Abstract
In this research, molybdenum disulfide (MoS2) and bismuth trisulfide (Bi2S3) particles were synthesized. Bi2S3 was loaded on MoS2 nanosheet and was characterized by several techniques. Degradation of metronidazole (MTZ) and Cefalexin (CFX) were investigated via MoS2, Bi2S3, and MoS2/Bi2S3 particles under near-infrared (NIR) light irradiation. The evaluations of electronic structures and molecular geometries of MoS2 monolayer, MoS2/MTZ, and MoS2/CFX complexes were implemented using the DFT method. According to the electronic density of states (DOS) graphs results, when the MTZ and CFX interact with the monolayer surface, the energy gap (Eg) decreases compared to the MoS2 nanosheet. In other words, the electronic features of the MoS2 monolayer were altered after interaction with MTZ and CFX, where a reduction in the Eg value was evaluated (from 1.973 eV in the bare MoS2 to 0.010 and 1.936 eV in states MTZ and CFX complexes, an alteration of 99.49 and 1.875%, respectively. It can be concluded that the band gap significantly changes when the MTZ interacts with the monolayer surface. The maximum removal efficiencies of MTZ and CFX in 40 min and at pH 7 were obtained by MoS2/Bi2S3 photocatalyst, 91.54 and 73.18%, respectively. The results showed that the particles of Bi2S3, MoS2, and MoS2/Bi2S3 under NIR light irradiation have antibacterial properties, and the highest antibacterial property is related to the MoS2/Bi2S3 particles.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pollutants in the pharmaceutical industry wastewater treatment are very complex, non-biodegradable, and very harmful to the environment [1, 2]. The most important effect of antibiotics is low biological biodegradability, high toxicity, specific carcinogenicity, and most importantly their lack of biodegradability, and mutagenic. Their entry into municipal wastewater treatment plants can cause the death of treated organisms and disrupt biological treatment systems [3, 4]. Metronidazole (MTZ) with anti-inflammatory and antibacterial properties is one of the most widely used antibiotics in the world [5]. Its applications include the treatment of infectious diseases caused by anaerobic bacteria and protozoa and its use as an antiparasitic in poultry and fish [6]. This antibiotic is due to its high potency in eliminating infections of the gastrointestinal tract, mouth, teeth, and genitals in the affected organ, and it is one of the most widely used drugs [7, 8].
Cefalexin (CFX) is a cephalosporin antibiotic that is effective in treating many groups of infectious diseases, such as respiratory infections, middle ear infections, skin and soft tissue infections, bone or joint infections, and urinary and genital infections [9]. It is also used for people who have had surgery and other people who are prone to infection for any reason. Therefore, its presence in household effluents, hospitals, and pharmaceutical industries is high. Therefore, these widely used antibiotics must be treated before entering the environment [10]. Various techniques, such as membrane, oxidation, solid phase extraction, sonochemical degradation, nanofiltration, and electrophoresis, have been performed. Advanced oxidation processes (AOPs), including photocatalytic methods [11,12,13,14,15,16,17,18,19,20], have shown good potential for the removal of biodegradable organic compounds from municipal and industrial wastewater [21]. Due to the properties of MTZ and CFX antibiotics, it is necessary to control and eliminate this contaminant. In recent years, a family of chemical compounds known as dichalcogen-mediated metals, which have shown interesting properties, have received much attention [22].
MOS2 is part of the family of dicalcogen intermediate metals. Unlike graphene, these materials offer a variety of electronic properties [23]. As the most famous member of the family of intermediate dicalcogenes, MoS2 has been extensively used in two-dimensional fields in various fields, such as biomedicine, sensing, catalytic, antibacterial, and field-effect transistors [24]. Recently, the use of MoS2 nanostructures as an adsorbent in aqueous solution, especially with the nanosheet structure, has received a lot of attention [25]. MoS2 as a p-type semiconductor band gap is shown to change from 1.9 to 2.8 eV by the dielectric environment [26, 27]. Meanwhile, the preparation of MoS2 nanostructures by various methods of preparing nanostructures in parallel with their application has received much attention. One of the most optimal methods for the production of nanostructures is the hydrothermal method [28].
Bismuth trisulfide (Bi2S3) as an n-type has gained considerable attention because of its nontoxicity, low cost, direct band gap (Eg = 1.1 and 1.6 eV), thermo-chemical stability, and good optoelectronic and thermoelectric properties. Bi2S3 is a layered semiconductor with an orthorhombic crystal structure [29, 30]. The applications of Bi2S3 include areas, such as solar cells, sensors, photodetectors, photocatalysis, antibacterial, and thermoelectric devices [31]. Bi2S3 has different morphologies, such as flower, wire, tube, and sphere [32]. Various methods are used for synthesis such as microwave, hydrothermal, and solvothermal. Semiconductors have conduction between the conductor and the insulator [33].
P-n junctions are very simple bonds with a p-type semiconductor compound on one side of the bond and an n-type semiconductor compound on the other side [34]. In n-type matter, electrons increase, and in p-type, holes predominate. In type n semiconductors, the number of electrons is much more than the number in p-type semiconductors, and also in type p semiconductors, the number of holes is much more than the number in n-type semiconductors. For this reason, when the two types of semiconductors n and p are connected to each other, electrons penetrate into the semiconductor p and holes penetrate into the semiconductor n. The penetration of an electron from the n region to the p region causes the formation of a positive ion in the n-type semiconductor and also similarly, the penetration of a hole causes a negative ion acceptor to form in the p region. It recombines with one of the holes on the p side [35].
Sulfide semiconductors with a small band gap have high photocatalytic activity by starting absorption in the visible and NIR regions. Due to synergy, MoS2/Bi2S3 particles have a higher reactive oxidation species (ROS) production capacity and more antibacterial properties [36].
This research aims to investigate the removal of commonly used MTZ and CFX antibiotics using MoS2, Bi2S3, and MoS2/Bi2S3 photocatalysts under NIR irradiation. Furthermore, the antibacterial activity of MoS2, Bi2S3, and MoS2/Bi2S3 photocatalysts under NIR irradiation and in the dark has been studied. To compare the experimental and theoretical results, electronic structures and molecular geometries of MoS2 monolayer, MoS2/MTZ, and MoS2/CFX complexes were implemented using the DFT method.
2 Materials and methods
2.1 Material
Ammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24, ≥ 99%), thiourea (CH4N2S, > 99%), and bismuth nitrate Bi(NO3) from Sigma-Aldrich company was prepared. Metronidazole (C6H9N3O3) and Cefalexin (C16H17N3O4S) antibiotics were purchased by Darou Pakhsh Company.
2.2 Synthesis of MoS2
First, 1.24 g of (NH4)6Mo7O24 and 2.28 g of CH4N2S were poured into 36 mL of distilled water and stirred for 30 min. The resulting solution was poured into an autoclave stainless steel for 7 h at a temperature of 170 °C. After performing the synthesis process, a precipitate is obtained which was washed several times with distilled water, and heated in an oven at 150 °C for 2 h to give the MoS2 product [28].
2.3 Synthesis of Bi2S3
0.25 g of CH4N2S and 0.61 g of bismuth nitrate Bi(NO3) were poured in 50 mL of distilled water and stirred for 30 min. Then, 5 mL of HCl was added to the solution. The resulting solution was poured into an autoclave for 20 h at 140 °C. After performing the synthesis process, a precipitate obtained was washed several times with distilled water [37].
2.4 Synthesis of MoS2/Bi2S3
MoS2/Bi2S3 photocatalyst was prepared with a weight ratio of 1:1. In this way, first 0.05 g of MoS2 and 0.05 g of Bi2S3 were poured into 100 mL of distilled water and stirred for 12 h at room temperature. The mixture was washed and dried at 60 °C for 6 h and was placed in a furnace at 400 °C for 3 h.
2.5 Degradation of MTZ and CFX
In this step, MTZ and CFX were prepared at a concentration of 100 mg/L at pH 7. The amount of 0.02 g of MoS2, Bi2S3, and MoS2/Bi2S3 was added to 50 mL of prepared drugs and placed on a magnetic stirrer exposed to NIR light (800 nm, 100 W/cm2) for 5–45 min. Then, the samples were centrifuged at 7500 rpm for 15 min. Finally, the absorption of MTZ and CFX was measured by spectrophotometer at 319 and 261 nm, respectively
where A0 is the initial adsorption of the drugs and At is its final adsorption on the photocatalytic degradation [20].
2.6 MoS2/Bi2S3 photocatalyst recycling
For this purpose, the MoS2/Bi2S3 photocatalyst used in the degradation process of MTZ and CFX antibiotics was collected by centrifugation and then washed once with ethanol and three times with distilled water. The collected photocatalyst was dried at ambient temperature and then the photocatalyst was reused in the degradation reaction of MTZ and CFX antibiotics.
2.7 Computational details
The evaluations of electronic structures and molecular geometries of MoS2 monolayer and MTZ/CFX were implemented by means of the DFT method [38, 39] using SIESTA simulation software [40, 41]. Conventional DFT technics do not discuss long-range attractive contributions to van der Waals (vdW) interactions between the adsorbent and adsorbate. Therefore, the dispersion-corrected DFT combined with generalized gradient approximation (GGA-PBE) [42] approaches were employed [43,44,45,46]. The PBE function is a good choice for studying MoS2 material, which is confirmed by the reported DFT work [47]. In the simulation, a two-dimensional periodic boundary condition was utilized for the super-cells with 15.75 × 13.64 × 25 Å for the MoS2 monolayer. The double zeta polarization (DZP) orbitals have been utilized for the relaxation of the MoS2 nanosheet in the present study [48]. Furthermore, the interaction between the nuclei and core electrons and the valence electrons was estimated via Troullier–Martin realistic scheme [49, 50]. Further, the complete geometrical optimization was carried out with a conjugate gradient (CG) algorithm until the Hellmann–Feynman force including Pullay-like corrections was converged to 0.01 eV/Å [51]. The mesh cutoff was set to 150 Ry which presents a good accuracy. To better comprehension of physical quantities, a Brillouin zone was sampled using Monkhorst–Pack 5 × 5 × 1 k point sampling for converging geometry, whereas 15 × 15 × 1 for the assessment of the electronic DOS and charge transfer (CT) analysis [52]. The unit cell is allowed to converge in xy directions, whereas the z path is kept frozen at 25°A, consequently to eschew unwanted interactions between replicas.
To calculate the binding features of MoS2/Drugs complexes, we computed the interaction energies via pseudopotential approach through van der Waals (vdW) correction suggested by Grimme (DFT-D2) scheme [53] as well applied OpenMX code which is an outstanding DFT code for evaluating the molecular systems [54, 55]. The numerical pseudo-atomic orbitals’ (PAOs) method was employed as the basis sets for the Kohn–Sham potential and orbitals [56]. PAO basis functions are specified by C-s2p2d1, H 5.0-s2p2d1, N-s2p2d1, O 7.0-s2p2d1, S 7.0-s2p2d1, and Mo 7.0-s2p2d1. The energy cutoff for MoS2/Drugs was selected at 200 Ry [57]. In structural optimizations, convergence was assumed around 10–4 Hartree/Bohr, which offers rational outcomes for under study complexes.
The interaction energy as called Eint for the interacting Drugs with the MoS2 is defined as
where E (MoS2/Drugs), E (MoS2), and E (Drugs) are the total energies of the relaxed MoS2/Drugs complex, the pure MoS2, and the isolated drugs, respectively.
It is worth mentioning that the basis set superposition error (BSSE) correction was utilized through the counterpoise correction (CC) approach [56]. The δBSSE statement corresponds to the corrections on the BSSE using the counterpoise technique.
Besides, to clarify the electronic sensitivity of the MoS2 monolayer toward the adsorption of the drug, the modification in Eg is assessed by
where Eg1 and Eg2 are the measures of Eg for the pure MoS2 and the drugs, respectively.
To investigate the nature of the interaction between drugs and MoS2, the CT was considered through the Mulliken technique [58, 59].
2.8 Antibacterial activities
In this method, each sterilized disk was loaded with a different solution of Bi2S3, MoS2, and MoS2/Bi2S3 particles at a concentration of 1000 mg/µL. The dried disks are then placed on the surface of the NA agar plates sprayed uniformly by ca 1 × 106 CFU/ml of Escherichia coli (E. coli) from the culture medium by the applicator and inoculated into the culture medium. Plates with no treatments were used as control. Petri dishes were then irradiated with NIR light (800 nm, 100 W/cm2) for 2 min and also without light and incubated at 37 °C for 12 h in a shaking incubator. After 12 h, the diameters of the growth halos created around the disks were measured in millimeters with a caliper.
3 Results and discussion
3.1 Characterization
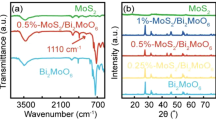
XRD technique was used to identify the phase and structure of MoS2 and Bi2S3, MoS2/Bi2S3 photocatalysts. Figure 1 shows the XRD pattern of samples. The XRD of MoS2 at the angles of 14.39, 32.91, 39.69, and 76.58° corresponds to plates (002), (100), (103), and (110), respectively; which indicates the hexagonal phase by the standard peaks of the characteristic MoS2 with JCPDS card No. 00-037-1492. The XRD spectrum of the Bi2S3 at the angles of 25.01, 28.66, 32.46, and 46.51° corresponds to plates (101), (130), (021), and (431), respectively. The results showed that the Bi2S3 were crystallized orthorhombic phase with the value in standard JCPDS card No. 00-017-0320. The peaks of MoS2/Bi2S3 at angles 14.39, 32.91, 39.69, and 76.58° are related to MoS2, and the peaks at angles of 25.01, 28.66, 32.46, and 46.5°, are related to Bi2S3, respectively (Fig. 1).
The average particle size was estimated based on the Debye–Scherrer equation using X’pert Highscore software
where B is the particle size, K is a constant number equal to 0.9, λ is the applied wavelength in terms of nanometers, and L is the peak full width at half maximum (FWHM) in terms of radians and θ is the angle corresponding to the highest peak [20].
The mean particle sizes calculated by the Debye–Scherrer method were MoS2, Bi2S3, and MoS2/Bi2S3 52.67 and 29.95, and 64.71 nm, respectively.
For the synthesized particles, the calculated crystal and lattice parameters are displayed in Table 1.
The interaction of infrared radiation with a sample changes the vibrational energy of the bond in its molecules and is a good way to identify functional groups and molecular structures. The FTIR spectra of the MoS2 in the range of 476.29 cm−1 are related to the Mo–S tensile vibration and in the peak range of 3424.21 cm−1 assigned to the O–H tensile vibration. The spectra of FTIR Bi2S3 in the range of 495.36 cm−1 are related to the tensile vibration of Bi–O, in the range of 618.16 cm−1 characteristic to C–S bond, and the peak located at 1117.73, 1271.87 and 2924.92 cm−1 corresponds to the bending vibration C–O, C–O–C, and C–H bond. Also, in the peak range 3436.05 cm−1, it is related to O–H tensile vibration (Fig. 2). In the MoS2/Bi2S3, the absorption bands at 467.93, 499.04, 528.85, and 613.33 cm−1 are assigned to the Mo–S, Bi–O, C–S, and C–S. The peak located at 1113.29, 1400.97, and 1631.65 cm−1 corresponds to the bending vibration C–O, C–S, and H–O–H bond. Also, in the peak range of 3436.05 cm−1, it is related to O–H tensile vibration (Fig. 2).
SEM technique was used to determine the surface characteristics and morphology of the synthesized particles. Based on the morphological results, MoS2 is like flower, Bi2S3 is Rod, and Bi2S3 particles are well stabilized on MoS2 (Fig. 3).
Based on the EDS diagram, Mo (36.97%), Bi (24.18%), and S (38.85%) elements are present in MoS2/Bi2S3. The Xmap technique examines point-by-point a specific area of the sample. In this technique, EDS analysis is performed from a large number of points in a specified area and the results of this analysis are displayed as a series of colored dots. Each color represents an element. Where the amount of this element is greater, the number of dots with that particular color is greater. In the Map images for MoS2/Bi2S3 the blue, green, and orange dots represent the presence of Bi, Mo, and S, respectively (Fig. 4).
BET/BJH results of MoS2, Bi2S3, and MoS2/Bi2S3 samples are shown in Fig. 5. Based on the results, by loading the Bi2S3 rod on MoS2 flower surface area and total pore volume increased. However, the average diameter of the average cavities decreased, which shows that Bi2S3 is well loaded on MoS2 (Table 2).
Using the absorption coefficient (α), the band gap of thin layers can be studied with the help of the Tauc equation [16]
where h is the Planck constant, ν is the applied frequency, α is the absorption coefficient, Eg is the band gap, and A is a proportional constant. The band-gap values for MoS2, Bi2S3, and MoS2/Bi2S3 1.90, 1.18, and 1.79 (eV) were determined by the Tauc method. The Tauc diagram is shown in Fig. 6. Based on the results as the MoS2 was bound to Bi2S3, the band gap decreased.
3.2 Theoretical section
In this section, we examine the interaction between the MoS2 monolayer and selected drugs in the aqueous environment. In our calculations, MoS2 monolayer with 17.75 Å × 13.64 Å super-cells has been modeled consisting of 25 and 50 molybdenum and sulfur atoms, respectively. The converged structure of the MoS2 has a Mo‒S bond length of around 2.42 Å. The separation between sulfur layers evaluated as 3.17 Å allows for direct comparison to the experimental data, 3.18 Å, calculated through STM images (Table 3). The obtained results are in good agreement with theoretical outcomes [60,61,62,63]. To discover the most stable structure of MET/CEPH drugs interacting with MoS2 monolayer, the GGA-PBE approach was implemented for optimization and DFT-D2 for total energies calculations of above-mentioned systems. According to the main goal in this study is the elimination of mentioned drugs in industrial wastewater, we performed the drugs and MoS2 monolayer interaction in the presence of water molecules as denoted in Fig. 7. After the optimization of the initial structures, the Eint were calculated with the DFT‒D2 scheme. The Eint were assessed around − 1.306 and − 1.060 eV for MTZ and CFX, respectively, as presented in Table 4. Simultaneously, we considered equilibrium distances in MoS2/Drugs complex. The obtained results reveal that the distance between the S atom of the MoS2 monolayer and the H atoms of CFX and MTZ is 2.507 and 2.513 Å, respectively (Fig. 8). Charge analysis is a vital analysis to consider the intermolecular and intra-molecular interactions, and as an appropriate source for the investigation of the charge transferring in molecular systems. In this stage of the investigation, we calculate the CT between drug molecules and the MoS2 monolayer. As depicted in Table 5, based on the Mulliken population, 0.01 and 0.010 and 0.121 e have been transferred from CFX and MTZ to MoS2 monolayer, respectively. The results show also that the MoS2 sheet gains electrons and drugs molecules lose electrons during the adsorption process. In other words, MoS2 behaves as an acceptor, and drug molecules act as donor counterpart. There is a negligible CT between the MoS2 and drug molecules, demonstrating that there is a weak interaction between drugs and MoS2 nanosheet. To better understand the modification of the electronic structures of the pure MoS2 monolayer and drug molecules’ adsorptions on the MoS2 monolayer, the electronic DOS diagrams were computed and plotted (Fig. 8). In electronic DOS plots, the Eg mentions the energy difference between the valence and the conduction levels. The electronic DOS graphs demonstrate that for selected complexes when the drugs interact with the monolayer surface, the band gap decreases compared to pure MoS2. In other words, the electronic features of the MoS2 monolayer were altered after adsorption with drugs, where a reduction in the Eg values was evaluated (from 1.973 eV in the pure MoS2 monolayer to 0.010 and 1.936 eV in states MTZ and CFX complexes, an alteration of 99.49 and 1.875%, respectively) (see Table 5). It can be concluded that the band gap significantly changes when the MTZ interacts with the monolayer surface. Moreover, the difference in the Fermi level of the MoS2 (EF = − 3.926 eV), MoS2/MTZ (EF = − 2.915 eV), and MoS2/CFX (EF = − 3.502 eV) clearly shows a CT between MoS2 and MoS2/Drugs in the adsorption process. To further insight into the adsorption process of drugs on the MoS2 monolayer, frontier molecular orbitals (FMO) including HOMO and LUMO are implemented. The HOMO denotes the ability to donate an electron and the LUMO as an electron acceptor represents the ability to obtain an electron. As shown in Fig. 9, the HOMO level for the MoS2/CFX complex is mainly located on the CFX drug surface, while the LUMO state is localized on the MoS2 surface. In the case of the MoS2/MTZ complex, both the HOMO and LUMO states are only focused on the MoS2 sheet as represented in Fig. 9. We also assessed the total electron density maps for mentioned complexes. As we can see from Fig. 10, there is an insignificant overlapping of electron clouds between the drug molecules and the MoS2 monolayer. In other words, there is no evidence for hybridization between two involving molecules.
3.3 Degradation of MTZ and CFX
Sulfide semiconductors with a small band gap have high photocatalytic activity by starting absorption in the visible and NIR regions. NIR photothermic can effectively absorb light energy, resulting in light-to-heat conversion, thereby increasing the reaction temperature. When the temperature increases, the rate of photocatalytic oxidation increases. To investigate the effect of MoS2, Bi2S3, and MoS2/Bi2S3 on the degradation efficiency of antibiotics, MTZ and CFX experiments were performed. According to the results, the performance of the Bi2S3 rod is higher than MoS2 flower, which can be attributed to the high surface area of Bi2S3 rod 151.96 (m2 g−1) and its small band gap of 1.18 eV. Among all the photocatalysts, the MoS2/Bi2S3 heterostructure had the highest removal efficiency. It can be concluded that MoS2/Bi2S3 heterostructure with a small band gap has high photocatalytic activity with absorption in the NIR region. Experimental results also showed that the degradation efficiency of MTZ is higher than that of CFX. Based on the results, the highest removal efficiencies of MTZ and CFX in 40 min and at pH 7 were obtained by MoS2 flower/Bi2S3 rod photocatalyst, 91.54 and 73.18%, respectively (Fig. 11a, b). The results of optical degradation curves of MTZ (λ = 319 nm) and CFX (λ = 261 nm), before and after degradation for 10, 20, 30, and 40 min, are shown in Fig. 12a, b. Based on the results, after 40 min, the absorption of MTZ and CFX has decreased, which can be concluded that MTZ and CFX have been removed from the aqueous media.
3.4 Proposed mechanism of degradation of MTZ and CFX
Several intermediates in the photocatalytic degradation pathways of MTZ and CFX are suggested. Possible photocatalytic degradation pathways for MTZ and CFX are plotted and shown in Fig. 13a, b. For the MTZ photocatalytic degradation pathway, intermediate (I) is related to oxidation and decarboxylation. In addition, N-denitration and hydroxylation lead to the formation of product (III). In CFX, intermediate (I) is created due to ring opening by hydroxylation and demethylation due to OH⋅/O2 attack on CFX and methyl exchange with hydroxyl. Decarboxylation and dealkylation can lead to the formation of a product (IV). Intermediate (V) output is due to CFX fragmentation. Deamination and oxidation of intermediate (V) lead to the production of the product (VI). Carboxylation and dealkylation form intermediate (II) and product (III). Previous researchers have suggested similar products for MTZ and CFX [64].
3.5 Recycled MoS2/Bi2S3 photocatalyst
Because the possibility of reusing the synthesized photocatalyst is economically important, therefore, it is necessary to evaluate the efficiency of the MoS2/Bi2S3 photocatalyst during successive recycling. The results obtained after five times recycling and reuse showed that MoS2/Bi2S3 photocatalyst decreased its photocatalytic activity by increasing the recycling frequency, the results of which are shown in Fig. 14a, b.
3.6 Antibacterial activities
In this study, the antibacterial properties of the synthesized particles were investigated. The results showed that disks loaded with a different solution of Bi2S3, MoS2, and MoS2/Bi2S3 particles under NIR light irradiation have antibacterial properties, and the highest antibacterial activity was found in MoS2/Bi2S3 particles under NIR irradiation. Based on the results, the inhibition rate for E. coli bacteria by the Bi2S3, MoS2, and MoS2/Bi2S3 particles under NIR light irradiation was recorded 2.6, 3.2, and 6.1 mm, respectively. The inhibition rate for E. coli bacteria by the Bi2S3, MoS2, and MoS2/Bi2S3 particles without NIR light irradiation was recorded 1.8, 2.7, and 4.8 mm, respectively (Table 6) [65]. The increase in temperature destroys bacterial membranes and proteins, and as a result, it leads to the inactivation of bacteria. Due to synergy, MoS2/Bi2S3 particles have a higher ROS production capacity and more antibacterial properties (Fig. 15a, b).
Based on the results obtained from this research, it can be concluded that the photocatalytic degradation of MTZ and CFX drugs under NIR light can be done with the lowest amount of photocatalyst consumed (0.02 g) in 40 min. Also, MoS2/Bi2S3 particles showed acceptable antibacterial properties under NIR light irradiation.
4 Conclusion
MoS2 flower-like and Bi2S3 rod-like were synthesized by hydrothermal method. Then, Bi2S3 rod was loaded on the MoS2 flower. MoS2, Bi2S3, and MoS2/Bi2S3 particles were characterized by different techniques. Degradation of MTZ and CFX were investigated onto MoS2, Bi2S3, and MoS2/Bi2S3 particles under NIR light. Based on the experimental results, the degradation efficiency of MTZ was higher than that of CFX. Also, the highest removal efficiencies of MTZ and CFX in 40 min and at pH 7 were obtained by MoS2 flower/Bi2S3 rod photocatalyst, 91.54 and 73.18%, respectively. Based on the results, it can be concluded that the MoS2/Bi2S3 photocatalyst has an acceptable performance in the removal of antibiotics. Based on the theoretical outcomes, the MoS2 nanosheet can be used as the appropriate platform for the removal of mentioned antibiotics drugs, especially MTZ. The obtained results reveal that the Eg of MoS2 was changed after the adsorption of antibiotic drugs. It can be concluded that the Eg significantly changes when the MTZ interacts with the MoS2 nanosheet. Also, in this study, the antibacterial properties of the synthesized particles were investigated. The results showed that the particles of Bi2S3, MoS2, and MoS2/Bi2S3 under NIR light irradiation have antibacterial properties, and the highest antibacterial property is related to the MoS2/Bi2S3 sample under NIR irradiation. In the present work, MoS2/Bi2S3 photocatalyst with antibacterial properties was made for the first time, which is a simple, cost-effective and environmentally friendly technique that has high efficiency for the degradation of antibiotics from aquatic environments.
Availability of data and materials
All data will be available if required.
References
S.S. Fekra, N.E. Fard, R. Fazaeli, Photocatalytic degradation of antibiotic norfloxacin aqueous solution by Ce/Bi2WO6: optimization and simulation of process by RSM. Russ. J. Appl. Chem. 94(6), 824–834 (2021)
M. Amanulla, C.M. Magdalane, G. Ramalingam, R. Sundaram, N. Tamam, H.H. Somaily, M.S. Al-Buriahi, Fabrication and characterization of Th (MoO4)2/TiO2 nanocomposite for potential use in photocatalytic degradation of toxic pollutants. Appl. Phys. A. 128(5), 1–21 (2022)
N. Askari, M. Farhadian, D. Mowla, M. Beheshti, Evaluating the binary Z-scheme Bi2S3/CuWO4 immobilized on FTO as a visible-light-driven photocatalyst for metronidazole degradation-response surface method. Appl. Phys. A. 127(10), 1–13 (2021)
M. Qian, L. Yang, X. Chen, K. Li, W. Xue, Y. Li, G. Shen, The treatment of veterinary antibiotics in swine wastewater by biodegradation and Fenton-like oxidation. Sci. Total Environ. 710, 136299 (2020)
N. Nasseh, F.S. Arghavan, S. Rodriguez-Couto, A.H. Panahi, M. Esmati, T.J.A. Musawi, Preparation of activated carbon@ ZnO composite and its application as a novel catalyst in catalytic ozonation process for metronidazole degradation. Adv. Powder. Technol. 31(2), 875–885 (2020)
B.M. Lee, L.K. Harold, D.V. Almeida, L. Afriat-Jurnou, H.L. Aung, B.M. Forde, C.J. Jackson, Predicting nitroimidazole antibiotic resistance mutations in Mycobacterium tuberculosis with protein engineering. PLoS Pathog. 16(2), e1008287 (2020)
A. Eguchi, S. Mizukami, M. Nakamura, S. Masuda, H. Murayama, M. Kawashima, T. Yoshida, Metronidazole enhances steatosis-related early-stage hepatocarcinogenesis in high fat diet-fed rats through DNA double-strand breaks and modulation of autophagy. Environ. Sci. Pollut. Res. 29(1), 779–789 (2022)
A. Nasiri, S. Rajabi, M. Hashemi, H. Nasab, CuCoFe2O4@ MC/AC as a new hybrid magnetic nanocomposite for metronidazole removal from wastewater: Bioassay and toxicity of effluent. Sep. Purif. Technol. 296, 121366 (2022)
Q. Wang, S. Tu, W. Wang, W. Chen, X. Duan, L. Chang, Optimized Indium modified Ti/PbO2 anode for electrochemical degradation of antibiotic cefalexin in aqueous solutions. Colloids Surf. A Physicochem. Eng. 628, 127244 (2021)
P. Chaturvedi, P. Shukla, B.S. Giri, P. Chowdhary, R. Chandra, P. Gupta, A. Pandey, Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 194, 110664 (2021)
N. Elmi Fard, R. Fazaeli, Fabrication of superhydrophobic CoFe2O4/polyaniline/covalent organic frameworks/cotton fabric membrane and evaluation of its efficiency in separation of olive oil from water. J. Chin. Chem. Soc. 69(12), 2014–2026 (2022)
N.E. Fard, R. Fazaeli, M. Yousefi, S. Abdolmohammadi, Oxidative Desulfurization of Dibenzothiophene Using M/TiO2/MWW (M= Cu, Ag, and Au) Composite. Russ. J. Phys. Chem. 95(1), S23–S32 (2021)
R. Fazaeli, N.E. Fard, Desulfurization of gasoline fuel via photocatalytic oxidation/adsorption using NaX zeolite-based under mild conditions: process optimization by central composite design. Russ. J. Appl. Chem. 93(7), 973–982 (2020)
L. Hao, Y. Zhang, Q. Zhao, S. Guan, T. Hu, Y. Lu, Comparative study of MoS2/MoO3, g-C3N4/MoO3 heterojunction films and their improved photocatalytic activity. Appl. Phys. A. 127(10), 1–10 (2021)
N.E. Fard, R. Fazaeli, M. Yousefi, S. Abdolmohammadi, Morphology-controlled synthesis of CuO, CuO Rod/MWW composite for advanced oxidation of indole and benzothiophene. Chem. Select 4, 9529–9539 (2019)
N.E. Fard, R. Fazaeli, M. Yousefi, S. Abdolmohammadi, Oxidation of carbazole by shape-controllable Cu2O on MWW catalysis. Appl. Phys. A. 125(9), 1–15 (2019)
N.E. Fard, R. Fazaeli, Optimization of operating parameters in photocatalytic activity of visible light active Ag/TiO2 nanoparticles. Russ. J. Phys. Chem. 92(13), 2835–2846 (2018)
N.E. Fard, R. Fazaeli, A novel kinetic approach for photocatalytic degradation of azo dye with CdS and Ag/CdS nanoparticles fixed on a cement bed in a continuous-flow photoreactor. Int. J. Chem. Kinet. 48(11), 691–701 (2016)
S. Abdolmohammadi, S.R. Nasrabadi, A. Seif, N.E. Fard, Ag/CdS nanocomposite: an efficient recyclable catalyst for the synthesis of novel 8-aryl-8H-[1, 3] dioxolo [4, 5-g] chromene-6-carboxylic acids under mild reaction conditions. Comb. Chem. High Throughput Screen. 21(5), 323–328 (2018)
N.E. Fard, R. Fazaeli, R. Ghiasi, Band gap energies and photocatalytic properties of CdS and Ag/CdS nanoparticles for Azo dye degradation. Chem. Eng. Technol. 39(1), 149–157 (2016)
A. Butkovskyi, A.H. Faber, Y. Wang, K. Grolle, R. Hofman-Caris, H. Bruning et al., Removal of organic compounds from shale gas flowback water. Water Res. 138, 47–55 (2018)
C.Z. Ning, L. Dou, P. Yang, Bandgap engineering in semiconductor alloy nanomaterials with widely tunable compositions. Nat. Rev. Mater. 2(12), 1–14 (2017)
Z. Wang, B. Mi, Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets. Environ. Sci. Technol. 51(15), 8229–8244 (2017)
X. Wang, J. He, L. Mao, X. Cai, C. Sun, M. Zhu, CsPbBr 3 perovskite nanocrystals anchoring on monolayer MoS2 nanosheets for efficient photocatalytic CO2 reduction. Chem. Eng. J. 416, 128077 (2021)
E. Benavente, F. Duran, C. Sotomayor-Torres, G. Gonzalez, Heterostructured layered hybrid ZnO/MoS2 nanosheets with enhanced visible light photocatalytic activity. J. Phys. Chem. Solids. 113, 119–124 (2018)
S. Liu, D. Chi, Q. Zou, Y. Ma, R. Chen, K. Zhang, MOFs-derived MoS2/C3N4 composites with highly efficient charge separation for photocatalytic H2 evolution. Inorganica. Chim. Acta 533, 120787 (2022)
A. Nipane, D. Karmakar, N. Kaushik, S. Karande, S. Lodha, Few-layer MoS2 p-type devices enabled by selective doping using low energy phosphorus implantation. ACS Nano 10(2), 2128–2137 (2016)
X. Zhang, X. Huang, M. Xue, X. Ye, W. Lei, H. Tang, C. Li, Hydrothermal synthesis and characterization of 3D flower-like MoS2 microspheres. Mater. Lett. 148, 67–70 (2015)
G. Jiang, X. Zhang, C. Liu, H. Ma, H. Yu, F. Wang, First-principles investigation on the vacancy dependence on the optical and electronic properties of Bi2S3 semiconductor ceramics. Vacuum 181, 109759 (2020)
R. Fortulan, S. Aminorroaya Yamini, C. Nwanebu, S. Li, T. Baba, M.J. Reece, T. Mori, Thermoelectric performance of n-type magnetic element doped Bi2S3. ACS Appl. Energy Mater 5(3), 3845–3853 (2022)
B. Weng, X. Zhang, N. Zhang, Z.R. Tang, Y.J. Xu, Two-dimensional MoS2 nanosheet-coated Bi2S3 discoids: synthesis, formation mechanism, and photocatalytic application. Langmuir 31(14), 4314–4322 (2015)
D.C. Onwudiwe, V.M. Nkwe, Morphological variations in Bi2S3 nanoparticles synthesized by using a single source precursor. Heliyon 6(7), e04505 (2020)
C. Zhang, D. Li, P. Pei, W. Wang, B. Chen, Z. Chu et al., Rod-based urchin-like hollow microspheres of Bi2S3: facile synthesis, photo-controlled drug release for photoacoustic imaging and chemo-photothermal therapy of tumor ablation. Biomaterials 237, 119835 (2020)
M. Li, J. Wang, P. Zhang, Q. Deng, J. Zhang, K. Jiang, J. Chu, Superior adsorption and photoinduced carries transfer behaviors of dandelion-shaped Bi2S3@ MoS2: experiments and theory. Sci. Rep. 7(1), 1–14 (2017)
W. Fei, H. Li, N. Li, D. Chen, Q. Xu, H. Li, J. Lu, Facile fabrication of ZnO/MoS2 pn junctions on Ni foam for efficient degradation of organic pollutants through photoelectrocatalytic process. Sol. Energy 199, 164–172 (2020)
L. Luo, X. Shen, L. Song, Y. Zhang, B. Zhu, J. Liu et al., MoS2/Bi2S3 heterojunctions-decorated carbon-fiber cloth as flexible and filter-membrane-shaped photocatalyst for the efficient degradation of flowing wastewater. J. Alloys Compd. 779, 599–608 (2019)
Y.R. Reynoso, A. Martinez-Ayala, M. Pal, F. Paraguay-Delgado, N.R. Mathews, Bi2S3 nanoparticles by facile chemical synthesis: Role of pH on growth and physical properties. Adv Powder Technol. 29(12), 3561–3568 (2018)
P. Hohenberg, W. Kohn, Inhomogeneous electron gas. Phys. Rev. 136(3B), B864 (1964)
J.R. Moreno, C. Giuseppe, G. Antoine, Deep learning the hohenberg-kohn maps of density functional theory. Phys Rev Lett. 125(7), 076402 (2020)
A. García, N. Papior, A. Akhtar, E. Artacho, V. Blum, E. Bosoni, P. Brandimarte, M. Brandbyge, J.L. Cerdá, F. Corsetti, R. Cuadrado, Siesta: Recent developments and applications. J Chem Phys 152(20), 204108 (2020)
J.M. Soler, E. Artacho, J.D. Gale, A. García, J. Junquera, P. Ordejón, D. Sánchez-Portal, The SIESTA method for ab initio Order-N materials simulation. J. Condens. Matter Phys. 14(11), 2745 (2002)
J.P. Perdew, K. Pbe, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev Lett. 77(18), 3865 (1996)
A. Aghahosseinin, L. Edjlali, S. Jamehbozorgi, M. Rezvani, E. Ghasemi, Theoretical investigations of functionalization of graphene and ZnO monolayers with mercaptopurine at aqueous media: a dispersion-corrected DFT calculations and molecular dynamic simulations. J. Mol. Liq. 369, 120865 (2023)
S. Tanreh, M. Rezvani, M. Darvish Ganji, Molecular simulation investigations on interaction properties of the teriflunomide–chitosan complex in aqueous solution. J Phys Chem Solids 174, 111171 (2023)
M. Sabet, S. Tanreh, A. Khosravi, M. Astaraki, M. Rezvani, M. Darvish Ganji, Theoretical assessment of the solvent effect on the functionalization of Au32 and C60 nanocages with fluorouracil drug. Diam. Relat. Mater. 126, 109142 (2022)
M. TaleMoghim, S. Jamehbozorgi, M. Rezvani, M. Ramezani, Computational investigation on the geometry and electronic structures and absorption spectra of metal-porphyrin-oligo- phenyleneethynylenes-[60] fullerene triads. Spectrochim. Acta A Mol. Biomol 280, 121488 (2022)
H. Li, M. Huang, G. Cao, Magnetic properties of atomic 3d transition-metal chains on S-vacancy-line templates of monolayer MoS2: Effects of substrate and strain. J. Mater. Chem. C. 5(18), 4557–4564 (2017)
A. Kumar, D. Banyai, P.K. Ahluwalia, R. Pandey, S.P. Karna, Electronic stability and electron transport properties of atomic wires anchored on the MoS2 monolayer. Phys. Chem. Chem. Phys 16(37), 20157–20163 (2014)
N. Troullier, J.L. Martins, Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B Condens. Matter 43(3), 1993 (1991)
Q. Zhang, T. Çaǧın, A. van Duin, W.A. Goddard, Y. Qi, L.G. Hector, Adhesion and nonwetting-wetting transition in the Al/α−Al2O3 interface. Phys. Rev. B 694, 4 (2004)
R.P. Feynman, Forces in molecules. Phys. Rev. 56(4), 340 (1939)
H.J. Monkhorst, J.D. Pack, Special points for Brillouin-zone integrations. Phys. Rev. B 13(12), 5188 (1976)
S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 132, 154104 (2010)
T. Ozaki, K. Nishio, H. Kino, Efficient implementation of the nonequilibrium Green function method for electronic transport calculations. Phys. Rev. B 8, 035116 (2010)
K. Lejaeghere, G. Bihlmayer, T. Björkman, P. Blaha, S. Blügel, V. Blum, D. Caliste, I.E. Castelli, S.J. Clark, A. Dal Corso, S. de Gironcoli, T. Deutsch, J.K. Dewhurst, I. Di Marco, C. Draxl, M. Dułak, O. Eriksson, J.A. Flores-Livas, K.F. Garrity, L. Genovese et al., Science 351, aad3000 (2016)
G. Hashemkhani Shahnazari, M. Darvish Ganji, Understanding structural and molecular properties of complexes of nucleobases and Au13 golden nanocluster by DFT calculations and DFT-MD simulation. Sci Rep 11, 435 (2021)
T. Ozaki, H. Kino, Efficient projector expansion for the ab initio LCAO method. Phys. Rev. B 72(4), 045121 (2005)
R.S. Mulliken, Electronic population analysis on LCAO–MO molecular wave functions. I. The J. Chem. Phys 23(10), 1833–1840 (1995)
P. Niknam, S. Jamehbozorgi, M. Rezvani, V. Izadkhah, understanding delivery and adsorption of Flutamide drug with ZnONS based on: dispersion-corrected DFT calculations and MD simulations. Physica E 135, 114937 (2022)
C. Li, B. Fan, W. Li, L. Wen, Y. Liu, T. Wang, Y. Yin, Bandgap engineering of monolayer MoS2 under strain: a DFT study. J. Korean. Phys. Soc 66(11), 1789–1793 (2015)
Y. Li, Z. Zhou, S. Zhang, Z. Chen, MoS2 nanoribbons: high stability and unusual electronic and magnetic properties. J. Am. Chem. Soc. 130(49), 16739–16744 (2008)
Q. Yue, J. Kang, Z. Shao, X. Zhang, S. Chang, G. Wang et al., Mechanical and electronic properties of monolayer MoS2 under elastic strain. Phys. Lett. A 376(12–13), 1166–1170 (2012)
A. Miralrio, E.R. Cortes, M. Castro, Electronic properties and enhanced reactivity of MoS2 monolayers with substitutional gold atoms embedded into sulfur vacancies. Appl. Sur. Sci 455, 758–770 (2018)
N. Askari, M. Beheshti, D. Mowla, M. Farhadian, Facile construction of novel Z-scheme MnWO4/Bi2S3 heterojunction with enhanced photocatalytic degradation of antibiotics. Mater. Sci. Semicond. Process. 127, 105723 (2021)
A.A.R. Niema, E.M. Abbas, N.K. Abdalameer, Enhanced antibacterial activity of selenium nanoparticles prepared by cold plasma in liquid. Dig. J. Nanomater. Biostruct. (DJNB) 16(4), 1479 (2021)
Author information
Authors and Affiliations
Contributions
Methodology, data collection, formal analysis, writing—original draft preparation, and writing—review and editing were performed by HP, NEF, and MR. Software and validation were carried out by MR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
We approved all ethics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pasdar, H., Elmi Fard, N. & Rezvani, M. Fabrication of MoS2/Bi2S3 heterostructure for photocatalytic degradation of Metronidazole and Cefalexin and antibacterial applications under NIR light: experimental and theoretical approach. Appl. Phys. A 129, 380 (2023). https://doi.org/10.1007/s00339-023-06663-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06663-5