Abstract
Dye/polymer composite is gradually emerging as an indispensable component of the modern optoelectronics technology due to their fascinating optical, mechanical and electrical properties. Here, we investigate the structural, mechanical, optical and electrical properties of the economical, self-standing, flexible alizarin red (AR)/poly (vinyl alcohol) (PVA) composites (AR concentration 0 wt %, 0.1 wt %, 1.0 wt %, 2.0 wt %) prepared by facile solution casting technique. The UV–Vis absorption spectroscopy results in higher absorption of the composites with increasing AR dye concentration in the UV–Vis region (200–600 nm). A 2.0wt % AR/PVA composite film of thickness 250 ± 30 µm can entirely block light in the 200–600 nm wavelength region and can effectively block high power laser irradiation (10.7 mW) of wavelength ̴ 544 nm for a period of 60 min without degrading itself, which makes AR/PVA a potential candidate for flexible, high power laser filter in 200–600 nm region. A significant reduction in the band gap (~ 3.1 eV) is also observed for the composites than that of the neat PVA. The dielectric constant (\(\varepsilon^{\prime}\)) is found to be increased significantly with incorporation of AR dye and an optimum value of \(\varepsilon^{\prime} =\) 208 (at 40 Hz and 300 K) is obtained for 2.0% AR/PVA with small \(\tan \delta\) value (~ 0.56). Temperature-dependent dielectric properties of the composites are also investigated which confirm the dominating role of the interfacial polarization and presence of multiple dielectric relaxation processes. The enhanced laser filtering and dielectric properties of the AR/PVA composites can be attributed to the formation of hydrogen bond-mediated complexes in the composites. The fabricated novel AR/PVA composites can be potentially used in flexible, low-cost laser filter and flexible energy storage devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dye/polymer composites have been widely used in multiple optoelectronic applications for the last few decades [1,2,3,4,5]. Rose Bengal (RB), coumarine red, congo red (CR) and other dye-incorporated poly (methyl methacrylate) (PMMA) and poly (vinyl alcohol) (PVA) polymer composites have been used as cut-off filters and other optoelectronic devices [5, 6].PVA, a transparent polymer, possesses the remarkable properties of an extraordinary host matrix and can host different optically and electrically active elements. PVA composites are actively used in recent developments of optoelectronic devices, laser blocking materials, polymer electrolytes and dielectric materials due to their fascinating optical and dielectric properties, high flexibility, low cost, light weight, better chemical stability and facile synthesis procedures [3, 6,7,8,9,10]. PVA is a polymer having carbon chain back bone with hydroxyl groups (OH−) attached to the methane carbon. These OH− groups can assist in the formation of complexes of the polymer chain and foreign elements via hydrogen bonding, hence modifying the structural, optical, electrical and mechanical properties of the PVA. Dyes are ionic materials, incorporation of which can cause an increase in the conductivity as well as the dielectric constant of PVA, but that does not significantly affect the tangent loss [11,12,13,14], increasing the possible applicability of the composite materials for charge storing devices. The incorporation of dye in PVA matrix also modifies the bandgap of the polymer, leading towards alteration of the optical properties of the PVA. Thus, dye/PVA composites have found manifold applications in optical and dielectric areas [6,7,8, 14,15,16,17]. Recently, 10 wt % rose bengal (RB) incorporated PVA film has been used to filter 200–610 nm wavelength [18]. Similarly, bandgap-modified CR/PVA composite films were used as optical filter in UV–Visible range [7]. But the limitations of these works are associated with the high doping percentage of dyes, reduced flexibility of the films and inability of withstanding the high incident laser power, which are the major challenges for fabricating laser filter. On the other hand, alizarin red (AR) or 1,2-dihydroxyanthraquinone is an interesting anionic dye and used frequently in photochemistry [19] and photo-sensitizers [20, 21]. Though AR is an economic compound having interesting optical absorption properties and remains stable as liquid solution against laser light [22], its contribution is still unexplored in dye/PVA composite films for engineering the laser filter devices. Furthermore, the dielectric properties of the anionic AR-incorporated PVA are still unexplored.
In this study, we report characterization of simplistic, economical AR/PVA composite films prepared by solution casting technique, which have been effectively used as UV–Vis laser cut-off filters. The structural, mechanical, optical and dielectric properties of the composites were studied thoroughly for a clear understanding of the material. The variations of the optical and dielectric characteristics of the composites have been described from the perspective of interfacial interactions between dye molecules and polymer chain. The laser filtering property of the prepared films has been studied using a considerably high power laser (10.7 mW) for a long period of time. The study of dielectric property of the solid polymer films has been used as a powerful tool to acquire adequate information about the characteristics of ionic and molecular interactions between AR dye molecules and PVA chain. Dielectric relaxation spectroscopy has been also used to understand the microscopic relaxation processes of the prepared PVA composites. The optimum value of dielectric constant \(\left( {\varepsilon \prime } \right)\sim\) 208 (at 40 HZ and 300 K) is obtained for 2.0% AR/PVA with small \(\tan \delta\) value (~ 0.56). The impedance spectroscopy of the samples has been also performed to describe the AR–PVA interactions.
2 Experimental
2.1 Materials
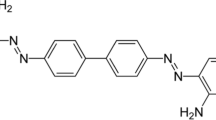
Poly(vinyl alcohol) (PVA) and alizarin red (AR) dye were purchased from Loba Chemie (India). All the substances were used without any additional refinement. The schematic of organic structures of AR and PVA is presented in Fig. 1a, b.
2.2 Preparation of AR/PVA composite films
PVA and AR/PVA composite films were prepared by modest solution casting procedure. Six gram of PVA was dissolved into 125 ml of water in a glass beaker through vigorously stirring (450 rpm) for 18 h at a fixed temperature of 50 °C. AR dye solutions were arranged by dissolving necessary quantity of AR dye into water at 300 K. The PVA solution was mixed with the AR dye solution of different strengths (0.1 wt %, 1.0 wt %, 2.0 wt % of PVA) for 2 h (at 450 rpm) with a magnetic stirrer to prepare the solutions of AR/PVA. The essential extent of the AR/PVA solution was casted into a cleaned Petri-dish and then the solvent was evaporated at 40 °C for 30 h to obtain the polymer composite film. The dehydrated PVA films of thickness 250 ± 30 µm containing 0.1 wt %, 1.0 wt %, 2.0 wt % AR dye were prepared following this method. The graphic representation of the synthesis route of the AR/PVA composite films is illustrated in Fig. 2a.On the other hand, PVA solution was simply casted into a Petri-dish to prepare the film of neat PVA. Figure 2b shows the digital image of the prepared AR/PVA composite film. The superior flexibility of the fabricated composite film is shown in Fig. 2c.
2.3 Characterization and measurements
X-Ray diffraction (Bruker D-8 X-Ray Diffractometer, Cu-Kα (1.5405 Å) radiation) technique was used to analyze the structural properties of the prepared films. Surface morphology of the films was explored by a field emission scanning electron microscope (ZEISS Sigma 300).A double-beam unpolarized Jasco V-730 BIO Spectrophotometer (resolution 1 nm) was used to record absorption and transmission bands of the prepared films in UV–Vis–NIR range (200–1100 nm) at 300 K. The laser cut-off filtering property of the prepared films was investigated by a laser cut-off testing setup, where the self-standing films (1.5 cm × 1.5 cm) were directly fixed at right angles to the path of transmission of the high power laser irradiation (HOLMARC, FWHM ~ 1.24 mm, Wavelength ~ 544 nm, Power ~ 100mW). The power of the incident light on a film sample was tuned by a normal filter arrangement placed between the film sample and laser source. Power of incident laser light and output laser light was measured by a optical multi-meter (ILX Lightwave (OMM-6810B), power measurement range—100nW–1 W, accuracy ~ ± 3.5%).Tinius Olsen 125 N load cell was used for stress–strain analysis of the prepared films. The samples of dimensions ~ 10 × 30 × 0.3 mm3 were riveted using a clip-type sample frame (gripping zone span of 10 mm) and tested under a stretching speed of 0.1 mm/min with a load cell of 125 N. The dielectric properties of the PVA based films were studied using a digital LCR meter (Agilent, E4980A). The dielectric constant (\(\varepsilon '\)), dielectric loss (\(\varepsilon ''\)) and total ac conductivity (\(\sigma_{ac}\)) of the samples were evaluated using the following equations [6]:
where the capacitance, thickness, effective area and dielectric loss of the samples are C, d, A and \(\varepsilon^{\prime\prime}\), respectively, f is the frequency of the applied electric field across the samples and \(\varepsilon_{0}\) is the free space permittivity (8.854 × 10−12 Fm−1). Copper electrodes (area ~ 1 cm2) were used for measuring the dielectric properties of the polymer films.
3 Results and discussion
3.1 XRD analysis
Figure 3a shows the XRD diffractogram of the crystalline AR dye which has a monoclinic configuration [19] as confirmed from the well-indexed peaks of the XRD pattern. Figure 3b illustrates the XRD diffractogram of neat PVA and AR/PVA composites self-standing films viz. 0.1%, 1.0%, 2.0% AR-doped PVA composite films. Neat PVA and AR/PVA composites films show semi-crystalline nature which is represented by the peak positioned at 2θ = 19.7o corresponding to the (200) plane of PVA and an amorphous hump positioned at 2θ = 13.5o corresponding to (101) plane of PVA. The semi-crystalline nature of the PVA is occurred due to the presence of strong intermolecular hydrogen bonds in the PVA chain causing a long-range ordering [7].The traces of peaks corresponding to crystalline AR dye are not present in XRD diffractogram of AR/PVA composite films, indicating AR is properly dispersed and captivated in polymer host matrix. Figure 3b suggests that the influence of AR incorporation within the PVA matrix becomes more distinguishable with greater quantity of AR, as the crystalline characteristic peak at 2θ = 19.7o is broadened with higher amount of doping of AR in PVA. Furthermore, the relative intensity of the peak at 2θ = 13.5o (corresponding to (101) plane of PVA) is increased in the diffractogram of AR/PVA composite films with enhanced dye percentage in polymer matrix. The crystallinity index (CI) can be calculated for the different samples using the relation [16,17,18]:
where If and Is are the peak intensities of the fundamental band (18o–22o) and the secondary band (12o–15o), respectively. The calculated CI value decreases from 61% for pure PVA to 58% for 0.1% AR/PVA sample; whereas for the 1.0% AR/PVA sample, CI value decreases to almost 10%. Therefore, it is evident from the XRD analysis that the crystallinity in the PVA composites is significantly decreased with incorporation of AR dye. The significant modifications in XRD histograms of AR/PVA composites are observed owing to the enhanced electrostatic interaction of AR with PVA (via hydrogen bond), which partially disturbs the long-range ordering of PVA chains. The proposed interaction between AR and PVA is shown in Fig. 3c.
3.2 Scanning electron microscopy
Figure 4a, b depicts the FESEM micrographs of neat PVA and 2.0 wt % AR dye-incorporated PVA composite films, respectively. Surface morphology of both the films exhibits an even and flat surface. However, after the doping of the dye molecules, the films revealed a surface with even more regularity in the composite films (Fig. 4b).Fig. 4b also assures the reasonable solubility between the AR dye and the polymer host. The AR/PVA composite structure offers great homogeneity and consistency which is indispensable for laser filter applications.
3.3 Stress–strain analysis
The stress–strain plots of neat PVA and 2.0% AR/PVA films are depicted in Fig. 5a, b. The Young’s modulus of neat PVA and 2.0% AR/PVA composite films are 800 MPa and 60 Mpa, respectively, as estimated from the figures. The outstanding stretching ability of the AR/PVA composite film is evident from the Fig. 5b which also confirms the fair mechanical stability of the composite film. The incorporation of the AR dye in the polymer host matrix enhances the ability of stretching of the composite films because the hydrogen bond-mediated AR dye–PVA interaction causes structural changes in the polymer.
3.4 Optical properties of AR/PVA thin films
UV–Vis transmittance spectra of neat PVA and AR/PVA composite films are shown in Fig. 6a. Typically, the neat PVA film shows high transparency in the entire visible light region, and its absorption edge lies in the deep-UV light region. Optical transparency of the AR/PVA composite films is reduced significantly with loading of the AR dye in PVA matrix as shown in the figure, indicating the prominent role of the AR dye in the high UV–Vis absorbance property of the composite film. 0.1 wt %AR/PVA composite shows two broad absorption bands centered at ~ 250 nm and ~ 500 nm, which are broadened rapidly with increase in AR loading. These two absorption bands are merged for 2.0 wt % AR composite film, enabling complete block of light of 200–600 nm wavelength. The optical absorption property of the AR/PVA composite film can be attributed to the hydrogen bond-mediated AR dye/polymer complexes [7]. Increase of the AR concentration in the AR/PVA composite leads to the increase in the number of AR dye/polymer complexes which are responsible for broadening the absorption bands of the composites by developing new absorption bands in 200–600 nm wavelength region. Figure 6a indicates that the AR/PVA composite films show very high transmittance for the light of wavelength region > 600 nm, similar to the neat PVA film. Transmittance values for the different AR/PVA composites at wavelength of 400 nm and 1050 nm are given in Table 2.The 2.0 wt % AR/PVA composite film is opaque, i.e., the transmittance value is almost zero (T ≈ 0) in the 200–600 nm wavelength region and the composite film acts as an effective optical filter in this wavelength region. The decrease in transmittance of the AR/PVA composites in the short wavelength region is attributed to the intermolecular interactions of dye molecules with PVA chains through hydrogen bonding [6].Previously, dye/PVA composites films such as rose bengal/PVA [18] and congo red/PVA [7] were used as optical filters to block light in different wavelength regions as mentioned in Table 1.
The indirect optical band gap (Eg) of neat PVA and AR/PVA composite films was calculated using Tauc’s equation [23,24,25,26,27,28]:
where A is a constant which is independent of energy, α is absorption coefficient \(\left(\alpha = 2.303 \frac{\text{Absorbance}}{\text{Thickness of the film}}\right)\), h is Planck’s constant, and ν is frequency. Figure 6b, c depicts the variation of (αhυ)1/2 with energy (hν) for neat PVA and AR/PVA composites. The figure shows that absorption edges of AR/PVA composites are noticeably shifted towards lower photon energy with respect to the absorption edge of the neat PVA, which infers the modification in bandgap energy of the AR/PVA composites. The bandgap values of neat PVA and AR/PVA composites films have been estimated and incorporated in Table 2. The bandgap of AR/PVA composites is reduced to ~ 1.61 eV for AR/PVA composites, whereas the band gap of neat PVA is ~ 4.7 eV. Captivated AR molecules in the PVA matrix cause the considerable reduction of bandgap energy of the composites in comparison to that of the neat PVA. Incorporation of AR molecules leads to new absorption centers for absorbing lights of different wavelengths in UV–Vis region, resulting band engineering within the composites. AR dye molecules and PVA chains interact with each other through hydrogen bond to create new molecular dipoles and these new absorption centers create new trapped levels within the HOMO–LUMO gap of the PVA [7]. The reduced band gap of the polymer composite system is also supported by the absorption spectra fitting (ASF) model [28], where the relation between absorbance and wavelength is given by the equation:
where \(D \, = \, \left[ {B\left( {\text{hc}} \right)^{{{\text{m}} - 1}} d/ 2. 30 3} \right].\)
The Eg can be calculated by obtaining the corresponding wavelength \(\lambda_{g}\) from the extrapolation of the linear portion of \(({\text{A}}/ \lambda )^{2}\) vs. \(( 1/ \lambda )\) plot to \(({\text{A}}/ \lambda )\) 2 = 0, as depicted in Fig. 6d. The Band gap can be attained using the relation,\(E_{g} = \frac{1240}{{\lambda_{g} \left( {nm} \right)}}\), which agrees with the previous values as described in Table 2.
3.5 Laser cut-off filter
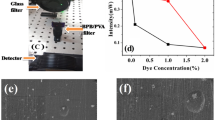
The experimental setup for the measurements of the optical filtering properties of the self-standing polymeric films is shown in Fig. 7. An optical detection and measuring system was adjusted to meet the necessities for observing the laser cut-off filter properties of the films bearing width 250 ± 30 µm. A laser light(wavelength ~ 544 nm) having power 10.7mW was used as source for the experiments. The optical filtering properties of the composite films are presented for different dye concentration in Fig. 7b. Figure 7b shows that the power of transmitted light through AR/PVA composite film is reduced with greater concentration of AR dye in PVA environment. The number of dye molecules per unit volume of the composite escalates with the upsurge in concentration of dye; therefore, active interaction among AR molecules and PVA is amplified. These enhanced extent of interfaces effectively involves into the absorption processes to diminish the transmission and improving the optical filtering effect [29, 30].While using 2.0 wt % AR/PVA composite film (thickness ~ 250 ± 30 µm),the output laser power from the composite film reaches 0.00017% of the incident power (~ 10.7mW) as shown in the Fig. 7b, which confirms the remarkable laser light blocking characteristics of the dye/polymer film. The composite film was continuously exposed to that high power laser for a time period of 60 min and then again UV–Vis absorption spectroscopy of the same film was studied. Figure 7c shows the transmittance spectrum of that 2.0 wt % AR/PVA composite film. The figure shows that the absorption property of the mentioned film is remain unaltered, confirming the 2.0 wt % AR/PVA composite film can sustain its performance even after uninterrupted blocking of high-laser power (10.7mW) for a long time period (60 min) without any degradation. Previously, methyl violate-6B-incorporated (3.33 wt %) PMMA composite film and rose bengal dye/PVA (20% filling of dye) films were reported for blocking 0.65 mW and 0.45 mW laser, respectively [17, 18].Methylene blue/PVA (3% MB) film was used to block 3.6 mW laser [31] and to the best of our knowledge, only Cr3+ ion-doped PVA is able to filter laser light with higher power(~ 15mW) [32].Hence, our fabricated laser cut-off filter having remarkable durability and stability is superior to other reported dye/polymer-based flexible laser cut-off filters in terms of thickness, capability to block high power laser, and concentration of dye in the polymer composite. Therefore, 2.0wt % AR/PVA composite film can be used as an efficient laser obstructing material in flexible, high power laser cut-off filter in 200–600 nm wavelength region [23, 31,32,33,34].
3.6 Dielectric properties
Figure 8a–b depicts variation of dielectric response of AR/PVA composites with frequency for different loading percentage of AR dye. Figure 8a shows that the dielectric constant of the AR/PVA composites is improved with increasing AR concentration in PVA matrix. The maximum value of dielectric constant (~ 208 at 40 Hz) is obtained for the 2.0% AR/PVA composite and the corresponding tangent loss (tan δ) value is 0.56. The dielectric constant of 2.0% AR/PVA composite is enhanced by 21 times in comparison to neat PVA. The dielectric properties of the AR/PVA samples can be elucidated on the basis of Maxwell–Wagner–Seller (MWS) interfacial polarization effect [35] which occurs at the interface of two different phases of a heterogeneous medium having different conductivity and permittivity. At lower loading of AR dye, the interfacial interaction between well-dispersed dye molecules and polymer is less. However, with increasing loading concentration of dye molecules, the interfacial interaction is increased, leading to enhanced charge accumulation at the dye/polymer interface. Consequently, the dielectric constant is increased via enhanced interfacial polarization. The prominent enhancement in dielectric constant of the 2.0% AR/PVA composite establishes the strong interfacial interaction between the AR and PVA molecules by the cross-linking formation inside the PVA host matrix [35,36,37]. A capacitor made with a polymer composite having high dielectric constant can store higher energy density (\(\eta \, = \,\frac{{\varepsilon^{\prime}\varepsilon_{0} E^{2} }}{2}\)) using comparatively lower electric field. Therefore, it is advantageous to use flexible AR/PVA composite films having high dielectric constant in capacitors applications [36, 37].
Evidently, the dielectric constant for all the samples decreases with increase in frequency as shown in the Fig. 8a. It has been suggested by Lopes et al. [35] that the reduced number of aligned dipoles per unit volume with increase in frequency causes this phenomenon. The change in the number of free oscillating dipoles in the alternating field gives rise to the variation in the dielectric permittivity. In the low-frequency region (\(\omega \ll \frac{1}{\tau }\), where τ is the relaxation time), the alignment of the dipoles tracks the applied electric field. With the increase in frequency (\(\omega < \frac{1}{\tau }\)), the dipoles start to lag behind the field, and consequentially dielectric constant decreases. At ω = \(\frac{1}{\tau }\), the dielectric constant reveals a relaxation process. At very high frequencies (\(\omega \gg \frac{1}{\tau }\)), the dipoles are unable to follow the applied electric field and therefore, the dielectric constant dies off. From the variation of the tanδ (Fig. 8b) with frequency, it is observed that the neat PVA and AR/PVA composites show a relaxation in the frequency range of 103–105 Hz, and the relaxation frequency is shifted towards lower frequency region with increase in AR dye content. The relaxation time is increased with increasing loading concentration of AR dye due to the larger size of dye–polymer complex at the higher filler concentration; consequently, the relaxation frequency moves towards lower frequency [38]. On the other hand, higher AR concentration in PVA matrix increases heterogeneity in the composite, produces larger interfacial area, and results in increased conductivity and higher loss [38, 39]. Therefore, the tanδ value is increased with increase in filler loading as shown in the Fig. 8b. Figure 8a, b shows that the AR/PVA composites exhibit high dielectric constant with considerably lower tan δ value (< 0.8) at low-frequency region than other polymer-based dielectric materials reported recently (compared in Table 3), confirming its potential applications in capacitors.
Figure 9a, b shows the temperature-dependent dielectric properties of the 2.0% AR/PVA composite. Figure 9a shows that the dielectric constant of the composite enhances with the increase in temperature. The value of dielectric constant at 303 K (at 40 Hz frequency) is ~ 208, which reaches up to ~ 480 at 403 K which is almost 49 times that of neat PVA. This temperature-dependent behavior of the dielectric constant can be explained in terms of the absorbed thermal energy by the composite film [40, 41].At lower temperature, absorbed thermal energy by the composite is small and comparatively a smaller number of dipoles can be oriented along the direction of the applied electric field. Due to higher absorbed thermal energy at higher temperature, the viscosity of the polymer composite decreases, and more number of dipoles become free to orient along the direction of the external electric field [41]. Consequently, dielectric constant of the polymer composite enhances significantly at higher temperature. Figure 9b depicts temperature-dependent tan δ of 2.0% AR/PVA composite. As seen from the figure, the composite undergoes a relaxation process in the 103–106 Hz frequency region at different temperatures (303–403 K). The relaxation process is designated as α-relaxation related to the glass transition. Pure PVA also exhibits similar type of glass transition related α-relaxation in the 103–106 Hz frequency range at 383–403 K temperature, which is reported earlier [42]. It is observed that with increasing temperature, the relaxation peak is shifted towards higher frequency which is characteristic of \(\alpha\) relaxation process [42, 43]. Further, above Tg (T > 363 K), the \(\alpha\) relaxation peak is split into two peaks. First peak is known as \(\alpha_{a}\) relaxation occurring at lower frequencies; it is associated with glass transition phenomenon, where micro-Brownian motion of the main polymer chain occurs in the amorphous region of PVA. The second peak is associated with \(\alpha_{c}\) relaxation occurring at higher frequencies due to molecular motion of the main polymer chain at crystalline phase or in the intermediate region between crystalline and amorphous phase of PVA.
In a heterogeneous medium, MWS polarization plays an important role as the interfacial polarization becomes dominating at the interfaces of two different phases having different conductivity and permittivity. But, at low-frequency and high-temperature conditions, the effect of electrode polarization may completely mask the effects of other polarizations. In this context, modulus formalism works as an interesting tool to analyze the dielectric response of an ionic polymeric material, where the signal intensity associated with electrode polarization can be eliminated successfully. The real (M’) and imaginary part (M’’) of dielectric modulus (M*) are calculated using the relations [44, 45]:
The frequency-dependent variations of real and imaginary parts of dielectric modulus of 2.0% AR/PVA composite are depicted in Fig. 10a, b. From Fig. 10a, it is evident that M’ increases with increasing frequency following a ‘S’-shaped nature. At low frequency M’ has a near zero value, confirming the negligible contribution of electrode polarization in the overall dielectric response. The increase in the value of M’ is associated with the interfacial polarization occurring at the dye–polymer interface. The cross-linking formations in the polymer matrix due to the dye–polymer complexes may play a significant role in the enhancement of M’ value. Figure 10b depicts the variation of the imaginary part (M’’) of dielectric modulus as a function of frequency at different temperatures. The peak in the figure represents a relaxation process, and it also determines the frequency range in which charge carriers are mobile over long distances. While temperature is increased it can be seen that the relaxation peak is shifted towards higher frequencies. At the frequency region beyond the peak position, the charge carriers are confined in potential wells and, therefore, carriers are mobile for only short distances. The conductivity relaxation time (\(\tau\)) is defined by the relation [44,45,46]:
where fm is the frequency at which M’’ reaches at its maximum value. Figure 10b shows that the frequency dependence of M’’ at different temperatures is used to find out \(\tau\) at different temperatures. Typically, the temperature-dependent conductivity relaxation time can be explained by Arrhenius behavior and defined as [47,48,49]:
where Ea, K and \(\tau_{0}\) are activation energy of the relaxation process, Boltzmann constant and relaxation time at infinite temperature, respectively. Figure 10c shows the ln(\(\tau\)) versus 1000/T curve which has been used to calculate Ea. The calculated value of Ea for the 2.0 %AR/PVA sample is 0.19 eV, whereas the pure PVA has the Ea value of 0.45 eV [44]. This decrease in activation energy with the increasing doping concentration of AR dye in the PVA matrix infers that the conduction mechanism is favored in the composite due to the ions provided by the dye.
Figure 11 shows the impedance spectrum (or Cole–Cole plot) of 2.0% AR/PVA polymer composite film at 303 K. The characteristic ac impedance spectrum depicts two distinct regions; the high-frequency semi-circle and the low-frequency spike. The high-frequency semi-circle is associated with the ionic conduction process in the bulk of the polymer composite and the low-frequency spike can be ascribed to the effect of blocking electrodes [45]. The observed angle of inclination of spike is less than 90o instead of a vertical spike, which infers the formation of the dye/polymer complexes causing the inhomogeneity within the polymer composite [45].The bulk conductivity was calculated from Fig. 11 by the following relation [46, 47]:
where l and A are the thickness of the film and the electrode area of the sample, respectively. The calculated value of the bulk conductivity of 2.0% AR/PVA polymer composite is 6.66 × 10−6 S/m.
4 Conclusion
The present work describes fabrication of low-cost, flexible AR/PVA composite films for flexible high power laser filter and flexible energy storage devices. The incorporation of AR dye in the PVA matrix causes formation of hydrogen bond-mediated complexes which lead towards significant enhancement of dielectric and optical irradiation absorbing properties of the composites. The semi-crystalline structure of the AR/PVA composites is confirmed by the XRD results. Addition of AR dye in PVA matrix develops broader absorption bands in UV–Vis range, which facilitate the absorption of incident irradiation of almost entire UV–Vis range (200–600 nm) by the composite film. The band gap of the AR/PVA composite, estimated using Tauc’s formula and ASF model (~ 1.6 eV for 2.0% AR/PVA), is also significantly modified in comparison to the neat PVA due to addition of AR dye. The self-standing 2.0% AR/PVA films (thickness ~ 250 ± 30 µm)were directly employed as laser cut-off filters for blocking a 10.7 mW incident laser radiation of wavelength 544 nm and the composite film was remained highly stable after exposing the laser irradiation for a long period of time (60 min.). Inclusion of AR dye does not reduce the flexibility of the AR/PVA composite film and the stretching ability of the 2.0% AR/PVA composite films is found to be increased significantly than the neat PVA, making it even a better candidate for multiple uses in optoelectronic devices. The dye-doped films show very high dielectric constant (~ 208 for 2.0% AR/PVA at 303 K) at 40 Hz with very low tangent loss (~ 0.56). The dielectric relaxation phenomena are investigated from the viewpoint of temperature dependence which shows that α-type relaxation peak splits into two peaks beyond the glass transition temperature of PVA. The modified dielectric property of the composite is attributed to the MWS interfacial polarization occurring between the two different phases of the formed AR–PVA complexes. The dielectric modulus formalism also shows key role of the MWS interfacial polarization behind the significant enhancement of the dielectric constant of the composite. The impedance spectroscopy results of the 2.0 % AR/PVA composite support the formation of complexes within the AR/PVA composite. The bulk conductivity was calculated to be 6.66 × 10−6 S/m. The fabricated AR/PVA composite films can be potentially used in the field of flexible, economic, polymeric laser cut-off filter, protective windows, optical filtering, flexible capacitors and other flexible optoelectronic applications.
References
C.K. Madsen, J.H. Zhao, Optical measurements and filter analysis, Optical filter design and analysis: a signal processing approach (Wiley, Hoboken, 1999)
L.I. Epstein, The design of optical filters. J. Opt. Soc. Am. 42, 806 (1952)
H.A. Macleod, Thin-film optical filters (CRC Press, London, 2001)
G. Wyszecki, W.S. Stiles, Color science (Wiley, New York, 1982)
N. Sultanova, S. Kasarova, I. Nikolov, Dispersion properties of optical polymers. Acta Phys. Pol. A 116, 585 (2009)
M.M. Abutalib, I.S. Yahia, Selective CUT-OFF laser filters using brilliant green-doped PMMA polymeric composite films: sensing approach. J. Mater. Sci. 29, 19798–19804 (2018)
I.S. Yahia, A. Bouzidi, H.Y. Zahran, W. Jilani, S. AlFaify, H. Algarni, H. Guermazi, Design of smart optical sensor using polyvinyl alcohol/Fluoresce in sodium salt: laser filters and optical limiting effect. J. Mol. Struct. 1156, 492–500 (2018)
D.A. Hora, A.B. Andrade, N.S. Ferreira, V.C. Teixeira, M.V. Rezende, Effect of the PVA (polyvinyl alcohol) concentration on the optical properties of Eu-doped YAG phosphors. Opt. Mater. 60, 495–500 (2016)
V.K. Gupta, A. Mittal, D. Jhare, J. Mittal, Batch and bulk removal of hazardous coloring agent Rose Bengal by adsorption techniques using bottom ash as adsorbent. RSC Adv. 2, 8381–8389 (2012)
C. Åkerlind, H. Arwin, F. Jakobsson, H. Kariis, K. Järrendahl, Optical properties and switching of a Rose Bengal derivative: a spectroscopic ellipsometry study. Thin Solid Films 519, 3582–3586 (2011)
F.H.M. Al-Kadhemy, W.H. Abaas, Absorption spectrum of crystal violetin chloroform solution and doped PMMA thin films. Atti Della Fondazione Giorgio Ronchi 3, 359–367 (2012)
H.M. Zidon, A. Tawansi, M. Abu-Elnader, Miscibility, optical and dielectric properties of uv–irradiated poly(vinylacelate)/poly methyl methacrylate. Phys. B 339, 78–86 (2003)
R. Bisen, J. Tripathi, A. Sharma, A. Khare, Y. Kumar, S. Tripathi, Optical behaviour of coumarin dye in PVA and PMMA film matrices. Vacuum 152, 65–69 (2018)
T. Martins, R. Weiss, T. Atvars, Synthesis and photo physical properties of poly(methyl methacrylate) polymer with carbazolyl Side groups. J. Braz. Chem. Soc. 19, 1450–1461 (2008)
Z. Khodair, M. Saeed, H.A. Allah, Synthesis and characterization of wide-scale UV–vis CUT-OFF laser filter using methyl violet-6B/PMMA polymeric composite films. Iraq. J. Phys. 12, 47–51 (2018)
D. Xu, D. Neckers, Aggregation of rose Bengal molecules in solution. J. Photochem. Photobiol. A 40, 361–370 (1987)
I.S. Yahia, S.M.A.S. Keshk, Preparation and characterization of PVA/Congo red polymeric composite films for a wide scale laser filters. Opt. Laser Technol. 90, 197–200 (2017)
S. Yahia, S.M.A.S. Keshk, S. AlFaify, A.M. El-Naggar, Synthesis and characterization of wide-scale UV–vis CUT-OFF laser filter using methyl violet-6B/PMMA polymeric composite films. Sens. Actuators, A 269, 388–393 (2018)
S.M. El-Bashir, I.S. Yahia, M.A. Binhussain, M.S. AlSalhi, Designing of PVA/Rose Bengal long-pass optical window applications. Results Phy. 7, 1238–1244 (2017)
M.L. de Souza, P. Corio, Effect of silver nanoparticles on TiO2-mediated photo-degradation of Alizarin Red S. Appl. Catal. B 136–137, 325–333 (2013)
M. Ghaedi, A. Hassanzadeh, S.N. Kokhdan, Multiwalled carbon nanotubes as adsorbents for the kinetic and equilibrium study of the removal of Alizarin Red S and Morin. J. Chem. Eng. Data 56, 2511–2520 (2011)
M. Jean Mcleod, Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299–301 (1980)
A. Kimoto, S. Shimada, Electrochemical degradation of anthraquinone dye Alizarin Red S by anodic oxidation on boron-doped diamond. Dyes Pigments 76, 86–89 (2007)
S. Chan, M. Yunus, W. Mahmood, W.M.Z. Yunus, Measurement of thermal diffusivity, optical transmission and optical absorption peaks of laser dyes R6G doped in poly (methylmethacrylate) using photoacoustic technique and fibre optics spectrophotometer, Pertanika. J. Sci. Technol. 11, 83–91 (2003)
K.S. Hemalatha, K. Rukmani, N. Suriyamurthy, B.M. Nagabhushana, Influence of ZnO nanoparticles on thermal behavior of poly vinyl alcohol films. Mater. Res. Bull. 51, 438–446 (2014)
G. Omed, S.B. Aziz, K.M. Omer, Y.M. Salih, Reducing the optical band gap of polyvinyl alcohol (PVA) based nanocomposite. Mater. Sci. Mater. Electron. 26, 5303–5309 (2015)
H. Esfahani, M. Ghanipour, D. Dorranian, Effect of dye concentration on the optical properties of red-BS dye-doped PVA film. J. Theor. Appl. Phys. 8, 117–121 (2014)
T.S. Moss, Optical properties of semiconductors (Butterworths, London, 1959)
F.M. Ali, Structural and optical characterization of [(PVA:PVP)-Cu2þ] composite films for promising semiconducting polymer devices. J. of Mol. Struct. 1189, 352–359 (2019)
K.M. Manikandan, A. Yelilarasi, P. Senthamaraikannan, S.S. Saravanakumar, A. Khan, M.A. Abdullah, A study on optical limiting properties of Eosin-Y and Eriochrome Black-T dye-doped poly (vinyl alcohol) composite film. Int. J. Polym. Anal. Charact. 24, 326–333 (2019)
R. Manshad, Q. Hassan, Optical filtering properties of magenta doped PMMA under CW laser illumination. Adv. Appl. Sci. Res. 3, 3696–3702 (2012)
D. Ghoshal, D. Bhattacharya, D. Mondal, S. Das, N. Bose, M. Basu, Methylene blue/PVA composite film for flexible, wide-scale UV–Vis laser cut-off filter. Mater. Res. Express 6, 075332 (2019)
H. Elhosiny Ali, Y. Khairy, H. Algarni, H.I. Elsaeedy, A.M. Alshehri, H. Alkharis, I.S. Yahia, The visible laser absorption property of chromium doped polyvinyl alcohol films: synthesis, optical and dielectric properties. Opt. Quant. Electron. 51, 47 (2019)
M. Aslam Manthrammel, A.M. Aboraia, I.S.Yahia MohdShkir, Mohammed A. Assiri, H.Y. Zahran, V. Ganesh, S. AlFaify, V. Alexander, S.M. Aslam, Optical analysis of nanostructured rose bengal thin films using Kramers-Kronig approach: new trend in laser power attenuation. Opt. Laser Technol. 112, 207–214 (2019)
R. Bisen, J. Tripathi, A. Sharma, Y. Kumar, A. Khare, S. Tripathi, Optical behaviour of coumarin dye in PVA and PMMA film matrices. Vacuum 152, 65–69 (2019)
A.C. Lopes, C.M. Costa, R. Sabateri Serra, I.C. Neves, J.L. Gomez Ribelles, S. Lanceros-Mendez, Dielectric relaxation, ac conductivity and electric modulus in poly(vinylidene fluoride)/NaY zeolite composites. Solid State Ion. 235, 42–50 (2013)
Y. Li, X. Hung, Z. Hu, P. Jing, S. Li, T. Tanaka, Large dielectric constant and high thermal conductivity in poly (vinylidene fluoride)/barium titanate/silicon carbide three-phase nanocomposites. ACS Appl. Mater. Interfaces. 3, 4396–4403 (2011)
E. Kar, N. Bose, S. Das, N. Mukherjee, S. Mukherjee, Enhancement of electroactive β phase crystallization and dielectric constant of PVDF by incorporating GeO2 and SiO2 nanoparticles. Phys. Chem. Chem. Phys. 17, 22784–22798 (2015)
G.M. Tsangaris, N. Kouloumbi, S. Kyvelidis, Interfacial relaxation phenomena in particulate composites of epoxy resin with copper or iron particles. Mater. Chem. Phys. 44, 245–250 (1996)
J. Mijovic, J.W. Sy, T.K. Kwei, Reorientational dynamics of dipoles in poly (vinylidene fluoride)/poly (methyl methacrylate)(PVDF/PMMA) blends by dielectric spectroscopy. Macromolecules 30, 3042–3050 (1997)
H.M. Zidan, N.A. El-Ghamaz, A.M. Abdelghany, A. Lotfy, Structural and electrical properties of PVA/PVP blend doped with methylene blue dye. Int. J. Electrochem. Sci. 11, 9041–9056 (2016)
T.A. Abdel-Baset, AHassen. Phys. B 499, 24–28 (2016)
T.A. Hanafy, Dielectric relaxation and alternating-current conductivity of gadolinium-doped poly(vinyl alcohol). J.Appl. Polym. Sci. 108, 2540–2549 (2008)
S. Kumar, G.K. Prajapati, A.L. Saroj, P.N. Gupta, Structural, electrical and dielectric studies of nano-composite polymer blend electrolyte films based on (70–x) PVA–x PVP–NaI–SiO2. Phys. B 554, 158–164 (2018)
P.B. Bhargav, B.A. Sarada, A.K. Sharma, V.V.R.N. Rao, Electrical conduction and dielectric relaxation phenomena of PVA Based polymer electrolyte films. J. Macromol. Sci. 47, 131–137 (2009)
M. Arous, I. Ben Amor, A. Kallel, Z. Fakhfakh, G. Perrier, Crystallinity and dielectric relaxations in semi-crystalline poly(ether ether ketone). J. Phys. Chem. Solids 68, 1405–1414 (2007)
M.H. Makled, E. Sheha, T.S. Shanap, M.K. El-Mansy, Electrical conduction and dielectric relaxation in p-type PVA/CuI polymer composite. J. Adv. Res. 4, 531–538 (2013)
E. Sheha, E. Sheha, H. Khoder, T.S. Shanap, M.G. El-Shaarawy, M.K. El Mansy, Structure, dielectric and optical properties of p-type (PVA/CuI) nanocomposite polymer electrolyte for photovoltaic cells. Optik 123, 1161–1166 (2012)
E. Kar, N. Bose, S. Das, N. Mukherjee, S. Mukherjee, Temperature dependent dielectric properties of self-standing and flexible poly(vinylidene fluoride)films infused with Er3+ doped GeO2 and SiO2 nanoparticles. J. Appl. Polym. Sci. 44016, 1–20 (2016)
Acknowledgements
Authors are extremely thankful to Dr. Pallab Dutta, CHST, IIEST, Shibpur for providing stress–strain measurement facility.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghoshal, D., Bhattacharya, D., Mondal, D. et al. Flexible alizarin red/PVA composites with colossal dielectric and high power laser filtering properties. Appl. Phys. A 126, 28 (2020). https://doi.org/10.1007/s00339-019-3210-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-3210-8