Abstract
In this paper, electrical and photoluminescence properties of the (Bi0.5−x/0.94Erx/0.94Na0.5)0.94Ba0.06TiO3 lead-free ceramics with x ≤ 0.02 were investigated. All ceramics have pure perovskite phase. Lattice distortion induced by the doping of Er3+ was observed. The ceramics show average grain sizes around 0.6–1.0 µm. The doped ceramics exhibit decreased dielectric constant, which are still higher than 1200 at room temperature. Curie temperatures of the samples do not fluctuate dramatically with changing the doping amounts, which are around 250 °C. The piezoelectric constant d33 is closely related to the Er3+ content. Under an excitation of 487 nm, the ceramics exhibit visible luminescent emissions at 528 nm (green), 548 nm (green), and 670 nm (red).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, ferroelectric perovskites have been widely used in many fields [1,2,3]. Due to regulations about environmental protection, studies on lead-free materials become very hot [4,5,6]. Bismuth sodium titanate (BNT), as one of most promising candidates, has high Curie temperature and good ferroelectric properties [7, 8]. But, high coercive field of BNT ceramics always induces low piezoelectric performance. As has been reported, the solid solution of (Na0.5Bi0.5Ba0.06)TiO3 exhibits a tetragonal–rhombohedral morphotropic phase boundary (MPB) [7, 9], which facilitates improving piezoelectric performance of the ceramics [10,11,12]. With the development of modern apparatus, it is very important to realize multifunction in one material. For example, the materials possessing optical and electrical properties can be used in solid-state laser, LED, dynamic random access memory, and photoelectric integrator, etc [13]. For realizing versatility of BNT-based materials, one frequently-used method is to add trivalent elements of lanthanide [14]. Herabut et al. reported that the doping of La3+ could improve dielectric, piezoelectric, and electromechanical properties of the BNT system [15]. Sum et al. found that the adding of Pr3+ improved ferroelectric properties and increased intensity of red emission [16]. Hu et al. reported that the Er3+-doped BNT-based materials exhibit excellent optical and electrical properties [17]. Lau et al. observed that point defects have great influence on the photoluminescence properties of the Er3+-doped BNT-based ceramics [18]. These results demonstrate that the elements of lanthanide are effective in affecting both electrical and optical performances. In this work, conventional solid-state reaction method was used to prepare (Bi0.5−x/0.94Erx/0.94Na0.5)0.94Ba0.06TiO3 ceramics. Their electrical and photoluminescence properties were investigated.
2 Experimental procedure
(Bi0.5−x/0.94Erx/0.94Na0.5)0.94Ba0.06TiO3 (abbreviated as BN6BT:xEr, where x = 0, 0.0025, 0.005, 0.0075, 0.01, and 0.02) ceramics were obtained by means of a solid-state sintering method. Dried Na2CO3 (99.8%), Bi2O3 (98.9%), BaCO3 (99.0%), TiO2 (98.0%), and Er2O3 (99.9%) powders were chosen as starting materials. For each composition, the oxides and carbonates were weighed according to the chemical formula after drying. The powders were calcined at 900 °C after ball-milling for 24 h. The powders were pulverized via adding 5 wt% polyvinyl alcohol. The pellets were pressed under 280 MPa. The diameter of the pellets is 10 mm and the thickness is approximate 1.3 mm. The binder in green pellets was burned out at 500 °C before sintering. The pellets were sintered at 1150 °C and dwelling time was 2 h. During sintering, the pellets were covered by the corresponding powders.
X-ray diffraction (XRD) data were collected via a D/Max2550 XRD meter (Rigaku Co) and crystallite structure was refined via the General Structure Analysis System (GSAS) software package [19,20,21]. For microstructural analysis, the ceramics were thoroughly polished, thermally etched and then characterized using a Nova Nano 450 scanning electron microscope (SEM, FEI). Average size of grains was estimated by using software Nano Measurer and more than 200 grains were used to calculate grain size distribution. Agilent E4980A LCR meter was used to measure dielectric properties. The samples were poled at 50 kV/cm for 20 min at room temperature and then placed for 24 h. The piezoelectric coefficient d33 were measured via by means of a ZJ-4A d33 meter. Ferroelectric behavior was characterized on a ferroelectric analyzer (Precision Premier II) at room temperature. Fluorescence spectrophotometer (FluoroMax-4, HORIBA, USA) was used to measure photoluminescence of the ceramics.
3 Results and discussion
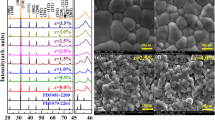
The theoretical density (ρt) of the ceramics was determined from the lattice volume (Table 1). The relative density (ρr) was calculated based on the equation of ρr = ρb/ρt × 100%, where ρb is bulk density and was obtained by using Archimedes method. The dependences of ρb, ρt, and ρr as a function of x are shown in Fig. 1. The ρb values of the ceramics are higher than 5.8 g/cm3 and change slightly with increasing x. All samples show ρr values around 98%, implying these ceramics were well sintered. The SEM images of the samples are displayed in Fig. 2, which exhibit dense microstructures. The grain boundaries are distinguishable. The size distribution of grains is shown in the insets of Fig. 2. The average grain sizes (D) are around 0.6–1.0 µm.
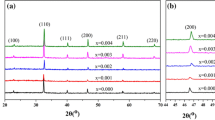
The XRD curves of the ceramics demonstrate that all ceramics were crystallized into single perovskite structure (Fig. 3a). Figure 3b presents the XRD peaks around 30°. The peak positions move toward right hand side, confirming the entrance of Er3+ into the lattice. It is well known that the composition of (Na0.5Bi0.5Ba0.06)TiO3 exhibits a tetragonal–rhombohedral MPB [11, 26]. Crystallite structure of the ceramics was refined with a two-phase mixture model of R3c + P4bm and the results are shown in Table 1. As a typical example, the refinement result for the ceramics with x = 0.0025 is shown in Fig. 3c. The lattice parameters exhibit a decrease trend with the increase in Er3+ content, which can be attributed to the different radii between Bi3+ and Er3+. Here, we want to mention that the radii of Bi3+ and Er3+ with coordination number (CN) of 12 are not available in Shannon’s table [22]. The radius of Bi3+ with CN = 12 can be determined by extrapolation of the lower CN values [23], which is 1.36 Å, as reported in our previous work [24]. By means of the same method, the obtained radius of Er3+ with CN = 12 is below 1.23 Å. The smaller radius of Er3+ than that of Bi3+ causes the lattice shrinkage of the ceramics.
Figure 4a shows dielectric spectra of the unpoled samples. The dielectric loss (tanδ), dielectric constant (εr) at room temperature, maximum dielectric constant (εm), and Curie temperature (Tc) are exhibited in Table 2. Curie temperatures of the samples do not fluctuate dramatically with changing the doping amount, which are around 250 °C. The ceramic with x = 0 shows εr of 1853 at room temperature. The doped ceramics exhibit decreased εr values, which are still higher than 1200 at room temperature. The tanδ values are around 0.04. The peaks of dielectric constant around Tc are obviously broadened, indicating diffuse character. The depolarization temperature (Td) can be determined via the dielectric spectrum of the poled ceramics [24]. The changes of εr and tanδ of the poled ceramics measured at 1 kHz as a function of temperature (T) are shown in Fig. 4b. The poled samples show a new dielectric anomaly on the εr ~ T curves at the temperature denoted as Td. Correspondingly, a new peak appears on the tanδ ~ T curves. The obtained Td values for the ceramics with x = 0, 0.0025, 0.005, 0.0075, 0.01 are 89 °C, 83 °C, 78 °C, 83 °C, and 78 °C, respectively. For the ceramic with x = 0.02, the dielectric anomaly was not observed. The Curie–Weiss (CW) law was used to explore diffuse behavior:
where C and T0 are the CW constant and CW temperature, respectively [25]. As a typical example, the fitting result for the ceramic with x = 0.0025 via the CW law is shown in Fig. 4c. At temperature denoted as TB, the εr value deviates from the fitting line. The obtained fitting values are displayed in Table 3. The values of ΔT′ = TB − Tc reflect the deviation from the CW law. All samples exhibit high ΔT′ values (≥ 100 °C), demonstrating remarkable deviation. The values of ΔT = Tc − T0 denotes phase transition character, which is positive for first-order phase transition, and zero for second-order phase transition [26]. Here, all ΔT values are larger than zero, indicating the former behavior. The fitting result for the ceramic with x = 0.0025 according to the modified CW law is shown in Fig. 4d. The modified CW equation is as following:
where γ and C′ are diffusion coefficient and Curie-like constant, respectively [24]. The γ values are always between 1 and 2. Ideal normal ferroelectrics have γ = 1 and ideal relaxor ferroelectrics have γ = 2. All samples have γ values approaching 2 (Table 3), indicating remarkable diffuse behavior. The diffuse character of dielectric behavior in BNT-based ceramics is suggested to be associated with the existence of complex cations, which possess similar radii but different charges and electronic configurations at an equivalent crystallographic site [11, 24, 26].
The polarization (P)–electric field (E) loops of the ceramics measured under various electric fields are shown in Fig. 5. With increasing measurement electric field, hysteresis loops become clear. For the ceramics with high Er3+ content, the P–E loops become thinner at a given measurement electric field. The obtained coercive field (Ec), maximum polarization (Pm), and remnant polarization (Pr) at 80 kV/cm are shown in Table 4. For the samples with x ≤ 0.01, the values of Pm are higher than 40 µC/cm2, Pr values are higher than 30 µC/cm2, and the Ec values are between 35 and 38.2 kV/cm. For the sample with x = 0.02, both polarization and Ec decrease due to the constriction of the P–E loops. The current density (J)–electric field (E) curves measured at 80 kV/cm are also shown in Fig. 5. For the ceramics with low Er3+ doping concentration (x < 0.01), two distinct peaks (P1 and P2) can be observed on the J–E curves, which is related to the domain switching with changing E [27]. For the ceramic with x > 0.01, there appear two other weak peaks (P3 and P4), which are related to the transition between ferroelectric order and weak polar phases, as reported by others [28]. The ceramic with x = 0.0025 exhibits Ec=36.2 kV/cm, Pm=43.7 µC/cm2, and Pr=34.7 µC/cm2, showing relatively good ferroelectric properties. The d33 values are also shown in Table 4. The ceramics with x ≤ 0.0075 exhibit high d33 values between 132 and 137 pC/N. As x increases to 0.02, the d33 of the ceramic decreases to 61 pC/N. The piezoelectric constant and polarization have the relationship of d33∝ε33ε0QPr, where ε0 is vacuum permittivity, Q is electrostrictive coefficient, ε33 is intrinsic dielectric permittivity in the direction of the polarization [29]. Generally, high polarization corresponds to high piezoelectric constant. The low polarization of the ceramic with x = 0.02 should be responsible for its low d33.
Fluorescence emission spectra of the ceramics under the excitation of 487 nm are shown in Fig. 6a. All ceramics exhibit two luminescent emission bands around 650–675 nm (red light) and 520–560 nm (green light). Figure 6b shows the schematic energy level of Er3+. Electrons in ground state 4I15/2 can jump to 4F5/2 and 4F7/2 under the excitation of 450 nm and 487 nm, respectively. Energy radiative relaxation of the electrons in these two states can happen to the mixed energy (2H11/2, 4S3/2, 4F9/2) under the action of multi-phonon radiation. In this case, red (528 nm, 549 nm) and green (670 nm) light can be observed under excitation at 450 and 487 nm, respectively. The radiative relaxation down to 2H11/2, 4S3/2, 4F9/2 and the followed transition to the ground state cause the emission of red (528, 549 nm) and green (670 nm) light. But, the transits of 4F7/2 + 4I11/2 → 4F9/2 and 4H11/2 + 4I15/2 → 4F9/2 + 4I13/2 result in reduction of electrons in 4F7/2 and 2H11/2. The green emission has two obvious strong peaks, corresponding to the wavelength of 528 nm and 548 nm, which are attributed to the transitions of 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2, respectively. The red emission (670 nm) is attributed to the transition of 4F9/2 → 4I15/2. The inset displays the emission intensity of the ceramics with the different Er3+ amounts. The intensity of green emission increases first as x is between 0 and 0.0075, and then decreases with further increasing x. The intensity of red emission increases continuously with increasing x. With the increase in the Er3+ concentration, the absorbing pumping source increases, and thus the intensity of emission spectra of different bands increases. But, in the case of higher Er3+ doping concentration, the distance between neighboring Er3+ ions should shorten. The complex crossover relaxation and energy transfer processes become more efficient, which enhances quenching of the green emission and thus weakens the green emission [30, 31]. However, such processes cause more electrons at 4F9/2 level, and then induce more transitions from 4F9/2 to 4I15/2, which increases red intensity [17, 31].
4 Conclusions
The (Bi0.5−x/0.94Erx/0.94Na0.5)0.94Ba0.06TiO3 lead-free ceramics with high density were successfully obtained. All samples have pure phase and average grain sizes between 0.6 and 1.0 µm. The Curie temperature of the ceramics are between 243 and 256 °C. The ceramic with x = 0.0025 exhibits Ec of 36.2 kV/cm, Pm of 43.7 µC/cm2, Pr of 34.7 µC/cm2, and d33 of 137 pC/N. The green emission on luminescent emission spectrum shows two distinct peaks at 528 nm and 548 nm. The ceramic with x = 0.0075 exhibits the maximum intensity of green emission. The red emission between 650 and 675 nm shows an obvious peak corresponding to the transition from 4F9/2 to 4I15/2. With increasing x, the intensity of red emission increases continuously.
References
G.H. Haertling, J. Am. Ceram. Soc. 82, 797 (1999)
L.J. Liu, M. Knapp, H. Ehrenberg, L. Fang, L.A. Schmitt, H. Fuess, M. Hoelzel, M. Hinterstein, J. Appl. Crystallogr. 49, 574 (2016)
D.W. Wang, F. Hussain, A. Khesro, A. Feteira, Y. Tian, Q.L. Zhao, I.M. Reaney, J. Am. Ceram. Soc. 100, 627 (2017)
C. Pascual-Gonzalez, G. Schileo, S. Murakami, A. Khesro, D.W. Wang, Appl. Phys. Lett. 110, 172902 (2017)
C. Pascual-Gonzalez, G. Schileo, A. Khesro, I. Sterianou, D.W. Wang, I.M. Reaney, A. Feteira, J. Mater. Chem. C 5, 1990 (2017)
S.Z. Erika Odendo, L.J. Liu, D.P. Shi, Y.M. Huang, L.L. Fan, J. Chen, L. Fang, B. Elouadi, J. Appl. Phys. 113, 094102 (2013)
T. Takenaka, K. Maruyama, K. Sakata, J. Appl. Phys. 30, 2236 (1991)
L.J. Liu, D.P. Shi, M. Knapp, H. Ehrenberg, L. Fang, J. Chen, J. Appl. Phys. 116, 184104 (2014)
F. Li, G.R. Chen, X. Liu, J.W. Zhai, B. Shen, S.D. Li, P. Li, K. Yang, H.R. Zeng, H.X. Yan, Appl. Phys. Lett. 110, 182904 (2017)
X.S. Qiao, X.M. Chen, H.L. Lian, J.P. Zhou, P. Liu, J. Eur. Ceram. Soc. 36, 3995 (2016)
X.S. Qiao, X.M. Chen, H.L. Lian, W.T. Chen, J.P. Zhou, P. Liu, J. Am. Ceram. Soc. 99, 198 (2016)
J. Glaum, H. Simons, M. Acosta, M. Hoffman, J. Am. Ceram. Soc. 96, 2881 (2013)
H.Q. Sun, D.F. Peng, X.S. Wang, M.M. Tang, Q.W. Zhang, X. Yao, J. Appl. Phys. 110, 016102 (2011)
H.L. Pan, J.J. Zhang, X.R. Jia, H.J. Xing, J.Y. He, J.Y. Wang, F. Wen, Ceram. Int. 44, 5785 (2018)
A.S. Herabut, J. Am. Ceram. Soc. 80, 2954 (1997)
H.Q. Sun, Q.W. Zhang, X.S. Wang, Y. Zhang, Ceram. Int. 40, 15669 (2014)
B. Hu, Z. Pan, M. Dai, F.F. Guo, H.P. Ning, Z.B. Gu, J. Chen, M.H. Lu, S.T. Zhang, B. Yang, W.W. Cao, J. Am. Ceram. Soc. 97, 3877 (2014)
C.M. Lau, X. Wu, K.W. Kwok, J. Appl. Phys. 118, 034107 (2015)
B.H. Toby, J. Appl. Cryst. 34, 210 (2001)
D.W. Wang, Z.M. Fan, D. Zhou, A. Khesro, S. Murakami, A. Feteira, Q.L. Zhao, X.L. Tan, I.M. Reaney, J. Mater. Chem. A 6, 4133 (2018)
D.W. Wang, Z.M. Fan, W.B. Li, D. Zhou, A. Feteira, G. Wang, S. Murakami, S. Sun, Q.L. Zhao, X.L. Tan, I.M. Reaney, ACS Appl. Energy Mater. 1, 4403 (2018)
R.D. Shannon, Acta Crystallogr. A 32, 751 (1976)
C.W. Tai, S.H. Choy, H.L.W. Chan, J. Am. Ceram. Soc. 91, 3335 (2008)
R.Y. Jing, X.M. Chen, H.L. Lian, X.S. Qiao, X.J. Shao, J.P. Zhou, J. Eur. Ceram. Soc. 38, 3111 (2018)
T. Wang, X.M. Chen, Y.Z. Qiu, H.L. Lian, W.T. Chen, Mater. Chem. Phys. 186, 407 (2017)
Z.D. Yu, X.M. Chen, Physica B 503, 7 (2016)
L. Jin, F. Li, S. Zhang, J. Am. Ceram. Soc. 97, 1 (2014)
G. Dong, H. Fan, J. Shi et al., J. Am. Ceram. Soc. 98, 1150 (2015)
Q.M. Zhang, H. Wang, N. Kim, L.E. Cross, J. Appl. Phys. 75, 454 (1994)
D.F. Peng, X.S. Wang, C.N. Xu, X. Yao, J. Lin, T.T. Sun, J. Am. Ceram. Soc. 96, 184 (2013)
S. Chen, M.J. Wu, L.Q. An, Y.X. Li, S.W. Wang, J. Am. Ceram. Soc. 90, 664 (2007)
Acknowledgements
This work was supported by Shaanxi Province Science and Technology Foundation (2018JM1009), Fundamental Research Funds for the Central Universities (Nos. GK201803017, GK201901005), and National Innovation and Entrepreneurship Training Program for College Students (No. CX2018105).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Ln., Chen, Xm., Jing, Ry. et al. Electrical and photoluminescence properties of (Bi0.5−x/0.94Erx/0.94Na0.5)0.94Ba0.06TiO3 lead-free ceramics. J Mater Sci: Mater Electron 30, 5233–5239 (2019). https://doi.org/10.1007/s10854-019-00822-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-00822-2