plant proximity perception dynamically modulates hormone levels and sensitivity in Arabidopsis. J Exp Bot 65:2937–2947
Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150:482–493
Brewer PB, Dun EA, Gui R, Mason MG, Beveridge CA (2015) Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiol 168:1820–1829
Casal JJ (2012) Shade Avoidance. Arabidopsis Book 10:e0157. https://doi.org/10.1199/tab.0157
Chatfield SP, Stirnberg P, Forde BG, Leyser O (2000) The hormonal regulation of axillary bud growth in Arabidopsis. Plant J 24:159–169
Cline MG (1996) Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot 78:255–266
Cline MG, Oh C (2006) A reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Ann Bot 98:891–897
de Wit M, Ljung K, Fankhauser C (2015) Contrasting growth responses in lamina and petiole during neighbor detection depend on differential auxin responsiveness rather than different auxin levels. New Phytol 3:198–209
Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12:211–221
Dong Z, Alexander M, Chuck G (2019) Understanding grass domestication through maize mutants. Trends Genet 35:118–128
Effendi Y, Rietz S, Fischer U, Scherer GFE (2011) The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. Plant Journal 65:282–294
Fichtner F, Barbier FF, Feil R, Watanabe M, Annunziata MG, Chabwika TG, Höfgen R, Stitt M, Beveridge CA, Lunn JE (2017) Trehalose 6-phosphate is involved in triggering axillary bud outgrowth in garden pea (Pisum sativum L.). Plant J 92:611–623
Finlayson SA, Krishnareddy SR, Kebrom TH, Casal JJ (2010) Phytochrome regulation of branching in Arabidopsis. Plant Physiol 152:1914–1927
Gocal GFW, Pharis RP, Yeung EC, Pearce D (1991) Changes after decapitation in concentrations of indole-3-acetic-acid and abscisic-acid in the larger axillary bud of Phaseolus vulgaris L. cv tender green. Plant Physiol 95:344–350
González-Grandío E, Poza-Carrión C, Sorzano COS, Cubas P (2013) BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 25:834–850
González-Grandío E, Pajoro A, Franco-Zorrilla JM, Tarancon C, Immink RGH, Cubas P (2017) Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc Natl Acad Sci USA 114(2):E245–E254
Hall SM, Hillman JR (1975) Correlative inhibition of lateral bud growth in Phaseolus vulgaris L. timing of bud growth following decapitation. Planta 123:137–143
Halliday KJ, Martizez-Garcia JF, Josse E-M (2009) Integration of light and auxin signaling. Cold Spring Harb Perspect Biol 1:a001586
Hersch M, Lorrain S, de Wit M, Trevisan M, Ljung K, Bergmann S, Fankhauser C (2014) Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc Natl Acad Sci U S A 111:6515–6520
Holalu SV, Finlayson SA (2017) The ratio of red light to far red light alters Arabidopsis axillary bud growth and abscisic acid signalling before stem auxin changes. J Exp Bot 68:943–952
Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, Lopez-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, Xenarios I, Fankhauser C (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J 71:699–711
Kebrom TH (2017) Growing stem inhibits bud outgrowth–The overlooked theory of apical dominance. Frontiers in Plant Science 8:1874. https://doi.org/10.3389/fpls.2017.01874
Kebrom TH, Burson BL, Finlayson SA (2006) Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol 140:1109–1117
Kebrom TH, Chandler PM, Swain SM, King RW, Richards RA, Spielmeyer W (2012) Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiol 160:308–318
Keuskamp DH, Pollmann S, Voesenek LA, Peeters AJ, Pierik R (2010) Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA 107:22740–22744
Lauri PE, Costes E, Regnard JL, Brun L, Simon S, Monney P, Sinoquet H (2009) Does knowledge on fruit tree architecture and its implications for orchard management improve horticultural sustainability? an overview of recent advances in the apple. Acta Hort 817:243–250
Li C-J, Bangerth F (1999) Autoinihibition of indoleacetic acid transport in the shoot of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol Plant 106:415–420
Li L, Ljung K, Breton G, Schmitz RJ, Pruneda-Paz J, Cowing-Zitron C, Cole BJ, Ivans LJ, Pedmale UV, Jung H-S, Ecker JR, Kay SA, Chory J (2012) Linking photoreceptor excitation to changes in plant architecture. Genes Dev 26:785–790
Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53:312–323
Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci 111:6092–6097
Morris SE, Cox MCH, Ross JJ, Krisantini S, Beveridge CA (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol 138:1665–1672
Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O (2009) Control of bud activation by an auxin transport switch. Proc Nat Acad Sci USA 106:17431–17436
Pucciariello O, Legris M, Rojas CC, Iglesias MJ, Hernando CE, Dezar C, Vazquez M, Yanovsky MJ, Finlayson SA, Prat S, Casal JJ (2018) Rewiring of auxin signaling under persistent shade. Proc Natl Acad Sci USA 115:512–5617
Reddy SK, Finlayson SA (2014) Phytochrome B promotes branching in Arabidopsis by suppressing auxin signaling. Plant Physiol 164:1542–1550
Reddy SK, Holalu SV, Casal JJ, Finlayson SA (2013) Abscisic acid regulates Arabidopsis axillary bud outgrowth responses to the ratio of red: far red light. Plant Physiol 163:1047–1058
Remington DL, Figueroa J, Rane M (2015) Timing of shoot development transitions affects degree of perenniality in Arabidopsis lyrate (Brassicaceae). BMC Plant Biol 15:226–239
Sachs T (1981) The control of the patterned differentiation of vascular tissues. Adv Bot Res 9:151–262
Su H, Abernathy SD, White RH, Finlayson SA (2011) Photosynthetic photon flux density and phytochrome B interact to regulate branching in Arabidopsis. Plant Cell Environ 34:1986–1998
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45:1028–1103
Tao Y, Ferrer J, Ljung K, Pojer F, Hong F, Long J, Li L, Moreno J, Bowman M, Ivans L, Cheng Y, Lim J, Zhao Y, Ballaré CL, Sandberg G, Noel JP, Chory J (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133:164–176
Thimann KV, Skoog F (1933) Studies on the growth hormone of plants. III. The inhibitory action of the growth substance on bud development. Proc Natl Acad Sci USA 19:714–716
Tucker DJ (1977) Effects of far-red light on lateral bud outgrowth in decapitated tomato plants and associated changes in levels of auxin and abscisic acid. Plant Sci Lett 8:339–344
Tucker DJ, Mansfield TA (1972) Effects of light quality on apical dominance in Xanthium strumarium and associated changes in endogenous levels of abscisic acid and cytokinins. Planta 102:140–151
van Rongen M, Bennett T, Ticchiarelli F, Leyser O (2019) Connective auxin transport contributes to strigolactone-mediated shoot branching control independent of the transcription factor BRC1. PLoS Genet. https://doi.org/10.1371/journal.pgen.1008023
Waldie T, Hayward A, Beveridge CA (2010) Axillary bud outgrowth in herbaceous shoots: how do strigolactones fit into the picture? Plant Mol Biol 73:27–36
Yao C, Finlayson SA (2015) Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol 169:611–626
Funding
This work was supported by Texas A&M AgriLife Research (S.A.F.).
Author information
Authors and Affiliations
Contributions
All of the authors contributed to the design, experimentation, interpretation and writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Holalu, S.V., Reddy, S.K. & Finlayson, S.A. Low Red Light:Far Red Light Inhibits Branching by Promoting Auxin Signaling. J Plant Growth Regul 40, 2028–2036 (2021). https://doi.org/10.1007/s00344-020-10253-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10253-7