Abstract
Loquat is considered as a non-climacteric fruit; however, there is an evidence of a climacteric-like maturation. Therefore, it seems its ripening behavior has yet to be satisfactory classified. Because autocatalytic regulation of ethylene production during fruit ripening is one of the primary features defining climacteric-like fruit maturation, we examined its ability of autocatalysis during ripening by applying the ethylene-releasing compound ethephon to the on-tree-fruit or ethylene to detached fruit of ‘Algerie’ loquat and measuring its ethylene and CO2 production. We also analyzed indoleacetic acid (IAA), gibberellin, cytokinin, and abscisic acid (ABA) contents as plant hormones involved in fruit ripening. The fruit response to ethephon (500 mg l−1) applied at color change was immediate producing increasing amounts of ethylene during the 4 h following the treatment, but 24 h after treatment onward values were similar to those produced by untreated fruit. Similar results were obtained when applying ethylene to detached fruit (10 µl l−1). Accordingly, applying ethephon (200 mg l−1) did not advance harvest; neither the color nor the percentage of fruit harvested at the first picking date differed significantly from the untreated fruit. Flesh firmness, total soluble solid concentration, and acidity of juice were not significantly altered either. IAA concentration reached the maximum value when fruit stopped growing, declining sharply at fruit color change; active gibberellins and cytokinins declined continuously during the fruit growth period, and ABA content sharply increased during ripening, peaking after fruit color break. Results indicate that ‘Algerie’ loquat lacks a ripening-associated autocatalytic rise in ethylene production, and suggest that a decline in gibberellin, cytokinin, and IAA concentrations might be needed to allow its ripening process to proceed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Loquat (Eriobotrya japonica Lindl.) is a pome fruit whose ripening behavior has yet to be satisfactorily classified. Although considered a non-climacteric fruit (Kader 2002), there is an evidence of a climacteric-like maturation. Under Mediterranean environmental conditions, a peak in ethylene production is associated with an increased respiration rate in fruits of five loquat cultivars (Amorós and others 2003). Hirai (1980) also found increased respiration during ripening of the Tanaka cultivar. Analysis of the expression of ethylene biosynthetic genes revealed an increase in ACC-synthase 2 (EjACS2) that paralleled a peak in ethylene production at the time of color change in the Luoyangqing cultivar. The rate of expression of other genes, such as EjACS1 and EjACO1, increased progressively during ripening. These genes, however, did not undergo major changes in detached fruits. These results suggest a climacteric-like maturation of this cultivar regulated mainly by EjACS2 (Jiang and others 2011).
Other studies revealed that loquat behaves as a non-climacteric fruit because ethylene and CO2 production gradually declined during fruit maturation in Algerie (González and others 2003) and Mogi (Ding and others 1998) cultivars. In the Golden Nugget cultivar, Undurraga and others (2011) found an increase in ethylene production but not in respiration rate at the time of color break. These authors also reported an increase in the activity of the peroxidase enzyme, but a constant reduction in that of pectinmethylesterase, polygalacturonase, and cellulose, and thus they could not confirm that enzyme activity was linked to ethylene production. Accordingly, and although in some cases there is a well-defined peak in ethylene production, there is not enough data available to classify loquat as a climacteric fruit. Besides, there is no starch accumulation in the flesh tissues of the Algerie cultivar (Gariglio and others 2002), the firmness of Luoyangqing cultivar is increased during ripening due to tissue lignification (Cai and others 2006), and there is no evidence of a general response to ethephon promoting fruit ripening (Undurraga and Olaeta 2003; Reig and others 2007). Moreover, some reports indicate that after harvest there is a very low level of ethylene production (Blumenfeld 1980; Ding and others 1998; Amorós and others 2003; Cai and others 2006), and others report no concomitant increase in ethylene production and respiration rate (Blumenfeld 1980; Zheng and others 1993; Ding and others 1998; González and others 2003; Kader 2002). These results illustrate the controversy concerning loquat ripening and ethylene production, and it is risky to conclude that loquat is a fruit with climacteric-like behavior. For further information, see the review by Pareek and others (2014).
Autocatalytic regulation of ethylene production during fruit ripening is one of the major features defining climacteric-like fruit maturation (Yang and Hoffman 1984; Giovannoni 2001). In climacteric fruits, ethylene is able to induce its own biosynthesis through a positive feedback regulation of specific members of the ACS gene family and ACO genes as well, thus leading to a massive increase in ethylene production triggering the onset of ripening, and all the ethylene-related events occurring during ripening (see reviews by Grierson 2014; Cherian and others 2014). Despite loquat’s ability to produce small amounts of ethylene during ripening (Blumenfeld 1980; Ding and others 1998; Amorós and others 2003; Cai and others 2006), the fact that fruit ripening is not stimulated by the ethylene-releasing compound ethephon suggests that autocatalysis of ethylene production does not operate in loquat fruit. However, there is still no evidence of changes in ethylene production and respiration rate in response to ethylene or ethylene-releasing compounds.
Besides ethylene, other plant hormones influence fruit ripening. According to McAtee and others (2013) and Cherian and others (2014), a dynamic interplay between phytohormones and metabolites is required for fruit to mature and ripen. In a number of both climacteric and non-climacteric fruit crops, a reduction in auxin levels is required for ripening to commence (Given and others 1988; Zaharah and others 2012). Furthermore, in fruits for which it is not strictly associated with ethylene, auxin treatment delays ripening (Jones and others 2002), and the initiation of ripening is prevented in transgenic apples, which can maintain high levels of auxin (Schaffer and others 2013). Cytokinins are a powerful antisenescence factor (Richmond and Lang 1957), and there is an evidence suggesting that these plant hormones play some role in fruit maturation (Kumar and others 2014). For example, in loquat, kinetin applied prior to fruit color change significantly delays the loss of chlorophyll (Lou and others 2012). Gibberellins have also been found to delay fruit ripening in both climacteric (Martínez-Romero and others 2000; Singh and others 2007) and non-climacteric fruit (Agustí and others 1981). Finally, an increase in abscisic acid (ABA) has also been associated with color change. In non-climacteric fruit, where no peak of ethylene production during ripening takes place, there is an increase in ABA content (McAtee and others 2013), and any treatment that delays this increase reduces fruit color intensity (Gambetta and others 2014). Therefore, studies integrating the pattern of changes in the most important plant hormones during loquat fruit development and ripening may shed light on its ripening behavior.
The aim of this research is to provide an evidence for the behavior of loquat fruit ripening especially that regarding its ability of autocatalysis of ethylene production during ripening. To this end, we used exogenous ethylene and the ethylene-releasing compound ethephon to examine the effect on the fruit inducing ethylene production and respiration rates. We also studied the time-course of the endogenous content of the plant hormones (abscisic acid, indole-3-acetic acid, gibberellin, and cytokinin) during the stages prior to fruit ripening (703-709 growth stage on the BBCH-scale; Martínez-Calvo and others 1999) and their role on the initiation of the process (growth stage 801 BBCH-scale).
Materials and Methods
Plant Material and Treatments
Experiments were conducted over four consecutive years (2011–2014) in a commercial orchard located in Callosa d’En Sarriá, Alicante, Spain (38°39′N, 00°07′W), and in an experimental orchard at the Universitat Politècnica de València, Spain (39°28′N; 00°22′W). The 20–25-year-old ‘Algerie’ loquat (E. japonica Lindl.) trees were budded onto loquat seedling rootstock, grown in a loamy-clay soil, pH 7.5–8.0, planted 4 × 4 m apart, with drip irrigation (two drippers per tree), and pruned to a vase shape. Fertilization, annual pruning, thinning, as well as pest and disease management were in accordance with commercial practices. The experiments used different trees each year, with no significant differences among years.
To evaluate the effect of ethephon (2-chloroethylphosponic acid, Ethrel® 48 % SL, Bayer Crop Science, Valencia, Spain) on fruit ripening, 200 and 500 mg l−1 were applied at the onset of fruit color change (growth stage 709 on the BBCH-scale), or slightly earlier, to the whole tree with a hand-gun at a pressure of 2.5–3.0 MPa, wetting the tree to the point of run-off. A non-ionic wetting agent (alkyl polyglycol ether) was added at a rate of 0.01 %. A randomized complete-block design with single-tree plots of 6–8 replications each, depending on the year, was performed. At harvest, the number of fruits harvested per tree was recorded, and commercial fruit characteristics were analyzed. This experiment was carried out in Callosa during the years 2011–2013.
In 2014, from early March, when fruit size was about 40 % of its final size (growth stage 704 on the BBCH-scale), to senescent fruit [BBCH stage beyond (+) 809] two fruiting shoots per tree bearing at least three fruits each were randomly selected from 6 control trees. The fruits, attached to the shoot, were enclosed in hermetic 3-l plastic bottles with a 1.5 cm-diameter rubber stopper (septum). After 2–3 h, a triplicate 1 ml air sample was withdrawn with a hypodermic syringe through the septum for ethylene and CO2 analysis. Afterward, the bottle was removed, fruits were aired to allow a free gas exchange with the atmosphere, and fruit color was measured. Three h later, fruits were detached from the shoot and sealed for another 2–3 h at 20 °C in 1.7 l jar, provided with a septum, for ethylene and CO2 production analysis. Hence, ethylene and CO2 production was analyzed from the same fruit attached to the tree and detached from the tree. This experiment was also performed in Callosa.
To evaluate fruit ability of autocatalysis of ethylene production, 200, 500, 750, and 1000 mg l−1 of ethephon were sprayed on the whole tree during ripening, that is, at 709, 801, and 803 phenological growth stage on the BBCH-scale, using the same surfactant at the same concentration as done in the previous experiment. A set of 15 trees were used for each phenological stage, treating three trees per concentration and using the three remaining trees as controls for comparison. Before treatment and 0.5, 1, 2, 4, 8, 24, and 168 h after treatment, 4 fruits per tree were detached from the tree and enclosed in 1.7 l jars for 2 h as above for ethylene production analysis. Besides, ethylene produced by fruits detached 4 h after treatment (when a peak in ethylene production occurred) was also analyzed at 1, 2, 3, 5, 12, and 24 h after detachment.
To evaluate fruit response to exogenous ethylene, a set of 60 fruits from 6 untreated trees were sampled at growth stage 801 and 803 BBCH-scale, delivered to the laboratory, and samples of 20 fruits were incubated in an ethylene-free atmosphere (air) or in one of 10 µl l−1 ethylene or 1 µl l−1 1-methylcyclopropene (1-MCP), an inhibitor of ethylene action, as described by Lafuente and others (2001). Fruits were incubated in 27 l tanks, at 20 °C and 85–90 % RH, in the dark, for up to 7 days. To avoid an excess of respiratory CO2, Ca(OH)2 powder was added to the tanks and fruits were ventilated everyday. On days 0, 1, 3, and 7 of incubation, 5 fruits per treatment were enclosed in jars for ethylene production measurement as described above.
Finally, 10 fruits per tree from 3 untreated trees were sampled from early developmental stage (growth stage 703 of the BBCH-scale) to color change (growth stage 801 of the BBCH-scale), frozen immediately with N2, and stored at −80 °C until gibberellin (GA), indoleacetic acid (IAA), abscisic acid (ABA), and cytokinins (CK) dihydrozeatin (DZ), N6-(∆2-isopentenyl)adenine (iP), and trans-zeatin (tZ) content analysis. These last three experiments were performed in 2014 in Valencia orchard.
Harvest and Fruit Characteristic Analyses
Fruits were harvested in accordance with conventional commercial color and size standards. The number of fruits per tree was recorded on each harvest date, and results were recorded as the percentage of fruits harvested on the first picking date. At harvest, 20 fruits per treatment were sampled at random, from all around the canopy at 1.5 m above ground level to evaluate fruit characteristics. Fruit firmness was assessed using a fruit pressure tester FT-011 (Facchini, Italy) with a 1.5 mm-diameter flat cylinder probe. Total soluble solid (TSS) concentration of juice (°Brix) was assessed with a digital refractometer (Atago, Japan), and free acidity was analyzed by titration with 0.1 N NaOH.
Fruit color was evaluated by determining the a and b Hunter co-ordinates; three measurements were made per fruit at the equatorial area using a Minolta Chroma Meter CR-300 (Tokyo, Japan).
Ethylene and CO2 Analyses
For ethylene production, a 1 ml gas sample was withdrawn from the headspace of the container and injected into a Trace™ Ultra Gas Chromatograph (ThermoFisher Scientific Inc., Waltham, MA, USA) equipped with a 2 m × 1.8 mm alumina column and a flame ionization detector. Nitrogen was used as carrier gas at a flow rate of 30 ml min−1, and the column temperature was maintained at 140 °C.
Carbon dioxide concentration inside the containers was determined in a Trace™ Ultra Gas Chromatograph (ThermoFisher Scientific Inc., Waltham, MA, USA) equipped with a Carbowax column and a thermal conductivity detector. Temperature was maintained at 60 °C. The carrier gas was helium at a flow rate of 45 ml min−1.
In all cases, gas production values represent the mean of three replicates.
Plant Hormones Analysis
Frozen material was lyophilized and ground into fine powder. Aliquots (about 50 mg dry matter-DW) of ground material were extracted with 80 % methanol containing 1 % acetic acid. Internal standards were added and mixed with the aliquots at 4 °C for 1 h. Deuterium-labeled hormones were used as internal standards for plant hormone quantification.
The extraction protocol was carried out according to Seo and others (2011) with some modifications. In brief, for desaltation, the extracts were passed through reverse phase columns HLB (Waters Cromatografía, S.A., Barcelona, Spain). The plant hormones were eluted by 80 % methanol containing 1 % acetic acid and consecutively applied to cation exchange MCX columns (Waters Cromatografía, S.A., Barcelona, Spain). The fraction containing the acidic ABA, GAs, and IAA hormones was applied through the ion exchange WAX columns (Waters Cromatografía, S.A., Barcelona, Spain). The final residue was dissolved in 5 % acetonitrile-1 % acetic acid, and the hormones were separated using an autosampler and reverse phase UPHL chromatography (2.6 µm Accucore RP-MS column, 50 mm length × 2.1 mm i.d.; ThermoFisher Scientific Inc., Waltham, MA USA) with a 5–50 % acetonitrile gradient containing 0.05 % acetic acid, at 400 µl min−1 during 14 min. To obtain the basic fraction containing cytokinins, the MCX cartridge was eluted with 60 % methanol–5 % NH4OH, and the final eluate was dried and dissolved in 5 % acetonitrile–1 % acetic acid. Cytokinins were separated using an autosampler and reverse phase UPHL chromatography (2.6 µm Accucore RP-MS column, 50 mm length × 2.1 mm i.d.; ThermoFisher Scientific Inc., Waltham, MA USA) with a 5–50 % acetonitrile gradient during 7 min.
The hormones were analyzed with a Q-Exactive mass spectrometer (Orbitrap detector; ThermoFisher Scientific Inc., Waltham, MA, USA) by targeted Selected Ion Monitoring (SIM). The concentrations of hormones in the extracts were determined using embedded calibration curves and the Xcalibur 2.2 SP1 build 48 and TraceFinder programs.
Statistical Analysis
Analysis of variance was performed on the data, using the Student–Newman–Keuls’ multi-range test for means separation. Percentages were analyzed after arc-sine transformation.
Results
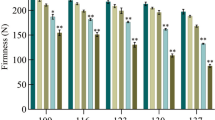
Ethephon applied at 200 mg l−1 just prior to fruit color break (growth stage 709 on the BBCH-scale) slightly advanced color development. However, neither the color of the fruit skin at harvest nor the percentage of fruit harvested at the first picking date (early May) differed significantly with regard to untreated control fruit in the 3 years of the experiment (Table 1). Flesh firmness, TSS concentration, and titrable acidity of juice were not significantly affected by the treatment either (Table 1). A higher concentration of ethephon, 500 mg l−1 in our experiments, did not influence the response, nor did earlier or later applications (data not shown).
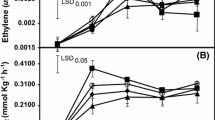
The time-course of ethylene and CO2 production was not modified by ethephon. In control and 200 mg l−1 treated fruit, a well-defined peak of ethylene, similar in amount, was found when fruit changed color (801 BBCH-scale) (Fig. 1a). It is worth noting that the very low ethylene production (<3 nl g−1 h−1) found for ‘Algerie’ loquat. The respiration rate for both control and treated fruit was irregular during ripening, and coincided in time and amount of CO2 production (Fig. 1b). In both treatments, the maximum CO2 production was associated with that of ethylene production.
Effect of ethephon (200 mg l−1) applied before fruit changed color (5 April; growth stage 707 BBCH-scale) on the time-course of ethylene production rate (a) and respiration rate (b) of ‘Algerie’ loquat fruit. Each value is the average ± SE of 6 fruits per tree and 6 trees. In some cases, SE is smaller than symbol size. Results are for 2012
These results reveal a transient effect of both natural and ethephon-induced ethylene production, as shown by the study of hourly ethylene production during 0.5-168 h after treatment by fruit treated with ethephon at color change (growth stage 801 on the BBCH-scale). The fruit response to treatment was immediate, and 30 min after the application of 500 mg l−1 ethephon fruit produced significantly higher amounts of ethylene (3.1 nl g−1 h−1) than the untreated fruit (2.0 nl g−1 h−1), the difference increasing over time, and reaching a maximum 4 h after treatment (4.7 and 2.3 nl g−1 h−1, respectively) (Fig. 2). But 24 h after treatment, values from treated and control fruit were similar (2.2 and 1.9 nl g−1 h−1, respectively) (Fig. 2), and remained almost constant to at least 168 h after treatment (2.1 and 1.8 nl g−1 h−1, respectively), when the experiment was stopped. Furthermore, accumulated ethylene production declined during the 24 h after harvest from 4.7 to 1.2 nl g−1 h−1 in treated fruit collected 4 h after treatment (r = −0.989) and from 2.3 to 0.9 nl g−1 h−1 in untreated fruit (r = −0.991) (Fig. 3). This kind of response was not dependent on the phenological growth stage at treatment (709 to 803 BBCH-scale) or the concentration applied from 200 to 1000 mg l−1 (data not shown). Values from control fruit did not differ statistically over time.
Ethylene production rate by ‘Algerie’ loquat fruit during the 24 h after treatment with the ethylene-releasing compound ethephon (500 mg l−1) applied at the onset of fruit color change (growth stage 801 BBCH-scale). Ethephon was applied to entire tree at 8:00 a.m. (02 May) and fruits for ethylene production analysis were collected just prior to treatment, and at 0.5, 1, 2, 4, 8, and 24 h after treatment. Each value is the average ± SE of 4 fruits per tree and 3 trees. In some cases, SE is smaller than symbol size. Results are for 2014
Accumulated ethylene production by ‘Algerie’ loquat fruit treated with ethephon (500 mg l−1) for 24 h after collected. Treatment was applied to the whole tree at the onset of fruit color change (growth stage 801 BBCH-scale), at 8:00 a.m. (02 May), collected 4 h after treatment, and analyzed at 1, 2, 3, 5, 10, and 24 h after collection without removing the fruits from the 1.7 l jar used. Each value is the average ± SE of 4 fruits per tree and 3 trees symbols for treatments as in Fig. 2. In all cases, SE is smaller than symbol size. Results are for 2014
The rate of ethylene production showed a similar trend in both on-tree and detached fruit (Fig. 4a), peaking (1.6 and 1.4 nl g−1 h−1, respectively) at color break (12 April; 801 BBCH-scale) (Fig. 4b). However, the respiration rate differed significantly between on-tree and detached fruit (Fig. 4c). On-tree CO2 production increased until 12 April, coinciding with the peak in ethylene production, remaining almost constant until fruit senescence. In detached fruit, a higher and irregular amount of CO2 was produced throughout the period studied, ranging 71–41 µl g−1 h−1 (Fig. 4c), with a slight increase also coinciding with the peak in ethylene production (12 April; 801 BBCH-scale), and similar to that found in the previous experiment (see Fig. 1b). Interestingly, a sharp increase in ABA content took place during ripening, peaking after fruit color break, that is, 12 days after the peak in ethylene production (807 BBCH-scale) (Fig. 4a).
Time-course of ethylene production and ABA (a), fruit coloration (a/b Hunter co-ordinates ratio) (b), and respiration rate (c) of on-tree and detached-tree ‘Algerie’ loquat fruit. ABA values are for detached fruit. Each value is the average ± SE of 3 fruits, at least, from 2 fruiting shoots per tree and 6 trees. In some cases, SE is smaller than symbol size. BBCH phenological growth stages are given. Results are for 2014
As expected, applying ethylene (10 µl l−1) during ripening to fruit detached from the tree did not increase endogenous ethylene production with regard to control (air-treated) fruit measured 3 days after treatment and up to 1 week later, regardless the phenological fruit growth stage at treatment (801 or 803 BBCH-scale) (Table 2). A pre-treatment with the ethylene action inhibitor 1-MCP (1 µl l−1) at color break (stage 801 BBCH-scale) did not modify ethylene production either, but when applied at early ripening (stage 803 BBCH-scale) ethylene production of treated fruit 1 day after treatment was significantly higher (4.7-fold) than that of untreated fruit. This difference lessened over time, but lasted for at least 7 days after treatment (Table 2).
To elucidate the potential involvement of plant growth regulators other than ethylene in the ripening process of loquat fruit, the endogenous concentrations of IAA and CK as well as GA-biosynthetic and catabolic intermediates during fruit growth and ripening were also determined. Indole-3-acetic acid concentration remained almost constant during fruit development until stage 706 on the BBCH-scale (56–64 ng g−1 DW) increasing rapidly afterward up to 156 ng g−1 DW when fruit stopped growing (709 BBCH-scale), and declining to values similar to those during the active fruit growth period (65 ng g−1 DW) at fruit color change (801 BBCH-scale) (Fig. 5a).
Time-course of the indoleacetic acid (a) and the bioactive cytokinin trans-zeatin (tZ; b), N6-(Δ2-isopentenyl)adenine (iP; c), and dihydrozeatin (DZ; d) concentration during ‘Algerie’ loquat fruit development. Values are averages ± SE (n = 3). In some cases, SE is smaller than symbol size. BBCH phenological growth stages are given. Results are for 2014
Cytokinin concentrations dropped during the active fruit growth period. Particularly noteworthy was that of tZ (Fig. 5b), the most abundant CK in loquat fruit, which decreased from 3.80 to 0.42 µg g−1 DW (703 to 705 BBCH-scale), remaining almost constant until fruit stopped growing (709 BBCH-scale) (Fig. 5b). At color change (801 BBCH-scale), tZ fruit content was almost undetectable. iP and DZ showed a trend similar to tZ, although with a much lower concentration (Fig. 5c, d).
The values for the earliest precursor of bioactive gibberellins, GA12, declined dramatically during the fruit growth period, from 14 ng g−1 DW at fruit growth stage 703 of the BBCH-scale to 0.4 ng g−1 DW at growth stage 705 BBCH-scale, remaining close to zero until color break (Fig. 6). The subsequent GA-intermediates in the non-hydroxilated pathway, GA15 and GA24, showed a pattern similar to that of the downstream bioactive GA4, while the GA15 concentration was considerably lower. The precursor GA9, however, failed to show the same declining profile, as it decreased slightly from 8.7 ng g−1 DW (growth stage 703 BBCH-scale) to 7.5 ng g−1 DW (706 BBCH-scale), and then dropped sharply until fruit color break (<0.05 ng g−1 DW, 801 BBCH-scale) (Fig. 6). The concentrations of the GA51- and GA34-catabolite intermediates were below the detection level. The GA-intermediates in the 13-hydroxilation pathway, GA53 and GA44, also declined sharply from fruit growth stage 703 to 705 BBCH-scale, followed by an almost constant value, close to zero, until fruit changed color (Fig. 6). The values of GA19 were high during the entire period of fruit growth, ranging 2.8–3.6 ng g−1 DW from growth stages 703 to 709 on the BBCH-scale (Fig. 6), declining sharply afterward to 0.8 ng g−1 DW at fruit color break (growth stage 801 BBCH-scale), whereas GA20 (the precursor of GA1) declined continuously during the period studied (Fig. 6). The bioactive GA1 slightly reduced content from growth stage 703 (3.2 ng g−1 DW) to 705 BBCH-scale (2.8 ng g−1 DW), dropping sharply 1 week later (1.5 ng g−1 DW), and declining somewhat again until fruit stopped growing (1.1 ng g−1 DW) and changed color (0.7 ng g−1 DW) (Fig. 6). GA-catabolic intermediates showed similar endogenous concentration profiles, although differing in their concentrations, being higher for GA29. Both GA29 and GA8 declined dramatically from 703 to 706 BBCH-scale, the former from 1.8 to 0.8 ng g−1 DW, the latter from 4.2 to 0.0 ng g−1 DW, and remaining almost constant up to fruit color change (Fig. 6).
Time-course of the concentration of metabolites in the gibberellin metabolic non-hydroxilation (left) and 13-hydroxilation (right) pathway during ‘Algerie’ loquat fruit development. Values are averages ± SE (n = 3). In some cases, SE is smaller than symbol size. BBCH phenological growth stages are given. Results are for 2014
To study the role of active GA catabolism, the accumulation rates were calculated for the GA1 and its precursors and catabolites, based on their concentrations, during the active fruit growth period and at the onset of ripening. Rates of precursors GA19 and GA20 became negative as fruit completed growth and changed color. The bioactive GA1 showed the same pattern (Table 3). However, GA-catabolic intermediates revealed an opposite trend, remaining close to zero but positive (≤0.005 ng g−1 DW day−1) for GA29 and being nil for GA8.
Discussion
In loquat, there is some controversy concerning the climacteric or non-climacteric behavior of fruit at ripening, and despite having been considered as a non-climacteric fruit, there are physiological (Amorós and others 2003) and molecular evidence (Jiang and others 2012) suggesting a climacteric-like ripening behavior of some cultivars. This research contributes to our knowledge on this subject showing that ‘Algerie’ loquat fruit lacks the ability of autocatalysis of ethylene production, which is an essential feature characterizing climacteric fruit.
Climacteric fruit differs from non-climacteric fruit in its increasing respiration and ethylene biosynthesis rates during ripening (Brady 1987; Lelievre and others 1997). In climacteric fruit, ethylene triggers the onset of ripening, and as a consequence there is a massive increase in ethylene production (Peacock 1972), that is, its action is continuously required for the progress of the ripening processes. Moreover, exposing the fruit to ethylene triggers endogenous ethylene biosynthesis and the autocatalytic ethylene production is stimulated (Yang 1981). Some of these aspects have been observed in loquat ripening behavior, but others have not. For example, the peak in ethylene production prior to color break reported for some loquat cultivars (Amorós and others 2003) is not consistent with an increase in respiration rate (Zheng and others 1993; Ding and others 1998; Amorós and others 2003; González and others 2003; Undurraga and others 2011). Our results agree with those reported previously, both for ethylene production and respiration rate, even after applying ethephon.
The release of ethylene produced by ethephon when applied to ‘Algerie’ loquat just prior to color break did not induce an over-production of endogenous ethylene by the fruit, as expected for a climacteric fruit (McMurchie and others 1972; Downs and others 1991; Yamane and others 2007). Moreover, the patterns of ethylene production and respiration rate in ethephon-treated were similar to those of untreated fruit, suggesting that ethylene released by ethephon might be a transient effect incapable of triggering the autocatalytic biosynthesis of ethylene and, thus, the ripening process. In fact, the application of ethephon at the time of peak of ethylene, i.e., growth stage 801 on the BBCH-scale, increased transiently ethylene production, with a maximum being reached after 4 h and declining gradually to 24 h, revealing that ethephon reached the pulp and releases ethylene, but fruit fails to induce ethylene production, as non-climacteric fruit does. As for ethylene (Lurie and Klein 1989), the upsurge in respiration rates after applying ethephon seems a transient event as well. Accordingly, it may be argued that other ethylene-independent regulatory factors, probably upstream of ethylene, may be responsible for the control of ripening (Leng and others 2014).
The over-production of ethylene by fruit treated with 1-MCP at early ripening reinforces the non-climacteric ripening behavior of ‘Algerie’ loquat. Sisler and others (1996) showed that 1-MCP binds irreversibly to ethylene receptors and, thus, inhibits many ripening-related events such as fruit firmness, color change, aroma, etc., and even blocks the normal autocatalytic rise in ethylene production of climacteric fruits (Sisler and Blankenship, 1993) by down-regulating both ACC synthase and ACC oxidase genes (Grierson 2014). But for fruits in which ethylene inhibits its own production, such as Citrus fruits, 1-MCP eliminates the negative feedback control of ethylene biosynthesis and it is over-induced (Mullins and others 2000; Lado and others 2015). Our results for loquat agree with this, and fruit treated with 1-MCP during ripening over-produced ethylene, whereas those treated with ethylene did not, which further reinforces the hypothesis of an auto-inhibition of ethylene production by loquat, the opposite of that expected for climacteric fruit.
Nevertheless, in our experiments, this transient production of ethylene by applying ethephon advanced loquat fruit coloration slightly, but not significantly, suggesting that ethylene may play a role at specific stages of ripening, and this effect is shared with non-climacteric fruits, such as Citrus fruits (Purvis and Barmore 1981). This effect, which is common to most climacteric and non-climacteric fruits, has been interpreted by Giovannoni (2001) as an additional regulatory strength of climacteric fruit maturation in addition to general ethylene biosynthesis and signaling. Hence, some ripening regulatory genes may be operating separately from and in addition to ethylene, representing regulatory mechanisms common to both climacteric and non-climacteric fruit species (Giovannoni 2004). In accordance, Undurraga and others (2011) found that cellulose, pectinmethylesterase, and polygalacturonase activities did not change during ripening (from color change onward) in loquat fruit cv. Golden Nugget suggesting that the transient production of ethylene did not affect enzyme activities. This last finding agrees with our results for which ethephon did not alter flesh firmness in loquat fruit ‘Algerie’.
ABA content follows the increase in ethylene production, when its concentration decreases. The involvement of ABA in the induction of ethylene production and other ripening processes has been inferred from some climacteric fruit such as apple, peach, or persimmon, for which a sharp increase in ABA accumulation precedes ethylene production and its inhibition delays color change and fruit firmness (Leng and others 2014). By contrast, in non-climacteric fruit ABA deficiency may cause a delay in different ripening-related processes (Rodrigo and others 2003). Then, the involvement of ABA in the ripening of loquat is not clear, as it may be the result and not the cause of the process since ABA is the last product in the carotenoid biosynthetic pathway, and may accumulate as carotenoid concentration increase paralleling fruit coloration. It is also likely that in loquat ethylene may induce ABA accumulation, as in the non-climacteric fruit sweet orange (Rodrigo and others 2006), explaining the different timings in the peak of each hormone. Hence, the role of ABA in the ripening process of loquat fruit, as for non-climacteric Citrus fruits, is not well understood and there is no evidence as to whether the ABA triggers the process or simply parallels color development (Zhang and others 2009; Gambetta and others 2014).
Other plant hormones besides ethylene are involved in the regulation of fruit ripening. For example, in sweet orange, a non-climacteric fruit, fruit changes color by reducing active GA concentrations in the exocarp (Gambetta and others 2012), and a sudden decline in GA-like activity coincides with fruit ripening in Satsuma mandarin (Kuraoka and others 1977; García-Luis and others 1985), indicating that the presence of GA in the exocarp prevents fruit coloration, and may explain why the application of gibberellic acid delays the process in Citrus sp. (El-Otmani and others 2000). When applied prior to color break gibberellic acid also delays ripening and increases flesh firmness of persimmon (Ben-Arie and others 1986), mango (Khader 1991), apple (Looney and others 1992), and peach (Southwick and others 1995) among other fruits.
In our experiments, the time-course of GA content in developing loquat fruit ‘Algerie’ shows, an average, a continuous decline until color change for both non-hydroxylation and 13-hydroxylation pathway. This result agrees to that reported for sweet orange (Gambetta and others 2012), and suggests that such a reduction is required for the fruit to change color. The remarkable decline in the bioactive GA1 during fruit growth and at color break showed a pattern similar to that of GA-intermediate GA53, GA44, and GA20, indicating that these early biosynthesis steps regulate the GA1 levels. The different GA19-intermediate accumulation profiles suggest that this is subjected to turnover by enzymatic activity in the bioactive GA1, and the accumulation of GA29 together with the dramatic decrease in GA8 when levels of GA1 are declining leads us to examine in more detail the role of GA catabolism in the time-course of GA1 at the onset of fruit color change. The study of the active GA catabolism and the bioactive GA1 accumulation rate shows (i) accumulation rate of GA1 became significantly negative at the end of the fruit growth and at fruit ripening, (ii) the accumulation rate of the precursor GA20 showed a pattern similar to the downstream GA1, suggesting that this is an active precursor not subjected to turnover in the bioactive GA1, (iii) the sharp decline of GA8 and the relative constant level of GA29 during growth stage 709–801 BBCH-scale suggest that catabolism does not play a role in maintaining GA1 levels, and that during fruit ripening, once the cell stops growing and no dilution by cell growth occurs, active export of GA1 takes place rather than catabolic activity, in accordance with results reported for fruit color change in other non-climacteric fruits (Gambetta and others 2012).
Active cytokinin, mainly tZ, and in a lower proportion, iP (DH content is extremely low), showed a similar declining profile during fruit growth and ripening. However, iP content declined close to undetectable levels from growth stage 705 BBCH-scale onward indicating that its activity may be likely constitutive, and probably plays a minor role in ripening. tZ completely declined to be almost nil when fruit stopped growing (709 BBCH-scale) suggesting that it could be involved in the process. Kinetin applied prior to fruit color break significantly delayed coloration in loquat (Lou and others 2012).
The delay in fruit color development by gibberellin and cytokinin is due to the delay of chlorophyll degradation and the carotenoid biosynthesis (Agustí and others 1981; Gross and others 1984; Trebitsch and others 1993) thus maintaining temporarily the tissues in a relative juvenile stage. Actually, GA regulates chloroplast division and grana stacking through DELLA repressors (Jiang and others 2012). Besides, many physiological and biochemical responses to ethylene can be counteracted by gibberellin and cytokinin (Goldschmidt and others 1977) by reducing the tissue’s sensitivity to ethylene (Ben-Arie and others 1989). Therefore, a decline in gibberellin and cytokinin concentrations in the fruit to very low values prior to the ripening process seems to be necessary for the fruit to change color. In this way, some kind of balance between ethylene production and the reduction of gibberellin and cytokinin concentrations might be responsible for the ripening process in loquat.
The increase in IAA concentration during fruit growth (from stage 703 to 709 BBCH-scale) is in accordance with its role promoting fruit cell elongation (Rayle and Cleland 1972). The decline prior to color break agrees with low auxin concentration required for the initiation of ripening (Manning 1993), which is consistent with the effect of auxin inhibiting the expression of ripening-related genes (Manning 1994). It has been proposed that timing for the onset of ripening might be modulated by changes in auxin biosynthesis and activity (Paul and others 2012).
In conclusion, ‘Algerie’ loquat fruit has a limited ripening-related response to exogenous ethylene since it lacks a ripening-associated autocatalytic rise in ethylene production, reinforcing the concept that its ripening may be classified as non-climacteric. Nevertheless, small amounts of endogenous ethylene production seem to be required to trigger the ripening process. Besides, the results suggest that a decline in gibberellin, cytokinin, and IAA concentrations might be needed prior to allow the ripening process to proceed.
References
Agustí M, Guardiola JL, Almela V (1981) The regulation of fruit cropping in mandarins through the use of growth regulators. Proc Int Soc Citric 1:216–220
Amorós A, Zapata P, Pretel MT, Botella MA, Serrano M (2003) Physicochemical and physiological changes during fruit development and ripening of five loquat (Eriobotrya japonica Lindl.) cultivars. Food Sci Technol Int 9:43–51
Ben-Arie R, Bazak H, Blumenfeld A (1986) Gibberellin delays harvest and prolongs life of persimmon fruits. Acta Hortic 179:807–813
Ben-Arie R, Roisman Y, Zuthi Y, Blumenfeld A (1989) Gibberelllic acid reduces sensitivity of persimmon fruits to ethylene. In: Clijsters H, De Proft M, Marcelle R, Van Poucke M (eds) Biochemical and physiological aspects of ethylene production in lower and higher plants advances in agricultural technologies, vol 26. Springer, Netherlands, pp 165–171
Blumenfeld A (1980) Fruit growth of loquat. J Am Soc Hortic Sci 105:747–750
Brady CJ (1987) Fruit ripening. Ann Rev Plant Physiol 38:155–178
Cai C, Xu CJ, Li X, Ferguson I, Chen KS (2006) Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharv Biol Technol 40:163–169
Cherian S, Figueroa CR, Nair H (2014) ‘Movers and shakers’ in the regulation of fruit ripening: a cross-dissection of climacteric versus non-climacteric fruit. J Exp Bot 65:4705–4722
Ding CK, Chachin K, Ueda Y, Mochioka R (1998) Changes in polyphenol concentrations and polyphenol oxidase activity of loquat (Eriobotrya japonica Lindl.) fruit in relation to browning. J Jpn Soc Hortic Sci 676:360–366
Downs CG, Brady CJ, Campbell J, McGlasson WB (1991) Normal ripening cultivars of Pyrus serotina are either climacteric or non-climacteric. Sci Hortic 48:213–221
El-Otmani M, Coggins CW, Agustí M, Lovatt CJ (2000) Plant growth regulators in citriculture: world current uses. Crit Rev Plant Sci 19:395–447
Gambetta G, Martínez-Fuentes A, Betancour O, Mesejo C, Reig C, Gravina A, Agustí M (2012) Hormonal and nutritional changes in the flavedo regulating rind color development in sweet orange [Citrus sinensis (L.) Osb.]. J Plant Growth Regul 31:273–282
Gambetta G, Mesejo C, Martínez-Fuentes A, Reig C, Gravina A, Agustí M (2014) Gibberellic acid and norflurazon affecting the time-course of flavedo pigment and abscisic acid content in ‘Valencia’ sweet orange. Sci Hortic 180:94–101
García-Luis A, Agustí M, Almela V, Romero E, Guardiola JL (1985) Effect of gibberellic acid on ripening and peel puffing in Satsuma mandarin. Sci Hortic 27:75–86
Gariglio N, Juan M, Castillo A, Almela Agustí M (2002) Histological and physiological study of purple spot of loquat fruit. Sci Hortic 92:225–263
Giovannoni JJ (2001) Molecular biology of fruit maturation and ripening. Ann Rev Plant Physiol Mol Biol 52:725–749
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16:S170–S180
Given NK, Venis MA, Grierson D (1988) Hormonal-regulation of ripening in the strawberry, a non-climacteric fruit. Plant 174:402–406
Goldschmidt EE, Aharoni Y, Eilati SK, Riov J, Monselise SP (1977) Differential counteraction of ethylene effects by gibberellin A3 and N6 benzyladenine in senescing citrus peel. Plant Physiol 59:193–195
González L, Lafuente MT, Zacarías L (2003) Maturation of loquat fruit (Eriobotrya japonica Lindl.) under Spanish growing condition and its postharvest performance. Options Mediterr 58:171–179
Grierson D (2014) Ethylene biosynthesis. In: Nath P, Bouzayen M, Matoo AK, Pech JC (eds) Fruit ripening: physiology, signaling and genomics. CAB International, Wallinford, pp 178–192
Gross J, Bazak H, Blumenfeld A, Ben-Arie R (1984) Changes in chlorophyll and carotenoid pigments in the peel of ‘Triumph’ persimmon (Diospyros kaki L.) induced by pre-harvest gibberellin (GA3) treatment. Sci Hortic 24:305–314
Hirai M (1980) Sugar accumulation and development of loquat fruit. J Jpn Soc Hortic Sci 49:347–353
Jiang TM, Wang P, Yin XR, Zhang B, Xu CJ, Li X, Chen KS (2011) Ethylene biosynthesis and expression of related genes in loquat fruit at different developmental and ripening stages. Sci Hortic 130:452–458
Jiang X, Li H, Wang T, Peng C, Wang H, Wu H, Wang X (2012) Gibberellin indirectly promotes chloroplast biogenesis as a means to maintain the chloroplast population of expanded cells. Plant J 72:768–780
Jones B, Frasse P, Olmose Zegzouti H, Li ZG, Latche A, Pech JC, Bouzayen M (2002) Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J 32:603–613
Kader AA (2002) Biology and technology: an overview. In: Kader AA (ed) Postharvest technology and horticultural crops. University of California. Agriculture and Natural Resources, Publication 3311, pp 39–48
Khader SESA (1991) Effect of preharvest application of GA3 on postharvest behaviour of mango fruits. Sci Hortic 47:317–321
Kumar R, Khurana A, Sharma AK (2014) Role of plant hormones and their interplay in development and ripening of fleshy fruits. J Exp Bot 65:4561–4575
Kuraoka T, Iwasaki K, Ishii T (1977) Effects of GA3 on puffing and levels of GA3-like substances and ABA in the peel of Satsuma mandarin (Citrus unshiu Marc.). J Am Soc Hortic Sci 102:651–654
Lado J, Rodrigo MJ, Zacarías L (2015) Analysis of ethylene biosynthesis and perception during postharvest cold storage of Marsh and Star Ruby grapefruits. Food Sci Technol Int. doi:10.1177/1082013214553810
Lafuente MT, Zacarías L, Martínez-Téllez MA, Sánchez-Ballesta MT, Dupille E (2001) Phenylalanine ammonia-lyase as related to ethylene in the development of chilling symptoms during cold storage of citrus fruits. J Agric Food Chem 49:6020–6025
Lelievre JM, Latche A, Jones B, Bouzayen M, Pech JC (1997) Ethylene and fruit ripening. Physiol Plant 101:727–739
Leng P, Yuan B, Guo Y, Chen P (2014) The role of abscisic acid in fruit ripening and responses to abiotic stress. J Exp Bot 65:4577–4588
Looney NE, Granger RL, Chu CL, McArtney SJ, Mander LN, Pharis RP (1992) Influences of gibberellins A4, A4+7, and A4 +iso-A7 on apple fruit quality and tree productivity. I. Effects on fruit russet and tree yield components. J Hortic Sci 67:613–618
Lou H, Chen P, Sheng H, Xu C, Lu H (2012) Effect of kinetin on quality and harvest date of loquat fruit. Afr J Agric Res 7:1577–1583
Lurie S, Klein JD (1989) Cyanide metabolism in relation to ethylene production in climacteric and non-climacteric fruits. J Plant Physiol 135:518–521
Manning K (1993) Soft Fruit. In: Seymour GB, Taylor JE, Tucker GA (eds) Biochemistry of fruit ripening. Chapman & Hall, London, pp 347–378
Manning K (1994) Changes in gene expression during strawberry fruit ripening and their regulation by auxin. Planta 194:62–68
Martínez-Calvo J, Badenes ML, Llácer G, Bleiholder H, Hack H, Meier U (1999) Phenological growth stages of loquat tree (Eriobotrya japonica (Thunb) Lindl.). Ann App Biol 134:353–357
Martínez-Romero D, Valero D, Serrano M, Burló F, Carbonell A, Burgos L, Riquelme F (2000) Exogenous polyamines and gibberellic acid effects on peach (Prunus persica L.) storability improvement. J Food Sci 65:288–294
McAtee P, Karim S, Schaffer R, David K (2013) A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci 4:1–7
McMurchie EJ, McGlasson WB, Eaks IL (1972) Treatments of fruit with propylene gives information about the biogenesis of ethylene. Nature 237:235–236
Mullins ED, McCollum TG, McDonald RE (2000) Consequences on ethylene metabolism of inactivating the ethylene receptor sites in diseased non-climacteric fruit. Posharvest Biol Technol 19:155–164
Pareek S, Benkeblia N, Janick J, Cao S, Yahia EM (2014) Postharvest physiology and technology of loquat (Eriobotrya japonica Lindl.) fruit. J Sci Food Agric 94:1495–1504
Paul V, Pandey R, Srivastava GC (2012) The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—an everview. J Food Sci Technol 49:1–21
Peacock BC (1972) Role of ethylene in the initiation of fruit ripening. Queensl J Agric Anim Sci 29:137–145
Purvis AC, Barmore CR (1981) Involvement of ethylene in chlorophyll degradation in peel of citrus fruits. Plant Physiol 68:854–856
Rayle DL, Cleland R (1972) The in vitro acid-growth response: relation to in vivo growth responses and auxin action. Planta 104:282–296
Reig C, Martínez-Fuentes A, Juan M, Gariglio N, Marti G, Mesejo, Agustí M (2007) Técnicas para anticipar la recolección del fruto del níspero japonés (Eriobotrya japonica Lindl.). XI Cong. Nal. SECH, Abstract 4D01
Richmond AE, Lang A (1957) Effect of kinetin on protein content and survival of detached Xanthium leaves. Science 125:650–651
Rodrigo MJ, Marcos JF, Alferez F, Mallent MD, Zacarías L (2003) Characterization of pinalate, a novel Citrus sinensis mutant with a fruit specific alteration that results in yellow pigmentation and decreased ABA content. J Exp Bot 54:727–738
Rodrigo MJ, Alquezar B, Zacarias L (2006) Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J Exp Bot 57:633–643
Schaffer RJ, Ireland HS, Ross JJ, Ling TJ, David KM (2013) SEPALLATA1/2-suppressed mature apples have low ethylene, high auxin and reduced transcription of ripening-related genes. AoB PLANTS 5:pls47. doi:10.1093/aobpla/pls047
Seo M, Jikumaru Y, Kamiya Y (2011) Profiling of hormones and related metabolites in seed dormancy and germination studies. Methods Mol Biol 773:99–111
Singh R, Singh P, Pathak N, Singh VK, Dwivedi UN (2007) Modulation of mango ripening by chemicals: physiological and biochemical aspects. Plant Growth Regul 53:137–145
Sisler EC, Blankenship SM (1993) Diazocyclopentadiene, a light sensitive reagent for the ethylene receptor. Plant Growth Regul 12:125–132
Sisler EC, Serek M, Dupille E (1996) Comparison of cyclopropene, 1-methylcyclopropene and 3,3-dimethylcyclopropene as ethylene antagonists in plants. Plant Growth Regul 18:169–174
Southwick SM, Weis KG, Yeager JT (1995) Controlling cropping in ‘Loadel’ cling peach using gibberellin: effects on flower density, fruit distribution, fruit firmness, fruit thinning, and yield. J Am Soc Hortic Sci 120:1087–1095
Trebitsch T, Goldschmidt EE, Riov J (1993) Ethylene induces de novo synthesis of chlorophyllase, a chlorophyll degrading enzyme, in Citrus fruit peel. Proc Natl Acad Sci 90:9441–9445
Undurraga P, Olaeta JA (2003) Effect of ethephon (2-chloro ethylphosphonic acid) applied to the trees on fruit ripening in ‘Golden Niugget’ loquat (Eriobotrya japonica Lindl.). Options Mediterr 58:123–128
Undurraga P, Olaeta JA, Cancino C (2011) Ethylene, enzymatic and respiratory patterns evolution in loquat (Eriobotrya japonica (Thumb.) Lindl.) cv. Golden Nugget in the last four sequential stages of maturation. Chil J Agric Res 71:530–535
Yamane M, Abe D, Yasui S, Yokotani N, Kimata W, Ushijima K, Nakano R, Rubo Y, Inaba A (2007) Differential expression of ethylene biosynthetic genes in climacteric and non-climacteric Chinese pear fruit. Postharvest Biol Technol 44:220–227
Yang SF (1981) Biosynthesis of ethylene and its regulation. In: Friend J, Rhodes MJC (eds) Recent advances in the biochemistry of fruit and vegetables. Academic, London, pp 89–106
Yang SF, Hoffman NE (1984) EWthylene biosynthesis and its regulation in higher plants. Ann Rev Plant Physiol 35:155–189
Zaharah SS, Singh Z, Symons GM, Reid JB (2012) Role of brassinosteroids, ethylene, abscisic acid, and infloe-3-acetic acid in mango fruit ripening. J Plant Growth Regul 31:363–372
Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60:1579–1588
Zheng YH, Xi YF, Ying TJ (1993) Studies on postharvest respiration and ethylene production of loquat fruits. Acta Hortic Sin 2:111–115
Acknowledgments
We thank Dr. Isabel López-Díaz and Dr. Esther Carrera, IBMCP-UPV (Valencia, Spain) for hormone quantification, and Dr. Debra Westall (UPV) for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reig, C., Martínez-Fuentes, A., Mesejo, C. et al. Loquat Fruit Lacks a Ripening-Associated Autocatalytic Rise in Ethylene Production. J Plant Growth Regul 35, 232–244 (2016). https://doi.org/10.1007/s00344-015-9528-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-015-9528-3