Abstract
The lead-free ceramics K0.5Na0.5NbO3: x wt% Er3+ (x = 0, 1, 2, 3 and 4) were produced via solid-state method. The ceramic was sintered at 1100 °C to produce a pure-phase perovskite with an orthorhombic structure. No extra phases in the XRD spectra demonstrate that all the Er3+ ions have dispersed into the host lattice. At room temperature, PL emission spectra were examined under the wavelengths 488 and 980 nm. In both emission spectra, green emission bands (528 and 549 nm) and slightly faint red emission bands (662 nm) were found. Observing the effect of pump power revealed that two photons are involved in the emission process. The time decay profile indicates an average lifetime of Er3+ ions is 25.05 μs. The ferroelectric hysteresis loop at room temperature showed decent shapes with good remnant. Thus, by combining the optical and ferroelectric properties, KNN may have potential applications to be employed in optoelectronic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A large amount of poisonous lead is present, and lead-based ceramics have produced major environmental and health issues. The WEEE (Waste from Electrical and Electronic Equipment) and RoHS (Restriction of Hazardous Substances) regulations have therefore prohibited the use of these ceramics [1, 2]. Researchers concentrated on lead-free ceramics such as Na0.5Bi0.5TiO3, BaTiO3, bismuth layer-structured ferroelectrics (BLSF), and various other compounds to substitute the harmful lead found in lead-based ceramics. BaTiO3 (BT), Na0.5Bi0.5TiO3 (NBT), and BLSF are some examples of lead-free ceramics. Their unique characteristics include optical transmission, ferroelectricity, dielectricity, piezoelectricity, and electro-optics, making them an exciting research topic [3]. The search for one particular lead-free alternative that is compatible with all applications in terms of its characteristics and applications has consistently been unsuccessful [4]. The previous reports on lead-free ceramics with excellent dielectric, piezoelectric, and ferroelectric properties and their comparatively low phonon energy (750 cm−1) and strong physical–chemical stability were found. Among them, sodium potassium niobate (KNN) ferroelectric ceramics are the better substrates for upconversion luminescence [5,6,7,8,9]. From the literature survey, it has been confirmed that ferroelectric materials can display electrical and luminescence properties after being doped with rare-earth (RE3+) ions [10, 11]. KNN has an ABO3 perovskite structure [12]. It is a composition in a 1:1 ratio of ferroelectric and antiferroelectric, KNbO3 and NaNbO3, respectively [12, 13].
The choice of the host material is essential for achieving above-average mid-infrared (MIR) emission and vis-up-conversion efficiency for Er3+. Halide glasses, particularly fluoride because of their significantly low phonon energies, have been thoroughly examined in the previous investigation as the host materials of Er3+. Among RE3+, finding appropriate host materials is becoming more crucial to achieve optimum up-conversion efficacy in the mid-IR and visible regions. It is discovered that the host matrix’s interactions with particle dimension, RE3+ doping, phase purity, position replacement, and ion diffusion affect the upconversion (UC) emission [14]. Particularly for luminescent thermometry applications, there is a need for appropriate host materials that can maintain their heat stability and responsiveness throughout a wide temperature range (around the ambient temperature) [15]. Er3+ is found to be the most cost-effective and abundant among all the lanthanide ions and a decent activator because of its broad photoluminescence (PL) emission range, from mid-infrared (MIR) to visible regions [4, 16, 17]. Er3+-based solid-state lasers have shown promising results in laser output and can be used in the eye-safe MIR region. These lasers have many potential applications, including atmosphere pollution monitoring, medical surgery, military counter-measures, and remote sensing [10]. Due to its energy level configuration with a 4I15/2 to 4I11/2 transition, which is easily activated by low-energy photons, Er3+ is the most popular ion for upconversion [18].
In the field of optoelectronics and photovoltaics, the photo-electronic characteristics of ceramics play a vital role in determining their appropriateness. Lanthanide elements improve electrical and optical performance, enabling efficient and eco-friendly electronics [19]. Transparent ceramics like (K0.5Na0.5)NbO3-ErBiO3 have good optical and ferroelectric properties, making them potential candidates for optical modulation, switchers, and electro-optical devices [20]. Sm3+-doped lead-free 0.85(K0.5Na0.5)NbO3-0.15SrZrO3 (KNN-SZ: Sm3+) transparent ferroelectric ceramics have a unique photochromic phenomenon and reversible luminescence modulation, making them ideal for information storage, displays, anti-counterfeiting, and photo-switching devices [21]. Studies also demonstrate that (Bi0.5Na0.5)0.94Ba0.06TiO3 lead-free ferroelectric ceramics control the dielectric constant by light exposure while maintaining superior electrical insulation. This makes these ceramics suitable for photo-dielectric devices [22].
Based on the design technique mentioned above, in this research, the structural and ferroelectric properties of Er3+ substituted KNN ceramics were explored to enhance radiative upconversion emission. Using Cu-Kα radiations, an X-ray diffractometer was utilized to analyze the crystalline manner and identify the KNN ceramic’s phase. Further, FTIR spectroscopy was done to confirm the perovskite structure. P-E loops were investigated to study the ferroelectric properties. The optical properties, PL, and UCL emission spectra were also examined under the two excitation wavelengths, 488 nm and 980 nm, respectively.
2 Experimental procedure
2.1 Sample preparation
The solid-state reaction is a commonly used synthesis technique for creating polycrystalline materials from solid reagents. This method typically involves using very high temperatures to initiate the reaction. The chemical and morphological properties of the reagents, including their reactivity, surface area, and free energy, as well as the reaction conditions, such as temperature, pressure, and environment, can all affect the solid-state reaction. The solid-state reaction method helps produce a high volume of product is extremely easy to use compared to other techniques, and is economical [23]. The solid-state reaction was chosen in this study because it is simple, and the apparent lack of solvents makes the process economical. The solid-state reaction is preferable for commercial applications. However, to maintain stoichiometry, the starting materials, particularly K2CO3, must be dried due to their sensitivity to moisture [24].
The K0.5Na0.5NbO3 (KNN) ceramics added with x wt% Er3+ ion abbreviated as (KNN: xEr, x = 0, 1, 2, 3, and 4). This study used a conventional solid-state reaction route to prepare the KNN ceramics. High purity (99.99%) precursors K2CO3, Na2CO3, Nb2O5, and Er2O3 from Sigma Aldrich were measured in stoichiometric ratio. Mixed powders in the stoichiometric ratio were hand-ground in a mortar pestle for 5 h using ethanol. In a small crucible made from alumina, the hand-ground powders were calcined for 3 h at varying temperatures (700–900 °C). Then, the powders with the least unreacted residue were combined with 5 wt% polyvinyl alcohol (PVA), a standard binder. After mixing, the resulting blend was compressed into a 1 mm thick pellet with a 10 mm diameter using a manual hydraulic press. The press had a pressure of 50 MPa and a resting period of 1 min. The pellets were then sintered in a furnace at 1100 °C for 2 h with intermittent heat treatment at 500 °C for 1 h to eliminate the binder from the pellets [25]. Similar steps were repeated for producing KNN: Er3+ ceramics. A schematic diagram is given in Fig. 1 to explain the synthesis process. Equation (1) provides the synthesis with reaction [26]:
2.2 Sample characterization
X-ray diffraction (XRD) spectra were collected on a Bruker D-8 advance X-ray diffractometer with Cu-Kα radiation in the range 2θ (10°—80°), and the phase formation of synthesized samples was analyzed. The HORIBA PTI Quanta-master (8450–11) at an excitation wavelength of 980 nm was utilized to investigate the upconversion luminescence (UC) spectra. A silver paste was coated (area ~ 8.5–11 mm2) on the prepared pellets (thickness ~ 0.55–0.65 mm). The coated pellets were baked for 10 min at 500 °C so that the paste adhered utterly to the pellets. The P-E hysteresis loops were marked out by an automatic loop tracer under 50 Hz. SHIMADZU RF-5301 PC-spectro fluorophotometer and Xenon arc lamp were acquired to study the PL spectra under the excitation of 488 nm at room temperature. The FTIR spectra were obtained by the Perkin Elmer spectrum-II.
3 Result and discussion
3.1 XRD analysis
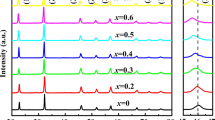
Figure 2a–e displays the XRD pattern of pure KNN powder calcined at various temperatures (700 °C, 750 °C, 800 °C, 850 °C, 900 °C). There are some small peaks at 15.88°, 27.76°, 30.24°, and 34.44° (depicted by symbol *), indicating that the material is not solely single-phased. Still, the slight impurities did not significantly impact the X-ray diffraction pattern. With the gradual increase in calcination temperature (700–900 °C), there is an apparent rise in X-ray reflection intensity from various planes and the intensity of secondary peaks (depicted by symbol *) reduces significantly.
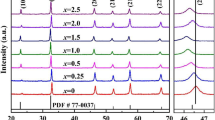
At 900 °C, the unit cell structure of the crystal of the KNN sample is observed to have a strong reflection arising from the [020] plane. The peak splitting near 46° and broadening in the diffraction patterns implies that the structure is orthorhombic perovskite [27, 28]. Particularly, the (022) and (200) peaks splitting around 46°, with the (022) peak having a higher value, confirms the orthorhombic lattice [28]. The XRD test demonstrates the existence of all anticipated Amm2 space group reflections, which matches the reference number 98–024-7571 from the inorganic crystal structure database (ICSD), which has an orthorhombic crystalline arrangement. For further investigation, 900 °C was chosen to be the optimum calcination temperature. Figure 3a depicts the XRD pattern of KNN: xEr with various concentrations of Er3+ sintered at 1100 °C after being calcined at 900 °C [29]. The perovskite structure is present in all the ceramics, which indicates that Er3+ ions have successfully diffused into the KNN host lattice. Due to the incorporation of erbium ions, the (022) and (200) peaks appear to merge indicating there may be a phase conversion [10]. The intense peak (020) moves to higher angles due to the incorporation of Er3+ ions, as shown in Fig. 3b. Er3+ ions with a smaller ionic radius of 1.23 Å occupy the A sites (K, Na) (K+ ionic radius = 1.64 Å, Na+ ionic radius = 1.39 Å) [9, 30]. This decreases the interplanar spacing (d), causing the XRD peak (002) to shift to higher angles, resulting in peak shifting. Furthermore, the decrement in interplanar spacing (d) was confirmed by calculating the a, b, and c lattice parameters of the KNN: xEr (x = 0, 1, 2, 3, 4) using the Topaz software given in Table 1. A decrease in cell volume and lattice parameters a, b, c is observed for KNN: xEr (x = 0, 1, 2, 3). Dopants possessing ionic radii that are less than those of the host material result in a reduction of the lattice parameters. This decrease is attributed to the combined influence of the small dopant and the contraction generated by the development of oxygen vacancies [31]. A slight lattice expansion is observed at x = 4, this is attributed to erbium ions not being diffused properly into the host lattice [32].
3.2 Fourier transform infrared (FTIR) spectroscopy
FTIR studies were carried out on KNN ceramics after being sintered at 1100 °C temperatures to discover more about their chemical interactions and functional groups. The FTIR spectra obtained for KNN: xEr (x = 0, 1, 2, 3, and 4) are represented in Fig. 4a. The high-intensity transmittance band at 486 cm−1 is accredited to the stretching and bending of the NbO6 bond [13]. The less dominant band at 720 cm−1 is attributed to the oxygen octahedral which are the characteristic peaks of KNN [12]. In Fig. 4b, there is a peak shift from 471 cm−1 to 486 cm−1, which leads to the conclusion that the FTIR spectra are exceptionally prone to changes in the local structure of the substance when a foreign ion is introduced [11].
3.3 Vis-down conversion photoluminescence
The excitation spectra of KNN are given in Fig. 5a and the dominant peak was observed at 488 nm via transition from ground state to 4F7/2. Figure 5b displays the emission spectra of visible down conversion photoluminescence; the 2H11/2 → 4S3/2 and 2H11/2 → 4I15/2 transitions are responsible for the dominant green radiation near 528 nm and 549 nm, correspondingly. The relatively faint red color radiation at 662 nm is attributed to the transition 4F9/2 → 4 I15/2 [31, 33]. Under excitation by a wavelength of 488 nm, the electrons are directly excited to the 2H11/2 and 4S3/2 levels, which accounts for the significant intensity of both green and red color emissions via down-conversion. These levels simultaneously experience radiative and non-radiative emissions. Er3+ ions are stimulated to the 4F7/2 level when the light source is 488 nm. The preponderance of stimulated Er3+ ions first reaches the 4S3/2 level via non-radiative relaxation due to the highest multi-photon relaxation (MPR) rate, and then they reach the 4I15/2 ground level via radiation relaxation. This demonstrates that Er3+, and not any other particles in the KNN lattice, is the source of the green luminescence detected in the KNN [8]. The magnified view of the red band at 662 nm can be seen in the inset of Fig. 5. Therefore, the most effective illumination sources for Er-doped PL materials should have a wavelength of 488 nm for operations involving down-conversion [34].
3.4 Vis-up conversion photoluminescence
The UC emission bands that have been detected are consistent with past studies on various hosts that have been doped with Er3+ [35,36,37,38]. Figure 6a displays the vis-up-conversion luminescence spectrum of KNN: xEr using a source of a diode laser lamp with a 980 nm wavelength. There are two prominent fluorescence peaks of a green color observed at 528 nm and 549 nm and a weak red band at 662 nm. The red color can be attributed to the 4f-4f transition within the Er3+ (inset Fig. 6a). The two primary peaks at 528 nm and 549 nm correspond with the 2H11/2 energy level and the 4S3/2 energy level of Er3+, respectively. This causes Er3+ to transition to the ground state 4I15/2 and release photons. The faint fluorescence peak of red at 662 nm corresponds to the transition from the 4F9/2 energy level of Er3+ to the ground state 4I15/2. The UCL intensity enhances with Er3+ concentration but subsequently reaches its maximum strength, i.e., at x = 3, the UCL intensity diminishes with further insertion of Er3+ content in the host lattice. This occurs due to the concentration-quenching effect. Figure 6b shows the variation of UCL intensity with varying Er3+ concentrations. The dopant concentration reaches an edge where the distance between erbium (dopant) ions becomes so minute that their energy transference becomes simpler, contributing to non-radiative relaxation. The radiative transitions reduce; hence the strength of the PL emissions diminishes [13].
Figure 6c shows the mechanism of the down-conversion process of Er3+ ions under the excitation of 488 nm. It has been observed that Er3+ ions in the ground state absorb photons and directly go to a higher energy level of 4F7/2. However, under 488 nm excitation, the red and green emissions are low relative to the emission under 980 nm excitation. Excitation at a wavelength of 488 nm causes the electrons to be directly excited to the 4F7/2 level. The multi-phonon relaxation (MPR) of the 4F7/2 energy level results in non-radiative transmission and occupies the mixed 2H11/2 and 4S3/2 energy levels at the same time. A restricted number of electrons reach the 4F9/2 level simultaneously. The electrons then radiatively transition to the ground state 4I15/2, emitting photons of the colors green and red with wavelengths of 549 and 662 nm, respectively. It demonstrates that the transitions involved in up-conversion at 980 nm differ significantly from those in excitation at 488 nm. As shown in Fig. 6d, Er3+ ions in the 4I15/2 ground state are excited by the incident 980 nm photons to the 4I11/2 level by the ground state absorption process (GSA). Some of the Er3+ ions in the 4I11/2 level absorb one more incident photon to populate the 4F7/2 level by an excited state absorption (ESA) process. Under 980 nm stimulation, numerous Er3+ ions are driven to the transitional level, thereby increasing the population of the 4F7/2 energy level via energy transfer (ET), which is not possible at 488 nm [15].
3.5 Impact of different pump power on UCL
The total intensity of each emission band has been incorporated, and its relationship to pumping power has been plotted in Fig. 7a–d. With the rising pump power, the UCL intensity increases for all the concentrations of Er3+. A power law mathematical expression among the intensity I and pump power P is suggested by theoretical analysis of the multi-photon absorption process is represented in Eq. (2) [39,40,41]:
where n signifies the amount of photons needed to complete the UCL mechanism. In the log–log plot shown in Fig. 8(a-d), three straight lines with positive slopes (denoted by n) for each dopant concentration have been calculated for all emission bands. The characteristics of the upconversion phenomenon, or the number of photons absorbed during the upconversion phenomenon, can be determined using a log–log scale to plot emission intensity versus excitation power. For the emission bands nearly at 528, 549, and 662 nm, the slope (n) is calculated by linearly fitting the experimental data and it comes around 2. This shows that at least 2 pump photons are required in UC emissions [42].
3.6 Time decay analysis
The time-resolved photoluminescence (TRPL) decay curve revealed the emission at 549 nm of Er3+ ions when stimulated with a Xenon laser at 980 nm. Figure 9 illustrates the time decay profile associated with the 4S3/2 → 4I15/2 level at room temperature. It was adequate to fit the decay patterns of singular doped systems with a tri-exponential decay function for Er3+, as described by Eq. (3):
Here, I(t) represents the time variation of luminous intensity; Ai represents the scalar quantities; t represents the measured time; and τi represents the decay time when the excited state population falls to 1/e of its initial value. The generalized equation with i = 1, 2, and 3 for tri-exponential fit is used to determine the average lifespan [43]. After substituting all the values in Eq. (4), the average time was calculated to be 25.05 µs [44].
3.7 Ferroelectric properties
When describing the electrical behavior of a ferroelectric ceramic, the hysteresis loop (polarization versus electric field) is the single most crucial measurement that can be made. The name “ferroelectric” comes from the fact that this loop resembles the magnetic loop (magnetization versus magnetic field) obtained from a ferromagnetic material, even though ferroelectrics do not contain a significant amount of ferro or iron. The hysteresis loop provides information about the switching behavior of the ferroelectric material and is used to determine important parameters such as coercive field, remnant polarization, and saturation polarization. This information is essential for designing and optimizing ferroelectric devices such as capacitors, actuators, and sensors [28, 32]. Since among all doped ceramics, KNN:xEr (x = 3) showed the best upconversion intensity and XRD plot, its hysteresis loop was compared with undoped KNN. Figure 10a, b displays the P-E hysteresis loop of KNN: xEr (x = 0, 3). The hysteresis loop holds good shape for both samples, indicating KNN is a good ferroelectric. For the undoped KNN, i.e., (x = 0), the remnant polarization and coercive field increase up to 5.1 μC/cm2 and 8.5 kV/cm respectively, when applied electric field increases up to 31 kV/cm. Above 31 kV/cm, the undoped KNN could not achieve the shape, resulting in lossy loops. For doped KNN: xEr (x = 3), the hysteresis loop holds its shape for the higher values of the applied electric field (31 kV/cm < E < 37 kV/cm). The remnant polarisation and coercive field decrease to 3.17 μC/cm2 and 6.24 kV/cm, respectively. The observed reduction in remnant polarization is linked to the associated relaxation in the structural distortion, which can be observed from XRD and FTIR analysis. The loss of K/Na can create vacancies of oxygen, which have been observed to migrate to areas of low energy at domain walls and electrode interfaces under high electric fields. This can impede polarization switching due to domain pinning and increase coercive field [40, 45]. Table 2 elaborates on the measured values of remnant polarization and coercive field for KNN: xEr (x = 0 and 3) at various electric fields.
4 Conclusion
The lead-free ceramic potassium sodium niobate (K0.5Na0.5NbO3) mixed with the rare earth element Er3+ abbreviated KNN: xEr (where x = 0, 1, 2, 3, and 4) is made by solid-state method to investigate the effect that Er3+ have on structural, ferroelectric, and photoluminescence characteristics. To generate a distinct phase perovskite crystal with an orthorhombic crystal structure, the undoped KNN was first calcined at temperatures gradually increasing from 700 to 900 °C and then sintered at a temperature of 1100 °C for 3 h. Because no other phases are present in the XRD spectra of the Er3+-enriched KNN, it can be deduced that all of the Er3+ ions have been incorporated into the host lattice. The development of a perovskite structure could be seen in the FTIR spectrum observed near 486 cm−1 and 720 cm−1. The PL emission spectra of Er3+ concentrated KNN particles were analyzed at room temperature to an illumination wavelength of 488 nm and 980 nm. Both emission spectra contained bands of green emission that were quite intense (528 nm and 549 nm) and bands of red emission that were slightly less intense (662 nm). At an illumination wavelength of 980 nm, it was discovered that the upconversion PL spectra exhibited a significantly greater emission intensity than the vis-down conversion PL. It was determined that ceramics use a two-photon pumping process for the emission process after observing the influence of pump strength on the emission process. The ferroelectric PE loop was observed to have good loop shapes at room temperature and satisfactory remnant polarization for the doped and undoped KNN ceramic. This type of material possesses outstanding intrinsic piezoelectric capabilities, and by combining its optical and ferroelectric properties, it may also have the potential to be utilized as a multifunctional optoelectronic device.
Data availability
The above-mentioned authors have all the relevant data associated with this research work and will be dedicated to sharing that if they are asked to do so in the future.
References
R. Groß, D. Bunke, C.-O. Gensch, S. Zangl, and A. Manhart, Study on Hazardous Substances in Electrical and Electronic Equipment, Not Regulated by the RoHS Directive Final Report Content, 2008
X. Vendrell, J.E. García, X. Bril, D.A. Ochoa, L. Mestres, G. Dezanneau, Improving the functional properties of (K0.5Na0.5)NbO3 piezoceramics by acceptor doping. J. Eur. Ceram. Soc. 35(1), 125–130 (2015). https://doi.org/10.1016/j.jeurceramsoc.2014.08.033
H. Wu, S. Shi, X. Liu, H. Wang, J. Xu, L. Yang, W. Qiu, S. Zhou, Photoluminescence and outstanding transparent in Er3+-doped (K0.5Na0.5)NbO3-(Sr0.5Ba0.5)(Bi0.5Nb0.5)O3 ceramics. J. Mater. Sci. Mater. Electron. 34, 612 (2023). https://doi.org/10.1007/s10854-023-10021-9
J.P. Sharma, D. Kumar, A.K. Sharma, Structural and dielectric properties of pure potassium sodium niobate (KNN) lead-free ceramics. Solid State Commun. (2021). https://doi.org/10.1016/j.ssc.2021.114345
Q. Zhang, L. Luo, J. Gong, P. Du, W. Li, G. Yuan, Photoluminescence, thermoluminescence and reversible photoluminescence modulation of multifunctional optical materials Pr3+ doped KxNa1-xNbO3 ferroelectric ceramics. J. Eur. Ceram. Soc. 40(12), 3946–3955 (2020). https://doi.org/10.1016/j.jeurceramsoc.2020.05.003
Y.Y.S. Cheng, L. Liu, Y. Huang, L. Shu, Y.X. Liu, L. Wei, J.F. Li, All-inorganic flexible (K, Na)NbO3-based lead-free piezoelectric thin films spin-coated on metallic foils. ACS Appl. Mater. Interfaces 13(33), 39633–39640 (2021). https://doi.org/10.1021/acsami.1c11418
M. Dolhen, A. Mahajan, R. Pinho, M.E. Costa, G. Trolliard, P.M. Vilarinho, Sodium potassium niobate (K0.5Na0.5NbO3, KNN) thick films by electrophoretic deposition. RSC Adv. 5(6), 4698–4706 (2015). https://doi.org/10.1039/c4ra11058g
W. Li, D. Wang, X. Li, P. Li, P. Fu, C. Hu, J. Hao, W. Li, Q. Zhang, Optical temperature sensing properties and thermoluminescence behavior in Er-modified potassium sodium niobate-based multifunctional ferroelectric ceramics. J. Mater. Chem. C. Mater. 10(33), 11891–11902 (2022). https://doi.org/10.1039/d2tc01268e
L. Xiang, H. Gangbin, W. Huangtao, S. Shaoyang, W. Hua, X. Jiwen, Y. Ling, Q. Wei, Transmittance, photoluminescence and electrical properties in Er-doped 0.98K0.5Na0.5NbO3-0.02Sr(Yb0.5Ta0.5)O3 ferroelectric ceramics. J. Electron. Mater. 51(7), 3476–3484 (2022). https://doi.org/10.1007/s11664-022-09626-3
X. Wu, S. Lu, K.W. Kwok, Photoluminescence, electro-optic response and piezoelectric properties in pressureless-sintered Er-doped KNN-based transparent ceramics. J. Alloys Compd. 695, 3573–3578 (2017). https://doi.org/10.1016/j.jallcom.2016.11.409
A. Banwal, R. Bokolia, Efficient tunable temperature sensitivity in thermally coupled levels of Er3+/Yb3+ co-doped BaBi2Nb2O9 ferroelectric ceramic. J. Lumin. 263, 120071 (2023). https://doi.org/10.1016/j.jlumin.2023.120071
S. Kumar, N. Thakur, Effect of alkali metal (Na, K) ion ratio on structural, optical and photoluminescence properties of K0.5Na0.5NbO3 ceramics prepared by sol-gel technique. Bull. Mater. Sci. (2021). https://doi.org/10.1007/s12034-020-02341-x
S. Bairagi, S.W. Ali, Effects of surface modification on electrical properties of KNN nanorod-incorporated PVDF composites. J. Mater. Sci. 54, 11462–11484 (2014). https://doi.org/10.1007/s10853-019-03719-x
Y. Zhang, L. Duan, A. Zhang, D. Wang, R. Chu, Z. Xu, G. Li, C. Zhang, Photoluminescence, electrical properties and electron band structure of (Ho, Yb)3+ co-doped SrBi4Ti4O15 multifunctional ceramics. Ceram. Int. 48(7), 9248–9257 (2022). https://doi.org/10.1016/j.ceramint.2021.12.111
R. Bokolia, O.P. Thakur, V.K. Rai, S.K. Sharma, K. Sreenivas, Dielectric, ferroelectric and photoluminescence properties of Er3+ doped Bi4Ti3O12 ferroelectric ceramics. Ceram. Int. 41(4), 6055–6066 (2015). https://doi.org/10.1016/j.ceramint.2015.01.062
X. Wu, C.M. Lau, K.W. Kwok, Effect of phase transition on photoluminescence of Er-doped KNN ceramics. J. Lumin. 155, 343–350 (2014). https://doi.org/10.1016/j.jlumin.2014.07.005
M. Narwan, A. Banwal, R. Sharma, R. Bokolia, Non-invasive thermal sensing and improved recoverable energy storage density of Bi0.5Na0.5TiO3: Er3+ doped multifunctional ferroelectric ceramic. J. Lumin. 265, 120236 (2024). https://doi.org/10.1016/j.jlumin.2023.120236
J. Wangi, Y. Sun, S. Shi, H. Wang, J. Xu, L. Yang, W. Qi, Effects of Er3+ doping on the structure and electro-optical properties of 0.94(K0.5Na0.5)NbO3–0.06Sr(Zn1/3Nb2/3)O3 ceramics. Bull. Mater. Sci. (2022). https://doi.org/10.1007/s12034-021-02596-y
L.N. Liu, X.M. Chen, R.Y. Jing, H.L. Lian, W.W. Wu, Y.P. Mou, P. Liu, Electrical and photoluminescence properties of (Bi0.5−x/0.94Erx/0.94Na0.5)0.94Ba0.06TiO3 lead-free ceramics. J. Mater. Sci. Mater. Electron. 30, 5233–5239 (2019). https://doi.org/10.1007/s10854-019-00822-2
C. Lin, X. Wu, M. Lin, Y. Huang, J. Li, Optical, luminescent and optical temperature sensing properties of (K0.5Na0.5)NbO3-ErBiO3 transparent ceramics. J. Alloy. Compd. 706, 156–163 (2017). https://doi.org/10.1016/j.jallcom.2017.02.245
Y.J. Du, L.I.D.J. Martin, D. Poelman, Reversible yellow-gray photochromism in potassium-sodium niobate-based transparent ceramics. J. Eur. Ceram. Soc. 41, 1925–1933 (2021). https://doi.org/10.1016/j.jeurceramsoc.2020.10.046
L.N. Liu, X.M. Chen, Y.D. Xu, H.L. Lian, P. Liu, Light-triggered “on-off” switchable dielectric constant in (Bi0.5Na0.5)0.94Ba0.06TiO3 lead-free ceramics. Appl. Phys. Lett. 123, 022904 (2023). https://doi.org/10.1063/5.0153351
M.S. El-Eskandarany, Mechanically induced solid-state reduction. Mech. Alloy. (2015). https://doi.org/10.1016/B978-1-4557-7752-5.00006-1
L. Kozielski, K. Feliksik, B. Wodecka-Duś, D. Szalbot, S. Tutu, Hot pressed K0.5Na0.5NbO3 material for piezoelectric transformer for energy harvesting. Arch. Metall. Mater. 63(3), 1275–1280 (2018). https://doi.org/10.24425/123801
A. Banwal, R. Bokolia, Phase evolution and microstructure of BaBi2Nb2O9 ferroelectric ceramics. Mater. Today Proc. 46, 10121–10124 (2021). https://doi.org/10.1016/j.matpr.2020.09.380
H.C. Thong, C. Zhao, Z. Zhou, C.F. Wu, Y.X. Liu, Z.Z. Du, J.F. Li, W. Gong, K. Wang, Technology transfer of lead-free (K, Na)NbO3-based piezoelectric ceramics. Mater. Today 29, 37–48 (2019). https://doi.org/10.1016/j.mattod.2019.04.016
A. Dahiya, O. P. Thakur, and J. K. Juneja, Sensing and actuating applications of potassium sodium niobate: Use of potassium sodium niobate in sensor and actuator, in Proc. of the Int. Conf. on Sens. Tech. ICST, pp. 383–386, (2013), doi: https://doi.org/10.1109/ICSensT.2013.6727680
Q. Jin, M. Jiang, S. Han, Y. Yan, Microstructure, optical and electrical properties of Bi and Ba co-doped K0.52Na0.48NbO3 transparent ceramics. J. Mater. Sci. Mater. Electron. 29(15), 13407–13417 (2018). https://doi.org/10.1007/s10854-018-9466-5
S. Dwivedi, T. Pareek, S. Kumar, Structure, dielectric, and piezoelectric properties of K0.5Na0.5NbO3-based lead-free ceramics. RSC Adv. 8(43), 24286–24296 (2018). https://doi.org/10.1039/c8ra04038a
L. Gao, Z. Liu, P. Ren, R. Liang, T. Li, K. Guo, B. Xie, J. Lu, P. Mao, J. Tian, L. Shu, Inhibiting oxygen vacancies and twisting NbO6 octahedron in erbium modified KNN-based multifunctional ceramics. J. Materiomics (2023). https://doi.org/10.1016/j.jmat.2023.05.007
E. Makagon, O. Kraynis, R. Merkle, J. Maier, I. Lubomirsky, Non-classical electrostriction in hydrated acceptor doped BaZrO3: proton trapping and dopant size effect. Adv. Funct. Mater. 31, 2104188 (2021). https://doi.org/10.1002/adfm.202104188
H. Sun, Q. Zhang, X. Wang, M. Gu, Green and red upconversion luminescence of Er3+-doped K0.5Na0.5NbO3 ceramics. Ceram. Int. 40(2), 2581–2584 (2014). https://doi.org/10.1016/j.ceramint.2013.10.089
A. Banwal, R. Bokolia, Enhanced upconversion luminescence and optical temperature sensing performance in Er3+ doped BaBi2Nb2O9 ferroelectric ceramic. Ceram. Int. 48(2), 2230–2240 (2022). https://doi.org/10.1016/J.CERAMINT.2021.09.314
W.U. Xiao, Rare-earth-doped KNN-based ceramics for photoluminescent and electro-optic applications (The Hong Kong Polytechnic College, Hong Kong, 2015)
D. Peng, H. Zou, Upconversion luminescence, ferroelectrics and piezoelectrics of Er doped SrBi4Ti4O15. AIP Adv. (2012). https://doi.org/10.1063/1.4773318
W. Li, Z. Xu, R. Chu, P. Fu, G. Zang, Improved piezoelectric property and bright upconversion luminescence in Er doped (Ba0.99Ca0.01)(Ti0.98Zr0.02)O3 ceramics. J. Alloys Compd. 583, 305–308 (2014). https://doi.org/10.1016/j.jallcom.2013.08.103
H. Liu, J. Wang, H. Wang, J. Xu, C. Zhou, W. Qiu, Er3+ and Sr(Bi0.5Nb0.5)O3-modified (K0.5Na0.5)NbO3: a new transparent fluorescent ferroelectric ceramic with high light transmittance and good luminescence performance. Ceram. Int. 48(3), 4230–4237 (2022). https://doi.org/10.1016/j.ceramint.2021.10.215
Z. Cao, J. Wang, C. Zhang, X. Mao, L. Luo, Flexible piezoelectric nanogenerator based on the Er3+ doped lead-free (Na0.5Bi0.5)TiO3-BaTiO3 piezoelectric nanofibers with strong upconversion luminescence. J. Alloys Compd. (2022). https://doi.org/10.1016/j.jallcom.2022.165766
M.K. Mahata, T. Koppea, T. Mondal, C. Brüsewitza, K. Kumar, V.K. Rai, H. Hofsässa, U. Vetter, Incorporation of Zn2+ ions into BaTiO3:Er3+/Yb3+ nanophosphor: an effective way to enhance upconversion, defect luminescence and temperature sensing. PCCP 17(32), 20741–20753 (2015). https://doi.org/10.1039/c5cp01874a
D.B. Paulaviciene, N. Traskina, R. Vargalis, A. Katelnikovas, S. Sakirzanovas, Thermal decomposition synthesis of Er3+-activated NaYbF4 upconverting microparticles for optical temperature sensing. J. Lumin. (2019). https://doi.org/10.1016/j.jlumin.2019.116672
R. Bokolia, O.P. Thakur, V.K. Rai, S.K. Sharma, K. Sreenivas, Electrical properties and light up conversion effects in Bi3.79Er0.03Yb0.18Ti3-xWxO12 ferroelectric ceramics. Ceram. Int. 42(5), 5718–5730 (2016). https://doi.org/10.1016/j.ceramint.2015.12.103
A. Banwal, R. Bokolia, Thermometric sensing performance in Erbium modified SrBi2-xNb2ErxO9 ferroelectric ceramic for optoelectronic devices. Ceram. Int. 48(23), 34405–34414 (2022). https://doi.org/10.1016/j.ceramint.2022.08.019
Q. Zhang, K. Chen, L. Wang, H. Sun, X. Wang, X. Hao, A highly efficient, orange light-emitting (K0.5Na0.5)NbO3:Sm3+/Zr4+ lead-free piezoelectric material with superior water resistance behavior. J. Mater. Chem. C. Mater. 3(20), 5275–5284 (2015). https://doi.org/10.1039/c4tc02995j
L. Wang, B. Lua, X. Liu, Y. Shi, J. Li, Y. Liu, Fabrication and upconversion luminescence of novel transparent Er2O3 ceramics. J. Eur. Ceram. Soc. (2019). https://doi.org/10.1016/j.jeurceramsoc.2019.11.048
Y.A. Genenko, J. Glaum, M.J. Hoffmann, K. Albe, Mechanisms of aging and fatigue in ferroelectrics. MSEB 192(C), 52–82 (2015). https://doi.org/10.1016/j.mseb.2014.10.003
Acknowledgements
We thank the Department of Applied Physics, Delhi Technological University, for letting us use research facilities. The authors, MV and SS, would like to extend their sincere appreciation to their supervisor, Dr. RB, for her guidance, patience, and constant encouragement throughout their research work. They also wish to express their gratitude to AB for her valuable contributions to the experiment and manuscript writing, her kindness and friendship, and for creating a positive research environment. Finally, the authors would like to thank their family and friends for their unwavering support, understanding, inspiration, and love.
Author information
Authors and Affiliations
Contributions
Muskan Varshney: Conceptualization, data curation, writing-original draft, made all the measurements, Shreya Soni: Conceptualization, data curation, writing-original draft, made all the measurements, Ankita Banwal: Data curation, writing-editing, Megha Narwan: Data curation, writing-editing, Manoj Verma: XRD analysis, writing-editing, Renuka Bokolia: Supervision, reviewing and editing of the original draft.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Varshney, M., Soni, S., Banwal, A. et al. Effect of Er3+ ion incorporation on the structural, photoluminescence, and ferroelectric properties of K0.5Na0.5NbO3 ceramic for optoelectronic applications. Appl. Phys. A 130, 267 (2024). https://doi.org/10.1007/s00339-024-07447-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07447-1