Abstract
Due to greater consumption of poultry products and an increase in exports, more poultry houses will be needed. Therefore, it is important to investigate ways that poultry facilities can coexist in close proximity to residential areas without odors and environmental challenges. Ammonia (NH3) is the greatest concern for environmental pollution from poultry production. When birds consume protein, they produce uric acid, ultimately converted to NH3 under favorable conditions. Factors that increase production include pH, temperature, moisture content, litter type, bird age, manure age, relative humidity, and ventilation rate (VR). NH3 concentration and emissions in poultry houses depend on VR; seasons also have effects on NH3 production. Modern ventilation systems can minimize NH3 in enclosed production spaces quickly but increase its emissions to the environment. NH3 adversely affects the ecosystem, environment, and health of birds and people. Less than 10 ppm is the ideal limit for exposure, but up to 25 ppm is also not harmful. NH3 can be minimized by housing type, aerobic and anaerobic conditions, manure handling practices, litter amendment, and diet manipulation without affecting performance and production. Antibiotics can minimize NH3, but consumers have concerns about health effects. Administration of probiotics seems to be a useful replacement for antibiotics. More studies have been conducted on broilers, necessitating the need to evaluate the effect of probiotics on NH3 production in conjunction with laying hen performance and egg quality. This comprehensive review focuses on research from 1950 to 2018.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The USA is the second largest egg producer in the world after China. According to data published by the USDA in the World Agricultural Supply and Demand Estimate (June, 2017, WASDE), total egg production in the USA was 104.988 billion (8.749 billion dozen) which was 2.1% more than 2016. According to this report, US egg production is expected to increase by 1.6% to 8.890 billion dozens in 2018. USDA also expects an increase in egg exports in 2017 and 2018. It was predicted that 302.8 million dozen (8.5% more than previous year) and 320.0 million dozen (up 5.7% from 2017) would be exported. If these trends in the US continue, more laying hens will be needed to meet the demand for eggs, prompting the need for more poultry houses. Historically, people living in close proximity to poultry houses have complained about associated foul odors. Gases such as ammonia (NH3), hydrogen sulfide, and volatile sulfur compounds are responsible for some of these complaints. Odorless methane is often associated with the volatile gases.
Presently, NH3 produced in poultry houses is a concern for the health of poultry, human, and environment. In this comprehensive review, we discuss major factors leading to formation of NH3. These factors include determinants of NH3 in poultry facilities, seasonal and geological effects, NH3 in the environment, its effects on human and poultry health, and techniques for NH3 reduction in poultry production including housing type, aerobic and anaerobic conditions, litter amendments, and diet manipulation. We conclude by discussing the most important strategies to reduce NH3 in poultry production.
Formation of NH3 in poultry
Uric acid is the major source of NH3 formation in poultry, mostly occurring in the ceca. The microbial breakdown of large amounts of uric acid in feces and urine results in urea in the presence of an enzyme, uricase, and eventually into NH3 (Almuhanna et al., 2011; Schefferle 1965; O’Dell et al. 1960; Mahimairaja et al. 1994; Whyte 1993; Kim and Patterson 2003a,b; Bachrach 1957; Moore 1998; Anderson et al. 1964; Li et al. 2013; Santoso et al. 1999; David et al. 2015; Creek and Vasaitis 1961).
The hydrolysis of urea to NH3 and carbon dioxide (CO2) by urease activity is shown in the following reaction (Figs. 1 and 2).
Probable pathway of aerobic breakdown of uric acid by the pseudomonads used (Bachrach 1957)
Bachrach (1957) showed the following schematic representation of degradation of uric acid into NH3.
The following is the overall reaction suggested by Bachrach (1957).
Bacillus pasteurii (a ureolytic bacteria that facilitates NH3 production) has no growth in acidic conditions. Thus, NH3 formation from uric acid is more favorable at a pH higher than 7 (Li et al. 2013; Elliott and Collins 1982).
Several studies have shown that NH3 formation depends on the amount of urea, urease activity, pH, temperature, relative humidity (RH) air velocity/ventilation rate (VR), manure handling practice, litter, bird age, and moisture content (MC).
Determinants of NH3 formation in poultry facilities
Table 1 is a compilation of results for effects of various determinants of NH3 production.
Results of all studies in Table 1 are in agreement with findings of Almuhanna et al. (2011), Schefferle (1965), O’Dell et al. (1960), Mahimairaja et al. (1994), Whyte (1993), Kim and Patterson (2003a), Bachrach (1957), Moore (1998), Anderson et al. (1964), Li et al. (2013), Santoso et al. (1999), David et al. (2015), and Creek and Vasaitis (1961).
For instance, a 2-year study was conducted to find the relationship between pH and NH3 volatilization. Three different diets [a control, EcoCal (natural mixture of zeolite and gypsum) and DDGS (corn-dried distiller grain with solubles)] were fed to laying hens. Results showed a direct relationship between pH and NH3 emissions. EcoCal, DDGS and the control had a pH of 8.0, 8.9, and 9.3, respectively. The acidifier (gypsum) content of EcoCal decreased the pH and ultimately less N to convert into aerial NH3. MC also had an important role in NH3 production. Based on the results of this study, it was illustrated that EcoCal had a higher MC (50.2%) than the control (46.1%) and DDGS (43.5%). As shown in Table 2, no significance difference in organic nitrogen (Org-N) and total Kjeldahl nitrogen (TKN) was recorded for all three diets while on a dry matter (DM) basis, 68% more NH3-N was measured in the EcoCal diet than in the control (Li et al. 2012).
Generally, they show the direct relationship between pH and NH3 production. pH at more than 7 is responsible for NH3 production and its volatilization from poultry manure while nitrogen (N) stays in the form of ammonium (NH4+) when pH is less than 7.
The relationship between NH3 and hydrogen ion concentration ([H+]) was elucidated by Xue et al. (1998) who trapped NH3 from manure storage facilities. Calculations showed that less [H+] produced more free NH3 as shown in the following equation. pH is the negative log of [H+]; therefore, a higher pH indicates higher free NH3 (Xue et al. 1998).
Bird age is also important in NH3 production. As the chicken’s age increase, they produce more NH3; this is clearly shown in Table 1.
Results of another study revealed the relationship between bird age and NH3 production. Laying hens of three different age groups (21-, 38-, and 59-week-old) were housed to observe the effect of bird age on NH3 volatilization. Manure from the youngest hens volatilized less NH3 as compared to other two groups (Wu-Haan et al. 2007).
VR also affects NH3 concentration and emissions. Various studies reported a positive relationship between VR and emissions while an inverse relationship was reported for concentration and VR. This means that NH3 emissions increases with the increase of VR in summer and vice versa in winter. Ultimately, greater water content is associated with more NH3 production.
Seasonal and geological effects
It has been reported that NH3 production also depends on seasons and geological sites. Weather and temperature cause various seasons and seasonality affects volatilization. Eighteen consecutive flocks of broilers were studied for seasonal effects. These birds were observed until 40–42 days of age and consumed a commercial diet. Significant difference in volatilization for winter and summer were observed as shown in Table 3 (Coufal et al. 2006).
The dependence of NH3 reduction on seasons was observed in a 2-year study. NH3 volatilization by the EcoCal diet varied from − 7.1% in September 2008 to 72.2% in February 2009 while it varied from 16.3% in September 2008 to 51.0% in October 2009 with the DDGS diet. More (p < 0.01) NH3 concentration was found in winter than in summer (Li et al. 2012).
NH3 in the environment
Adverse effects of NH3 on environment; ecosystem; and health of humans, animals, and birds were revealed in many studies. Environmental groups/agencies have also pressured producers to lower NH3 emissions (Li et al. 2013; Liang et al. 2005).
NH3 is a precursor of secondary particulate matter (PM2.5) and contributes to the production of PM2.5 (Xin et al. 2011; Baek and Aneja 2004). It produces these particles when combined with oxides of N and sulfur. These very small particles affect human health as discussed below (NH3 effects on human health). As well as producing PM2.5, atmospheric NH3 can alter oxidation rates in clouds and can also elevate acid rain production (Xin et al. 2011; Baek and Aneja 2004; Baek et al. 2004; van Breemen et al. 1982; ApSimon et al. 1987; Sharma et al. 2007).
NH3 contributes to acidification in soil and N deposition in ecosystem (Li et al. 2013; Liang et al. 2005; Jones et al. 2013). Moreover, nitrifying bacteria in the soil convert it into nitrates which lower pH of ground water and increase concentrations of nitrates in drinking water (Santoso et al. 1999; Adams et al. 1994). Van Breemen, 1988 and Angus et al. (2003) reported N contribution in eutrophication, acidification, and nitrification of groundwater and leaching.
NH3 effects on human health
NH3 is a known irritant of the mucous membranes in the upper respiratory tract, nose, and eyes (Santoso et al. 1999; Ihrig et al. 2006; Almuhanna et al. 2011; Pratt et al., 1998); thus, it can damage the respiratory system of workers (Fig. 3) at all levels (Nararaja et al. 1983; Whyte 1993; Charles and Payne 1966). This was confirmed in an epidemiological study by Hartung (2005).
Effects on respiratory system (http://hotnewsnaija.ng/wp-content/uploads/2016/10/Respiratory.png)
As mentioned above, NH3 is responsible for the production of PM2.5 which can penetrate deeper into the respiratory system of humans and animals where they damage tissues. Birds (feather and skin dander), feed particles, litter, and feces can also be responsible for production of different sizes of inhalable PM2.5. Higher concentrations of NH3 in air affect the respiratory system of humans while cough, nose, and throat irritation can also be caused by lower concentrations. Sundblad et al. (2004) also found increased symptoms of irritation and central nervous system effects upon NH3 exposure. Poultry workers are adversely affected by NH3 as compared to non-poultry workers; this is supported by many epidemiological studies. Workers exposed to NH3 in poultry confinements experienced burning and watery eyes, sneezing, stuffy and running noses, and also coughs (Sanderson et al. 1995; Rees et al. 1998).
Respiratory symptoms during and after work in poultry houses has increased in recent years. All studies showed acute and chronic effects on poultry workers’ health (Kirychuk et al. 2003; Zuskin et al. 1995; Reynolds et al. 1993; Santoso et al. 1999; Close et al. 1980; Morris et al. 1991). It was also reported that respiratory symptoms were greater in winter months and pulmonary function also decreased the number of work days (Reynolds et al. 1993). Extensiveness of chronic cough, chronic phlegm, chronic bronchitis, and chest tightness were higher in poultry workers and chicken catchers than the control and non-exposed blue-collar workers (Zuskin et al. 1995; Morris et al. 1991). A recent study revealed that a water-based sprinkler cooling system did not reduce NH3 concentration nor did it improve worker health (Ischer et al. 2017).
NH3 effects on the health of poultry
Almuhanna et al. (2011) reported that NH3 is the most abundant toxic gas in poultry houses (Fig. 4).
Measured concentration of toxic gases (NH3, SO2, NO2, H2S) (Almuhanna et al. 2011)
NH3 is a colorless gas with a characteristic pungent smell. It is the most common, noxious, and highly water-soluble gas. NH3 is alkaline and corrosive, adversely affecting the chicken’s nasal cavity and eyes. It reacts with nasal moisture to produce the corrosive effect shown below.
As shown in the equation, the NH4+ solution formed corrodes the respiratory system of chicken and consequently results in paralyzed or lost cilia. Mucus on the mucosal surface of the trachea becomes unclear due to corrosion of cilia which leads trapped bacteria to air sacs and lungs and ultimately causes infection (Aziz and Barnes 2010; Maliselo and Nkonde, 2015; Quarles and Kling 1974; Anderson et al. 1964; Oyetunde et al. 1978; David et al. 2015; Nararaja et al. 1983). Contradictory results were reported by Al-Mashhandani and Beck (1985) who noted no observable effects of NH3 on the appearance of lungs and trachea. As shown in Fig. 5, production of NH3 also affects both the performance and health of birds by preventing mobility.
Different levels of NH3 affect birds’ health and performance as shown in Table 4.
Beker et al. (2004) mentioned that no significant differences were recorded in final body weight, body weight gain, and feed consumption when 1-day-old broiler chicks were exposed to 0, 30, and 60 ppm NH3 concentrations. In addition, or perhaps related to health, Yi et al. (2016b) found insignificant differences in average daily gain, average daily feed intake, and feed conversion ratio when chicken were exposed to 3 and 25 ppm NH3. No significant differences in feed conversion and mortality with 25 and 50 ppm NH3 exposure were reported by Reece et al. (1981). Average body weight, air sac scores, lung weights, and Bursa of Fabricius did not differ significantly in ammoniated birds when compared to the control (Caveny et al. 1981).
Many experiments on the effects of NH3 on birds’ health were conducted in the middle of twentieth century and continue. While reviewing the literature, it was difficult to discern the meaning of low, medium, and high because actual quantities in ppm were not provided.
Generally, recommendation of NH3 concentration in poultry houses is less than 25 ppm. Ideally, NH3 exposure should be less than 10 ppm but temporarily exceeding the limit to 25 ppm is not harmful (NOISH, 2016; Animal Husbandry Guidelines for US Egg Laying Flocks, 2010; Miles et al. 2006; Kristensen et al. 2000). According to the Occupational Safety and Health Administration (Aziz and Barnes 2010), 50 ppm for 8-h exposure is the recommended concentration of NH3 in a poultry house or OSHA has recommended no more than 35 mg/m3 for 8-h daily exposure (EPA 2016). It is the lowest concentration which can irritate eyes, nose, and throat.
Techniques for NH3 reduction in poultry production
Modern ventilation systems in the poultry houses can reduce NH3 concentration quickly but also increase its emissions in the atmosphere simultaneously. Poultry health is improved by exhausting NH3 to the outside; however, exhausted NH3 affects the surrounding environment and, ultimately, the ecosystem. Therefore, actions are necessary to control both concentration and emissions of this toxic gas. A review of the literature discusses many techniques to reduce N production and consequently, NH3.
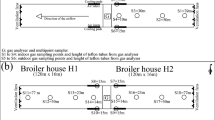
Mitigation, without affecting birds’ production performance, includes housing type (Fig. 6a–j), bird age, manure age or handling practices, building VR, and diets [low crude protein (CP), synthetic amino acids, addition of fiber, and use of probiotics]. These strategies are discussed below.
a Cross section of high-rise (https://www.researchgate.net/figure/221971812_fig2_Figure-2-Cross-section-view-of-the-monitored-high-rise-laying-hen-houses-and-sampling). b Manure-belt (https://www.alibaba.com/product-detail/poutry-Manure-belt-conveyor-belt-for_60499340942.html). c Cross section of manure-belt (https://www.researchgate.net/figure/274345115_fig1_Figure-1-Cross-section-of-the-aviary-hen-house-one-side-of-the-double-house-monitored). d Deep-pit (http://www.thepoultrysite.com/poultrynews/37795/midwest-deep-pit-layer-house/). e Cage free (https://lpelc.exposure.co/layer-chicken-housing-and-manure-management, http://www.onegreenplanet.org/animalsandnature/think-you-know-free-range-and-cage-free-chicken-think-again/). f Stilt house (https://www.pinterest.com/pin/368521181983914852). g Conventional cage (http://www.thepoultrysite.com/articles/3543/the-laying-hen-housing-research-project/). h Enriched colony (http://www.thepoultrysite.com/articles/3543/the-laying-hen-housing-research-project/). i Aviary house (http://www.thepoultrysite.com/articles/3543/the-laying-hen-housing-research-project/). j Broiler house (https://www.wright.ie/case-study/modern-poultry-house-facility-broiler-house-co-monaghan/)
Housing type
There are two common house styles - [high-rise (HR) (Fig. 6a) and manure-belt (MB) (Fig. 6b, c)] in the US egg industry. Manure is removed once a year in HR while in MB, it is removed two to seven times per week. A study was conducted to measure the concentration and emissions rate in both house styles. Commercial layer houses in Iowa and Pennsylvania were used in this study. After 1 year, it was concluded that MB significantly lowered both NH3 concentration and its emission rate as compared to HR. In addition, a comparison between daily and semi-weekly removal was made. Daily removal showed 74% less NH3 emission rate in comparison to semi-weekly (Liang et al. 2005). Findings from Green et al. (2009), Koerkamp (1994), Li and Xin (2010), Ni et al. (2012, 2017a), Keener et al. (2002), Roumeliotis and Van Heyst (2008), and Mendes (2010) also support these results. Appropriate temperature and NH3 levels were found both in HR and MB in winter while in summer, no difference was observed in NH3 level in all house types. House temperature was slightly, though not significantly, higher than the ambient temperature (Liang et al. 2005).
A review conducted by Xin et al. (2011) also reported less NH3 release from MB houses due to less surface area and less MC.
Green et al. (2009) conducted a field study to determine the best housing type for reduction of NH3. Cage-free floor-raised (FR) (Fig. 6e), HR (Fig. 6a), and MB (Fig. 6b, c) were used in this study. Results illustrated that NH3 emissions was higher in FR (46 ppm) than HR (14 ppm) and MB (7 ppm). Similar findings were noted by Nimmermark et al. (2009), Koerkamp and Bleijenberg (1998), and Costa et al. (2012).
Three houses were used to accommodate laying hens by Nicholson et al. (2004). One of three houses had a belt-scraped design (Fig. 6b, c). Deep-pit (Fig. 6d) and stilt designs (Fig. 6f) were used for the second and third houses, respectively. Daily removal of manure for the belt-scraped design reduced NH3 more than 2× when compared to weekly removal; these results were confirmed by Koerkamp et al. (1996). In the deep-pit house, more NH3 was volatilized than in the belt-scraped and stilt houses. Koerkamp et al. (1996) and Keener et al. (2002) also reported comparable findings. Additionally, Fournel et al. (2012) reported lower NH3 concentration and emissions from manure for the belt-scraped design than for the HR house (Nicholson et al. 2004; Zhao et al. 2013; Fabbri et al. 2007).
The effect of housing type on NH3 emission rate (ER) was observed in the study of Shepherd et al. (2015). Three different houses [conventional cage (CC) (Fig. 6g), enriched colony (EC) (Fig. 6h), and aviary house (AV) (Fig. 6i)] were used to monitor gas volatilization. The results indicated that NH3 in CC and EC was significantly less compared to AV.
Housing type with other factors
Nicholson et al. (2004) conducted a study to find the relationship between manure handling practices and NH3 volatilization in both broiler and laying hen houses. It was reported that NH3 emissions was greatly influenced by housing type, manure removal, drinker (Fig. 7a, b), and litter type. Housing type and land spreading are the most important factors in NH3 losses. In broiler houses, two different types of litter (straw and wood shaving) were used. There was no difference in NH3 losses in summer from both types of litter, and similar findings were also discussed by Elwinger and Svensson (1996) and Tasistro et al. (2007). Moreover, it was mentioned that houses with bell drinkers emitted more NH3 (numerically) than houses having nipple drinkers, and these results were also similar to that of Elwinger and Svensson (1996) and Da Borso and Chiumenti (1999). It was reported that most of the gas lost occurred during transportation, but no differences were recorded during storage and land spreading.
a Bell drinker (http://www.wesstron.pl/drob.php?lang=en&id_strony=82). b Nipple drinker (https://www.indiamart.com/proddetail/poultry-nipple-drinker-system-)
Aerobic and anaerobic conditions
Mahimairaja et al. (1994) performed an experiment to investigate the effect of four carbon-rich bedding materials, one acidifying material, and two adsorbents on N transformation and its loss in poultry manure under aerobic and anaerobic conditions. Anaerobic conditions showed a significant reduction in NH3 volatilization in comparison to the aerobic environment after 12-week incubation period of poultry manure (Table 5). Bachrach (1957) also discussed the similar results.
Findings for NH3 production in aerobic and anaerobic environments were also supported by Kirchmann and Witter (1989). A study was conducted on fresh chicken manure combined with oat straw for both aerobic and anaerobic conditions. Less NH3 was volatized in an anaerobic condition due to low pH; possibly NH3 losses to some extent, were dependent on quantity of straw present in aerobic condition but no effect was observed in an anaerobic environment. Significantly higher NH3 volatilization in aerobic versus anaerobic decomposition was also reported by Kirchmann and Lundvall (1998).
Litter amendments
A review of several studies reported that NH3 emissions can be minimized by litter amendment. It was helpful in all poultry houses, especially in laying hen facilities (Roumeliotis and Van Heyst 2008). As shown in Table 6, significant effects of litter amendments on NH3 reduction were observed.
In addition to the negative effect of acidic electrolyzed water, several studies showed that litter amendment was the best way to control NH3 concentration and emissions. Different types of adsorbents, inhibitors, and bedding materials were applied in poultry houses. Alum and zeolite were used most commonly. When these materials were added to the litter, they lowered pH and produced more NH4+ rather than NH3. Some inhibitors reduced urease activity, responsible for NH3 production.
Uricase-specific antibody (IgY) from hens immunized with uricase by triplicate injections suppressed microbial uricase activity (Kim and Patterson, 2003b). The investigators suggested that more work was needed to ascertain the effect of the uricase-specific IgY on microbial uricase, possibly reducing NH3 volatilization from poultry manure. In contrast to results of Nakaue et al. (1981), no effect of clinoptilolite (zeolite) on ammonia reduction was observed when laying hens were fed in a 28-day study (Nakaue and Koelliker 1981). Litter amendment does not commonly affect birds' performance and production. Ali et al. (2000) reported contradictory results indicating that alum affected broilers performance.
Figure 8a–c shows the different types of litter amendments in poultry houses.
a Litter amendment with gypsum (https://www.usagypsum.com/gypsum-products/gypsum-poultry-litter-amendment). b Litter amendment with pine shaving (http://www.thepoultrysite.com/articles/3554/alternatives-to-pine-shavings-for-poultry-bedding/). c Litter amendment with wood shaving and poultry additive, zeotec (https://www.bpmnz.co.nz/en/products/zeotec/)
Diet manipulation
Several studies showed that NH3 production can be decreased by diet manipulation (Hale 2005; Roberts et al. 2015). Positive results without affecting birds’ performance and production were obtained in most of them.
Low crude protein
High protein in the diet of layers is mainly responsible for elevated production of NH3. Poultry cannot store excess amino acids, resulting in released N, mostly in the form of uric acid (Kristjan and Roberts 2006).
Liang et al. (2005) explained the influence of reduced CP on NH3 emissions. Birds were fed a diet containing 1% low CP and essential amino acid supplements. There was significantly lower NH3 concentration and its emissions was low in houses with low CP. Liang et al. (2003), Gates (2000), Ji et al. (2014), Meluzzi et al. (2001), and Nahm (2007) found similar results. Contradictory findings were shown in the study of Burley (2009).
Results of another study revealed that by adding low CP (and lysine), NH3 gas concentration decreased by 31% and litter N by 16.5% (DM basis) as shown in Table 7. Broilers were fed with three different diets: high CP (High), low CP with additional synthetic amino acids (Low), and an equal blend (1:1) of High and Low CP treatments (Medium). Low CP reduced NH3 and litter N by lowing pH and MC (Ferguson et al. 1998). Similar results were obtained in studies by Blair et al. (1999), Gates et al. (2000), and Namroud et al. (2008).
Direct relationships between dietary protein and NH3 emissions, litter N, or total N were found in several other studies. Results showed that these factors increased with the increase of CP (Elwinger and Svensson 1996; Summers 1993; Keshavarz and Austic 2004; Robertson et al. 2002; Aletor et al. 2000; Rezaei et al. 2004).
The effects of dietary protein were also observed in laying hen houses. A 2-year study was conducted using three commercial HR houses (Fig. 6a). Three different diets included a control, 7% EcoCal, and DDGS. Results showed that Ecocal and DDGS significantly lowered NH3 volatilization compared to a control diet. EcoCal and DDGS had 39.2% and 14.3% less NH3 emissions, respectively. Manure obtained from the EcoCal diet had higher NH3-N retention (68%) than the control. NH3 emission rates are shown in Table 8 (Li et al. 2012). The findings of Roberts (2009) showed contrasting results for DDGS.
Wu-Haan et al. (2007) confirmed findings of others when two different diets were fed to three different age groups of hens. Diets were a reduced-emission (RE) diet, containing a mixture of 6.9% of CaSO4-zeolite and slightly reduced CP and a commercial diet (CM). Laying hens (21-, 38-, and 59-week-old) were used in this study. Significant reduction (p < 0.01) in NH3 emissions was observed when hens were fed the RE diet. Other investigators reported similar findings (Romero et al. 2012; Xin et al. 2005; Cabuk et al. 2004, Wu-Haan 2006). Lon-Wo (2010) also reported less NH3 volatilization when hens were fed a diet containing 3% natural zeolite (Clinoptilolite) instead of a control diet. After 10 days, NH3 emissions from manure of hens fed zeolite was only 30.6 ppm while 937 ppm NH3 emissions was reported for the control (Nakaue et al. 1981; Hale 2005).
Findings of Nakaue and Koelliker (1981) and Karamanlis et al. (2008) were not in agreement with the results discussed above. Ferguson et al. (1998) also reported an insignificant effect of dietary protein on NH3 gas emissions, MC, and pH; but, litter N was lowered significantly by adding low CP and P in a broiler diet. Results also showed that gaseous NH3 production was inversely proportional to dietary P, and this relationship was previously explained by Taraba et al. (1980) as well. Based on the results, it was suggested that NH3 concentration depended on pH and litter surface moisture which were not sensitive response variables as compared to chemical analyses (Ferguson et al. 1998; Taraba et al. 1980). Analysis by Burley et al. (2013) showed no significant difference in NH3 emissions when laying hens were fed diets containing different levels [low, intermediate, and high (control)] of CP.
Fiber
According to Roberts et al. (2007b), NH3 emissions can be diminished by feeding a fibrous diet because (1) amino acids in ingredients of a highly fibrous diet are less digestible as compared to that of those in a low fibrous diet and (2) amino acids in a fibrous diet are not available to degrade to urea and consequently to NH3. Additionally, investigators stated that fermentable fiber can change the form of N excretion from urea (urine) to microbial protein (feces). Microbial protein is more stable and less degradable to NH3. Fiber also helps in minimizing pH of the manure by production of volatile fatty acids. This is important because low pH retards production of NH3 and produces more NH4+ ions which are not volatile (Roberts et al. 2006, 2015).
Roberts et al. (2006) also reported that hens digest less fiber, so diets with high fiber decrease protein digestibility and increase excretion of N (Fig. 9). In this study, three types of fiber (soy hulls, wheat middlings, and DDGS) were added to the diet. Inclusion rate for DDGS was 10% and wheat middlings were also included to obtain the same neutral detergent fiber. Researchers found no difference in N excretion both in the control and the diet with fiber. Wheat middlings caused a significant reduction in uric acid excretion which is the main source of NH3 production in birds. pH is also important in NH3 production, and addition of fiber decreased pH of the manure without affecting hen production performance. Reduced uric acid and decreased pH were factors in minimizing NH3 volatilization (Pineda et al. 2008).
Total ammonia emission from manure over 7 days. Data are means ± pooled SEM, n = 6. *Different from control (p < 0.05) (Roberts et al. 2006)
Roberts et al. (2007a) also supported findings in Fig. 10. This study was conducted with 17-week-old laying hens. Hens were fed eight different diets [normal crude protein = corn and soybean meal control diet, control with 10% DDGS, 7.3% wheat middlings (WM), and 4.8% soybean hulls (SH); reduced CP = corn and soybean meal control diet, control with 10% DDGS, 7.3% wheat middlings (WM), and 4.8% soybean hulls (SH)] . Addition of higher fibers lowered NH3 emissions from manure over 7 days in comparison to the control. Reduced CP by 1% did not lower NH3 emissions as supported by results from previous studies with reduced CP (Bregendahl et al. 2002; Roberts et al. 2007b; Bregendahl and Roberts 2007).
Ammonia emission rate from manure. Data are means ± pooled SEM, n = 6 (Roberts et al. 2006)
One possible high fiber dietary addition for reduction of NH3 is meal made from sunflower seed, a globally grown oil crop having high fiber and fat. Meal is the by-product of seed processing (with or with part of the hull) left after oil extraction and could be an important protein source in animal diets (Kalmendal et al. 2011; Laudadio et al. 2014; Selvaraj and Purushothaman 2004, Ditta and King, 2017). Although it provides very low utilizable carbohydrate and low lysine, 25% sunflower seed meal (SFM) could be used in a balanced diet without affecting weight gain or feed efficiency of growing chicks (Rodriguez et al. 1998). It contains linoleic acid which is a fat source for laying hens (San Juan and Villamide 2001). Abdelrahman and Saleh 2007 reported that SFM can be effectively used rather than soybean meal. It contains about the same average CP (30–32%), a higher quantity of methionine, and less lysine as compared to soybean meal. SFM (10%) could be used to improve average body weight and lessen feed cost for production. Another advantage of SFM is that it is free of most antinutritional factors (Senkoylu and Dale 1999; Deaton et al. 1979; Rose et al. 1972; Walter et al. 1959; Villamide and San Juan 1998).
Bamboo charcoal
Maliselo and Nkonde (2015) recommended the use of bamboo charcoal particles in diet manipulation to minimize NH3 emissions. These results indicated that bamboo charcoal had adsorption properties to bind NH3.
Probiotics
Along with decreasing CP, feeding essential synthetic amino acids, and adding fiber to reduce N excretion, use of antibiotics also created positive results; but, consumers have concerns as antibiotics are deposited in eggs and thereby, may cause antibiotic resistance in humans. Odors may be decreased by administration of live microorganisms (probiotics), which are non-pathogenic and non-toxic. They help hosts maintain health by (1) fortifying the digestive system and (2) lowering NH3 concentration by competitive exclusion of other bacteria.
Several studies have been conducted to analyze the effect of probiotics on humans. Along with humans, many investigations were conducted to reduce NH3 production without affecting birds' health and performance.
Probiotic (Lactobacillus casei) suppressed NH3 production in the GI tract of broiler chicken in a 6-week study. Lactobacillus casei at 0.1%, chloroxytetracycline (antibiotic) at 0.1%, and yucca extract at 0.2% were added in the diet. Two-day-old broiler chicks were randomly assigned to a control and treated diets. Diets containing probiotic showed significant effects on feed intake and weight gain. Urease activity also decreased in the GI tract of broilers which were fed the diet containing probiotic. These findings concluded that dietary probiotic restrained bacterial growth which was responsible for urease activity and, ultimately, NH3 production (Yeo and Kim 1997). Isshiki (1979) also found positive effects of dietary Lactobacillus casei on reduction of non-protein N and urea N which resulted in decreased uric acid and NH3 level (Isshiki 1979).
Environmental NH3 levels in the broiler house were decreased by feeding a lactobacilli probiotic (ecozyme). Two experiments were conducted on 56-day-old-male broilers. Probiotic contained Lactobacilli ecozyme with a minimum of 6.0 CFU per gram of the product in experiment 1 while 3.0 CFU per gram of the product was added in experiment 2. Birds in one treatment were fed a control diet without any probiotic while birds in the other treatment were fed a control diet containing 5% ecozyme. There was a significant difference in NH3 concentration between two treatments after week 3 (Fig. 11).
Environment ammonia concentration of broiler room as affected by ecozyme diet supplementation. Exp. 1: log 6 Lactobacilli cfu/g feed; exp. 2: log 3 Bactobacilli cfu/g feed (Chang and Chen 2003)
Statistically similar results were obtained by feeding ecozyme with 3.0 CFU/g. Earlier studies illustrated that NH3 production depended on pH and MC, so lower pH and lower MC in both experiments were observed in the fecal matter of treated birds (Chang and Chen 2003).
Ahmed et al. (2014) also conducted a study to analyze the effect of dietary probiotics (Bacillus amyloliquefaciens, BAP) on NH3 production. Four hundred 1-day-old male broiler chicks were fed with commercial broiler feed containing five different levels of BAP (0, 1, 5, 10, and 20 g/kg of BAP). Table 9 shows the relationship between different levels of BAP and NH3 volatilization. Higher NH3 emissions from the control at all the incubation times were recorded. It was also observed that NH3 volatilization reduced as the probiotic level (20 g/kg of BAP) was increased. BAP lowered the pH and ultimately NH3 volatilization (Ahmed et al. 2014).
Santoso et al. (1999) conducted two experiments on 65-week-old Hyline W36 and 2-week-old broiler chicks to determine the effect of dried Bacillus subtilis culture (DBSC) on body weight, feed intake, protein intake, feed conversion ratio, NH3 gas, total N, urate N, NH3-N, N utilization, and serum urea-N. It was reported that DBSC significantly decreased NH3 gas without affecting chicken body weight, feed intake, and egg production. It was also observed that DBSC did not decrease total N in feces. Possibly, DBSC produced subtilin which helped to inhibit urease producing microflora in the gastrointestinal lumen and eventually NH3. DBSC also produced a substance which helped to reduce NH3 gas by binding N present in the feces.
The above findings were also supported by the results of Tanaka and Santoso (2000) in a 3-week study. Seven-day-old female broiler chicks were fed different levels of fermented product from Bacillus subtilis. A significant decrease in NH3 production was recorded in treated chicks when compared to the control; but, there was no difference in total N, urate N, NH3-N in fecal matter, pH, and NH3-N of cecum in both groups of chicks.
Dried Bacillus subtilis natto reduced NH3 level in chicken blood when it was fed at different levels in two experiments. Diets having 0, 0.5, 1, and 3% levels of Bacillus subtilis natto were fed to White Leghorn chickens for 3 days in experiment 1 and 0, 0.2, 0.5, and 1% levels were fed for 28 days in experiment 2. It was reported that 0.5% dietary Bacillus subtilis natto depressed the NH3 concentration in chicken blood in experiment 1 and in all levels in experiment 2. It was concluded that Bacillus subtilis natto restrained the growth of urease-producing bacteria and consequently NH3 concentration (Samanya and Yamauchi 2002).
Hmani et al. (2017) reported less NH3 production when Bacillus subtilis HB2 was fed to chicken. Bacillus subtilis UBT-MO2 was found to be effective in decreasing NH3 emissions in broiler fecal matter. Mixed sex broilers were fed two levels of enramycin (0 or 5 ppm) and Bacillus subtilis (0 or 105 cfu/kg). The NH3 gas was significantly (p = 0.03) lowered in broilers fed diets containing Bacillus subtilis in contrast to that for bird sans Bacillus subtilis. Enramycin or Bacillus subtilis did not impact Escherichia coli and Lactobacillus in ceca and small intestine. Bacillus subtilis showed more effective results when added alone as shown in Table 10 (Zhang et al. 2013).
Zhang and Kim (2013) reported less NH3 emissions when 40-week-old laying hens were fed a diet supplemented with 0.01% probiotic (Enterococcus faecium DSM 7134). It was reported that probiotic improved intestinal microbial balance of treated hens and ultimately less NH3 volatilization. Excreta of probiotic treated hens had more lactobacillus counts and less Escherichia coli counts in comparison to that of hens fed no probiotics.
According to the literature, multistrain probiotics provided better results and improved functionality when compared to monostrain (Timmerman et al. 2004; Chapman et al. 2011). Yoon et al. (2004) reported the effect of multiple probiotics on NH3 gas emissions in broiler chicks. One-day-old male broiler chicks were fed with two levels of diets containing probiotics (0 and 0.2%) and three levels of drinking water containing probiotics (0, 0.01, and 0.1%). Drinking water with a 0.1% level significantly decreased NH3 gas volatilization from broiler fecal matter in contrast to the 0% level. Overall, it was noticed that dietary probiotics reduced this noxious gas emissions from fecal matter. In a similar manner, Hassan and Ryu (2012) illustrated the effect of multiprobiotics [Lactobacillus plantarum (5 × 107 cfu/g), Saccharomyces cerevisiae (6 × 107 cfu/g), and Bacillus subtillis (2 × 107 cfu/g)] on NH3 gas emissions. The work of Chiang and Hsieh (1995) supported these findings. There was no indication in reduction of malodor by their results, but NH3 level in fecal matter was lowered by feeding probiotics containing Lactobacillus acidophilus, Streptococcus faecium, and Bacillus subtilits. The effect of dietary probiotics on NH3 emissions was also observed when broilers were fed Bacillus subtilis and Lactobacillus acidophilus versus the control and antibiotics. NH3 emissions was measured after feeding probiotics and antibiotics for 15 and 35 days. The results showed significant difference in NH3 reduction in fecal matter of broilers fed probiotics after 15 and 35 days as compared to the control plus antibiotics. Malodor in probiotic fecal matter was detected as lighter than the control and antibiotics. The findings of this study proved the importance of probiotics’ use in NH3 reduction (Chen et al. 2012).
Zhang and Kim (2014) conducted a study to examine the effects of multistrain probiotics on excreta odor for broilers. Lactobacillus acidophilus, Bacillus subtilis, and Clostridium butyricum were added in the control diet with different levels [control diet + 1 × 105 cfu of multistrain probiotics/kg of diet (P1) and control diet + 2 × 105 cfu of multistrain probiotics/kg of diet (P2)]. In another treatment, antibiotic avilamycin (5 mg/kg of avilamycin) was added to the control diet. P2 significantly lowered NH3 concentration in contrast to all other treatments. P1 also decreased NH3 concentration as compared to the control. At days 3 and 5, both P1 and P2 reduced NH3 production in broiler fecal matter over that for the control as shown in Table 11.
Results from Hossain et al. (2015) supported the above findings when tri-strain probiotics (TSP, Bacillus subtilis, Clostridium butyricum, and Lactobacillus acidophilus) were fed to chicken. DM and N digestibility were improved by feeding TSP which led to less NH3volatilization.
In 1999, probiotic consisting of Bacillus, Lactobacillus, Streptococcus, Clostridium, Saccharomyces, and Candida species were fed to both male and female broilers to analyze their effect on caecal flora and metabolites, lipid metabolism, meat components, productivity, and raising environment. In the probiotic group, NH3 in the cecum was significantly (p < 0.05) lower than that for the control group. Additionally, it was reported that pH in cecum was decreased significantly in the probiotic group which was responsible for NH3 production according to earlier studies (Endo and Nakano 1999).
Conclusion
The following are the most important strategies used to reduce NH3 production in poultry houses (Table 12).
NH3 is the most noxious gas in poultry houses needing control. pH, moisture content, litter, bird age, manure age, relative humidity, ventilation rate, and temperature play important roles in NH3 production. Seasonality and geological sites are also responsible for its production.
Housing type, manure handling practices, aerobic and anaerobic conditions, and litter amendment are postdigestive strategies. Dietary manipulation for reduction of NH3 uses low crude protein, synthetic amino acids, supplementation of fiber, and probiotics (single and multistrains).
Many studies have been conducted to evaluate the effect of probiotics on broilers and less with laying hens. More laying hens are needed to fulfill the demand for eggs; therefore, there will be more malodor and environmental pollution produced by NH3. One possibility includes use of a combination of strategies for reducing NH3 in layer house. Researchers need to conduct studies on laying hens to minimize the NH3 production and emissions in poultry houses to protect animal and human health and also to protect the environment from its harmful effects.
References
Abdelrahman MM, Saleh FH (2007) Performance of broiler chickens fed on corn-sunflower meal diets with ß-Glucanase enzyme. Jordan J Agric Sci 3
Adams PL, Daniel TC, Edwards DR, Nichols DJ, Pote DH, Scott HD (1994) Poultry litter and manure contributions to nitrate leaching through the vadose zone. Soil Sci Soc Am J 58:1206–1211
Ahmed ST, Islam MM, Mun HS, Sim HJ, Kim YJ, Yang CJ (2014) Effects of bacillus amyloliuefaciens as a probiotic strain on growth performance, cecal microflora and fecal noxious gas emissions of broiler chickens. Poult Sci 93:1963–1971
Aletor VA, Hamid II, Niess E, Pfeffer E (2000) Low-protein amino acid-supplemented diets in broiler chickens: effects on performance, carcass characteristics, whole-body composition and efficiencies of nutrient utilisation. J Sci Food Agric 80:547–554
Ali MM, Moubarak ST, Badawy MF, Zahran OK, Badawy ES (2000) Effect of litter treatment on broiler performance and litter quality. Vet Med J Giza 48:309–318
Al-Mashhandani EH, Beck MM (1985) Effect of atmospheric ammonia on the surface ultrastructure of the lung and trachea of broiler chicks. Poult Sci 64:2056–2061
Almuhanna EA, Ahmed AS, Al-Yousif YM (2011) Effect of air contaminants on poultry immunological and production performance. Int J Poult Sci 10:461–470
Amer AH, Pingel H, Hillig J, Soltan M, von Borell E (2004) Impact of atmospheric ammonia on laying performance and egg shell strength of hens housed in climatic chambers. Archiv fur Geflugelkunde 68:120–125
Anderson DP, Beard CW, Hanson RP (1964) The adverse effects of ammonia on chickens including resistance to infection with Newcastle disease virus. Avian Dis 8:369–379
Angus AJ, Hodge ID, McNally S, Sutton MA (2003) The setting of standards for agricultural nitrogen emissions: a case study of the Delphi technique. J Environ Manag 69:323–337
Animal Husbandry Guidelines for U.S. Egg Laying Flocks. 2010 edition. http://www.uepcertified.com/media/pdf/UEP-AnimalWelfare-Guidelines
ApSimon HM, Kruse M, Bell JNB (1987) Ammonia emissions and their role in acid deposition. Atmos Environ 21:1939–1946
Armstrong KA, Burns RT, Walker FR, Wilhelm LR, Raman DR (2003) Ammonia concentrations in poultry broiler production units treated with liquid alum. In Air Pollution from Agricultural Operations-III, Proceedings of the 12-15 2003 Conference, Research Triangle park, North Carolina USA, American Society of Agricultural and Biological Engineers, pp 116–122
Atapattu NSBM, Senaratna D, Belpagodagamage UD (2008) Comparison of ammonia emission rates from three types of broiler litters. Poult Sci 87:2436–2440
Aziz T, Barnes HJ (2010) Harmful effects of ammonia on birds. World Poultry 26:28–30
Bachrach U (1957) The aerobic breakdown of uric acid by certain pseudomonads. Microbiology 17:1–1
Baek BH, Aneja VP (2004) Measurement and analysis of the relationship between ammonia, acid gases and fine particles in eastern North Carolina. J Air Waste Manage Assoc 54:623–633
Baek BH, Aneja VP, Tong Q (2004) Chemical coupling between ammonia, acid gases and fine particles. Environ Pollut 129:89–98
Becker JG, Graves RE (2004) Ammonia emissions and animal agriculture. In Proceedings Mid-Atlantic Agricultural Ammonia, Mid-Atlantic CSREES Regional Water Quality Project, 2004
Beker A, Vanhooser SL, Swartzlander JH, Teeter RG (2004) Atmospheric ammonia concentration effects on broiler growth and performance. J Appl Poult Res 13:5–9
Benton CE Jr, Brake J (2000) Effects of atmospheric ammonia on albumen height and pH of fresh broiler breeder eggs. Poult Sci 79:1562–1565
Blair R, Jacob JP, Ibrahim S, Wang P (1999) A quantitative assessment of reduced protein diets and supplements to improve nitrogen utilization. J Appl Poult Res 8:25–47
Bloomington MN, Loch FC, Oliveira MCD, Silva DD, Goncalves BN, Faria BFD, Menezes JFS (2011) Quality of poultry litter submitted to different treatments in five consecutive flocks. Rev Bras Zootec 40:1025–1030
Bregendahl K, Roberts S (2007) nutritional strategies to reduce Ammonia emissions from laying hens. InProc. Midwest Poultry Federation Convention, St. Paul. 2006, pp. 21–23
Bregendahl K, Sell JL, Zimmerman DR (2002) Effect of low-protein diets on growth performance and body composition of broiler chicks. Poult Sci 81:1156–1167
Bullis KL, Snoeyenbos GH, Van Roekel H (1950) A keratoconjunctivitis in chickens. Poult Sci 29:386–399
Burgess RP, Carey JB, Shafer DJ (1998) The impact of pH on nitrogen retention in laboratory analysis of broiler litter. Poult Sci 77:1620–1622
Burley HK (2009) Effects of reduced crude protein, amino acid balanced diets on performance, economics and ammonia emission in a large-scale commercial laying hen flock. Thesis, The Pennsylvania State University
Burley HK, Patterson PH, Elliot MA (2013) Effect of a reduced crude protein, amino acid-balanced diet on hen performance, production costs and ammonia emissions in a commercial laying hen flock. J Appl Poult Res 22:217–228
Burns RT, Xin H, Gates RS, Li H, Overhults DG, Moody L, Earnest J (2007) Ammonia emissions from broiler houses in the Southeastern United States. In International Symposium on Air Quality and Waste Management for Agriculture, American Society of Agricultural and Biological Engineers, 16–19 September 2007, Broomfield, Colorado 2007, pp 76
Cabuk M, Alcicek A, Bozkurt M, Akkan S (2004) Effect of yucca schidigera and natural zeolite on broiler performance. Int J Poult Sci 3:651–654
Calvet S, Cambra-Lopez M, Estelles F, Torres AG (2011) Characterization of gas emissions from a Mediterranean broiler farm. Poult Sci 90:534–542. https://doi.org/10.3382/ps.2010-01037
Carey JB, Coufal CD, Chavez C, Niemeyer PL (2005) Long term studies of nitrogen balance in broiler production. Available at http://www.cals.ncsu.edu/waste_mgt/natlcenter/sanantonio/proceedings.htm. Verified 26 July 2007
Carr LE, Wheaton FW, Douglass LW (1990) Empirical models to determine ammonia concentrations from broiler chicken litter. Trans ASAE 33:1337–1342
Carr LE, Nicholson JL (1980) Broiler response to three ventilation rates. Trans ASAE 23:414–0418
Casey KD, Gates RS, Wheeler EF, Xin H, Zajaczkowski JS, Topper P, Liang Y (2004) Ammonia emissions from Kentucky broiler houses during winter and spring. National CASANZ Conference: Linking Air Pollution Science Policy and Management, Newcastle, NSW Australia 23–27 November 2007
Caveny DD, Quarles CL, Greathouse GA (1981) Atmospheric ammonia and broiler cockerel performance. Poult Sci 60:513–516
Chai L, Zhao Y, Xin H, Wang T, Atilgan A, Soupir M, Liu K (2017) Reduction of particulate matter and ammonia by spraying acidic electrolyzed water onto litter of aviary hen houses: a lab-scale study. Trans ASABE 60:497–506
Chang MH, Chen TC (2003) Reduction of broiler house malodor by direct feeding of a lactobacilli containing probiotic. Int J Poult Sci 2:313–317
Chapman CM, Gibson GR, Rowland I (2011) Health benefits of probiotics: are mixture more effective than single strains? Eur J Nutr 50:1–7
Charles DR, Payne CG (1966) The influence of graded levels of atmospheric ammonia on chickens. I. Effects on respiration and on the performance of broilers and replacement growing stock. Br Poult Sci 7:177–187
Chen H, Yan FF, Hu JY, Wu Y, Tucker CM, Green AR, Cheng HW (2017) Immune response of laying hens exposed to 30 ppm ammonia for 25 weeks. Int J Poult Sci 16:139–146. https://doi.org/10.3923/ijpa.2017.139.146
Chen K, Gao J, Li J, Huang Y, Luo X, Zhang T (2012) Effects of probiotics and antibiotics on diversity and structure of intestinal microflora in broiler chickens. Afr J Microbiol Res 6:6612–6617
Chiang SH, Hsieh WM (1995) Effect of direct-fed microorganisms on broiler growth performance and litter ammonia level. Asian-Aust J Anim Sci 8:159–162
Choi IH, (2004) A study on reducing the environmental pollutants from animal feces and urine. Thesis, Taegu University
Choi IH, Moore PA (2008a) Effect of various litter amendments on ammonia volatilization and nitrogen content of poultry litter. J Appl Poult Res 17:454–462
Choi IH, Moore PA (2008b) Effects of liquid aluminum chloride additions to poultry litter on broiler performance, ammonia emissions, soluble phosphorus, total volatile fatty acids and nitrogen contents of litter. Poult Sci 87:1955–1963
Close LG, Catlin FI, Cohn AM (1980) Acute and chronic effects of ammonia burns on the respiratory tract. Arch Otolaryngol 106(3):151–158
Cook KL, Rothrock MJ, Warren JG, Sistani KR, Moore PJ Jr (2008) Effect of alum treatment on the concentration of total and ureolytic microoganisms in poultry litter. J Environ Qual 37:2360–2367
Costa A, Ferrari S, Guarino M (2012) Yearly emission factors of ammonia and particulate matter from three laying-hen housing systems. Anim Prod Sci 52:1089–1098
Cotterill OJ, Nordsog AW (1954) Influence of ammonia on egg white quality. Poult Sci 33:432–434
Cotterill OJ, Winter AR (1953) Some nitrogen studies on built-up litter. Poult Sci 32:365–366
Coufal CD, Chavez C, Niemeyer PR, Carey JB (2006) Nitrogen emissions from broilers measured by mass balance over eighteen consecutive flocks. Poult Sci 85:384–391
Creek RD, Vasaitis V (1961) Uric acid excretion in the chicks as related to the intake of its precursors and nitrogen. Poult Sci 40:283–288
Da Borso F, Chiumenti R (1999) Poultry housing and manure management systems: recent development in Italy as regards ammonia emissions. Proceedings of the 8th international conference of the FAO ESCORENA network on recycling of agricultural, municipal and industrial residues in agriculture, RAMIRAN 98, vol. 2, poster presentation, pp 15-21
David B, Mejdell C, Michel V, Lund V, Moe RO (2015) Air quality in alternative housing systems may have an impact on laying hen welfare. Part II-ammonia Animals 5:886–896
Deaton JW, McNaughton JL, Burdick D (1979) High-fibre sunflower meal as a replacement for soyabean meal in layer diets. Br Poult Sci 20:159–162
Deaton JW, Reece FN, Lott BD (1982) Effect of atmospheric ammonia on laying hen performance. Poult Sci 61:1815–1817
Deaton JW, Reece FN, Lott BD (1984) Effect of atmospheric ammonia on pullets at point of lay. Poult Sci 63:384–385
DeLane PB, Moore PA, Daniel TC, Lemunyon JL (2004) Effect of chemical and microbial amendments on ammonia volatilization from composting poultry litter. J Environ Qual 33:728–734
Demmers TGM, Burgess LR, Short JL, Phillips VR, Clark JA, Wathes CM (1999) Ammonia emissions from two mechanically ventilated UK livestock buildings. Atmos Environ 33:217–227
Ditta YA, King AJ (2017) Recent advances in sunflower seed meal as an alternate source of protein in broilers: an overview. World’s Poultry Sci J 73:527–542
Do JC, Chai IH, Nahm KH (2005) Effects of chemically amended litter on broiler performance, atmospheric ammonia concentration and phosphorus solubility in litter. Poult Sci 84:679–686
Eleroğlu H, Yalçın H (2005) Use of natural zeolite-supplemented litter increased broiler production. S Afr J Anim Sci 35:90–97
Elliott HA, Collins NE (1982) Factors affecting ammonia release in broiler houses. Trans ASAE 25:413–0418
Elwinger K, Svensson L (1996) Effect of dietary protein content, litter and drinker type on ammonia emission from broiler houses. J Agric Eng Res 64:197–208
Endo T, Nakano M (1999) Influence of a probiotic on productivity, meat components, lipid metabolism, caecal flora and metabolites and raising environment in broiler production. Anim Sci J 70:207–218
EPA (2016) Toxicological review of ammonia noncancer inhalation. https://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=529126
Eugene B, Moore PA, Li H, Miles DM, Trabue SL, Burn R, Buser M (2015) Effect of alum additions to poultry litter on in-house ammonia and greenhouse gas concentrations and emissions. J Environ Qual 44:1530–1540
Fabbri C, Valli L, Guarino M, Costa A, Mazzotta V (2007) Ammonia, methan, nitrous oxide and particulate matter emissions from two different buildings for laying hens. Biosyst Eng 97:441–455
Faddoul GP, Ringrose RC (1950) Avian keratoconjuncitivitis. Vet Med 45:492–493
Ferguson NS, Gates RS, Tarba JL, Cantor AH, Pescatore AJ, Ford MJ, Burnham DJ (1998) The effect of dietary crude protein on growth, ammonia concentration and litter composition in broilers. Poult Sci 77:1481–1487
Fournel S, Pelletier F, Godbout S, Lagace R, Feddes JJR (2012) Odour emission, hedonic tones and ammonia emissions from three cage layer housing systems. Biosyst Eng 112:181–191
Gates RS (2000) Poultry diet manipulation to reduce output of pollutants to environment. Simpósio sobre Resíduos da Produção Avícola 12:62–74
Gates RS, Casey KD, Wheeler EF, Xin H, Pescatorea AJ (2008) U.S. broiler housing ammonia emissions inventory. Atmos Environ 42:3342–3350
Gates RS, Pescatore AJ, Taraba J, Cantor AH, Liberty K, Ford MJ, Burnham DJ (2000) Dietary manipulation of crude protein and amino acids for reduced ammonia emission from broiler litter. ASAE Paper No. 004024. St. Joseph, MI, 9-12 July 2000, pp 1-16
Golbabaei F, Islami F (2000) Evaluation of workers’ exposure to dust, ammonia and endotoxin in poultry industries at the province of Isfahan, Iran. Ind Health 38:41–46
Green AR, Wesley IV, Trampel DW, Xin H (2009) Air quality and bird health status in three types of commercial egg layer houses. J Appl Poult Res 18:605–621
Gustafsson G, Wachenfelt EV (2005) Measures against ammonia release in a floor housing system for laying hens. Agricultural Engineering International: CIGR Ejournal Manuscript BC 05 003. Vol. VII. December, 2005
Hale EC (2005) Reduction of ammonia emission and phosphorus excretion in laying hen manure through feed manipulation. In: Proceedings of the Symposium on the State of the Science: Animal Manure and Waste Management. San Antonio, Texas, USA: National Center for Manure and Animal Waste Management; 2005, pp 1-6
Hartung J (2005) Luftverunreinigungen in der Nutztierhaltung. KTBL Schrift 436:7–19 (in German)
Haslam SM, Brown SN, Wilkins LJ, Kestin SC, Warriss PD, Nicol CJ (2006) Preliminary study to examine the utility of using foot burn or hock burn to assess aspects of housing conditions for broiler chicken. Br Poult Sci 47:13–18. https://doi.org/10.1080/00071660500475046
Hassan MR, Ryu KS (2012) Naturally derived probiotic supplementation effects on physiological properties and manure gas emission of broiler chickens. J Agric Life Sci 46:119–127
Hayes ET, Curran TP, Dodd VA (2006) Odour and ammonia emissions from intensive poultry units in Ireland. Bioresour Technol 97:933–939
Hmani H, Daoud L, Jlidi M, Jalleli K, Ali MB, Brahim AH, Bargui M, Dammak A, Ali MB (2017) A Bacillus subtilis strain as probiotic in poultry: selection based on in vitro functional properties and enzymatic potentialities. J Ind Microbiol Biotechnol 44:1157–1166
Hossain MM, Begum M, Kim IH (2015) Effect of Bacillus subtilis, Clostridium butyricumand Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Veterinarni Medicina 60:77–86
Hunolt AE, Maguire RO, Ogejo JA, Badgley BB, Frame WH, Reiter MS (2015) Multiple applications of sodium bisulfate to broiler litter affect ammonia release and litter properties. J Environ Qual 44:1903–1910
Ihrig A, Hoffmann J, Triebig G (2006) Examination of the influence of personal traits and habituation on the reporting of complaints at experimental exposure to ammonia. Int Arch Occup Environ Health 79:332–338
Isshiki Y (1979) Effect of lactobacilli in the diet on the concentration of nitrogenous compounds and minerals in blood of chickens. Jpn Poultry Sci 16:254–258 (in Japanese)
Ji F, Fu SY, Ren B, Wu SG, Zhang HJ, Yue HY (2014) Evaluation of amino-acid supplemented diets varying in protein levels for laying hens. J Appl Poult Res 23:384–392
Jiang JK, Sands JR (2000) Odour and ammonia emission from broiler farms. Rural Industries Research and Development Corporation, Kingston, Australia. Publication no. 00/2. February, pp. 94
Johnson TM, Murphy B (2008) Use of sodium bisulfate to reduce ammonia F(PLT) as a litter treatment. Ala Coop Ext Serv ANR-1208
Jones L, Nizam MS, Reynolds B, Bareham S, Oxley ERB (2013) Upwind impacts of ammonia from an intensive poultry unit. Environ Pollut 180:221–228
Kalmendal R, Elwinger K, Holm L, Tauson R (2011) High-fibre sunflower cake affects small intestinal digestion and health in broiler chickens. Br Poult Sci 52:86–96
Kaoud HA (2013) Removal of ammonia gas emission from broiler litter. Global J Sci Res 1:42–47
Karamanlis X, Fortomaris P, Arsenos G, Dosis I, Papaioannou D, Batzios C, Kamarianos A (2008) The effect of a natural zeolite (Clinoptilolite) on the performance of broiler chickens and the quality of their litter. Asian-Aust J Anim Sci 21:1642–1650
Keener HM, Elwell DL, Grande D (2002) NH3 emissions and N-balances for a 1.6 million caged layer facility: manure belt/composting vs. deep pit operation. Transactions of the ASAE 45:1977
Keshavarz K, Austic RE (2004) The use of low-protein, low-phosphorus, amino acid- and phytase-supplemented diets on laying hen performance and nitrogen and phosphorus excretion. Poult Sci 83:75–83
Kilic L, Yaslioglu E (2014) Ammonia and carbon dioxide concentrations in a layer house. Asian Australas J Anim Sci 27:1211
Kim WK, Patterson PH (2003a) Effect of minerals on activity of microbial uricase to reduce ammonia volatilization in poultry manure. Poult Sci 82:223–231
Kim WK, Patterson PH (2003b) Production of an egg yolk antibody specific to microbial uricase and its inhibitory effects on uricase activity. Poult Sci 82:1554–1558
Kirchmann H, Lundvall A (1998) Treatment of solid animal manures: identification of low NH3 emission practices. Nutr Cycl Agroecosyst 51:65–71
Kirchmann H, Witter E (1989) Ammonia volatilization during aerobic and anaerobic manure decomposition. Plant Soil 115:35–41
Kirychuk SP, Senthilselvan A, Dosman JA, Juorio V, Feddes JJ, Willson P, Classen H, Reynolds SJ, Guenter W, Hurst TS (2003) Respiratory symptoms and lung function in poultry confinement workers in western Canada. Can Respir J 10:375–380
Kithome M, Paul JW, Bomke AA (1999) Reducing nitrogen losses during simulated composting of poultry manure using adsorbents or chemical amendments. J Environ Qual 28:194–201
Kling HF, Quarles CL (1974) Effect of atmospheric ammonia and the stress of infectious bronchitis vaccination on leghorn males. Poult Sci 53:1161–1167
Knížatová M, Brouček J, Mihina Š (2010b) Seasonal differences in levels of carbon dioxide and ammonia in broiler housing. Slovak J Anim Sci 43:105–112
Knížatová M, Mihina Š, Brouček J, Karandušovská I, Mačuhová J (2010a) Ammonia emissions from broiler housing facility: influence of litter properties and ventilation. XVIIth World congress of the International Commission of Agricultural and Biosystems Engineering (CIGR), June 13-17 Canadian Society for Bioengineering (CSBE/SCGAB) Québec City June 2010
Knížatová M, Mihina Š, Brouček J, Karandušovská I, Mačuhová J (2010c) The influence of litter age, litter temperature and ventilation rate on ammonia emissions from a broiler rearing facility. Czech J Anim Sci 55:337–345
Kocaman B, Esenbuga N, Yildiz A, Lacin E, Macit M (2006) Effect of environmental conditions in poultry houses on the performance of laying hens. Int J Poult Sci 5:26–30
Koerkamp PG (1994) Review on emissions of ammonia from housing systems for laying hens in relation to sources, processes, building design and manure handling. J Agric Eng Res 59:73–87
Koerkamp PG, Bleijenberg R (1998) Effect of type of aviary, manure and litter handling on the emission kinetics of ammonia from layer houses. Br Poult Sci 39:379–392
Koerkamp PG, Keen A, van Niekerk T, Smit S (1996) The effect of manure and litter handling and indoor climate conditions on ammonia emissions from a battery cage and an aviary housing system for laying hens. NJAS Wageningen J Life Sci 43:351–373
Koerkamp PG, Speelman L, Metz JHM (1999) Litter composition and ammonia emission in aviary houses for laying hens: part II, modelling the evaporation of water. J Agric Eng Res 73:353–362
Kristensen HH, Burgess LR, Demmers TGH, Wathes CM (2000) The preference of laying hens for different concentrations of atmospheric ammonia. Appl Anim Behav Sci 68:307–318
Kristensen HH, Wathes CM (2000) Ammonia and poultry welfare: a review. World’s Poultry Sci J 56:235–245
Kristjan B, Roberts S (2006) Nutritional strategies to reduce ammonia emissions from laying hens. InProc Midwest Poultry Federation Convention St Paul M March 2006 21 pp. 21–23
Laudadio V, Ceci E, Lastella NMB, Tufarelli V (2014) Effect of feeding low-fiber fraction of air-classified sunflower (Helianthus annus L.) meal on laying hen productive performance and egg yolk cholesterol. Poult Sci 93:2864–2869
Leonard JJ, Feddes JJR, McQuitty JB (1984) Air quality in commercial broiler housing. Can Agric Eng 26:65–72
Li H, Lin C, Collier S, Brown W, White-Hansen S (2013) Assessment of frequent litter amendment application on ammonia emission from broilers operations. J Air Waste Manage Assoc 63:442–452
Li H, Xin H (2010) Lab-scale assessment of gaseous emissions from laying hen manure storage as affected by physical and environmental factors. Trans ASABE 53:593–604
Li H, Xin H, Burns RT (2006) Reduction of ammonia emission from stored poultry manure using additives: zeolite, Al+ clear, ferix-3 and PLT. ASAE Annual International Meeting, American Society of Agricultural and Biological Engineers, Oregon Convention Center Portland, Oregon, paper no. 064188, 9-12 July 2006, pp 1
Li H, Xin H, Burns RT, Roberts SA, Li S, Kliebenstein J, Bregendahl K (2012) Reducing ammonia emissions from laying-hen houses through dietary manipulation. J Air Waste Manage Assoc 62:160–169
Liang Y, Xin H, Gates RS, Wheeler EF (2003) Updates on ammonia emission from Iowa layer houses. Proceedings of the Iowa industry symposium Ames IA
Liang Y, Xin H, Wheeler EF, Gates RS, Li H, Zajaczkowski JS, Topper PA, Casey KD, Behrends BR, Burnham DJ, Zajaczkowski FJ (2005) Ammonia emissions from U.S. laying hen houses in Iowa and Pennsylvania. Trans ASAE 48:1927–1941
Lim TT, Heber AJ, Ni JQ (2003) Air quality measurements at a laying hen house: ammonia concentrations and emissions. In American Society of Agricultural and Biological Engineers Conference 2003
Liu Z, Wang L, Beasley D, Oviedo E (2007) Effect of moisture content on ammonia emissions from broiler litter: a laboratory study. J Atmos Chem 58:41–53
Lon-Wo E (2010) Effect of natural zeolite (Clinoptilolite) on the laying hen diet. Its influence on ammonia release through the feces. Cuban Journal of Agricultural Science 44
Madelin TM, Wathes CM (1989) Air hygiene in a broiler house: comparison of deep litter with raised netting floors. Br Poult Sci 30:23–37
Mahimairaja S, Bolan NS, Hedley MJ, Macgregor AN (1994) Losses and transformation of nitrogen during composting of poultry manure with different amendments: an incubation experiment. Bioresour Technol 47:265–273
Maliselo PS, Nkonde GK (2015) Ammonia production in poultry houses and its effect on, the growth of gallus gallus domestica (broiler chickens): a case study of a small scale poultry in riverside, Kitwe, Zambia. Int J Sci Technol Res 4:141–145
Maliselo SP, Mwaanga P (2016) Effects of pH, moisture and excreta age on ammonia emission in a poultry house: a case study for Kitwe, Zambia. International Journal of Scientific and Research Publications volume 6
Manunza B, Deiana S, Pintore M, Gessa C (1999) The binding mechanism of urea, hydroxamic acid and N-(N-butyl)-phosphoric triamide to the urease active site. A comparative molecular dynamics study. Soil Biol Biochem 31:789–796
McQuitty JB, Feddes JJR, Leonard JJ (1985) Air quality in commercial laying barns. Can Agric Eng 27:13–19
McWard GW, Taylor DR (2000) Acidified clay litter amendment. J Appl Poult Res 9:518–529
Meluzzi A, Sirri F, Tallarico N, Franchini A (2001) Nitrogen retention and performance of brown laying hens on diets with different protein content and constant concentration of amino acids and energy. Br Poult Sci 42:213–217
Mendes LB (2010) Ammonia emissions, feeding and defecation dynamics of W36 pullets and laying hens as affected by stocking density and manure accumulation time. Thesis, Iowa State University
Mihina Š, Knížatová M, Brouček J (2010) Effect of season on carbon dioxide and ammonia production in broiler housing. In: Selected problems of soil tillage systems and operations, Warsaw: Faculty of Production Engineering, Warsaw University of life Sciences, pp. 123−133
Miles BM, Braton SL, Lott BD, Simmons JD (2002) Quantified detriment of ammonia to broilers. Poultry Sci 81:54–55
Miles DM, Branton SL, Lott BD (2004) Atmospheric ammonia is detrimental to the performance of modern commercial broilers. Poult Sci 83:1650–1654
Miles DM, Miller WW, Branton SL, Maslin WR, Lott BD (2006) Ocular responses to ammonia in broiler chickens. Avian Dis 50:45–49
Miles DM, Rowe DE, Moore PA Jr (2012) Litter ammonia losses amplified by higher airflow rates. J Appl Poult Res 21:874–880
Moore Jr PA (1998) Best management practices for poultry manure utilization that enhance agricultural productivity and reduce pollution. Anim Waste Util: Effective use of manure as a soil Res 89
Moore Jr PA, Burns R, Miles DM (2008) Reducing ammonia emissions from poultry litter with alum. In: Proceeding of mitigation air emissions from animal feeding operations conference, May19–21 2008 Mar 19, pp 90-94
Moore PA Jr, Daniel TC, Edwards DR (1999) Reducing phosphorus runoff and improving poultry production with alum. Poult Sci 78:692–698
Moore PA Jr, Daniel TC, Edwards DR (1995) Effect of chemical amendments on ammonia volatilization from poultry litter. J Environ Qual 24:293–300
Moore PA Jr, Daniel TC, Edwards DR (2000a) Reducing nonpoint source phosphorus runoff from poultry manure with aluminum sulfate. In: Balázs E et al (eds) Biological resource management connecting science and policy. Springer Berlin, Heidelberg, pp 117–127
Moore PA Jr, Daniel TC, Edwards DR, Miller DM (1996) Evaluation of chemical amendments to reduce ammonia volatilization from poultry litter. Poult Sci 75:315–320
Moore PA Jr, Huff WE, Daniel TC, Edwards DR, Sauer TC (1997) Effect of aluminum sulfate on ammonia fluxes from poultry litter in commercial broiler houses. In: Proceed Fifth Int Symp Livestock Environ Trans ASAE 2:883–891
Moore PA, Daniel TC, Edwards DR (2000b) Reducing phosphorus runoff and inhibiting ammonia loss from poultry manure with aluminum sulfate. J Environ Qual 29:37–49
Moore PA, Edwards DR (2005) Long-term effects of poultry litter, alum-treated litter and ammonium nitrate on aluminum availability in soils. J Environ Qual 4:2104–2111
Moore PA, Edwards DR (2007) Long-term effects of poultry litter, alum-treated litter and ammonium nitrate on phosphorus availability in soils. J Environ Qual 36:163–174
Morris PD, Lenhart SW, Service WS (1991) Respiratory symptoms and pulmonary function in chicken catchers in poultry confinement units. Am J Ind Med 19:195–204
Mulhausen JR, McJilton CE, Redig PT, Janni KA (1987) Aspergillus and other human respiratory disease agents in Turkey confinement houses. Am Ind Hygiene Assoc J 48:894–899
Nadier A, Allouim MN, Bennoune O, Bouhentala S (2013) Effect of ventilation and atmospheric ammonia on the health and performance of broiler chickens in summers. J World's Poult Res 3:54–56
Nagaraj M, Wilson CAP, Saenmahayak B, Hess JB, Bilgili SF (2007) Efficacy of a litter amendment to reduce pododermatitis in broiler chickens. J Appl Poult Res 16:255–261
Nahm KH (2007) Feed formulations to reduce N excretion and ammonia emission from poultry manure, review. Bioresour Technol 98:2282–2300
Nakaue HS, Koelliker JK (1981) Studies with clinoptilolite in poultry: I. Effect of feeding varying levels of clinoptilolite (zeolite) to dwarf single comb white leghorn pullets and ammonia production. Poult Sci 60:944–949
Nakaue HS, Koelliker JK, Pierson ML (1981) Studies with clinoptilolite in poultry. II. Effect of feeding broilers and the direct application of clinoptilolite (zeolite) on clean and reused broiler litter on broiler performance and house environment. Poult Sci 60:1221–1228
Namroud NF, Shivazad M, Zaqhari M (2008) Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level and excreta characteristics of broiler chicks. Poult Sci 87:2250–2258
Nararaja KV, Emery DA, Jordan KA, Newman JA, Pomeroy BS (1983) Scanning electron microscopic studies of adverse effects of ammonia on tracheal tissues of turkeys. Am J Vet Res 44:1530–1536
National Institute of Occupational Safety and Health (NOISH), 2016, United Egg Producers
Ni J-Q, Chai L, Chen L, Bogan BW, Wang K, Cortus EL, Heber AJ, Lim T, Diehl CA (2012) Characteristics of ammonia, hydrogen sulfide, carbon dioxide an particulate matter concentrations in high-rise and manure-belt laying hen houses. Atmos Environ 57:165–174
Ni JQ, Diehl CA, Chai L, Chen Y, Heber AJ, Lim TT, Bogan BW (2017a) Factors and characteristics of ammonia, hydrogen sulfide, carbon dioxide and particulate matter emissions from two manure-belt layer houses. Atmos Environ 156:113–124
Ni JQ, Liu S, Diehl CA, Lim TT, Bogan BW, Chen L, Chai L, Wang K, Heber AJ (2017b) Emission factors and characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter at two high-rise layer hen houses. Atmos Environ 154:260–273
Nicholson FA, Chambers BJ, Walker AW (2004) Ammonia emissions from broiler litter and laying hen manure management systems. Biosyst Eng 89:175–185
Nimmermark S, Gustafsson G (2005) Influence of temperature, humidity and ventilation rate on the release of odour an ammonia in a floor housing system for laying hens. Agricultural Engineering International: the CIGR Ejournal. Vol. VII. Manuscript BC 04 008, March 2005
Nimmermark S, Lund V, Gustafsson G, Eduard W (2009) Ammonia, dust and bacteria in welfare-oriented systems for laying hens. Ann Agric Environ Med 16:103–113
O’Dell BL, Woods WD, Laerdal OA, Jeffay AM, Savage JE (1960) Distribution of the major nitrogenous compounds and amino acids in chicken urine. Poult Sci 39:426–432
Olanrewaju HA, Miller WW, Maslin WR, Thaxton JP, Dozier WA III, Purswell J, Branton SL (2007) Interactive effects of ammonia and light intensity on ocular, fear and leg health in broiler chickens. Int J Poult Sci 10:762–769
Olanrewaju HA, Thaxton JP, Dozier WA III, Purswell J, Collier SD, Branton SL (2008) Interactive effects of ammonia and light intensity on hematochemical variables in broiler chickens. Poult Sci 87:1407–1414
Oliveira MC, Ferreira HA, Cancherini LC (2004) Effect of chemical conditioners on chickens bed quality. Brazilian Archives of Veterinary Medicine and Animal Science. Federal University of Minas Gerais, School of Veterinary Medicine, v. 56, n. 4, p. 536–541. Available at: < http://hdl.handle.net/11449/28558 > (in Portuguese)
Oliveira MCD, Almeida CV, Andrade DO, Rodrigues SMM (2003) Dry matter content, pH and volatilized ammonia from poultry litter treated with different additives. Rev Bras Zootec 32:951–954 (in Portuguese)
Oyetunde OOF, Thomson RG, Carlson HC (1978) Aerosol exposure of ammonia, dust and Escherichia coli in broiler chickens. Can Vet J 19:187
Pineda L, Roberts S, Kerr B, Kwakkel RP, Verstegen M, Bregendahl K (2008) Maximum dietary content of corn dried distiller's grains with solubles in diets for laying hens. Effects on nitrogen balance, manure excretion, egg production, and egg quality. Anim Ind Rep 654:83
Pope MJ, Cherry TE (2000) An evaluation of the presence of pathogens on broilers raised on poultry litter treatment-treated litter. Poult Sci 79:1351–1355
Pratt EV, Rose SP, Keeling AA (1998) Atmospheric nitrogen losses from poultry excreta. Br Poult Sci 39:12–13
Quarles CL, Kling HF (1974) Evaluation of ammonia and infectious bronchitis vaccination stress on broiler performance and carcass quality. Poult Sci 53:1592–1596
Redwine JS, Lacey RE, Mukhtar S, Carey JB (2002) Concentration and emission of ammonia and particulate matter in tunnel-ventilated broiler under summer conditions in Texas. Trans ASAE 45:1101
Reece FN, Bates BJ, Lott BD (1979) Ammonia control in broiler houses. Poult Sci 58:754–745
Reece FN, Lott BD, Deaton JW (1980) Ammonia in the atmosphere during brooding affects performance of broiler chickens. Poult Sci 59:486–488
Reece FN, Lott BD, Deaton JW (1981) Low concentrations of ammonia during brooding decrease broiler weight. Poult Sci 60:937–940
Rees D, Nelson G, Kielkowski D, Wasserfall C, da Costa A (1998) Respiratory health and innunological profile of poultry workers. S Afr Med J 88:1110–1117
Reynolds SJ, Parker D, Vesley D, Smith D, Woellner R (1993) Cross-sectional epidemiological study of respiratory disease in Turkey farmers. Am J Ind Med 24:713–722
Rezaei M, Moghaddam HN, Reza JP, Kermanshah H (2004) The effects of dietary protein and lysine levels on broiler performance, carcass characteristics and N excretion. Int J Poult Sci 3:148–152
Ritz CW, Mitchell BW, Fairchild BD, Czarick M III, Worley JW (2006) Improving in-house air quality in broiler production facilities using an electrostatic space charge system. J Appl Poult Res 15:333–340
Ritz CW, Tasistro AS, Kissel DE, Fairchild BD (2011) Evaluation of surface-applied char on the reduction of ammonia volatilization from broiler litter. J Appl Poult Res 20:240–245
Roberts S, Bregendahl K, Xin H, Kerr BJ, Russell JR (2006) Adding fiber to the diet of laying hens reduces ammonia emission. Animal Industry Report 652:49
Roberts SA (2009) Effect of dietary corn distiller’s dried grains with solubles on ammonia emission, production performance, manure characteristics and economic efficiency for laying hens. Dissertation, Iowa State University
Roberts SA, Xin H, Kerr BJ, Russell JR, Bregendahl K (2007b) Effects of dietary fiber and reduced crude protein on nitrogen balance and egg production in laying hens. Poult Sci 86:1716–1725
Roberts SA, Xin H, Kerr BJ, Russell JR, Breqendahl K (2007a) Effects of dietary fiber and reduced crude protein on ammonia emission from laying-hen manure. Poult Sci 86:1625–1632
Roberts SA, Xin H, Li H, Burns RT, Bregendahl K, Hale EC (2015) Dietary manipulations to lower ammonia emission from laying-hen manure. In the Proceed Mitigating Air Emissions Anim Feed Oper Conf 2015
Robertson AP, Hoxey RP, Demmers TGM, Welch SK, Sneath RW, Stacey KF, Forthergill A, Filmer D, Fisher C (2002) Commercial-scale studies of the effect of broiler-protein intake on aerial pollutant emissions. Biosyst Eng 82:217–225
Rodriguez ML, Ortiz LT, Trevino J, Rebole A, Alzueta C, Centeno C (1998) Studies on the nutritive value of full-fat sunflower seed in broiler chickens diets. Anim Feed Sci Technol 71:341–349
Romero C, Onyango EM, Powers W, Angel R, Applegate TJ (2012) Effect of a partial replacement of limestone by a CaSO4-zeolite mixture combined with a slight protein reduction on production indices, egg quality and excreta pH in laying hens. J Appl Poult Res 21:325–334
Rose RJ, Coit RN, Sell JL (1972) Sunflower seed meal as a replacement for soybean meal protein in laying hen rations. Poult Sci 51:960–967
Rothrock MJ, Cook KL, Warren JG, Eiteman MA, Sistani K (2010) Microbial mineralization of organic nitrogen forms in poultry litters. J Environ Qual 39:1848–1857
Roumeliotis TS, Van Heyst BJ (2008) Summary of ammonia and particulate matter emission factors for poultry operation. J Appl Poult Res 17:305–314
Samanya M, Yamauchi K (2002) Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto. Comp Biochem Physiol A Mol Integr Physiol 133:95–104
Sampaio MAPM, Schocken-Iturrino RP, Sampaio AAM, Berchielli SCP, Biondi A (1999) Study of the microbial population an ammonia release of treated broiler litter with agricultural gypsum. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 51:559–564 (in Portuguese)
San Juan LD, Villamide MJ (2001) Nutritional evaluation of sunflower products for poultry as affected by the oil extraction process. Poult Sci 80:431–437
Sanderson WT, Weber A, Echt A (1995) Epidemic eye and upper respiratory irritation in poultry processing plants. Appl Occup Environ Hyg 10:43–49
Santoso U, Ohtani S, Tanaka K, Sakaida M (1999) Dried Bacillus subtilis culture reduced ammonia gas release in poultry house. Asian Austr J Anim Sci 12:806–809
Schefferle HE (1965) The decomposition of uric acid in built up poultry litter. J Appl Microbiol 28:412–420
Schneider AF, De Almeida DS, Yuri FM, Zimmermann OF, Gerber MW, Gewehr CE (2016) Natural zeolites in diet or litter of broilers. Br Poult Sci 57:257–263. https://doi.org/10.1080/00071668.2016.1150962
Selvaraj RK, Purushothaman MR (2004) Nutritive value of full-fat sunflower seeds in broiler diets. Poult Sci 83:441–446
Senkoylu N, Dale N (1999) sunflower meal in poultry diets: a review. World’s Poultry Sci J 55:153–174
Sharma M, Kishore S, Tripathi SN, Behera SN (2007) Role of atmospheric ammonia in the formation of inorganic secondary particulate matter: a study at Kanpur, India. J Atmos Chem 58:1–7
Shepherd TA, Zhao Y, Li H, Stinn JP, Hayes MD, Xin H (2015) Environmental assessment of three egg production systems—part II. Ammonia, greenhouse gas, and particulate matter emissions. Poult Sci 94:534–543. https://doi.org/10.3382/ps/peu075
Shreve BR, Moore PA, Daniel TC, Edwards DR, Miller DM (1995) Reduction of phosphorus in runoff from field-applied poultry litter using chemical amendments. J Environ Qual 24:106–111
Sims JT, Luka-McCafferty NJ (2002) On-farm evaluation of aluminum sulfate (alum) as a poultry litter amendment: effects on litter properties. J Environ Qual 31:2066–2073
Singh A, Casey KD, King WD, Pescatore AJ, Gates RS, Ford MJ (2009) Efficacy of urease inhibitor to reduce ammonia emission from poultry houses. J Appl Poult Res 18:34–42
Slobodzian-Ksenicz O, Kuczynski T (2002) Effect of litter type on ammonia emission in Turkey housing. Agricultural Engineering International: CIGR Journal of Scientific Research and Development. Manuscript BC 01 006. Vol. IV
Steiner C, Das KC, Melear N, Lakly D (2010) Reducing nitrogen loss during poultry litter composting using biochar. J Environ Qual 39:1236–1242
Summers JD (1993) Reducing nitrogen excretion of the laying hen by feeding lower crude protein diets. Poult Sci 72:1473–1478
Sundblad BM, Larsson BM, Acevedo F, Ernstgård L, Johanson G, Larsson K, Palmberg L (2004) Acute respiratory effects of exposure to ammonia on healthy persons. Scand J Work Environ Health 1:313–321
Tanaka K, Santoso S (2000) Fermented product from Bacillus subtilis inhibits lipid accumulation and ammonia production of broiler chicks. Asian Austr J Anim Sci 13:78–80
Taraba JL, Driver RH, Ross IJ, Lacey RE (1980) Microbial protein production from anaerobically fermented poultry manure. In Winter Meeting of ASAE, Paper No. 804548, 1980 December 2-5, pp. 2-5
Tasistro AS, Ritz CW, Kissel DE (2007) Ammonia emissions from broiler litter: response to bedding materials and acidifiers. Br Poult Sci 48:399–405
Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC (2004) Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy. Int J Food Microbiol 96:219–233
Turan NG (2009) Nitrogen availability in composted poultry litter using natural amendments. Waste Manag Res 27:19–24
Valentine H (1964) A study of the effect of different ventilation rates on the ammonia concentrations in the atmosphere of broiler houses. Br Poult Sci 5:149–159
Van Breemen N (1988) Ecosystem effects of atmospheric deposition of nitrogen in the Netheland. Environ Pollut 54:249–274
Van Breemen N, Burrough PA, Velthorst EV, Van Dobben HF, de Wit T, Ridder TD, Reijnders HF (1982) Soil acidification from atmospheric ammonium sulphate in forest canopy throughfall. Nature 299:548–550
van Harn J, Aarnink AJA, Mosquera J, Van Riel JW, Ogink NW (2012) Effect of bedding material on dust and ammonia emission from broiler houses. Trans ASABE 55:219–226
Villamide J, San Juan LD (1998) Effect of chemical composition of sunflower seed meal on its true metabolizable energy and amino acid digestibility. Poult Sci 77:1884–1892
Vučemilo M, Matković K, Vinković B, Jakšić S, Granić K, Mas N (2007) The effect of animal age on air pollutant concentration in a broiler house. Czech J Anim Sci 52:170–174
Walter ED, Lindblad GS, Aitken JR (1959) The value of sunflower seed oil meal as a protein supplement for laying hens. Can J Anim Sci 39:45–49
Wang YM, Meng QP, Guo YM, Wang YZ, Wang Z, Yao ZL, Shan TZ (2010) Effect of atmospheric ammonia on growth performance and immunological response of broiler chickens. J Anim Vet Adv 9:2802–2806
Wathes CM, Holden MR, Sneath RW, White RP, Phillips VR (1997) Concentrations and emission rates of aerial ammonia, nitrous oxide, methane, carbon dioxide, dust and endotoxin in UK broiler and layer houses. Br Poult Sci 38:14–28
Weaver DW Jr, Meijerhof R (1991) The effect of different levels of relative humidity and air movement on litter conditions, ammonia levels, growth and carcass quality for broiler chickens. Poult Sci 70:746–755
Wei FX, Hu XF, Sa RN, Liu FZ, Li SY, Sun QY (2014) Antioxidant capacity and meat quality of broilers exposed to different ambient humidity and ammonia concentrations. Genet Mol Res 13:3117–3127
Wei FX, Hu XF, Xu B, Zhang MH, Li SY, Sun QY, Lin P (2015) Ammonia concentration and relative humidity in poultry houses affect the immune response of broilers. Genet Mol Res 14:3160–3169
Weiss A (2015) Effects of acidic litter amendments with multiple application on ammonia, microbial environment, production performance and health of broilers. Dissertation, University of Delaware
Wheeler EF, Casey KD, Gates RS, Xin H, Topper PA, Liang Y (2008) Ammonia emissions from USA broiler chicken barns managed with new bedding, built-up litter, or acid-treated litter. In; Livestock Environment VIII, American Society of Agricultural and Biological Engineers, 31 August–4 September 2008, Iguassu Falls, Brazil 2009
Wheeler EF, Casey KD, Gates RS, Xin H, Zajaczkowski JL, Topper PA, Liang Y, Pescatore AJ (2006) Ammonia emissions from twelve US broiler chicken houses. Trans ASABE 49:1495–1512
Whyte RT (1993) Aerial pollutants and the health of poultry farmers. World's Poultry Sci J 49:139–156
Ischer SW, Farnell MB, Tabler GT, Moreira M, O’Shaughnessy PT, Nonnenmann MW (2017) Evaluation of a sprinker cooling system on inhalable dust and ammonia concentrations in broiler chickens production. J Occup Environ Hyg 14:40–48
Worley JW, Risse LM, Cabrera ML, Nolan MP Jr (1999) Bedding for broiler chickens: two alternative systems. Appl Eng Agric 15:687–693
Worley JW, Cabrera ML, Risse LM (2000) Reduced levels of alum to amend broiler litter. Appl Eng Agric 16:441
Wu YN, Yan FF, Hu JY, Chen H, Tucker CM, Green AR, Cheng HW (2017) The effect of chronic ammonia exposure on acute-phase proteins, immunoglobulin, and cytokines in laying hens. Poult Sci 96:1524–1530
Wu-Haan W (2006) Dietary strategies to reduce air emissions from laying hen operations. Dissertation, Iowa State University
Wu-Haan W, Powers WJ, Angel CR, Hale CE III, Applegate TJ (2007) Effect of an acidifying diet combined with zeolite and slight protein reduction on air emissions from laying hens of different ages. Poult Sci 86:182–190
Xin H, Gates RS, Green AR, Mitloehner FM, Moore PA Jr, Wathes CM (2011) Environmental impacts and sustainability of egg production systems. Poult Sci 90:263–277
Xin H, Li H, Liang Y (2005) Update on ammonia emission mitigation from laying hen facilities. In: Iowa egg industry symposium. Ames, IA, pp 38–46
Xue SK, Chen S, Hermanson RE (1998) Measuring ammonia and hydrogen sulfide emitted from manure storage facilities. Trans ASAE 41:1125
Yang P, Lorimor JC, Xin H (2000) Nitrogen losses from laying hen manure in commercial high-rise layer facilities. Trans ASAE 43:1771
Yeo J, Kim KI (1997) Effect of feeding diets containing an antibiotic, a probiotic, or yucca extract on growth and intestinal urease activity in broiler chicks. Poult Sci 76:381–385
Yi B, Chen L, Sa R, Zhong R, Xing H, Zhang H (2016a) High concentrations of atmospheric ammonia induce alterations of gene expression in the breast muscle of broilers (Gallus gallus) based on RNA-Seq. BMC Genomics 17:598
Yi B, Chen L, Sa R, Zhong R, Xing H, Zhang H (2016b) Transcriptome profile analysis of breast muscle tissues from high or low levels of atmospheric ammonia exposed broilers (Gallus gallus). PLoS One 11:e0162631
Yoon C, Na CS, Park JH, Han SK, Nam YM, Kwon JT (2004) Effect of feeding multiple probiotics on performance and fecal noxious gas emission in broiler chicks. Korean J Poultry Sci 31:229–235
Zhang J, Li C, Tang X, Lu Q, Sa R, Zhang H (2015) High concentrations of atmospheric ammonia induce alterations in the hepatic proteome of broilers (Gallus gallus): an iTRAQ-based quantitative proteomic analysis. PLoS One 10:e0123596
Zhang ZF, Cho JH, Kim IH (2013) Effects of Bacillus subtilis UBT-MO2 on growth performance, relative immune organ weight, gas concentration in excreta and intestinal microbial shedding in broiler chickens. Livest Sci 155:343–347
Zhang ZF, Kim IH (2013) Effects of probiotic supplementation in different energy and nutrient density diets on performance, egg quality, excreta microflora, excreta noxious gas emission and serum cholesterol concentrations in laying hens. J Anim Sci 91:4781–4787
Zhang ZF, Kim IH (2014) Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding and excreta odor contents in broiler. Poult Sci 93:364–370
Zhao Y, Shepherd TA, Li H, Xin H (2015) Environmental assessment of three egg production systems–part I: monitoring system and indoor air quality. Poult Sci 94:518–533
Zhao Y, Xin H, Shepherd TA, Hayes MD, Stinn JP, Li H (2013) Thermal environment, ammonia concentrations, and ammonia emissions of aviary houses with white laying hens. Trans ASABE 56:1145–1156
Zuskin E, Mustajbegovic J, Schachter EN, Kern J, Rienzi N, Goswami S, Marom Z, Maayani S (1995) Respiratory function in poultry workers and pharmacologic characterization of poultry dust extract. Environ Res 70(1):11–19
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Naseem, S., King, A.J. Ammonia production in poultry houses can affect health of humans, birds, and the environment—techniques for its reduction during poultry production. Environ Sci Pollut Res 25, 15269–15293 (2018). https://doi.org/10.1007/s11356-018-2018-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2018-y