Abstract
Fruit ripening is a complex physiological and metabolic process regulated by plant hormones. The ripening of climacteric fruits is accompanied by softening, especially ‘Nanguo’ pear. The importance of ethylene in fruit softening is well established; however, an understanding of its effects during the later stages of fruit development requires further investigation. In this study, ethylene was sprayed on ‘Nanguo’ pear fruits before harvest resulting in enhanced fruit quality by increasing the soluble solid and sugar contents while decreasing the stone cell content. Additionally, ethylene promoted the activities of polygalacturonase, pectin methylesterase, cellulase, and β-galactosidase enzymes that play a critical role in cell wall metabolism, by up-regulating PuPG and PuPG2 expression. This leaded to changes in the cell wall structure and breakdown of its components, a reduction of cellulose and original pectin content, and an increase in water-soluble pectin content. These results indicate that ethylene enhances fruit softening by up-regulating the expression of genes involved in cell wall metabolism to facilitate the activity of cell wall degrading enzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ‘Nanguo’ pear (Pyrus ussuriensis Maxim.) is a special fruit in northern China, and its unique flavor is deeply loved by people (Ling et al. 2020). It is a climacteric fruit that requires softening to achieve the best edible quality (Zhenru et al. 2007), and in the process of fruit softening, hormone substances are crucial. The mechanism of plant hormones regulating fruit softening requires further study to shorten the post-ripening time of ‘Nanguo’ pear and accelerate the softening process.
The most significant sign of fruit ripening and softening is soft texture (Andrews et al., 1995). This is primarily caused by changes in cell wall metabolism, such as alterations in cell wall structure and composition, degradation of fruit cell wall components, and modification of enzyme activities related to cell wall degradation. The action of cell wall metabolizing enzymes such as pectin methylesterase (PME), polygalacturonase (PG), cellulase (Cx), and β-galactosidase (β-Gal) causes the degradation of cell wall materials, dissociation of polymers, and decreased intercellular connections. Cell dispersion and softening of the cell wall tissue cause the flesh to soften (Brummell, 2004; Villarreal et al. 2010; Johnston et al., 2015; Tucker et al. 2017). These enzymes have been thoroughly investigated in a variety of fruits, including pear (Akiea et al., 2001; Lindo-Garcia et al. 2020; Kaur et al. 2021; Kaur et al. 2023), strawberry (Wang et al., 2020b), blueberry (Wang et al. 2019), and tomato (Bu et al. 2013). PpePL1/15 silencing inhibits pectin depolymerization to delay peach fruit softening (Xu et al. 2022). SlPG49 overexpression promotes tomato fruit softening, while FaPG1 silencing in strawberry has the opposite effect by inhibiting cell wall degradation, improving fruit firmness, and extending the shelf life of fruit (Posé et al. 2013; Li et al. 2021). Meanwhile, apricot softening is related to PG and PME activity and related gene expression (Li et al. 2022).
Ethylene is required for the ripening and softening of climacteric fruits. This hormone holds paramount significance in modulating the ripening and senescence of climacteric fruits, wielding a pivotal role in regulation of fruit maturation and decline (Xiucai and Jipeng, 2001). Ethylene can regulate fruit ripening and senescence through direct or indirect means. Treating fruit with extremely small amounts of exogenous ethylene can induce fruit respiratory intensity, enhance the peak of respiration, advance the arrival time of ethylene peak, promote the physiological metabolism of fruit after harvest, and accelerate the softening process (Kaur et al. 2021; Li et al. 2022). Some studies have reported that even at low temperature, a small amount of exogenous ethylene can accelerate the ripening process of fruits (Hertog et al. 2016). Ethylene inhibitor (1-Methylcyclopropene, 1-MCP) treatment at the pre-ripe stage during pear ripening markedly retarded the initiation of the ripening-related events (Hiwasa et al. 2010). 1-MCP treatment slowed down the loss of ‘Nanguo’ pear fruit firmness by inhibiting ethylene biosynthesis (ACS4, ACS1 and ACO) and the expression of PG1 and PG2 genes (Li et al. 2014). Exogenous ethylene treatment can also accelerate the softening process of plum (Zhang et al. 2019), kiwi (Yuan et al., 2018), apricot (Fan et al. 2018), and mango (Chidley et al. 2017) fruit. These studies suggest that ethylene and cell wall metabolism are important factors contributing to fruit softening. However, there are limited reports on exogenous ethylene treatment of ‘Nanguo’ pear, and the methods of exogenous ethylene supplementation are generally post-harvest soaking or spraying, with few studies on pre-harvest ethylene spraying.
This study investigated the impact of ethylene on fruit quality and softening of ‘Nanguo’ pear by administering ethylene treatment before harvest (100 days after anthesis). The softening effect of ethylene on ‘Nanguo’ pear fruits was confirmed by analyzing cell wall substances, cell wall enzyme activity, changes in cell wall structure, and the expression of cell wall metabolic genes. The findings from this study establish a theoretical framework for the judicious application of plant growth regulators in the modulation of fruit softening, offering insights for their rational utilization.
Materials and Methods
Plant materials and Treatments
‘Nanguo’ pear trees at Shenyang Agricultural University (123°34′12'' E, 41°49′48" N), China were used as test materials. Ethephon was sprayed 100 days after anthesis at the following concentrations: T1, 100 mg·L−1; T2, 200 mg·L−1; T3, 400 mg·L−1; T4, 600 mg·L−1; CK, Spray water. Ethephon was sprayed on the ‘Nanguo’ pear fruit growing on trees, and each concentration treatment was repeated three trees. The fruit samples were treated again before sampling and sampled at 109, 116, 123, 130, and 137 days after anthesis. Collected 100 samples of each treated fruit, and fruits of uniform size with no visible disease or insect pests were randomly chosen for sampling. Sampled fruit pulp was frozen in liquid nitrogen and stored at -80℃ for further analysis.
Firmness
Three points were selected at the equatorial position of each fruit. After peeling, the fruit firmness was measured in kg·cm−2 using a GY-4 (Tuopuyunong, China) type fruit durometer and the average value for a single fruit was recorded. The firmness measurements were repeated three times.
Soluble Solid, Soluble Sugar, Titratable Acid, and Stone Cell Contents
Soluble solids were determined by removing the skin and squeezing the flesh, a drop of this juice was analyzed using an Abbe PAL-1 refractometer (Atago, Japan). Each treatment was repeated three fruits, and each fruit was measured three times.
The determination of soluble sugar content was conducted employing a modified protocol adapted from Liu et al. (2020) using anthrone for colorimetric detection. The titratable acid content was performed according to a modified version of a previous method (Wang et al. 2021) and was calculated with malic acid (conversion coefficient 0.067). A previously reported method was used to determine the content of stone cells (Lee et al. 2006).
Cell Wall Material Content
The cell wall material content was determined with reference to Vicente et al. (2005). The crude enzyme liquids of PG, PME, Cx, and β-gal were extracted, and their activities were determined according to a modified version of a method described by Wang et al. (2019) and Zhou et al. (2021).
A 1 g homogenate of sample was mixed with 3.0 mL pre-cooled 95% ethanol in a mortar at 4 °C, transferred to a centrifuge tube and placed on ice for 5 min, then centrifuged at 9,728 × g for 15 min at 4 °C. The precipitate was retained and washed by adding 3.0 mL ethanol (80%), incubating at 4 °C for 5 min, and the mixture was then centrifuged. The enzymes present in the precipitate were extracted using 5.0 mL extraction buffer (50 mmol·L−1 sodium acetate, 1.8 mol·L−1 NaCl pH = 5.5), incubated at 4 °C for 10 min, and centrifuged at 9,728 × g for 15 min at 4 °C. The resulting supernatant was the crude enzyme solution.
Enzyme activity: The reaction mixture consisted of 1.0 mL 50 mmol·L−1 aceto-sodium acetate buffer (pH 5.5) and 0.5 mL substrate (PG substrate, 1% polygalacturonic acid; PME substrate, 1% pectin; Cx substrate, 1% carboxymethyl cellulose Na; and β-gal substrate, 1% o-nitrophenyl-β-D-pyran galactoside [ONPG]). One unit (U) of polygalacturonase activity refers to 1 μg of free D-( +) galacturonic acid produced per gram of fresh sample per minute at 37 °C. One unit (U) of pectin methylesterase activity refers to the consumption of 1 mmol NaOH per gram of fresh sample for 10 min at 37 °C. One unit (U) of Cx activity refers to 1 μg D-glucose produced per gram fresh sample per minute at 37 °C via CMC decomposition. One unit (U) of β-galactosidase (β-Gal) activity refers to the hydrolysis of 1 nmol p-nitrophenol per gram of fresh sample per minute at 37 °C.
Microstructure Assessment of Pear Fruit
Paraffin section analysis was conducted following the methodology outlined by Han et al. (2016). The fruit was sliced into 5-mm-thick sections and rapidly immersed in FAA solution (formaldehyde: acetic acid: and 70% alcohol, 1:1:18) for vacuum fixation, dehydration, and paraffin embedding. Paraffin slicing was performed using an RM2016 instrument (Leica Instruments, Shanghai, China), followed by staining with toluidine blue solution and covering with a cover slip. Samples were examined under an Eclipse E100 microscope (Nikon, Japan), and images were analyzed using a DS-U3 system (Nikon, Tokyo, Japan).
RNA Extraction and Gene Expression Analysis
Transcriptome sequencing was performed on control fruit samples and T3-treated fruit samples (Biomarker, Beijing). A p ≤ 0.01 and log2(FC) ≥ 2 was defined as a differentially expressed gene (DEG).
The cetyltrimethylammonium ammonium bromide (CTAB) method was used for total RNA extraction from ethylene-treated and untreated fruit flesh (Jaakola et al. 2001). Subsequently, cDNA synthesis and gene expression analyses were carried out following previously established protocols (Zhang et al. 2019). Quantitative real-time polymerase chain reaction (qRT-PCR) was determined using SYBR Green kit (Takara, Shiga, Japan).
According to the transcriptome data and the website of National Biotechnology Information Center (https://blast.ncbi.nlm.nih.gov/), the sequence information of gene coding regions such as PuPG and PuPG2 was obtained by analysis, and gene-specific expression primers were designed using Primer3 website (https://bioinfo.ut.ee/primer3-0.4.0/). The primer sequences used for gene expression analysis are presented in Table 1.
Statistical Analyses
A completely randomized design was used with three replicates per treatment. GraphPad Prism version 8.0.2 software (San Diego, CA, USA) was utilized to visualize all analyses. Statistical analyses (including determination of the mean, standard deviation, and significant differences between samples) were performed using IBM SPSS Statistics 26 (SPSS Inc., Chicago, IL, USA). The data are displayed as the mean ± standard deviation. To separate and compare means, a separate sample t test was applied. Statistics were judged significant at P < 0.05 or P < 0.01.
Results
Effect of Ethylene Treatment on Pear Firmness
The firmness of fruits gradually decreased during their five stages of development. Ethylene treatment significantly reduced fruit firmness (P < 0.05), with a greater reduction observed with increasing ethylene concentration. Notably, the T4 treatment resulted in a significant reduction in fruit firmness (P < 0.01), with the fruits reaching the edible state 137 days after anthesis. The fruit firmness of the T4 treatment was 26.0% lower than that of the control. Therefore, ethylene decreased fruit firmness and facilitated fruit softening (Fig. 1).
Changes in firmness of ‘Nanguo’ pear fruit submitted to water treatment (control) and ethylene treatment. The error bars show the three biological replicates, and vertical bars represent the standard errors of the means. Asterisks represent values that are significantly different (*P < 0.05 and **P < 0.01) between the ethylene treatment and the control
Effect of Ethylene Treatment on Pear Fruit Quality
The soluble solid content of ‘Nanguo’ pear fruit exhibited a slow increase during the developmental stages, whereas ethylene treatment resulted in a significant increase in soluble solid content compared to that in the control (P < 0.05). Furthermore, an increase in ethylene concentration led to a noticeable increase in soluble solid content in the fruit. In particular, the T4 ethylene treatment caused the highest significant increase (37.5%) in soluble solid content of fruit (P < 0.01) compared with control fruit (Fig. 2A).
Changes in soluble solid content (A), soluble sugar content (B), titratable acid content (C), and stone cell content (D) in ‘Nanguo’ pear fruit submitted to water treatment (control) and ethylene treatment after 109, 116, 123, 130, and 137 d. The error bars show the three biological replicates, and vertical bars represent the standard errors of the means. Asterisks represent values that are significantly different (*P < 0.05 and **P < 0.01) between the ethylene treatment and the control
The soluble fruit sugar content gradually increased, and ethylene treatment significantly increased the soluble sugar content compared to that in the control (P < 0.01). Ethylene treatment significantly increased the soluble sugar content of fruit (P < 0.01), with a greater increase observed with increasing ethylene concentration. Fruits treated with T3 and T4 showed a significant increase in soluble sugar content that remained stable in the later developmental stage. The soluble sugar content of T3 and T4 treated fruits increased by 63.2% and 68.6%, respectively, compared to control fruits at 137 days after anthesis (Fig. 2B). The titratable acid content of fruit exhibited a slow downward trend: T4 treated fruits showed a 17.0% decrease in titratable acid content compared to control fruits at 137 days after anthesis. However, ethylene treatment did not significantly reduce the titratable acid content of ‘Nanguo’ pear fruits (Fig. 2C).
The stone cell content in pear fruit plays a crucial role in determining the fruit’s taste; reducing the stone cell content can improve the pear fruit quality. Ethylene treatment significantly reduced the stone content of fruit, and the reduction was even greater with the increase of ethylene concentration. The T4 ethylene treatment yielded a 30.1% decrease in stone cell content of pear fruits compared with that in the control on day 109 after anthesis. There was little difference in the stone cell content in fruit treated with different ethylene concentrations during the later developmental stage; however, ethylene treatment exhibited a steady trend of reducing the content of stone cells content and enhancing the overall quality of ‘Nanguo’ pear fruits (Fig. 2D).
Effects of Ethylene Treatment on the Contents of Cell Wall Components in ‘Nanguo’ Pear Fruit
Fruit texture gradually softened with fruit development, and the water-soluble pectin content showed an increasing trend, while the raw pectin content gradually decreased (Table 2). The fruit content of water-soluble pectin treated with ethylene significantly increased (P < 0.05) compared with the control, and the water-soluble pectin content of the T3 treatment had the most pronounced increase and was at a consistently higher level that the others. The increased in water-soluble pectin (WSP) content was largest in fruits treated with T4, with an increase of approximately 24.8%. The original pectin content decreased with increased ethylene treatment concentration in the early stage, and showed a fluctuating trend in the late stage. The original pectin content in the T3 and T4 treatment gently decreased compared with those in T1 and T2. The cellulose content showed an overall decreasing trend, and gradually decreased with increasing ethylene concentration, with the T4 treatment resulting in a significant decrease (P < 0.05) compared with the control.
Effect of Ethylene Treatment on the Activity of Cell Wall Degrading Enzymes in ‘Nanguo’ Pear Fruit
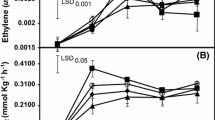
PG activity of control ‘Nanguo’ pear fruits gradually increased and then slowed down in the later stage of development. Meanwhile, PG activity increased in ethylene-treated fruit, but the trend of PG change was unchanged. There was no discernible difference between the ethylene-treated group and the control in the later stage of development (Fig. 3A). The PME activity of ‘Nanguo’ pear fruit increased slowly in the control with the late development of fruit, reaching its maximum activity at 123 days after anthesis before declining. Ethylene treatment promoted PME activity, with levels significantly higher than that of the control (P < 0.05). The changing trend of PME activity was consistent with that of the control, reaching peak activity at approximately 130 days. Activity levels in the T1 and T3 treatments were significantly higher than those in the control group (P < 0.01; Fig. 3B). The cellulase activity of ‘Nanguo’ pear fruit generally showed an upward trend with fruit ripening and softening, with the highest activity observed at 123 days, followed by a gradual decrease (Fig. 3C). Meanwhile, cellulase activity of T3 and T4 treatments was considerably higher than that of the control (P < 0.05). β-Gal activity of control ‘Nanguo’ pear fruit showed a decreasing trend, but the content remained at a high level. After ethylene treatment, its activity was enhanced, although the overall trend was variable. The β-Gal activity of fruits treated with T3 was considerably higher than that of the control (P < 0.05) (Fig. 3D).
Effects of ethylene treatment on activities of PG (A), PME (B), Cx (C), β-gal (D). The error bars show the three biological replicates, and vertical bars represent the standard errors of the means. Asterisks represent values that are significantly different (*P < 0.05 and **P < 0.01) between the ethylene treatment and the control
Effect of Ethylene Treatment on the Cell Wall Structure of ‘Nanguo’ Pear Fruit
Overall, the T3 treatment significantly improves ‘Nanguo’ pear fruit quality at the later stage of development, and promotes fruit softening. This suggests that it can meet marketing standards. Therefore, the T3-treated fruits were selected for comparison with the control to explore the internal effects of ethylene treatment on fruits.
Paraffin section analysis showed that the cell walls of untreated ‘Nanguo’ pear fruit were complete and continuous, with a small and compact shape of fruits. However, ethylene treatment increased the volume of ‘Nanguo’ pear fruit cells, and the cell wall started loosened, the structure was gradually deformed. This suggests that ethylene treatment acts on the cell wall to promote fruit softening (Fig. 4).
Effect of Ethylene Treatment on the Expression of Related Genes in ‘Nanguo’ Pear Fruit Cell Wall
Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis of transcript data identified 1550 differentially expressed genes in 50 pathways, including 36 metabolic pathways, 2 cellular processes, 3 environmental information processing, 8 genetic information processing, and 1 organismal systems. Further study found that the genes related to cell wall degradation were concentrated in pentose and gluconate interconversions metabolic pathways, and 18 different genes were screened. (Fig. 5), respectively, whose substrates were cellulose, hemicellulose, and pectinand. Eight genes were considerably up-regulated, including PuPG (LOC103958214), PuPG2 (LOC103935606), PuPL18 (LOC103950090), and Puβ-Gal (LOC103963535). Among these, PuPG (LOC103958214), PuPG2 (LOC103935606), PuPL18 (LOC103950090), and Puβ-Gal (LOC103963535) were high differentially expressed, indicating these may be the key genes associated with fruit softening.
The expression levels of PuPG, PuPG2, PuPL18, and Puβ-Gal in T3-treated fruits were significantly higher than that in the control according to RT-PCR (Fig. 6), with the expression levels of PuPG2 being the most significantly differentiated. The expression levels of PuPG, PuPG2, PuPL18, and Puβ-Gal were the highest at 137 days after anthesis in T3-treated fruits. T3 treatment inhibited PuPL18 expression and promoted the expression of PuPG, PuPG2, and Puβ-Gal at 123 days. These results suggest that ethylene treatment increases the expression levels of PuPG, PuPG2, PuPL18, and Puβ-Gal, but the action stages are different. Therefore, PuPG and PuPG2 may be the key genes in the regulation of ethylene on fruit softening.
Analysis of differential gene expression levels in ‘Nanguo’ pear fruit treated with ethylene and control. The error bars show the three biological replicates, and vertical bars represent the standard errors of the means. Asterisks represent values that are significantly different (*P < 0.05 and **P < 0.01) between the ethylene treatment and the control
Discussion
Fruit softening is a complex physiological and metabolic process. Climacteric fruits require softening by ethylene to achieve edible quality; however, excessive softening affects the quality of fruits during transportation and storage. In order to shorten the ripening time of climacteric fruits, ethylene treatment to promote fruit softening becomes particularly important. A large number of studies have expounded that ethylene can promote the softening process of fruits from the post-harvest point of view (Chen et al., 2020; Chidley et al. 2017; Yao et al. 2022), and then the application of inhibitor 1-MCP can extend the shelf life and storage time of fruits (Bai et al. 2022; Fan et al. 2018; Fan et al. 2018). This study supplemented the influence of ethylene on the later stage of fruit development from the pre-harvest point of view. According to the variety characteristics of' ‘Nanguo’ pear, exogenous ethylene treatment was applied in the later stage of fruit development, which not only promoted fruit ripening, but also enhanced fruit quality and shortened the post-ripening time, so that fruits could directly enter the market during commercial harvest.
Fruit softening is a prominent aspect of ripening that involves a reduction in firmness, and is primarily attributed to cell wall degradation (Posé et al. 2019). This study suggested that ethylene treatment reduced the firmness of ‘Nanguo’ pear in late development and brought the fruit to an edible state. Similar results were found in other fruits, including blueberries (Wang et al. 2019), mangoes (Li et al. 2022), and pulp (Soares et al. 2021). Numerous studies have shown that exogenous ethylene treatment can increase the endogenous ethylene content in fruits, which has been verified in mango (Chidley et al. 2017) and persimmon (Park et al., 2017). Previous studies on ‘Nanguo’ pear found that exogenous ethylene could increase the endogenous content of fruit (Yao et al. 2022), while inhibitor 1-MCP treatment decreased the endogenous ethylene content (Bai et al. 2022; Tao et al. 2019). It is therefore speculated that exogenous ethylene treatment in this study can also increase the endogenous ethylene content in ‘Nanguo’ pear fruits. Therefore, appropriate concentrations of ethylene improves fruit quality, increasing the soluble solid and soluble sugar contents of fruit, while decreasing titratable acid content. It was speculated that ethylene treatment promotes the fruit respiration rate, hydrolysis of starch and organic compounds, the conversion of starch to sugar, and the softening of fruit to ensure its quality. This is in line with the results of previous research on ‘Ruaner’ Pear (Zhang et al. 2019). Meanwhile, ethephon treatment reduces the stone cell content of ‘Nanguo’ Pear. This is consistent with the results of studies on ‘Korla Fragrant Pear’ and ‘Xinli No. 7’ (Chen et al., 2020).
Fruit softening is mainly manifested by changes in cell wall metabolism, including changes in cell wall structure and composition (Li et al. 2022). The molecular skeleton of the fruit cell wall is composed of cellulose and hemicellulose, and dispersed pectin substances are cross-connected to the cellulose-hemicellulose complex through polygalacturonic acid, arabosan, and galactose (Deng et al., 2016). Pectic substances, cellulose, and hemicellulose are the main components of primary cell wall polysaccharides, and their degradation causes the decrease of intercellular adhesion and cell wall decomposition (Brummell 2006; Fry 2004). Pectic substances that are the predominant polysaccharides found in the primary cell wall and middle lamella play a pivotal role in regulating cell-to-cell adhesion (Billy et al. 2007). This study found that the water-soluble pectin content of ‘Nanguo’ pear fruit showed an increasing trend, while the protopectin content and cellulose content gradually decreased. This indicated that the pectin gradually dissolved into water-soluble pectin and the cell wall structure was gradually loosened during the fruit softening process. Moreover, the cellulose content and protopectin content of fruit treated with ethylene significantly decreased compared with the control. The increased content of water-soluble pectin indicated that ethylene promoted the degradation of cell wall components to promote the fruit softening process. Microscopic examination of paraffin sections confirmed that ethylene promoted the ‘Nanguo’ pear fruit cell wall started loosened, and the structure was gradually deformed. The results confirmed previous findings that ethylene can induce changes in cell wall composition and structure to promote fruit softening (Fan et al. 2018; Shin et al. 2019; Wang et al. 2019; Soares et al. 2021).
The intricate process of fruit softening involves the breakdown of cell wall components through the action of various cell wall degrading enzymes, ultimately leading to compositional alterations in the cell wall structure (Brummell and Harpster 2001). The cell wall undergoes a series of metabolic reactions that contribute to softening throughout the process of fruit ripening. These include the demethylation of pectin catalyzed by PME, the decomposition of de-esterified polygalacturonic acid facilitated by PL, and the hydrolysis of α-1,4-galacturonic acid by PG, resulting in uronic acid production (Peng et al. 2022). The synergistic effect of PME and PG is widely believed to lead to pectin decomposition in post-harvest fruits and vegetables, and plays an important role in determining the fruit pectin content (Zhao et al. 2019). The activity of PG and PME in strawberry fruits decreases, resulting in the increased water-soluble pectin content and the downregulation of FaPG1 and FaPME1; this inhibits fruit softening (Wang et al. 2020a, b). In this study, ethylene treatment increased the PG and PME activities of fruit. This suggested that ethylene treatment may accelerate the degradation of protopectin and the formation of water-soluble pectin by increasing PME and PG activities, and promoting fruit softening. Ethylene treatment significantly increased the gene expression levels of PuPG and PuPG2 (P < 0.01), which was consistent with a study conducted in strawberry (Villarreal et al. 2010; Shin et al. 2019). Cellulase is a multicomponent enzyme complex involved in the depolymerization of cell wall polysaccharide that can hydrolyze β-D-glycosidic bonds in cellulose into monosaccharides or oligosaccharides; this results in cell wall damage and promotes fruit cell wall hydrolysis (Dong et al., 2020; Han 2016). This study showed that the cellulase activity of ‘Nanguo’ pear fruit generally showed an upward trend, with highest activity observed at 123 days. Ethylene treatment promoted the cellulase activity of fruit, a change that may be mainly attributed to the fact that ethylene treatment promoted the cellulase activity and reduced the cellulose content. This is consistent with study results in plum and strawberry (Villarreal et al. 2010; Lin et al. 2018). β-galactosidase belongs to the class of glycan hydrolases and is an important cellulose degrading enzyme. It mainly acts on the non-cut fibrous disaccharide molecules of β-glucoside to hydrolyze them into glucose molecules. It is thought to be related to the strengthening or relaxation of cell wall during plant cell growth and development (Chidley et al. 2017). The β-Gal activity of ethylene-treated fruits was significantly higher than that of the control (P < 0.05), and the expression of Puβ-Gal increased. Exogenous ethylene treatment increased the expression of genes related to cell wall degradation, and then increased the activity of cell wall degrading enzymes which changed the composition and structure of the cell wall and promoted fruit ripening and softening.
In general, ethylene changes the structure and component content of fruit cell walls by promoting the activity of cell wall degrading enzymes resulting in fruit softening during late development and ensuring that the quality of fruit to meet the marketable standards. Nonetheless, the precise molecular mechanisms underlying the regulation of fruit softening genes by ethylene warrant further investigation, and represent a key area of focus for our future research endeavors.
Conclusions
Ethylene treatment of ‘Nanguo’ pear fruit at the later stage of development increased the contents of soluble solids and soluble sugar in the fruit, decreased the titratable acid content and stone cell content, improved the fruit quality, and made it marketable. Ethylene increased the expression of cell wall degradation-related genes to enhance the activity of cell wall enzymes (PG, PME, Cx, and β-gal) to promote protopectin decomposition, cellulose degradation, and the generation of water-soluble pectin, and cellulose degradation, thus leading to cell wall degradation and promoting fruit softening. Ethylene treatment at 400 mg·L−1 significantly improved fruit quality, promoted fruit softening, and had the optimal overall effect.
Abbreviations
- 1-MCP:
-
1-Methylcyclopropene
- β-Gal:
-
β-Galactosidase
- CSP:
-
Chelator-soluble pectin
- CTAB:
-
Cetyltrimethylammonium ammonium bromide
- Cx:
-
Cellulase
- DEG:
-
Differentially expressed gene
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- NSP:
-
Na2CO3-soluble pectin
- ONPG:
-
O-nitrophenyl-β-D-pyran galactoside
- CMC:
-
Carboxymethyl cellulose Na
- PG:
-
Polygalacturonase
- PME:
-
Pectin methylesterase
- qRT-PCR:
-
Quantitative real time polymerase chain reaction
- WSP:
-
Water-soluble pectin
References
Akira T, Hiroaki I, Hajime S et al (2001) Molecular cloning of β-Galactosidase from Japanese pear (Pyrus pyrifolia) and its gene expression with fruit ripening. Plant Cell Physiol 5:492. https://doi.org/10.1016/j.jhep.2008.07.040
Andrews PK, Li SL (1995) Cell wall hydrolytic enzyme activity during development of nonclimacteric sweet cherry (Prunus avium L.) fruit. J Hortic Sci 70(4):561–567. https://doi.org/10.1080/14620316.1995.11515327
Bai L, Zhang L, Lv J et al (2022) Effects of 1-methylcyclopropene (1-MCP) treatment on ethanol fermentation of Nanguo pear fruit during ripening. J Food Biochem 46(5):e14035. https://doi.org/10.1111/jfbc.14035
Billy L, Mehinagic E, Royer G, Renard CMGC, Arvisenet G, Prost C, Jourjon F (2007) Relationship between texture and pectin composition of two apple cultivars during storage. Postharvest Biol Technol 47:315–324. https://doi.org/10.1016/j.postharvbio.2007.07.011
Brummell DA (2006) Cell wall disassembly in ripening fruit. Funct Plant Biol 33:103–119. https://doi.org/10.1071/FP05234
Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47:311–339. https://doi.org/10.1023/a:1010656104304
Brummell DA, Cin VD, Crisosto CH et al (2004) Cell wall metabolism during maturation, ripening and senescence of peach fruit. J Exp Bot 55(405):2029–2039. https://doi.org/10.2503/jjshs.58.801
Bu JW, Yu YC, Aisikaer G, Ying TJ (2013) Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol Technol. https://doi.org/10.1016/j.postharvbio.2013.07.026
Chidley HG, Deshpande AB, Oak PS et al (2017) Effect of postharvest ethylene treatment on sugar content, glycosidase activity and its gene expression in mango fruit. J Sci Food Agric 97(5):1624–1633. https://doi.org/10.1002/jsfa.7912
Fan X, Shu C, Zhao K, Wang X, Cao J, Jiang W (2018) Regulation of apricot ripening and softening process during shelf life by post-storage treatments of exogenous ethylene and 1-methylcyclopropene. Sci Hortic 232:63–70. https://doi.org/10.1016/j.scienta.2017.12.061
Fry SC (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol 161:641–675. https://doi.org/10.1111/j.1469-88137.2003.00980
Han Y, Ban Q, Li H, Hou Y, Jin M, Han S, Rao J (2016) DkXTH8, a novel xyloglucan endotransglucosylase/hydrolase in persimmon, alters cell wall structure and promotes leaf senescence and fruit postharvest softening. Sci Rep 6:39155. https://doi.org/10.1038/srep39155
Hertog MLATM, Jeffery PB, Gwanpua SG et al (2016) A mechanistic model to describe the effects of time, temperature and exogenous ethylene levels on softening of kiwifruit. Postharvest Biol Technol 121:143–150. https://doi.org/10.1016/j.postharvbio.2016.08.002
Hiwasa K, Rose JKC, Nakano R et al (2010) Differential expression of seven alpha-expansin genes during growth and ripening of pear fruit. Physiol Plant 117(4):564–572. https://doi.org/10.1034/j.1399-3054.2003.00064.x
Jaakola L, Pirttila AM, Halonen M, Hohtola A (2001) Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol 19:201–203. https://doi.org/10.1385/MB:19:2:201
Johnston JW (2015) Lower cell wall pectin solubilisation and galactose loss during early fruit development in apple (Malus domestica) cultivar ‘Scifresh’ are associated with slower softening rate. Plant Physiol 176:129–137. https://doi.org/10.1016/j.jplph.2014.12.012
Kaur A, Sharma S, Singh N (2021) Biochemical changes in pear fruits during storage at ambient conditions. Adv Hortic Sci. https://doi.org/10.36253/ahsc-10740
Kaur S, Gill MS, Gill P et al (2023) Influence of harvest date on storage quality of asian pear (Pyrus pyrifolia) fruit. Erwerbs-Obstbau. https://doi.org/10.1007/s10341-023-00896-7
Lee SH, Choi JH, Kim WS, Han TH, Park YS, Gemma H (2006) Effect of soil water stress on the development of stone cells in pear (Pyrus Pyrifolia cv. ‘Niitaka’) flesh. Sci Hortic 110(3):247–253. https://doi.org/10.1016/j.scienta.2006.07.012
Li T, Li X, Tan D, Jiang Z, Wei Y, Li J, Guodong Du, Wang A (2014) Distinct expression profiles of ripening related genes in the ‘Nanguo’ pear (Pyrus ussuriensis) fruits. Sci Hortic 171:78–82. https://doi.org/10.1016/j.scienta.2014.03.054
Li W, Xu L, Xia R, Shen Y, Zhu Z, Yu Y, Zang Y (2021) Cloning and functional identification of SlPG49 in Solanum lycopersicum. Appl Sci 11:11450. https://doi.org/10.3390/app112311450
Li Y, He H, Hou Y, Kelimu A, Fang W, Zhao Y, Shi L, Zhu X (2022) Salicylic acid treatment delays apricot (Prunus armeniaca L.) fruit softening by inhibiting ethylene biosynthesis and cell wall degradation. Sci Hortic. 300:111061. https://doi.org/10.1016/j.scienta.2022.111061
Li R, Ma J, Gu H, Jia W, Shao Y, Li W (2022) 1-Methylcyclopropene counteracts ethylene promotion of fruit softening and roles of MiERF2/8 and MiPG in postharvest mangoes. Front Plant Sci. https://doi.org/10.3389/fpls.2022.971050
Lin YF, Lin HT, Lin MS, Li H, Yuan F, Chen YH, Xiao JB (2018) Effects of paper containing 1-MCP postharvest treatment on the disassembly of cell wall polysaccharides and softening in Younai plum fruit during storage by atomic force microscopy. Trends Food Sci Technol 264:1–8. https://doi.org/10.1016/j.foodchem.2018.05.031
Lindo-Garcia V, Larrigaudiere C, Duaigues E et al (2020) Elucidating the involvement of ethylene and oxidative stress during on- and off-tree ripening of two pear cultivars with different ripening patterns. Plant Physiol Biochem 155:842–850. https://doi.org/10.1016/j.plaphy.2020.08.018
Ling X, Guangyan Z, Jinhui J et al (2020) Deep processing technology of ‘Nanguo Pear.’ Agric Prod Proc. https://doi.org/10.16693/j.cnki.1671-9646(X).2020.12.013
Liu X, Li S, Feng X et al (2020) Study on cell wall composition, fruit quality and tissue structure of hardened ‘Suli’ pears (Pyrus bretschneideri Rehd). J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10248-4
Park DS, Tilahun S, Heo JY, Jeong CS (2017) Quality and expression of ethylene response genes of ‘Daebong’ persimmon fruit during ripening at different temperatures. Postharvest Biol Technol 133:57–63. https://doi.org/10.1016/j.postharvbio.2017.06.011
Peng Z, Liu G, Li H, Wang Y, Gao H, Jemrić T, Fu D (2022) Molecular and genetic events determining the softening of fleshy fruits: a comprehensive review. Int J Mol Sci 23(20):12482. https://doi.org/10.3390/ijms232012482
Posé S, Paniagua C, Cifuentes M, Blanco-Portales R, Quesada MA, Mercado JA (2013) Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J Exp Bot 64:3803–3815. https://doi.org/10.1093/jxb/ert210
Posé S, Paniagua C et al (2019) A nanostructural view of the cell wall disassembly process during fruit ripening and postharvest storage by atomic force microscopy. Trends Food Sci Technol 87:47–58. https://doi.org/10.1016/j.tifs.2018.02.011
Sha Y, Huajia Li, Yongqing Z et al (2018) Ethylene ripening characteristics of ‘Hongyang’ kiwifruit following postharvest ethylene treatment. Food Sci 39(9):244–251. https://doi.org/10.7506/spkx1002-6630-201809037
Shin MH, Kwack Y, Kim Y, Kim JG (2019) Fruit ripening and related gene expression in ‘Goldone’ and ‘Jecy Gold’ kiwifruit by exogenous ethylene application. Korean J Hortic Sci Technol 37:54–64. https://doi.org/10.12972/kjhst.20190006
Soares CG, Do Prado SB, Andrade SCS, Fabi JP (2021) Systems biology applied to the study of papaya fruit ripening: the influence of ethylene on pulp softening. Cells 10:2339. https://doi.org/10.3390/cells10092339
Tao D, Wang J, Zhang L et al (2019) 1-Methylcyclopropene alleviates peel browning of ‘Nanguo’ pears by regulating energy, antioxidant and lipid metabolisms after long term refrigeration. Sci Hortic 247:254–263. https://doi.org/10.1016/j.scienta.2018.12.025
Tucker G, Yin XR, Zhang AD, Wang MM, Zhu QG, Liu XF, Xie XL, Chen KS, Grierson D (2017) Ethylene and fruit softening. Food Qual Safety 1:253–267. https://doi.org/10.1093/fqsafe/fyx024
Vicente AR, Costa ML, Martínez GA, Chaves AR, Civello PM (2005) Effect of heat treatments on cell wall degradation and softening in strawberry fruit. Postharvest Biol Technol 38:213–222. https://doi.org/10.1016/j.postharvbio.2005.06.005
Villarreal NM, Bustamante CA, Civello PM, Martínez GA (2010) Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J Sci Food Agric 90:683–689. https://doi.org/10.1002/jsfa.3868
Wang SY, Zhou Q, Zhou X, Zhang F, Ji SJ (2019) Ethylene plays an important role in the softening and sucrose metabolism of blueberries postharvest. Food Chem 310:125965. https://doi.org/10.1016/j.foodchem.2019.125965
Wang S, Zhou Q, Zhou X, Zhang F, Ji S (2020a) Ethylene plays an important role in the softening and sucrose metabolism of blueberries postharvest. Food Chem 310:125965. https://doi.org/10.1016/j.foodchem.2019.125965
Wang K, Li TT, Chen SQ et al (2020b) The biochemical and molecular mechanisms of softening inhibition by chitosan coating in strawberry fruit (Fragaria x ananassa) during cold storage. Sci Hortic 271:109483. https://doi.org/10.1016/j.scienta.2020.109483
Wang J, Zhang J, Li J et al (2021) Exogenous application of 5-aminolevulinic acid promotes coloration and improves the quality of tomato fruit by regulating carotenoid metabolism. Front Plant Sci 12:683868. https://doi.org/10.3389/fpls.2021.683868
Xiucai F, Jipeng Z (2001) Effects of 1-methylcyclopropene on physiological effects of postharvest monkey fruit. Acta Hortic Sinica 28(5):399–402. https://doi.org/10.1007/s11738-010-0703-7
Xu Z, Dai J, Kang T, Shah K, Li Q, Liu K, Xing L, Ma J, Zhang D, Zhao C (2022) PpePL1 and PpePL15 are the core members of the pectate lyase gene family involved in peach fruit ripening and softening. Front Plant Sci 13:844055. https://doi.org/10.3389/fpls.2022.844055
Yan C, Qian Z, Yuzhong D et al (2020) Effects of ethephon on decalyx rate and fruit quality of ‘Korla Fragrant Pear’ and ‘Xinli No.7.’ Journal of henan agricultural university. 54(6):949–955
Yao M, Zhou X, Ji Y et al (2022) Potential of ethylene in alleviating cold-induced volatile esters loss of ‘Nanguo’ pears by regulating the lipoxygenase pathway. Environ Exp Botany. https://doi.org/10.1016/j.envexpbot.2021.104723
Zhang HZ, Yang JL, Li WL, Chen YX, Lu H, Zhao SC et al (2019) PuHSFA4a enhances tolerance to excess zinc by regulating reactive oxygen species production and root development in Populus. Plant Physiol 180:2254–2271. https://doi.org/10.1104/pp.18.01495
Zhao YT, Zhu X, Hou YY et al (2019) Effects of nitric oxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill. Cv. Dongzao) fruit during storage. Postharvest Biol Technol 156:110954. https://doi.org/10.1016/j.postharvbio.2019.110954
Zhenru Li, Wenhui W, Xiaohui J et al (2007) Study on the suitable harvesting time of four western pear varieties. China Fruit Sci 5:22–25. https://doi.org/10.3969/j.issn.1000-8047.2007.05.009
Zhou Q, Zhang F, Ji S et al (2021) Abscisic acid accelerates postharvest blueberry fruit softening by promoting cell wall metabolism. Sci Hortic. 288:110325. https://doi.org/10.1016/j.scienta.2021.110325
Funding
This study was supported by the Earmarked Fund for the China Agriculture Research System (CARS-28), National Key Research and Development Program of China (2022YFD1600500), and the Key Technology of Calcium-regulated Quality Development in ‘Nanguo’ Pear (01042017005).
Author information
Authors and Affiliations
Contributions
Jiang Fan: Methodology; Data curation; Formal analysis; Writing—original draft. Mingyang Xu: Supervision; Validation; He Zhang: Writing—review & editing. Min Liu: Methodology; Software. Ling Zhao: Validation, Supervision. Guodong Du: Conceptualization, Supervision, Funding acquisition, Project administration.
Corresponding authors
Ethics declarations
Competing Interest
The authors state that they do not have any identifiable conflicts of interest or personal relationships that could have influenced the findings presented in this paper.
Additional information
Handling Author: Fabrizio Costa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, F., Xu, M., Zhang, H. et al. Ethylene Promotes Fruit Softening of ‘Nanguo’ Pear via Cell Wall Degradation. J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11432-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11432-6