Abstract
MnCexFe2-xO4 (x = 0.0, 0.6, 1.4) nanoparticles were successfully synthesized via sol–gel method. Their structural and magnetic properties were studied using X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray spectroscopy, and vibrating sample magnetometry techniques. The average crystallite size calculated using Scherrer equation was in the range of 26.90–60.44 nm. Scanning electron microscopy showed that the size of nanoparticles is less than 50 nm. Energy-dispersive X-ray results showed that the composition of the elements was relevant as expected from the synthesis. Vibrating sample magnetometry indicated that the samples have soft ferromagnetic properties. The highest saturation magnetization was related to the MnFe2O4 sample after annealing at 700 °C. The in vitro inhibitory activities of MnFe2O4 and MnCe1.4Fe0.6O4 nanoparticles were assessed against pathogenic fungi Aspergillus fumigatus, Candida albicans, and Fusariumoxysporum. Acceptable antifungal effects were observed in the presence of MnCe1.4Fe0.6O4 nanoparticles with minimum inhibition concentration and minimum fungicide concentration susceptibility determinations. As a result, these nanoparticles can be considered as potential antifungal agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The history of ferrite materials dates back to several centuries ago when rocks with the ability to absorb iron were discovered [1]. The study of magnetic nano-ferrites using chemical methods in the presence of environmentally friendly surfactants in a simple way is an important step toward the application of this group of nanoparticles in various practical fields such as drug delivery, water treatment, etc. Naturally occurring ferrites, such as magnetite, are soft magnets. The magnetic and electrical properties of ferrite-based materials make them suitable for a wide range of applications, such as high-frequency devices, microwave components, and biomedical applications [2,3,4]. Manganese ferrite (MnFe2O4) belongs to a group of soft magnetic materials with high magnetic permeability, low magnetic loss, high magnetic saturation, and relatively high temperature. Recently, MnFe2O4 nanostructures have been used in many applications such as microwave devices, computer memory chips, converter antennas, catalysts, sensors, and electronic devices. The shape, size, and morphology of nanostructured spinel materials play a vital role in determining the structural, morphological, optical, electrical, and magnetic properties. Due to their amazing properties in comparison with their bulk counterparts, manganese ferrite nanostructures with different morphologies such as nanoparticles, nanorods, and nanofibers have been synthesized via different methods including thermal decomposition, solvent hydrolysis, sol–gel, coprecipitation, and microwave methods [5].
Rare earth metals are used as promoter for active sites formation. Rare earth cations (trivalent cations such as Ce3+) affect the materials electronic distribution, favoring the adsorption of oxygen species [6,7,8]. So far, there is very little research on the application of these metals as dopants for the improvement of catalytic properties of ferrite materials. Therefore, in this research, cerium is considered as doping element. Cerium oxide or ceria with the unique ability to change oxidation state between + 3 and + 4 has various applications in UV absorbers, catalytic converters for car exhaust systems, solar cells, optics, and oxygen sensors [9]
The aim of this research is to prepare nanosized MnFe2O4 and Ce-doped manganese ferrite nanoparticles via sol–gel method and to study their structural and magnetic properties. The in vitro inhibitory activities of MnFe2O4 and Ce-substituted nanoparticles were also studies.
2 Experimental
2.1 Materials
Manganese nitrate [Mn(NO3)24H2O] (99%, Merck), iron nitrate [Fe(NO3)3.9H2O] (99%, Merck), cerium nitrate[Ce(NO3)3.6H2O] (99%, Merck), and citric acid (C6H8O7.H2O) were used as-received without further purification.
2.2 Preparation of nano-ferrites
The first step for the synthesis of MnCexFe2-xO4 (x = 0.0, 0.6, 1.4) nanoparticles via sol–gel method is the preparation of an aqueous solution containing different metal nitrates by dissolving Mn(NO3)2.4H2O, Fe(NO3)3.9H2O, and Ce(NO3)3.6H2O in distilled water containing citric acid under magnetic stirring at 80 °C. At the same time, ammonia was added dropwise to the solution until the pH of the solution reached 7.0. After 3 h, a concentrated solution was formed which was poured into a crucible and heated on an electric heater until a black deposit was obtained. The powder was converted to a puffy gray powder after annealing at 700 °C for 3 h.
2.3 In vitro antifungal susceptibility test
Antifungal drugs, i.e., terbinafine hydrochloride and fluconazole, as well as RPMI 1640 (Roswell Park Memorial Institute 1640) medium buffered to pH 7.0 with morpholine propane sulfonic acid (MOPS) were purchased from Sigma-Aldrich. Pathogenic yeast, i.e., Candida albicans (PTCC 5027), and molds, i.e., Fusariumoxysporum (PTCC 5115) and Aspergillus fumigatus (PTCC 5009), were provided from Persian Type Culture Collection (PTCC), Karaj, Iran. Yeast and mold suspensions were prepared with concentrations of 0.5–2.5 × 103 and 0.4–5 × 104 CFU mL−1, respectively. Broth microdilution and streak plate susceptibility tests were performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines M27-A2, M38-A2 and M26-A [1] and the average of three independent experiments were reported.
In MIC test, 20 μL of each NP (concentrations of 10,240, 5120, 2560, 1280, 640, 320, 160, 80 μg ml−1) in distilled water were added to wells 1–8 in a row of a 96-well microplate. 80 μL of RPMI 1640 and 100 μL of the prepared fungal suspensions were added to all the wells. Microplates were incubated under shaking at 100 rpm at 35 °C for 48 h. The MIC value was considered as the lowest concentration of nanoparticles (NPs) at which no visible turbidity was observed.
In MFC test, all non-turbid wells in the previous step were cultured in RPMI 1640 agar media plates. Dishes were incubated for 24 h at 35 °C at 45–55% relative humidity. The MFC was identified as the lowest concentration of NPs at which no microorganism was survived.
3 Characterization of ferrite nanoparticles
X-ray diffraction (XRD) patterns of ferrite nanoparticles were obtained by an X-ray diffractometer (Xpert PRO MPD) using CuKα radiation. The shape and size distribution of the nanoparticles were investigated using scanning electron microscope (SEM, KYKY-EM3900M). The magnetic properties of the samples were determined by vibrating sample magnetometer (VSM, model MDK, Iran).
4 Results and discussion
4.1 XRD patterns
One of the goals of this research is to understand the effect of substituting of different Ce concentrations on the structural properties of Mn ferrite nanoparticles.
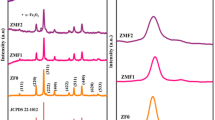
Figure 1 shows the XRD patterns of MnCexFe2-xO4 (x = 0.0, 0.6, 1.4) nanoparticles annealed at 700 °C. The peaks at 18.31°, 30.19°, 35.50°, 43.09°, 57.02°, and 62.49° are consistent with card no. 0465-017-00. The intense peak at 35.50˚ is related to (311) plane. The figure confirms that the diffraction peaks were sharp because of the nanometer size of the crystallites. The single-phase cubic spinel structure of undoped MnFe2O4 is clearly observed in the XRD patterns which is consistent with the results of Muthuraman et al. [10] and Islam et al. [6].The patterns also show that all the samples had formed the cubic single spinel phase. The size of the crystallites was determined by measuring FWHM of the most intense (311) diffraction peak using Debye Scherrer Eq. (1) [12].
where D is the size of the crystallite in nanometer, θ is the peak position (Bragg angle), λ is X-ray wavelength \((\lambda =1.54056\, \AA )\), and β is the full-width of the peak at half maximum (FWHM) in radian. The mean crystallite sizes of the samples are given in Table 1.The lattice parameter (a) \((\AA)\) was determined using Bragg's law (2) [10].
where d is the interplanar spacing, and h, k, \(l\) are the Miller indices of the mentioned planes. The lattice constants for all the prepared samples are also listed in Table 1. It can be seen that the lattice constants of individual phases did not change significantly by increasing the Ce concentration.
Further, the XRD patterns show the narrow reflections were exhibited due to the narrow size crystallites. The mean crystallite size of the samples lies in the range of 26.90 nm to 60.44 nm. The lattice parameter shows an increase for x = 0.6 and then decreases for x = 1.4. Since Ce ions have larger ionic radius (1.02 Å) than Fe+3 ions (0.64 Å), the lattice becomes more distorted by increasing the dopant concentration up to x = 0.6, leading to higher lattice parameter. By further increasing the concentration beyond x = 0.6, the lattice parameter decreases mainly due to the lower solubility of cerium ions compared to Fe+3 ions. This may also be due to the higher bonding energies of CeO2 in the spinel structure compared to iron oxide. These results are in good agreement with Meena et al. [12] and Kamran et al. [13]. The impure peak observed at about 32° for x = 0.0 can be associated with α-Fe2O3 phase.
4.2 Morphological analysis
SEM was used to study the morphology of nanoparticles. Typical SEM micrographs of MnCexFe2-xO4 nanoparticles with x = 0.6 and 1.4 prepared by sol–gel method after annealing at 700 °C are presented in Figs. 2a and b, respectively.
The annealing temperature of 700 °C was chosen as the optimum temperature, because the particles are almost spherical. The size of nanoparticles is less than 50 nm. The average size of nanoparticles in Fig. 2a is 40.61 nm. The nanoparticles in Fig. 2b are more uniform and distinguishable. The average size of these nanoparticles is 21.91 nm which is in good agreement with the crystallite size obtained from XRD patterns.
The surface morphology is clearly dependent on the cerium concentration. Figure 2a and b shows that by increasing the cerium concentration, the agglomeration and porosity of the particles vary without affecting the spherical morphology of the nanoparticles.
Field emission scanning electron microscopy (FESEM, MIRA, 3-TESCAN-XMU) images of Ce1.4MnFe0.6O4 nanoparticles annealed at 700 °C were also obtained (at 15 kV and 150 KX), as shown in Fig. 2c and d. Size distribution histogram of the nanoparticles is shown in Fig. 3. The solid line corresponds to the best fit of the data. It can be seen that the mean particle size of the MnCe1.4Fe0.6O4 nanoparticles is 30.53 nm. The results are almost equal to the crystallite sizes calculated according to Scherer equation.
4.3 Magnetic measurements
VSM was used to study the magnetic properties of MnCexFe2-xO4 (x = 0.0, 0.6, 1.4) nanoparticles. The magnetic hysteresis loops were measured at room temperature (RT) with maximal applied magnetic field of 1 T. Figure 4 shows magnetic hysteresis loops of the nanoparticles after annealing at 700 °C. It is clear that the soft ferromagnetic properties of MnCexFe2-xO4 nanoparticles depend on the Ce concentration. At this step, the samples were labeled as MnFe2O4 (S4), MnCe0.6Fe1.4O4 (S5), and MnCe1.4Fe0.6O4 (S6).
Different magnetic properties such as coercivity (Hc), saturation magnetization (Ms), and remanent magnetization (Mr) were measured for MnCexFe2-xO4 (x = 0.0, 0.6, and 1.4) nanoparticles based on the magnetization loops shown in Fig. 4. It can be seen from Table 2 that the saturation magnetization, remanent magnetization, and coercivity decrease with the addition of Ce. Elayakumar et al. [14] mentioned that the reduction in the saturation magnetization with increasing cerium content makes the Ce3+ substituted cobalt ferrites CoCexFe2−xO4 most suited for many technological potential such as the field of antenna construction and for reducing the size of the antenna.
M-H curve (Fig. 4a) for MnCexFe2-xO4 nanoparticles with x = 0.0, i.e., MnFe2O4, indicates that its saturation magnetization is 60.69 emu/g for the particle size of 60.44 nm at RT with an applied magnetic field of 1 T. Saturation magnetization of bulk manganese ferrite is 80 emu/g [11, 15, 16]. The smaller Ms value of MnFe2O4 with respect to the bulk may be due to the higher percentages of atoms located on the surface of nanoparticles.
4.4 Energy dispersive X-ray (EDX)
Figures 5 and 6 show EDX spectra of MnCexFe2-xO4 with x = 0.0 and x = 1.4, respectively. The concentration (weight percent and atomic percent) of the main elements presented in these two samples is also listed in Tables 3 and 4, respectively.
EDX spectrum in Fig. 6 shows the presence of cerium in the sample, confirming that Ce-doped MnFe2O4 has been successfully synthesized via sol–gel method.
4.5 Evaluation of antifungal activities
The in vitro inhibitory potential of MnFe2O4 and MnCe1.4Fe0.6O4 nanoparticles was studied against fungal pathogens (i.e., Candida albicans, Aspergillus fumigates, and Fusariumoxysporum), Gram-negative pathogenic bacteria (i.e., Escherichia coli, Acinetobacterbaumannii, and Pseudomonas aeruginosa), and Gram-positive pathogenic bacteria (i.e., Bacillus cereus, Staphylococcus epidermidis, and Streptococcus pneumonia). The results were reported as the minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC) values (Table 5). Antifungal drugs, i.e., terbinafine hydrochloride and fluconazole, were used as positive controls. Both MnFe2O4 and MnCe1.4Fe0.6O4 NPs were not able to block the growth of the tested bacterial strains even at concentrations of 1024 μg mL−1.
MIC and MFC values within the range of 256–512 μg mL−1 were recorded for MnCe1.4Fe0.6O4 NPs against all the pathogens. Metal oxide NPs can inhibit the growth of microorganisms via outer membrane and cell wall destruction, generation of reactive oxygen species, metal-ion release, and particle internalization [17]. It is predicted that the presence of cerium ion in the structure of MnCe1.4Fe0.6O4 nanoparticle is a major contributor to the antifungal activity. Cerium ions can cause oxidative stress of components of cell membrane of fungi, particularly Candida albicans [18]. The pH value on the cell membrane increases due to the reduction of Ce4+ to Ce3+, resulting in higher vascular permeability.
As can be seen from Table 5, no inhibitory activity was observed for MnFe2O4 NPs. Antimicrobial effects of NPs can be varied according to the type of carrier and species diversity, size and morphology of particles, as well as the nature of growth media. Manganese ferrite nanoparticles synthesized by Lopez-Abarrategui et al. did not show antifungal activity against Candida albicans [19]. Antifungal effects were observed when they were conjugated with citric acid or 12 aa peptide. It is believed that undoped MnFe2O4 is unable to generate reactive oxygen species via oxidation of water molecules. Even though MnFe2O4 NPs prepared with average crystallite size of 16 and 23 nm could inhibit Candida albicans strains [20]. This is probably due to the differences in the morphology of particles.
5 Conclusion
Manganese-cerium ferrite nanoparticles with different Ce concentrations were prepared by sol–gel method. The samples were analyzed using X-ray diffraction (XRD), scanning electron microscopy (SEM), field emission SEM (FESEM), and vibrating sample magnetometry (VSM) to obtain information on the sample phases, crystalline size, surface morphology, and magnetic properties of the samples. All the XRD peaks were indexed and the lattice constant (Å) was calculated for each Ce concentration. The crystallite size (nm) showed a decreasing trend with an increase in the Ce concentration. XRD patterns confirmed the presence of cubic α- Fe2O3 phase. Crystallite size of samples calculated using the Scherrer formula was in the range of 26.90 to 60.44 nm. SEM images showed that the particles are at the nanometer scale. EDX spectra revealed the presence of Ce in the composition, confirming the successful doping of Ce in the spinel structure. Magnetization curves revealed ferromagnetic behavior of MnCexFe2-xO4 (x = 0.0, 0.6, 1.4) nanoparticles. Pathogenic fungi cause infectious diseases in human, animals and plants. The results showed that the synthesized MnCeFe2O4 nanoparticles can effectively block the growth of both pathogenic yeast and mold. According to their inhibitory effects against Fusariumoxysporum, they can be considered as suitable fungicidal candidates. In addition, they can be applied in water treatment due to their low solubility.
The results can be summarized as follows:
-
1)
MnCexFe2-xO4 (x = 0.0, 0.6, 1.4) nanoparticles were synthesized via sol–gel method and their chemical, structural, and magnetic properties were studied using XRD, SEM, FESEM, EDX and VSM.
-
2)
The synthesized MnCeFe2O4 nanoparticles can effectively block the growth of both pathogenic yeast and molds. According to their inhibitory effects against Fusariumoxysporum, they can be considered as suitable fungicidal candidates.
-
3)
In addition, they can be applied in water treatment due to their low solubility.
References
K.H.J. Buschow, Magnetic Materials (University of Amsterdam, The Netherlands, 2015)
A. Goldman, Modern Ferrite Technology (Van Nostrand Reinhold, New York, 1990)
R. Langer, New methods of drug delivery. Science 249, 1527 (1990)
S. Rehman, M.A. Almessiere, N. Tashkandi, A. Baykal, Y. Slimani, R. Jermy, V. Ravinayagam, C. Yaman, Fabrication of spinel cobalt ferrite (CoFe2O4) nanoparticles with unique earth element cerium and neodymium for anticandidal activities. Chem. Sel. 4(48), 14329–14334 (2019)
J. Judith Vajaya, G. Sekaran, M. Bououdina, Ceram. Int. (2015). https://doi.org/10.1016/j.ceramint.2013.10.145
K. Islam, M. Haque, A. Kumar, A. Hoq, F. Hyder, S. ManjuraHoque, Nanomaterials (2020). https://doi.org/10.3390/nano10112297
I.T. Weber, A.P. Maciel, P.N. Lisboa-Filho, C.O. Paiva-Santos, W.H. Schreider, Y. Maniette, E.R. Leite, E. Longo, Nano Lett. 2, 969 (2002)
E.R. Leite, A.P. Maciel, I.T. Weber, P.N. Lisboa-Filho, E. Longo, C.O. Paiva-Santos, W.H. Schreider, Y. Maniette, C.A. Pascocimas, A.V.C. Andrade, Adv. Mater. 14(12), 905 (2002)
J.T. Dahle, Y. Arai, Int. J. Environ. Res. Public Health (2015). https://doi.org/10.3390/ijerph120201253
K. Muthuraman, V. Naidu, S.K. Ahamed, T. Vasudev, Int. J Comput. Appl. 65, 23 (2013)
P. Mathura, A. Thakura, M. Singha, J. Magn. Magn. Mater. 320, 1364–1369 (2008)
S. Meena, K.S. Anantharaju, Y.S. Vidya, L. Renuka, B. Uma, S.C. Sharma, B.S.S. Daruka Prasad et al., Ceram. Int. (2021). https://doi.org/10.1016/j.ceramint.2020.11.105
M. Kamran, M. Anis-ur-Rehman, J. Alloys Compd. 822, 153583 (2020)
K. Elayakumar, A. Dinesh, A. Manikandan, M. Palanivelu, G. Kavitha, S. Prakash, R. Thilak Kumar, S.K. Jaganathan, A. Baykal, Structural, morphological, enhanced magnetic properties and antibacterial bio-medical activity of rare earth element (REE) Cerium (Ce3+) doped CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 476, 157–165 (2019)
Q. Li, C.F. Conde, A. Conde, L.F. Kiss, Phys. Rev. B72(2005)
T. Suominen, J. Raittila, T. Salminen, K. Schlesier, J. Linden, P. Paturi, J. Magn. Magn. Mater. 309, 278–284 (2007)
H. Beyzaei, M. KamaliDeljoo, R. Aryan, B. Ghasemi, M.M. Zahedi, M. Moghaddam-Manesh, Chem. Cent. J. 12, 114 (2018)
S. Stankic, S. Suman, F. Haque, J. Vidic, J. Nanobiotechnol. 14, 73 (2016)
I. Albuquerque, P. Farias, C. Christiano Lima dos Santos, F. Correia Sampaio, Bio Med. Res. Int. (2018). https://doi.org/10.1155/2018/1923606
A.M. Jacintha, V. Umapathy, P. Neeraja, S. RexJeyaRajkumar, J. Nanostruct. Chem. (2017). https://doi.org/10.1007/s40097-017-0248-z
Acknowledgements
The authors are thankful the referees for their suggestions to bring the first edition of the paper in the present form and they are highly thankful to the Sistan and Baluchestan University and Zabol University, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohafez, F.S., Davarpanah, A.M., Rahdar, A. et al. Structural, magnetic, and in vitro inhibitory characteristics of Ce-substituted MnFe2O4 nanoparticles. Appl. Phys. A 127, 600 (2021). https://doi.org/10.1007/s00339-021-04759-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04759-4