Abstract
Effective biosensors are required to detect contagious viruses through a rapid test kit using electronic components. Field-effect transistor-based biosensors have been identified as potential candidates for label-free and rapid sensing applications. In this work, a dielectric modulated (DM) two-side underlap gate (U) Field-Effect Transistor (FET) biosensor device with a single gate structure is investigated for the label-free electrical detection of biomolecules. For detecting the presence of biomolecules immobilized in the underlap region, the change in subthreshold slope (SS), ON current (ION), ON current, OFF current ratio (ION/IOFF), and transconductance (gm) of the device have been considered. The proposed device is investigated for different underlap lengths, and its impact on device sensitivity is analyzed. All the characteristic trends have been validated through device simulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the present scenario, different viruses are a primary concern worldwide. For this, the detection of the protein and DNA of the virus is essential. Different researchers worldwide are developing a cost-effective, accurate, and high-performance biosensor. Many of them have developed biosensors to detect various viruses and proteins. Clark introduced the first biosensor in 1962 [1], which was later employed in various applications like the detection of the virus [2], toxicity [3], cancer [4], and can be used for health prognosis [5, 6], etc. Biosensors convert biological responses into electrical signals [7], and it works by combining two basic components, biological receptors and physical–chemical transducers [8]. The biological receptor converts the responses from the biochemical domain to a physical or chemical output signal with a defined sensitivity. Transducers convert this chemical or physical output signal to the electrical domain [8]. Bergveld, in 1970, invented ISFET, which started the use of FET for various biosensor applications [9].
The multiple benefits that can be achieved with such a design have recently boosted interest in using a field-effect transistor (FET) for various biosensors [10,11,12,13,14,15,16,17,18,19]. Janata and Caras, in 1980, used a FET biosensor for penicillin, showing the first practical uses of a FET biosensor [20]. Many other papers demonstrate FET biosensors' capabilities with different molecular targets [21], like nucleotides, cells [22], and amino acids [23]. These three papers [21,22,23] are based on experimental work. Rahman et al. simulated the junctionless double-gate MOSFET and its performance is analyzed. The device can be used to identify biomolecules like deoxyribonucleic acid (DNA), enzymes, cells, etc. [24]. The impact on sensitivity is seen by changing the biomolecule's position. Singh et al. simulated the Si0.5Ge0.5 Source dual-electrode doping less tunnel FET (DEDLTFET) biosensors and study the effect on performance by changing the cavity length, charge density (QF) and cavity thickness [25]. Kalra et al. worked on the tunability of the sensing performance of a FET biosensor by using electrostatic gating of the metal–semiconductor junction [26]. Hwang et al. explained using FET with a deformed monolayer graphene channel to detect nucleic acid [27]. All the above authors are trying to achieve better sensitivity for the proposed biosensor by using different structures and materials, but there is a limitation of sensitivity, as can be analyzed from their work, and also complex structure causes a difficult fabrication process [28]. Lower leakage current, better control over the channel, electrical insulation of substrate, fewer photolithography steps, and direct adaptation of the bulk IC design method are some advantages of using silicon on insulator (SOI) MOSFET [29,30,31,32,33,34]. In fully depleted SOI (FD-SOI) MOSFET, the parasitic capacitance between the source and drain, as exhibited by the bulk transistor, is reduced, which helps to provide better performance [35].
This manuscript proposes a dielectric modulated two-side underlap FET biosensor device (DMUFET) with a single gate structure to detect the biomolecule. The proposed device incorporated biomolecules as a biosensing material. The proposed biosensor's electrical response is analyzed by changing the dielectric constant of biomolecules in the underlap region. In addition, the change in sensitivity of SS, ION, ION/IOFF, and gm with respect to various dielectric constants are analyzed and discussed. Moreover, a comparison of sensitivity is also shown in the manuscript by varying the length of underlap region.
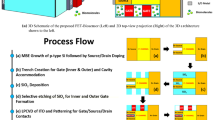
2 Device architecture and simulation
The Atlas SILVACO 2D device simulator is used for all the device simulations. The device parameter, its dimension value, and doping concentration for source, drain, and channel, which are used for simulation, are stated in Table I. The proposed biosensor device architecture and calibrated drain current characteristics curve are depicted in Fig. 1a, b. In Fig. 1a, Lu is the underlap region length, and Lg is the gate length. The thickness of the underlap region in which biomolecules are immobilized is considered 15 nm, the thickness of the oxide layer (SiO2) just above the channel is 5 nm, the thickness of the chromic oxide (Cr2O3) just below the gate is considered as 15 nm, and metal work function is considered as 5.1 eV. Properties of chromic oxides, such as low dielectric loss, high oxidation resistance, and high melting temperature, make it a better choice to be used as gate dielectric material in FETs than other high K materials [36, 37]. The SiO2 layer between the air and the silicon region is regarded under the cavity region. The presence of charges in the biomolecules significantly affects the biosensing action. However, for this work, two distinct oxide materials that have been used are Cr2O3 and SiO2, where SiO2 has low permittivity compared to Cr2O3. These two-oxide material stacking configurations can be chosen to reduce the interfacial trap density, which improves the device's performance [38, 39]. The open cavity region (underlap region) is used for sensing the immobilized biomolecules. The biomolecules are introduced in the underlap region by increasing the dielectric constant, i.e., K > 1. Many works of literature reported that biomolecules like protein, biotin, streptavidin, and APTES have a dielectric constant of 2.50, 2.63, 2.1, and 3.57, respectively [40,41,42]. The proposed biosensor's sensitivity is assessed at VDS = 0.5v and VGS = 1.5v (bias condition).
The various model, such as Field-Dependent Mobility Model (FLDMOB), Shockley–Read–Hall (SRH) Recombination Model, Concentration Dependent Mobility Model (CONMOB), Bandgap Narrowing Model (BGN), and Auger Model (AUGER) present in Atlas SILVACO tool has been used to simulate the electrostatic and current of the device [43]. The newton trap numerical solver is used during simulation to avoid convergence issues in the SOI structure [43].
3 Results and discussion
The Gate voltage (VGS) vs. Drain current (ID) plot is depicted in Fig. 2a. The figure reveals that the drain current reduces as the device's underlap length (Lu) increases. This reduction in drain current is due to the increase in series resistance introduced due to the increase in underlap length [44]. This restricts the inversion layer formation after gate voltage is applied, thus reducing the drain current. The underlap length is increased up to 350 nm, while the gate length (Lg) reduces to 300 nm, due to which controlling the channel becomes more difficult, which increases the leakage current or off current as depicted in Fig. 2b. The analysis is done keeping VDS = 0.5v, VGS=1.5v, and the underlap length is filled with air (K = 1). Figure 2c shows the change in transconductance with different gate lengths and underlap lengths. The graph clearly shows that the transconductance improves as the gate length increases due to an improved drain current. The device's efficiency in converting the gate voltage into drain current is provided by transconductance [45].
An increase in transconductance (\({g}_{m}\)) indicates high sensitivity for biomolecules. From Fig. 2c, the transconductance peak is observed at 0.9 Volts (VGS), which suggests that an improved sensitivity can be achieved at this biasing condition.
Figure 3 depicts the variation of an electric field, surface potential, energy band diagram, and electron concentration along the lateral length of the device when biomolecules are immobilized in the cavity region. Lu = 350 nm and Lg = 300 nm are selected for simulation since this is the optimum value for maximum device sensitivity. The surface potential is at the highest level for the empty cavity region, i.e. (K = 1). The surface potential of underlap section shifts downwards with the increase in biomolecules dielectric constant from one to a higher value, as shown in Fig. 3a. Figure 3b shows that the electric field is higher at the gate-underlap junction when air is immobilized in the cavity region (K = 1). The plot of energy band diagram variation with different dielectric constant values is shown in Fig. 3c, which indicates an energy band diagram rise for the higher dielectric constant value at the underlap region. In Fig. 3d, the electron concentration of the cavity region reduces as the constant dielectric increases, which is why surface potential shifts downwards in the cavity region as the K value increases. Figure 3e, f shows the ID–VGS characteristics on a linear scale (drain current) and on a log scale (subthreshold drain current), respectively. In both the figure, it is visible that the drain current improves with the increase in dielectric constant, and the subthreshold leakage current reduces with the increment in dielectric constant, so it can be analyzed that the ION/IOFF ratio is high for higher dielectric constant.
The DNA biomolecules are charged particles; therefore, for the detection of DNA, the impact of a charged biomolecule on the DMUFET biosensors is analyzed. The presence of charges in biomolecules affects the biosensing action of the DMUFET biosensor. Lu = 350 nm and Lg = 300 nm are selected for simulation since this is the optimum value for maximum device sensitivity. The effect of charged biomolecules on the surface potential of the DMUFET biosensor is presented in Fig. 4a. It is reflected from the figure that as the positive charge biomolecules are greater than before in the cavity, the surface potential shift upwards due to flat band voltage alteration. It makes the threshold voltage of the device to be lesser. The electric field increases with the positivity of charged biomolecules from 1e10 C/cm2 to 1e12 C/cm2 in the underlap region, as depicted in Fig. 4b. Figure 4c shows the variation in the energy band diagram with increased positivity of charged biomolecules; the figure clearly shows that the energy band diagram shifts downwards. As shown in Fig. 4d, the electron concentration of the cavity region improves as the positivity of charged biomolecules increases. That is why surface potential shifts upwards in the cavity region with the rise in the positivity of charged biomolecules. Drain current for different VGS in the presence of positively charged biomolecules is shown in Fig. 4e. This can be undoubtedly inferred from the graph that with the rise in the positivity of biomolecule from 1e10 C/cm2 to 1e12 C/cm2, the number of electrons in the channel region has improved significantly to a prominent attraction force. Hence, the ION has also been enhanced by a noticeable margin. Figure 4f shows the transfer characteristics of the device on the binding of the charged biomolecules for positively charged analytes OFF current rise.
From Figs. 3 and 4, it can be analyzed that the dielectric constant is less dominating than the charge effect. The impact of charge variation is more as compared with the dielectric constant variation on different parameters.
3.1 Sensitivity analysis
Percentage sensitivity analysis when biomolecules are immobilized in the gate-underlap section is done by the mathematical formulae as expressed [45].
\(V1\) is the value of quantity when underlap region is filled with air (K = 1), and \(V2\) is the value of quantity when underlap region is filled with the biomolecule (K value other than 1). Equation 2 expresses the absolute percentage deviation in the electrical parameters of the sensor with respect to air when the underlap region is immobilized with biomolecules (Table 1).
In biosensors based on FET, the ION/IOFF is also utilized as the sensing parameter to notice the sensitivity of the biosensor device when biomolecules are introduced into the device. Figure 5 shows that the absolute percentage sensitivity of SS improves with the increase in dielectric constant and the increase in underlap length. Figure 5a shows the percentage sensitivity of SS for different dielectric constants with various gate and underlap lengths. The SS sensitivity is maximum with Lu = 350 nm and Lg = 300 nm. Figure 5b shows the percentage sensitivity of ON current, which indicates the increase in ON current sensitivity with different dielectric constants. Figure 5b depicts that the sensitivity is better for underlap of 250 nm length, which means that the sensitivity for ON current is better for smaller underlap length and larger gate length, i.e. (Lu = 250 nm and Lg = 500 nm). This indicates poor control of the device due to a smaller gate length. The increment in OFF current is more significant than the decrement in ON, with the increase in constant dielectric results in better sensitivity in ION/IOFF, as shown in Fig. 5c. The absolute percentage sensitivity of transconductance increases with the increase in the K value. There is no significant change in percentage sensitivity at a lower dielectric constant for various underlap lengths. Still, the change in sensitivity is visible for a higher dielectric constant, as shown in Fig. 5d.
3.1.1 Improvement in sensitivity
Protein and DNA are essential biomolecules in nature. The dielectric constant of protein ranges between 2 and 4 [46, 47]. The dielectric constant of DNA is 8 [48]; therefore, to detect the protein, the K value of biomolecules in the sensing region is considered 2.1, 4.1, and for DNA, it is considered 8. DNA is a charged biomolecule, and sometimes protein also contains a charge, so a fixed charge, 1e11 C/cm2, at the Si–SiO2 interface region is considered. The proposed sensor exhibits a reproducible, specific detection of proteins and DNA, as shown in Fig. 6, showing potential for detecting viruses and cancer markers [49]. As shown in Fig. 6, the proposed sensor with underlap length of 350 nm showed improvement in sensitivity compared to underlap length of 250 nm for different dielectric constants. Underlap length of 350 nm shows 3.4 percent improvement in ION/IOFF sensitivity for dielectric constant (K = 2.1), it shows 28.9 percent improvement in ION/IOFF sensitivity for K = 4.1, and a percentage improvement of 55.5 in ION/IOFF sensitivity for K = 8 compared to 250 nm underlap length. This result indicates that more cavity regions contribute to better sensitivity, and the sensitivity is more elevated for a higher dielectric constant.
4 Conclusion
This manuscript shows a conclusive effect of increasing or decreasing the cavity region length. The increase in cavity region length provides more space for biomolecule immobilization, hence increased sensitivity. A decrease in underlap length results increase in gate length, which offers improved control over the channel, due to which the leakage current decrease (IOFF decreases). From the analysis, it can be determined that the sensitivity of the proposed device can be enhanced by increasing the underlap length where the biomolecules are immobilized. Changes in surface potential, energy band diagram, electric field, electron concentration, drain current, and subthreshold drain current has been inspected in-depth during the biomolecule's detection. From the analysis, it has been found that the sensitivity of the biosensor device is enhanced much more by increasing the positivity of charged biomolecules as compared to an increase in the dielectric constant. Also, the absolute percentage sensitivity of SS, ION, ON, and OFF current ratio and transconductance have been observed in the existence of charged biomolecules for different dielectric constants. The proposed device shows improvement in absolute percentage sensitivity of ION/IOFF by using 350 nm Lu compared to 250 nm Lu, which is 28.9 percent for K = 4.1, and improvement in absolute percentage sensitivity of transconductance is 4 percent for K = 64. Therefore, the manuscript clearly states that the suggested biosensor device can be a promising and good substitute for developing advanced and emerging bio-equipment because of its simple structure, higher sensitivity, and less thermal budgeting (low fabrication cost). The simulated results indicate that the proposed DMUFET can detect biomolecules such as protein and DNA. The proposed device can be a promising candidate to explore further and research label-free biosensing.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
L.C. Clark, C. Lyons, Ann. N. Y. Acad. Sci. 102, 29–45 (1962). https://doi.org/10.1111/j.1749-6632.1962.tb13623.x
M. Shariati, Biosens. Bioelectron. 105, 58–64 (2018). https://doi.org/10.1016/j.bios.2018.01.022
H. Gong, F. Chen, Z. Huang, Y. Gu, Q. Zhang, Y. Chen, Y. Zhang, J. Zhuang, Y.K. Cho, R.H. Fang, W. Gao, S. Xu, L. Zhang, ACS Nano 13, 3714–3722 (2019). https://doi.org/10.1021/acsnano.9b00911
D.S. Su, P.Y. Chen, H.C. Chiu, C.C. Han, T.J. Yen, H.M. Chen, Biosens. Bioelectron. 141, 111209 (2019). https://doi.org/10.1016/j.bios.2019.03.042
S.A. Fahliyani, A.A. Rastegari, A.N. Yadav, N. Yadav, New Fut. Dev. Microbial Biotechnol. Bioeng. (2020). https://doi.org/10.1016/b978-0-12-820528-0.00005-3
A. Pantelopoulos, N.G. Bourbakis, IEEE Trans Syst. Man Cybern. Part C Appl. Rev. 40, 1–12 (2010). https://doi.org/10.1109/TSMCC.2009.2032660
M. Torequl Islam, J. Gynecol. Womens Health 2, 2 (2017). https://doi.org/10.19080/jgwh.2017.05.555667
N. Bhalla, P. Jolly, N. Formisano, P. Estrela, Essays Biochem. 60, 1–8 (2016). https://doi.org/10.1042/EBC20150001
P. Bergveld, IEEE Trans. Biomed. Eng. 17, 70–71 (1970). https://doi.org/10.1109/TBME.1970.4502688
P. Singh, D.S. Yadav, Appl. Phys. A Mater. Sci. Process. 127(671), 1–15 (2021). https://doi.org/10.1007/s00339-021-04813-1
N.K. Singh, D. Mandal, R. Kar, Appl. Phys. A Mater. Sci. Process. 127(347), 1–9 (2021). https://doi.org/10.1007/s00339-021-04495-9
N.N. Reddy, D.K. Panda, Appl. Phys. A Mater. Sci. Process. 127(682), 1–9 (2021). https://doi.org/10.1007/s00339-021-04840-y
E.A. Kabaa, S.A. Abdulateef, N.M. Ahmed, Z. Hassan, F.A. Sabah, Appl. Phys. A Mater. Sci. Process. 125(753), 1–10 (2019). https://doi.org/10.1007/s00339-019-3056-0
B. Dewan, S. Chaudhary, M. Yadav, Appl. Phys. A Mater. Sci. Process. 127(791), 1–9 (2021). https://doi.org/10.1007/s00339-021-04933-8
M.K. Anvarifard, Z. Ramezani, I.S. Amiri, K. Tamersit, A.M. Nejad, J. Mater. Sci.: Mater. Electron. 31, 22699 (2020). https://doi.org/10.1007/s10854-020-04795-5
M. Kumari, N.K. Singh, M. Sahoo, H. Rahaman, Appl. Phys. A Mater. Sci. Process. 127(130), 1–8 (2021). https://doi.org/10.1007/s00339-020-04256-0
A. Lodhi, C. Rajan, A.K. Behra, D.P. Samajdar, D. Soni, D.S. Yadav, Appl. Phy. A Mater. Sci. Process. 126(837), 1–8 (2020). https://doi.org/10.1007/s00339-020-04008-0
S.I. Amin, L. Gajal, S. Anand, Appl. Phys. A Mater. Sci. Process. 124(578), 1–8 (2018). https://doi.org/10.1007/s00339-018-2003-9
M. Shariati, Appl. Phys. A Mater. Sci. Process. 123(370), 1–8 (2017). https://doi.org/10.1007/s00339-017-0994-2
S. Caras, J. Janata, Anal. Chem. 52, 1935–1937 (1980). https://doi.org/10.1021/ac50062a035
M. Fehr, D.W. Ehrhardt, S. Lalonde, W.B. Frommer, Curr. Opin. Plant Biol. 7, 345–351 (2004). https://doi.org/10.1016/j.pbi.2004.03.015
A.B. Kharitonov, M. Zayats, A. Lichtenstein, E. Katz, I. Willner, Sensors Actuators B Chem. 70, 222–231 (2000). https://doi.org/10.1016/S0925-4005(00)00573-6
C.H. Kim, J.H. Ahn, J.Y. Kim, J.M. Choi, K.C. Lim, T. Jung Park, N. Su Heo, H. Gu Lee, J.W. Kim, Y.K. Choi, Biosens Bioelectron. 41, 322–327 (2013). https://doi.org/10.1016/j.bios.2012.08.047
E. Rahman, A. Shadman, Q.D.M. Khosru, Sens. Bio-Sensing Res. 13, 49–54 (2017). https://doi.org/10.1016/j.sbsr.2017.02.002
A. Singh, S.I. Amin, S. Anand, SILICON 12, 2301–2308 (2020). https://doi.org/10.1007/s12633-019-00325-z
S. Kalra, M.J. Kumar, A. Dhawan, IEEE Sens. J. 20, 2261–2269 (2020). https://doi.org/10.1109/JSEN.2019.2952333
M.T. Hwang, M. Heiranian, Y. Kim, S. You, J. Leem, A. Taqieddin, V. Faramarzi, Y. Jing, I. Park, A.M. van der Zande, S. Nam, N.R. Aluru, R. Bashir, Nat. Commun. 11, 2 (2020). https://doi.org/10.1038/s41467-020-15330-9
N.N. Reddy, D.K. Panda, SILICON 13, 3085–3100 (2021). https://doi.org/10.1007/s12633-020-00657-1
J.P. Celis, B. Prakash, Surf. Process. by Dir. Energy Tech. 2, 111–119 (2006). https://doi.org/10.1016/B978-008044496-3/50005-8
A. Chaudhry, M.J. Kumar, IEEE Trans. Device Mater. Reliab. 4, 99–109 (2004). https://doi.org/10.1109/TDMR.2004.824359
W.Y. Lu, Y. Taur, IEEE Trans. Electron Devices 53, 1137–1141 (2006). https://doi.org/10.1109/TED.2006.871879
K. N. Singh and P. K. Dutta, Proc. 3rd Int. Conf. 2019 Devices Integr. Circuit, Dev 2019, 231–235 (2019). https://doi.org/10.1109/DEVIC.2019.8783714.
J.P. Colinge, Microelectron. Eng. 84, 2071–2076 (2007). https://doi.org/10.1016/j.mee.2007.04.038
V.P. Trivedi, J.G. Fossum, IEEE Electron Device Lett. 26, 26–28 (2005). https://doi.org/10.1109/LED.2004.839624
K. Cheng, A. Khakifirooz, Sci. China Inf. Sci. 59, 1–15 (2016). https://doi.org/10.1007/s11432-016-5561-5
C.P. Kwan, R. Chen, U. Singisetti, J.P. Bird, Appl. Phys. Lett. 106, 1–5 (2015). https://doi.org/10.1063/1.4915309
M.M. Abdullah, F.M. Rajab, S.M. Al-Abbas, AIP Adv. (2014). https://doi.org/10.1063/1.4867012
S.K. Parija, S.K. Swain, S. Adak, S.M. Biswal, P. Dutta, SILICON (2021). https://doi.org/10.1007/s12633-021-01118-z
M. Saxena, S. Haldar, M. Gupta, R.S. Gupta, Solid. State. Electron. 47, 2131–2134 (2003). https://doi.org/10.1016/S0038-1101(03)00221-1
M.S. Parihar, A. Kranti, Nanotechnology 26, 145201 (2015). https://doi.org/10.1088/0957-4484/26/14/145201
K. Talley, C. Ng, M. Shoppell, P. Kundrotas, E. Alexov, PMC Biophys. 1, 1–23 (2008). https://doi.org/10.1186/1757-5036-1-2
M.K. Gilson, B.H. Honig, Biopolymers 25, 2097–2119 (1986). https://doi.org/10.1002/bip.360251106
ATLAS user's manual, SILVACO Int, Santa Clara, CA (2014).
J.S. Lee, J.H. Seo, S. Cho, J.H. Lee, S.W. Kang, J.H. Bae, E.S. Cho, I.M. Kang, Curr. Appl. Phys. 13, 1143–1149 (2013). https://doi.org/10.1016/j.cap.2013.03.012
S. Kumar, R.K. Chauhan, M. Kumar, SILICON (2022). https://doi.org/10.1007/s12633-022-01865-7
T. Simonson, D. Perahia, Comput. Phys. Commun. 91, 291–303 (1995). https://doi.org/10.1016/0010-4655(95)00054-J
M. Amin, J. Küpper, Chem Open 9, 691–694 (2020). https://doi.org/10.1002/open.202000108
A. Cuervo, P.D. Dans, J.L. Carrascosa, M. Orozco, G. Gomila, L. Fumagalli, Proc. Natl. Acad. Sci. U. S. A. 111, 2 (2014). https://doi.org/10.1073/pnas.1405702111
H. Im, X.J. Huang, B. Gu, Y.K. Choi, Nat. Nanotechnol. 2, 430 (2007). https://doi.org/10.1038/nnano.2007.180
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Chauhan, R.K. Performance analysis of dielectric modulated underlap FD-SOI MOSFET for biomolecules detection. Appl. Phys. A 128, 1104 (2022). https://doi.org/10.1007/s00339-022-06260-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06260-y