Abstract
Ammonia detection is crucial for various applications such as environmental monitoring, industrial processes, and healthcare. Here, we explore the potential of La-doped ZnO thin films as a cost-effective, efficient, and environmentally friendly RT-NH3 sensor prepared by spray pyrolysis method. Pure and La-doped ZnO films were highly sensitive and selective to ammonia gas as compared to other gases. The 3% La-doped ZnO films were most sensitive to ammonia gas with a relatively high response of about 74.28%, with very fast response time and recovery time of 17 s and 30 s, respectively, at room temperature. The response of 3% La-doped ZnO thin films was about three times greater than that of the pure ZnO films when evaluated under the same conditions. Moreover, the selectivity, sensitivity, reproducibility and long-term stability were also examined. The 3% La-doped ZnO films show a gas-sensing response at a lower concentration of 0.5 ppm of ammonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ammonia (NH3) is a colourless gas with a distinct pungent odor that is widely used in various industries, such as agriculture and refrigeration processing. Despite its extensive use, ammonia can pose significant risks to human health and the environment due to its toxic and corrosive nature at elevated concentrations. The US Occupational Safety and Health Administration (OSHA) has set the Permissible Exposure Limit (PEL) for 50 ppm of exposure 8 h per working day. Exceeding this level of ammonia exposure can result in serious health issues such as respiratory discomfort, eye irritation, skin disorders, etc. Therefore, efficient and reliable detection of ammonia is essential for ensuring workplace safety, environmental protection, and process control [1,2,3,4].
Different materials such as carbon-based materials, metal oxide frameworks, and thin metal oxide films can be used as gas-sensing materials. Yuan et al. [5] and Peng et al. [6] reported the advances in a metal-organic framework for gas sensing applications owing to its unique physicochemical properties with specific area, morphology and pore size distribution. Moreover, the studies suggests that the generation of oxygen vacancies can tune the electronic structure of the material. Among these materials, metal oxide semiconductors such as SnO2 [7], CdO [8], In2O3 [9], Fe2O3 [10], WO3 [11], and ZnO [12] etc., with distinct morphologies, have gained considerable attention due to their potential in detecting various gases including ammonia. Among these materials, ZnO stands out due to its unique properties, such as high sensitivity, excellent chemical stability and ease of preparation. ZnO thin films have been extensively studied for gas-sensing applications. However, pure ZnO film performance in detecting ammonia can be limited by factors such as operating temperature, response time and selectivity. To overcome this limitation and improve the gas sensing performance of pure ZnO-based sensors, doping with suitable dopants has to be explored. Dopant can be chosen to effectively modify the energy band gap and surface morphology of the host material. Additionally, doping can increase defects and carrier concentration, which can improve the sensing performance of the materials [13,14,15,16].
A literature survey shows that various rare earth metals such as lanthanum (La), cerium (Ce), gadolinium (Gd), samarium (Sr), and yttrium (Y) have been significantly used as dopants due to their distinct characteristics. D. Alagarasan et al. [17] studied ammonia detection at room temperature using undoped ZnO, 2% Cu-doped ZnO, 2% La-doped ZnO, and 2% (Cu: La) co-doped ZnO thin films. The reported results show a sensor response that was remarkably enhanced to 341% for (Cu: La) co-doped ZnO thin film and a fast response/recovery time of 80/10 s for 250 ppm ammonia. Deva Kumar et al. [18] investigated ammonia gas sensing properties of Er-doped ZnO at RT and observed a sensor response of 97.07% for 125 ppm. Sikai Zhao et al. [19] studied the effect of Ce, Eu and Er doping on microstructural and ammonia gas sensing properties of ZnO nanowires. Jansi Santhosam et al. [20] reported the effect of La incorporation on ammonia sensing properties of ZnO films. The gas sensing results illustrate that 3% La-doped ZnO films show remarkable sensing response and quick response/recovery time (39 s/11 s) for 100 ppm of ammonia at room temperature. To the best of our knowledge, research on La-doped ZnO thin films for ammonia sensing is limited and not widely reported in the existing literature.
Researchers have explored various deposition methods such as thermal evaporation [21], chemical bath deposition [22], sol-gel dip coating [23], spray pyrolysis [24], sputtering [25] and SILAR [26] to synthesize ZnO thin films. Among these methods, the spray pyrolysis technique is one of the best techniques due to its ease of operation, cost-effective and safe approach, as well as its ability to deposit large surface areas. These methods also allow the control parameters of the process and ensure uniform thickness in the deposited films [27]. In this work, pure and La-doped ZnO thin films have been deposited using the spray pyrolysis method. The effect of La-doping on the structural, morphological, and optical properties of the deposited films has been investigated. Further, the gas sensing properties of pure ZnO and La-doped ZnO thin films are studied for ammonia gas at room temperature (RT). The gas sensing properties like selectivity, response, recovery times, reproducibility, detection limit and long-term stability of La-doped ZnO films are studied.
2 Experimental details
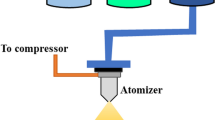
An automated spray pyrolysis apparatus (HOLMARC-TH-04) was used to prepare pure and La-doped ZnO thin films. Zinc acetate dihydrate (ZnCH3COO)2. 2H2O and lanthanum nitrate hexahydrate (La(NO3)3.6H2O) were used as precursors. Double distilled water was used as a solvent. All purchased chemicals and solvents were used without further purification. The Blue Star micro glass slides were used to deposit thin films. Before the deposition, the substrates were ultrasonically cleaned using chromic acid, soap solution, distilled water, and acetone. A solution of molarity 0.1 M was prepared using (Zn(CH3COO)2. 2H2O) and double distilled water as a solvent. The solution was kept on a stirrer for 10 min. to get a clear solution. For doping purposes, 1, 2 and 3 wt% of (La (NO3)3. 6H2O) was added to (Zn(CH3COO)2. 2H2O) precursor. The final solution was sprayed onto the pre-heated glass substrates using an atomizer. Compressed air was let into the gas inlet after passing through the dust filter, where the compressed air and solution flow rate were maintained at 30 psi and 2 ml/min. During the deposition, the substrate temperature was maintained at 400℃. The distance between the substrate and the atomizer was kept constant at 28 cm. All the prepared pure ZnO and La-doped ZnO thin films were uniform, transparent and adherent. These films were utilized for structural, surface morphological, optical, and gas sensing studies.
2.1 Characterizations
The crystallographic studies of the pure and La-doped ZnO thin films were carried out by Ultima IV, Rigaku Corporation, Japan X-ray diffractometer in the scanning range of 20-80o (2θ) using Cu-Kα radiation with a wavelength of 0.15046 nm. The morphology of the films was examined using field emission scanning electron microscopy (FESEM, Carl Zeiss supra-55, Germany). The surface chemical composition and valence states of the various elements were investigated using X-ray photoelectron spectroscopy (ESCALAB, Thermo-Fisher Scientific) using an Al kα source. The optical absorption analysis was carried out within the wavelength range of 350–700 nm for films using a UV-Vis spectrophotometer (a Shimadzu UV-1900i UV-VIS spectrophotometer).
2.2 Gas sensing measurements
A gas sensing experiment was performed with a home-built gas sensing setup at room temperature. The gas sensing setup consists of a cylindrical chamber and a computer-assisted Keithley electrometer (Model-6514). The sensor element of 1.5 cm X 1 cm (length x breadth) was utilised for gas sensing measurement. The silver paste was applied on the sides of the films to ensure good ohmic contact. Then, the prepared film was placed inside the sensing chamber, and its resistance was measured under the dry air to establish a baseline. Ammonia, in solution form, was then injected into the chamber using a microliter syringe. The introduction of ammonia vapour caused a noticeable change in the film’s resistance. The change in resistance was measured as a function of time using a Keithley electrometer. The chamber is opened to the ambient air once the resistance reaches saturation. The resistance values are measured relative to the baseline and recorded for various ammonia concentrations. The percentage response of the sensor towards the ammonia target gas was determined using the relation [28];
Ra and Rg denote the film resistance in air and gas atmospheres, respectively.
3 Results and discussion
3.1 Structural studies
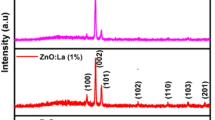
Figure 1 shows the X-ray diffraction patterns of pure and La-doped ZnO at concentrations of 1%, 2%, and 3% (wt%). From Fig. 1, it is found that all peaks at various 2θ values align closely with the JCPDS card # 79–0207, confirming the wurtzite (hexagonal) structure of the ZnO. All films have nine diffraction peaks located at 31.72, 34.38, 36.18, 47.50, 56.50, 62.76, 66.27, 67.78, 69.02 assigned to (100), (002), (101), (102), (110), (103), (200), (112), and (201) diffraction peaks respectively. Additionally, a decrease in the intensity of the XRD peaks and the broadening of the peaks can be related to the reduction in crystallite size. As the La doping concentration increases, the crystallite size decreases, leading to a broader peak width. This is consistent with the Scherrer equation, which indicates that smaller crystallite sizes result in broader XRD peaks. No secondary peaks related to La in XRD peaks were observed, which indicates the successful incorporation of La in ZnO lattices. The intensity of peak (002) shifted into (101) for La-doped ZnO films attributed to the nucleation process and crystal orientation effect of ZnO thin films [29]. The average crystallite size (D) and dislocation density (δ) can be calculated using the following formulae [30]:
The lattice constants (a and c) were calculated from the diffraction peaks using the following equation [31]:
where the interplanar spacing d is related to the diffraction angle θ by the relation \(\:1/d=\:2sin\theta\:/\lambda\:\:\). The (100) and (002) orientations are used to calculate the lattice parameters a = b and c, respectively.
where λ is the x-ray wavelength (1.5406 Å), and θ is the angle of diffraction in radians. The calculated values of average crystallite size, dislocation density and lattice constant are listed in Table 1. It is observed that the crystallite size decreases with increasing La-doping concentration. The decrease in crystallite size may be attributed to the incorporation of La3+ (∼ 1.02 Å) ions into Zn2+ (∼ 0.74 Å) ions, which causes deformation within the lattice system [32]. This deformation can result in a restriction of crystallite boundary growth. The observed increasing dislocation density as La-concentration suggested the formation of defects on the film surface [33]. This reduction in crystallite size and increase in defects can facilitate the absorption of more ammonia molecules. The obtained values of lattice constant (a and c) are listed in Table 1. From Table 1, an increase in lattice constant is observed after La-doping. This change may be due to differences in the ionic radii of Zn2+ and La3+ ions, resulting in the expansion of the lattice structure [34]. Moreover, a slight shift in the diffraction peaks towards lower diffraction angles is observed, as shown in Fig. 1(b).
3.2 Morphological and elemental studies
Pure and La-doped ZnO thin films were examined through field emission scanning electron microscopy (FE-SEM). Figure 2 shows the FESEM micrographs of spray-deposited pure and La-doped ZnO thin films. It is worth noting that the dopant has caused a significant alteration in the surface morphology of synthesized thin films. Figure 2(a) depicts the surface morphology of pure ZnO nanostructured films. Pure ZnO thin films possess a very compact and closely packed spherical nanograin distribution all over the film surface. A slight distortion is observed in the morphology on the further increase with La-doping. However, with the introduction of La dopant to the host material, the morphology changed to a distorted agglomerated spherical granular structure. Figure 2(d) represents the morphology of 3% La- ZnO thin films with more clear grains separated by grain boundaries. This type of nanostructured morphology is suitable for producing high sensitivity [35]. S. Fairsoe et al. [36] suggested that the porous surface of the film acts as the sensitive layer for ammonia vapour sensing when exposed to ammonia. G. K. Mani et al. [52] demonstrated the effect of morphology on sensing response and found that the smaller grain-sized particles have a higher surface area, leading to increased chemisorbed oxygen ions and decreases the height of the potential barrier.The mean grain size distribution was determined by using the Image Jand the corresponding histrograms for pure and La-doped ZnO thin films is as depicted in Fig. 2 (e)-(h). The average size was found to be decreased with increase in La-doping.
The stoichiometry of the synthesized films was studied by using the Energy Dispersive Spectroscopy (EDS) technique. Figure 3 shows typical EDS spectra for spray-deposited pure ZnO and La-doped ZnO thin films with various La-doping concentrations. An elementary analysis was performed to identify elements in the prepared samples. The unidentified minor peaks in the EDS spectra at 1.8 keV and 3.8 keV are due to Si and Ca, respectively, possibly originating from the glass substrates [37].
3.3 X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) was employed to examine the chemical states of each element. Figure 4(a) represents the complete XPS spectra of a 3% La-doped ZnO thin film sensor. The binding energies of the detected peaks were calibrated using the C 1s peak (284.6 eV) as a standard. Typical peaks correspond to the chemical state of the Zn, O and La elements. No impurity peaks other than C were detected, confirming the purity of the deposited film. The core levels of Zn 2p, O 1s and La 3d states of the deposited films were examined, as depicted in Fig. 4(b-d). According to Fig. 4(b), the Zn 2p3/2 and Zn 2p1/2 core levels of Zn ions have binding energies of 1021.48 eV and 1044.38 eV, respectively. The coupling energy between these two peaks is nearly 23 eV, attributed to spin-orbit coupling, which correlated to the + 2 oxidation state of Zn [38]. Figure 4(c) represents the deconvolution of an asymmetrical O 1s peak, indicating the existence of different chemical states of oxygen. In order to obtain precise information on O 1s spectra, Gaussian curve fitting was performed. Numerous investigations have demonstrated the existence of three broad peaks of O1s [39]. From Fig. 4(c), it can be seen that the peaks correspond to energies at 530.2, 531.8, and 532.4 eV, corresponding to the O atom. The peak at 530.2 eV is attributed to the stable O2− ions of the Zn-O bonds in the wurtzite structure of ZnO. The peak at 531.8 eV represents the adsorbed oxygen ions, which significantly impact the gas-sensing properties in oxygen-deficient regions, such as oxygen vacancies and oxygen interstitials within the deposited material. The peak corresponding to 532.4 eV is related to the chemisorbed species responsible for enhanced gas sensing performance [32, 40]. The typical core level peaks of La 3d XPS spectra are depicted in Fig. 4(d). The La 3d5/2 and La 3d3/2 are observed at 835.27 and 852.14 eV binding energies, respectively. The separation binding energy between these two peaks is nearly 16.87 eV. In accordance with the principle procedure and XPS instruments manual, La is present in a + 3 valence state. Additionally, the investigations reveal the successful substitution of Zn2+ ions with La3+ ions [41].
3.4 Optical properties
Figure 5(a) represents the optical transmittance spectra of prepared thin films in the wavelength region of 350 to 700 nm. All the synthesized films showed a transmittance of nearly 70% in the visible region. This may be attributed to the reduced average crystallinity, which allows them to pass more radiation [37]. Figure 5(b) represents the absorbance spectra for the prepared sample. The blue shift in absorbance spectra was observed with the introduction of the La-dopant element. This specifies the good incorporation of La ions into the ZnO matrix. Ayana et al. [42] also observed a similar trend of the blue shift of absorbance spectra for La-doped ZnO thin films, which was attributed to the concentration and nature of the dopant. The band gap of deposited films was determined from absorbance spectra by using the well-known Tauc’s relation [43].
where, \(\:{E}_{g}\) is the optical band gap, c is a constant known as the band tailoring parameter, \(\:h\nu\:\) is the energy of the incident photon, and \(\:\alpha\:\) is the absorption coefficient and n values depending upon the type of optical transition. The absorption coefficient was determined from the absorbance (A) and thickness (t) of the prepared thin film by using Lambert’s law\(\left(\alpha=\frac{2.303A}{t}\right)\)[44].
The ZnO is a direct bandgap semiconductor, thus a value of n = 1/2. The energy band gap was estimated by simply extrapolating the straight-line region of Tauc’s plot, as shown in Fig. 5(c). The band gap of prepared samples was observed to be 3.23, 3.25, 3.26 and 3.28 eV for pure, 1%, 2%, and 3% La-doped ZnO thin films, respectively. The increase in band gap values with the incorporation of La dopant was due to the Burstein-Moss effect ascribed to increased electron density [45]. One of the reasons for increased band gap values is the blueshift of absorbance spectra. The increase in band gap increases the concentration of defects in the ZnO attributed to La-doping, as reported by Santhosam et al. for La-doped ZnO thin films [20]. Similar results were also investigated by Ahmed et al. [46] and Mrabet et al. [47].
3.5 Gas sensing analysis
The gas sensing performance of pure and La-doped ZnO nanostructured films was examined by monitoring the change in resistance of the sensor element before and after introducing a target gas. The air resistance of the sensor element was made stable before exposure to the target gas. The sensor’s selectivity is an important parameter determining whether the sensor element can respond to several test gases or accurately sense one target gas.
3.5.1 Selectivity test of the sensor
In order to prevent interference from other gases during detection, gas sensors must also possess a high degree of selectivity. The gas responses of the pure and 3% La-doped ZnO sensors to 50 ppm concentration of various target gases such as NH3, NO2, H2S, SO2, CO, and H2 at room temperature were tested. The observed responses for the pure and 3% La-doped ZnO films are as represented in Fig. 6. It is observed that both pure and 3% La-doped ZnO sensors showed a higher response towards ammonia as compared to other test gases. The sensor based on 3% La-doped ZnO has the highest response to ammonia, and the value is 3 times higher than the pure ZnO film.
Table 2 summarises the selectivity coefficient for the target ammonia gas. The selectivity of the gas sensor element is determined by the selectivity coefficient (K) and refers to the ratio of sensor response to the target gas to the interfering gas. The selectivity coefficient value higher than 3 signifies that the sensor is better able to separate the target gas from the mixture of gases, as reported by Z. Wu et al. [48].
3.5.2 Ammonia gas sensing studies
The gas sensing response, response time, and recovery time were investigated at a 50 ppm concentration of ammonia gas at room temperature. The response time is defined as the time required for the sensor to reach 90% of its maximum signal change upon exposure to the target gas. Similarly, the recovery time is defined as the time needed for the sensor to return to 10% of its baseline signal after the removal of the target gas. These times were extracted from the dynamic response curves shown in Fig. 7(a)-(d). The response curves, characterized by sharp tower-like shapes, indicate a rapid response and recovery behavior. Pure ZnO sample responded 24.07% towards 50 ppm of ammonia with a 55 s response time and 67 s recovery time. The 1% and 2% La-doped ZnO samples showed response times of 31 s and 20 s and recovery times of 45 s and 36 s, respectively. However, 3% La-doped ZnO showed a 3 times higher response (74.28%) than pure ZnO at room temperature with a response time of 17 s and recovery time of 30 s. This phenomenon can be explained by the rapid adsorption and desorption of gas molecules on the highly reactive surface of La-doped ZnO films. The response and recovery times and % response of the prepared sensors are summarised in Table 3.
From Table 3, it is observed that the 3% La-doped ZnO sensor showed a higher response compared to other prepared films. The increase in response to ammonia is due to the reduced crystallite size of films and the modified surface morphology with an increasing La-doping concentration to the ZnO host material. La-doped ZnO films have a higher surface-to-volume ratio than pure ZnO films. Higher surface-to-volume ratios can result in the deficiency of surface atomic coordination and high surface energy as a result of the high number of surface atoms. Because of this, the surface is highly active, resulting in further adsorption of ammonia molecules [49], and hence the increased sensor response was observed. According to G. Biasotto et al. [50], the enhanced gas sensing performance is due to increased active sites ascribed to decreased crystallite size. Some of the literature reports on doped ZnO films towards ammonia gas are summarised in Table 4. The work reported in present study reports a higher response of 74.28% for 50 ppm of ammonia with response time of 17 s. However, the work presented by Jansi Santhosham reports the 55% response for 100 ppm with 39 s response time. Compared to the previously reported results, the 3% La-doped ZnO sensor showed an increased response towards 50 ppm concentration of ammonia gas operating at room temperature. Hence, it can be a promising material for developing an ammonia gas sensor operating at room temperature.
3.5.3 Sensitivity of the sensor
The sensitivity of the 3% La-doped ZnO sensor was tested for various concentrations from 0.5 ppm to 50 ppm of ammonia gas at room temperature and obtained response curves are depicted in Fig. 8(a). From Fig. 8(a), it can be seen that the sensor responses decreased with a decrease in concentration of ammonia. The observed sensor % responses are represented in Fig. 8(b). The obtained response and recovery times for each concertation of ammonia are summarised in Fig. 8(c). As the concentration of ammonia decreased, the response time increased, suggesting that fewer ammonia molecules were adsorbed on the surface of the sensor. The sensitivity studies suggested that the 3% La-doped ZnO sensor was able to detect ammonia at room temperature as low as 0.5 ppm.
3.5.4 Reproducibility and long-term stability
Reproducibility is one of the most imperative parameters in the studies of sensors in commercial usage. Thus, the reproducibility of the best sensor, 3% La-doped ZnO, was analysed for 5 repetitive cycles. All 5 cycles showed a very negligible change in the sensor response and thus identified the reproducibility of the sensor. Figure 9(a) depicts the reproducibility test for 3% La-doped ZnO nanostructured thin film towards 50 ppm ammonia concentration at ambient temperature, and excellent reproducibility was evidenced. This suggested that 3% La-doped ZnO thin films can be a suitable sensor.
Nevertheless, the long-term stability of the 3% La-doped ZnO sensor for 30 days was also evaluated. Figure 9(b) represents the long-term stability of the 3% La-doped ZnO sensor. The sensor showed a splendid response over the stability of the sensor.
3.5.5 Sensing mechanism
Figure 10 illustrates the gas sensing mechanism of pure and La-doped ZnO thin films in air and in an ammonia atmosphere. When the reducing gases are exposed to the sensor element, the interaction between gas molecules and adsorbed oxygen results in a desorption process that returns electrons to the film’s conduction band, reducing surface resistance. The mechanism of gas sensing is referred to as an adsorption-oxidation-desorption phenomenon. Figure 10(a) represents the mechanism of the thin films in air condition. As the thin films are kept in air condition, the oxygen molecules get adsorbed on the surface (Eqs. 8–9), which captures the electrons from the conduction band of ZnO and hence the oxygen ion molecules \(\:({O}^{-},\:{O}_{2}^{-})\) will be created and known as a chemisorbed oxygen species, forming the depletion layer [58]. These chemisorbed species interact with the ammonia introduced to the sensing chamber, causing the release of trapped electrons due to the decreased width of the sensor’s space charge region. Hence, the surface conductivity of films increases, and thus, the resistance decreases. Figure 10(b) represents the mechanism of thin film gas sensors in the presence of ammonia. At the initial stage of gas sensing, the air oxygen molecules get adsorbed on the surface of the thin film sensor. Depending upon the temperature at which the sensor is being operated, an electron is trapped and chemisorbed in one of the three different forms known as:\(\:{\text{O}}_{2}^{-},\:{\text{O}}^{-}\:\text{a}\text{n}\text{d}\:{\text{O}}^{2-}\)[59]. The sensing kinematics of chemisorbed oxygen is represented by the following equation;
Equation (10) represents the possible interaction between ammonia gas molecules and the oxygen species on the surface of the film [18],
Since ammonia has a reducing nature, the surface resistance of the ZnO film is reduced during the interaction reaction between NH3 molecules and the oxygen-adsorbed ZnO surface. Consequently, the trapped electrons are returned to the conduction band of the sensor material, resulting in a reduction in the potential barrier’s height and the depletion layer’s width. During this process, the generated electrons are released to the sensor’s conduction band. This results in the electron deletion layer having a reduced thickness and the sensors having a decreased resistivity. The larger specific surface areas on the doped samples correspond to more oxygen species being chemisorbed into the material than pure ZnO. An improved response to ammonia was achieved by reducing the grain size of the sensor element, which resulted in a larger surface area and contributed to the porous nature of the sensing layer.
4 Conclusion
Pure and La-doped ZnO thin films were successfully synthesized by spray pyrolysis. Structural characterization signifies that the hexagonal structure of ZnO was maintained even after La-doping. The average crystallite size was observed to be decreased from 23.51 nm to 16.98 nm. Also, a significant increase in lattice parameters was observed. Morphological studies revealed a formation of clear grain boundaries and more porous films with an increase in La-doping concentration. EDS spectra revealed stoichiometry of Zn, La, and O content in the pure and La-doped ZnO thin films. The optical band gap values determined from Tauc’s plot were found to increase from 3.23 to 3.28 eV with an increase in La-doping concentration. The gas sensing studies showed that 3% La-doped ZnO thin film has a 74.28% response with a 17 s response time and 30 s recovery time towards 50 ppm concentration of ammonia at room temperature. The reproducibility and 30 days of long-term stability are evidenced.
Moreover, the lower detection limit of the prepared 3% La-doped ZnO was observed to be 0.5 ppm ammonia at room temperature. The 3% La-doped ZnO samples showed excellent reproducibility, sensitivity and long-term stability towards ammonia gas at room temperature. Thus, the experimental results showed that the 3% La-doped ZnO thin film is promising for developing highly sensitive and stable ammonia gas sensor devices.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to legal or ethical reasons.
References
S. Naseem, A.J. King, Ammonia production in poultry houses can affect the health of humans, birds, and the environment—techniques for its reduction during poultry production. Environ. Sci. Pollut Res. 2018. 2516, 15269–15293 (2018). https://doi.org/10.1007/S11356-018-2018-Y
M.D. Oliveira, F.C. Sousa, J.O. Saraz, A.A. Calderano, I.F.F. Tinôco, A.P.S. Carneiro, Ammonia emission in poultry facilities: a review for tropical climate areas, atmosphere (Basel). 12 (2021) 1–12. https://doi.org/10.3390/atmos12091091
W. Tan, D. Lv, X. Guo, H. Du, L. Liu, Y. Wang, Accident consequence calculation of ammonia dispersion in the factory area. J. Loss Prev. Process. Ind. 67, 104271 (2020). https://doi.org/10.1016/j.jlp.2020.104271
S. Xiang, Y. Liu, G. Zhang, R. Ruan, Y. Wang, X. Wu, H. Zheng, Q. Zhang, L. Cao, New progress of ammonia recovery during ammonia nitrogen removal from various wastewaters. World J. Microbiol. Biotechnol. 36, 1–20 (2020). https://doi.org/10.1007/s11274-020-02921-3
H. Yuan, S. A.A.A. Aljneibi, J. Yuan, Y. Wang, H. Liu, J. Fang, C. Tang, X. Yan, H. Cai, Y. Gu, S.J. Pennycook, J. Tao, D. Zhao, ZnO nanosheets Abundant in Oxygen vacancies derived from Metal-Organic frameworks for ppb-level gas sensing. Adv. Mater. 31, 1807161 (2019). https://doi.org/10.1002/ADMA.201807161
X. Peng, X. Wu, M. Zhang, H. Yuan, Metal-Organic Framework Coated devices for Gas Sensing. ACS Sens. 8, 2471–2492 (2023). https://doi.org/10.1021/ACSSENSORS.3C00362/ASSET/IMAGES/MEDIUM/SE3C00362_0013.GIF
Y.C. Liang, C.M. Lee, Y.J. Lo, Reducing the gas-sensing performance of Ce-doped SnO2 thin films through a sputtering method. RSC Adv. 7, 4724–4734 (2017). https://doi.org/10.1039/c6ra25853k
J.K. Rajput, T.K. Pathak, V. Kumar, L.P. Purohit, Influence of sol concentration on CdO nanostructure with gas sensing application. Appl. Surf. Sci. 409, 8–16 (2017). https://doi.org/10.1016/j.apsusc.2017.03.019
D. Han, J. Yang, F. Gu, Z. Wang, Effects of rare earth element doping on the ethanol gas-sensing performance of three-dimensionally ordered macroporous In2O3. RSC Adv. 6, 45085–45092 (2016). https://doi.org/10.1039/c6ra06816b
S. Saritas, M. Kundakci, O. Coban, S. Tuzemen, M. Yildirim, Fe2O3, mg: Fe2O3 and Fe2O3 thin films gas sensor application. Phys. B Condens. Matter. 541, 14–18 (2018). https://doi.org/10.1016/j.physb.2018.04.028
S. Büyükköse, Highly selective and sensitive WO3 nanoflakes based ammonia sensor. Mater. Sci. Semicond. Process. 110 (2020). https://doi.org/10.1016/j.mssp.2020.104969
B. Ghanbari Shohany, A. Khorsand Zak, Doped ZnO nanostructures with selected elements - structural, morphology and optical properties: a review. Ceram. Int. 46, 5507–5520 (2020). https://doi.org/10.1016/j.ceramint.2019.11.051
K. Kasirajan, L. Bruno Chandrasekar, S. Maheswari, M. Karunakaran, P. Shunmuga Sundaram, A comparative study of different rare-earth (gd, nd, and sm) metals doped ZnO thin films and its room temperature ammonia gas sensor activity: synthesis, characterization, and investigation on the impact of dopant. Opt. Mater. (Amst). 121 (2021). https://doi.org/10.1016/J.OPTMAT.2021.111554
A.J. Jebathew, M. Karunakaran, K.D. Arun Kumar, S. Valanarasu, V. Ganesh, M. Shkir, I.S. Yahia, H.Y. Zahran, A. Kathalingam, An effect of Gd3 + doping on core properties of ZnS thin films prepared by nebulizer spray pyrolysis (NSP) method. Phys. B Condens. Matter. 574 (2019). https://doi.org/10.1016/j.physb.2019.411674
S.D. Ponja, S. Sathasivam, I.P. Parkin, C.J. Carmalt, Highly conductive and transparent gallium doped zinc oxide thin films via chemical vapour deposition. Sci. Rep. 10, 1–7 (2020). https://doi.org/10.1038/s41598-020-57532-7
F. Sarf, I. Karaduman Er, E. Yakar, S. Acar, The role of rare-earth metal (Y, Ru and Cs)-doped ZnO thin films in NH3 gas sensing performances at room temperature. J. Mater. Sci. Mater. Electron. 31, 10084–10095 (2020). https://doi.org/10.1007/s10854-020-03554-w
D. Alagarasan, S.S. Hegde, R. Naik, P. Murahari, H.D. Shetty, S. Prasad Hb, F. Maiz, M. Shkir, Fabrication of high-performance RT-NH3 gas sensor based on Cu and La co-doped ZnO films through a facile drop-casting method. Opt. Mater. (Amst). 147 (2024). https://doi.org/10.1016/J.OPTMAT.2023.114705
K.D. Arun Kumar, S. Valanarasu, J.S. Ponraj, B.J. Fernandes, M. Shakir, S. AlFaify, P. Murahari, K. Ramesh, Effect of Er doping on the ammonia sensing properties of ZnO thin films prepared by a nebulizer spray technique. J. Phys. Chem. Solids. 144, 109513 (2020). https://doi.org/10.1016/j.jpcs.2020.109513
S. Zhao, Y. Shen, A. Li, Y. Chen, S. Gao, W. Liu, D. Wei, Effects of rare earth elements doping on gas sensing properties of ZnO nanowires. Ceram. Int. 47, 24218–24226 (2021). https://doi.org/10.1016/j.ceramint.2021.05.133
A.J. Santhosam, K. Ravichandran, M. Shakir, M. Sridharan, Effect of La incorporation on the NH3 sensing behaviour of ZnO thin films prepared using low-cost nebulizer spray technique. J. Mater. Sci. Mater. Electron. 31, 13240–13248 (2020). https://doi.org/10.1007/s10854-020-03875-w
A. Alsulamei, A. Timoumi, Tailoring the physical and optical properties of Sn-doped In2S3 thin films obtained using VTE technique. Opt. Mater. X. 15, 100176 (2022). https://doi.org/10.1016/J.OMX.2022.100176
A.F. Abdulrahman, S.M. Ahmed, S.M. Hamad, A.A. Barzinjy, Effect of growth temperature on morphological, structural, and Optical properties of ZnO Nanorods using modified chemical bath deposition method. J. Electron. Mater. 50, 1482–1495 (2021). https://doi.org/10.1007/s11664-020-08705-7
Z.N. Kayani, M. Siddiq, S. Riaz, S. Naseem, Optical, magnetic and structural properties of Cr-doped ZnO thin films by sol-gel dip-coating method. Mater. Res. Express. 4 (2017). https://doi.org/10.1088/2053-1591/aa81f1
V.A. Owoeye, E. Ajenifuja, E.A. Adeoye, G.A. Osinkolu, A.P. Popoola, Microstructural and optical properties of Ni-doped ZnO thin films prepared by chemical spray pyrolysis technique. Mater. Res. Express. 6 (2019). https://doi.org/10.1088/2053-1591/ab26d9
M. Shaheera, K.G. Girija, M. Kaur, V. Geetha, A.K. Debnath, R.K. Vatsa, K.P. Muthe, S.C. Gadkari, Characterization and device application of indium doped ZnO homojunction prepared by RF magnetron sputtering. Opt. Mater. (Amst). 101 (2020). https://doi.org/10.1016/j.optmat.2020.109723
K.R. Devi, G. Selvan, M. Karunakaran, K. Kasirajan, M. Shakir, S. AlFaify, A SILAR fabrication of nanostructured ZnO thin films and their characterizations for gas sensing applications: an effect of ag concentration. Superlattices Microstruct. 143, 106547 (2020). https://doi.org/10.1016/j.spmi.2020.106547
S. Rahemi Ardekani, A. Sabour Rouh Aghdam, M. Nazari, A. Bayat, E. Yazdani, E. Saievar-Iranizad, A comprehensive review on ultrasonic spray pyrolysis technique: mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrol. 141, 104631 (2019). https://doi.org/10.1016/J.JAAP.2019.104631
S.D. Lokhande, M.B. Awale, G. Umadevi, V.D. Mote, A study on room temperature acetone sensing ability of Zn1–xNixO thin films and probing their properties for progressive sensor technology. Appl. Phys. Mater. Sci. Process. 129 (2023). https://doi.org/10.1007/s00339-023-07031-z
H. Ji, W. Zeng, Y. Li, Gas sensing mechanisms of metal oxide semiconductors: a focused review. Nanoscale. 11, 22664–22684 (2019). https://doi.org/10.1039/C9NR07699A
L.H. Kathwate, V.D. Mote, Optical and electrical properties of In-doped ZnO films via the Spray Pyrolysis Technique for Optoelectronics device applications. J. Electron. Mater. 51, 6894–6902 (2022). https://doi.org/10.1007/S11664-022-09918-8/METRICS
M. Awale, S.D. Lokhande, S.S. Jadhav, S.B. Kadam, V.D. Mote, A.B. Kadam, Effect on ethanol sensing ability of zinc oxide thin films with manganese doping. J. Mater. Sci. Mater. Electron. 34 (2023). https://doi.org/10.1007/s10854-023-10407-9
X.L. Xu, Y. Chen, S.Y. Ma, W.Q. Li, Y.Z. Mao, Excellent acetone sensor of La-doped ZnO nanofibers with unique bead-like structures. Sens. Actuators B Chem. 213, 222–233 (2015). https://doi.org/10.1016/J.SNB.2015.02.073
R. Sagheer, S.T. Khadija, Z.N. Kayani, S. Riaz, Structural, optical and magnetic properties of ZnO nanoparticles tailored by ‘La3+’ ions, Optik (Stuttg). 244 (2021). https://doi.org/10.1016/j.ijleo.2021.166816
P. Hemalatha, S.N. Karthick, K.V. Hemalatha, M. Yi, H.J. Kim, M. Alagar, La-doped ZnO nanoflower as photocatalyst for methylene blue dye degradation under UV irradiation. J. Mater. Sci. Mater. Electron. 27, 2367–2378 (2016). https://doi.org/10.1007/s10854-015-4034-8
S. Singh, A. Singh, B.C. Yadav, P.K. Dwivedi, Sensors and actuators B: Chemical fabrication of nanobeads structured perovskite type neodymium iron oxide film: its structural, optical, electrical and LPG sensing investigations. Sens. Actuators B Chem. 177, 730–739 (2013). https://doi.org/10.1016/j.snb.2012.11.096
S. Fairose, S. Ernest, Nanostructured ZnO sensor fabricated by successive ionic layer adsorption and reaction method for ammonia sensing application. Phys. B Condens. Matter. 557, 63–73 (2019). https://doi.org/10.1016/j.physb.2018.12.041
R. Thomas, T. Mathavan, M.A. Jothirajan, H.H. Somaily, H.Y. Zahran, I.S. Yahia, An effect of lanthanum doping on physical characteristics of FTO thin films coated by nebulizer spray pyrolysis technique. Opt. Mater. (Amst). 99, 109518 (2020). https://doi.org/10.1016/j.optmat.2019.109518
Y. Zhang, C. Wang, L. Zhao, F. Liu, X. Sun, X. Hu, G. Lu, Microwave-assisted synthesis of La/ZnO hollow spheres for trace-level H2S detection. Sens. Actuators B Chem. 334, 129514 (2021). https://doi.org/10.1016/j.snb.2021.129514
H.A. Ahsaine, A. Slassi, Y. Naciri, A. Chennah, C. Jaramillo-Páez, Z. Anfar, M. Zubair, A. Benlhachemi, J.A. Navío, Photo/Electrocatalytic Properties of Nanocrystalline ZnO and La–Doped ZnO: combined DFT Fundamental Semiconducting properties and experimental study, ChemistrySelect. 3 (2018) 7778–7791. https://doi.org/10.1002/slct.201801729
A. Koo, R. Yoo, S.P. Woo, H.S. Lee, W. Lee, Enhanced acetone-sensing properties of pt-decorated Al-doped ZnO nanoparticles. Sens. Actuators B Chem. 280, 109–119 (2019). https://doi.org/10.1016/j.snb.2018.10.049
H.N. Shivananjaiah, K. Sailaja Kumari, M.S. Geetha, Green mediated synthesis of lanthanum doped zinc oxide: study of its structural, optical and latent fingerprint application. J. Rare Earths. 38, 1281–1287 (2020). https://doi.org/10.1016/j.jre.2020.07.012
A. Ayana, N.B. Gummagol, P. Shankaragouda, P. Sharma, B.V. Rajendra, Materials Science in Semiconductor Processing Enhancement of optical limiting performance in nanocrystalline La 3 + doped ZnO film. Mater. Sci. Semicond. Process. 133, 105931 (2021). https://doi.org/10.1016/j.mssp.2021.105931
V.S. Chandak, M.B. Kumbhar, S.V. Talekar et al., Ultrasensitive and selective Cr-doped ZnO thin films synthesized via spray pyrolysis technique for detection of ammonia gas. Appl. Phys. A 130, 285 (2024). https://doi.org/10.1007/s00339-024-07425-7
G.K. Mani, J.B.B. Rayappan, A highly selective room temperature ammonia sensor using spray deposited zinc oxide thin film. Sens. Actuators B Chem. 183, 459–466 (2013). https://doi.org/10.1016/j.snb.2013.03.132
R.N. Bukke, J.K. Saha, N.N. Mude, Y. Kim, S. Lee, J. Jang, Lanthanum Doping in Zinc Oxide for highly Reliable Thin-Film transistors on Flexible substrates by Spray Pyrolysis. ACS Appl. Mater. Interfaces. 12, 35164–35174 (2020). https://doi.org/10.1021/acsami.0c05151
M.M.A. Ahmed, W.Z. Tawfik, M.A.K. Elfayoumi, M. Abdel-Hafiez, S.I. El-Dek, Tailoring the optical and physical properties of La-doped ZnO nanostructured thin films. J. Alloys Compd. 791, 586–592 (2019). https://doi.org/10.1016/j.jallcom.2019.03.340
C. Mrabet, O. Kamoun, A. Boukhachem, M. Amlouk, T. Manoubi, Some physical investigations on hexagonal-shaped nanorods of lanthanum-doped ZnO. J. Alloys Compd. 648, 826–837 (2015). https://doi.org/10.1016/j.jallcom.2015.07.009
Z. Wu, Z. Li, H. Li, M. Sun, S. Han, C. Cai, W. Shen, Y. Fu, Ultrafast Response/Recovery and high selectivity of the H 2 S Gas Sensor based on α-Fe 2 O 3 Nano-Ellipsoids from one-step hydrothermal synthesis. ACS Appl. Mater. Interfaces. 11, 12761–12769 (2019). https://doi.org/10.1021/acsami.8b22517
Y. Vijayakumar, P. Nagaraju, V. Yaragani, S.R. Parne, N.S. Awwad, M.V. Ramana Reddy, Nanostructured Al and Fe co-doped ZnO thin films for enhanced ammonia detection. Phys. B Condens. Matter. 581 (2020). https://doi.org/10.1016/j.physb.2019.411976
G. Biasotto, M.G.A. Ranieri, C.R. Foschini, A.Z. Simões, E. Longo, M.A. Zaghete, Gas sensor applications of zinc oxide thin film grown by the polymeric precursor method. Ceram. Int. 40, 14991–14996 (2014). https://doi.org/10.1016/j.ceramint.2014.06.099
V. Adimule, M.G. Revaigh, H.J. Adarsha, Synthesis and fabrication of Y-Doped ZnO nanoparticles and their application as a gas sensor for the detection of Ammonia. J. Mater. Eng. Perform. 2020 297. 29, 4586–4596 (2020). https://doi.org/10.1007/S11665-020-04979-4
M.R. Waikar, P.M. Raste, R.K. Sonker, V. Gupta, M. Tomar, M.D. Shirsat, R.G. Sonkawade, Enhancement in NH3 sensing performance of ZnO thin-film via gamma-irradiation. J. Alloys Compd. 830, 154641 (2020). https://doi.org/10.1016/j.jallcom.2020.154641
R.S. Ganesh, E. Durgadevi, M. Navaneethan, V.L. Patil, S. Ponnusamy, C. Muthamizhchelvan, S. Kawasaki, P.S. Patil, Y. Hayakawa, Controlled synthesis of Ni-doped ZnO hexagonal microdiscs and their gas sensing properties at low temperature. Chem. Phys. Lett. (2017). https://doi.org/10.1016/j.cplett.2017.09.057
R. Sankar Ganesh, E. Durgadevi, M. Navaneethan, V.L. Patil, S. Ponnusamy, C. Muthamizhchelvan, S. Kawasaki, P.S. Patil, Y. Hayakawa, Low-temperature ammonia gas sensor based on Mn-doped ZnO nanoparticle decorated microspheres. J. Alloys Compd. 721, 182–190 (2017). https://doi.org/10.1016/j.jallcom.2017.05.315
S.K. Sinha, Growth and ammonia sensing properties of Zn1-xSnxO nanofibers, sensors actuators. B Chem. 219, 192–198 (2015). https://doi.org/10.1016/j.snb.2015.04.129
A. Ramesh, D.S. Gavaskar, P. Nagaraju, S. Duvvuri, S.R.K. Vanjari, C. Subrahmanyam, Mn-doped ZnO microspheres prepared by solution combustion synthesis for room temperature NH3 sensing. Appl. Surf. Sci. Adv. 12, 100349 (2022). https://doi.org/10.1016/j.apsadv.2022.100349
K. Ravichandran, A.J. Santhosam, M. Sridharan, Effect of tungsten doping on the ammonia vapour sensing ability of ZnO thin films prepared by a cost-effective simplified spray technique. Surf. Interfaces. 18, 100412 (2020). https://doi.org/10.1016/j.surfin.2019.100412
D. Wei, W. Jiang, H. Gao, X. Chuai, F. Liu, F. Liu, P. Sun, X. Liang, Y. Gao, X. Yan, G. Lu, Facile synthesis of La-doped In2O3 hollow microspheres and enhanced hydrogen sulfide sensing characteristics. Sens. Actuators B Chem. 276, 413–420 (2018). https://doi.org/10.1016/j.snb.2018.08.130
S. Kanaparthi, S. Govind, Singh, Highly sensitive and ultra-fast responsive ammonia gas sensor based on 2D ZnO nanoflakes. Mater. Sci. Energy Technol. 3, 91–96 (2020). https://doi.org/10.1016/j.mset.2019.10.010
Acknowledgements
VSC is thankful to the Wise Kiran Division, Department of Science and Technology, Government of India, for Women Scientist Scheme-A, sanction order DST/WOS-A/PM-18/2021 (G). Authors are thankful to the department of Physics, Dayanand Science College, Latur for providing synthesis and characteristaion facility.
Author information
Authors and Affiliations
Contributions
VSC: Drafting, data curation, initial drafting, software, data collection. MBK: Software, Initial Analysis. LHK: Initial analysis. VDM: Initial analysis. PMK: Supervision, reviewing-editing, final writing.
Corresponding author
Ethics declarations
Supplementary information
Not applicable.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandak, V.S., Kumbhar, M.B., Kathwate, L.H. et al. Ultrasensitive and selective detection of ammonia gas at room temperature of La-doped ZnO thin films. Appl. Phys. A 130, 611 (2024). https://doi.org/10.1007/s00339-024-07774-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07774-3