Abstract
A series of Na2SiF6:xRe3+ (Re3+ = Eu3+, Tb3+, Ce3+) phosphors were prepared by co-precipitation method. The structure and luminescence performance of the materials were characterized by XRD, TG–DSC, TEM, HRTEM, EDS, FT-IR, fluorescence spectra and the calculated CIE coordinates. The results show that under the excitation of specific UV light, Na2SiF6:xEu3+, Na2SiF6:yTb3+ and Na2SiF6:zCe3+ single-doping materials exhibit the characteristic emission of Eu3+ (5D0 → 7F2, red), Tb3+ (5D4 → 7F5, green) and Ce3+ (5d → 4f, blue), respectively. In the Na2SiF6:xEu3+–yTb3+–zCe3+ three-doping system, Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material is representative. With the increase of exciting wavelength of UV light, the emission color of the material realizes tunable from white light to cool white light, warm white light and red orange light. And the luminescence of the material is closest to the ideal white light under excitation of UV light at 290 nm. So this phosphor can be promised as a single-component tunable white phosphor for application in white LEDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As a new generation of solid-state lighting source, white LED has been widely used in lighting, indicating, decoration and other fields, due to its energy saving, environmental protection, high efficiency and other performance [1, 2]. There are many kinds of processes for synthesizing white light [3, 4], e.g., in a combination of single red, green and blue LED chips, due to the different external voltages driving different chips, the electronic devices are complicated and difficult to simplify. This limits its development. Similarly, in a combination of a yellow phosphor and a blue LED chip, due to the lack of red light in the spectrum, the color temperature is high, the color is cold, and the color rendering index is poor; its development is also limited. Moreover, in a combination of three-color phosphors and a UV-LED chip, the white light of various color temperatures and color renderings can be obtained by adjusting the ratio of the three phosphors, thereby meeting the requirements of different lighting environments; however, this method has problems in color reabsorption and adjustment of the ratio. Therefore, developing a single-component white phosphor has become a hot topic in the research field of the current luminescent materials.
The phosphor consists of a host compound (i.e., a matrix moiety) and an active dopant (i.e., a luminescent center ion), sometimes doped with a sensitizer (i.e., another impurity ion to transfer energy) [5]. Rare earth ions have an unfilled 4f5d electronic configuration which is shielded from the outside. This leads to the rich electronic transitions. Among them, Eu3+, Tb3+, and Ce3+ are often selected as luminescent centers as rare earth ions capable of emitting red, green, and blue primary colors, respectively [6, 7]. Ren et al. [8] synthesized the Ce–Tb–Eu co-doped calcium borosilicate white light material, and studied the effect of Eu2O3 concentration on the luminescence performance of the material. Zhu et al. [9] studied the Ce–Tb–Eu co-doped silicate and phosphate white light materials. But the blue component of the photoluminescence spectrum was relatively strong, and the green and red components were relatively weak, resulting the color coordinates relatively poor. Ghosh et al. [10] synthesized the Ce–Tb–Eu co-doped zinc fluorosilicate white light material, which could obtain warm white light at 378 nm excitation. Subsequently, more researchers have studied fluorosilicate phosphors, and the results show that the doping of fluoride ion can improve the luminescence performance of the silicate phosphors. That is, the fluorosilicate phosphor is a new kind of promising luminescent materials.
Therefore, in this paper, the Na2SiF6 was used as the main matrix, and Eu3+, Tb3+, and Ce3+ were selected as luminescent centers. A series of rare earth ions single-doped red, green and blue monochromatic phosphors (i.e., Na2SiF6:xEu3+, Na2SiF6:yTb3+ and Na2SiF6:zCe3+) were synthesized by co-precipitation method, respectively. Then, by further analyzing the energy transfer process between the three ions to adjust their metering ratio, a white phosphor which could be excited by 290 nm was synthesized. Moreover, according to the difference of energy transfer efficiency between the three ions under the excitation of UV light of different excitation wavelengths, a white phosphor which could change the emitting color of the phosphor by changing the excitation wavelength of UV light was obtained. That is Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ phosphor.
2 Experimental part
2.1 Reagents and instruments
H2SiF6 and Na2SO4·10H2O (all AR) were, respectively, formulated into 0.20 mol/L H2SiF6 and Na2SO4 solutions; Eu2O3, Tb4O7 (≥ 99.999%), HCl (12 mol/L) and Ce(NO3)3 were, respectively, formulated into 0.10 mol/L EuCl3, TbCl3 and Ce(NO3)3 solutions; absolute ethanol (AR, ≥ 99.7%).
XRD (Japan Ultima VI type, ray source Cu Κα, radiation wavelength λ = 0.15406 nm, tube voltage 40 kV, tube current 40 mA, scanning range 2θ = 10°–80°, scanning speed 10°/min), TG–DSC (France Setaram Labsys Evo type, air flow 30 mL/min, heating rate 10 °C/min), TEM equipped with EDS (Japan JEOL-2010 type, magnification × 50–1,500,000, acceleration voltage 200 kv), FT-IR (USA Nicolet, 6700 type, scanning times 32, resolution 4 cm−1, KBr tablet method), fluorescence spectrometer (Japan Model FL-4500 type, Xe lamp source, PMT voltage 400 V, scanning speed 1200 nm/min, slit width 5.0 nm; UK Edinburgh, FLS-920 type, Xe lamp source, grating Ex/Em = 1/2, bandwidth Ex/Em = 10).
2.2 Preparation of materials
A series of Na2SiF6:xRe3+ (Re3+ = Eu3+, Tb3+, Ce3+) materials were synthesized by co-precipitation method. We prepared the matrix Na2SiF6 according to the reaction principle of Na2SO4 + H2SiF6 = Na2SiF6\(\downarrow\) + H2SO4. First, the known quantities Na2SO4 and H2SiF6 were reacted in a thermostat water bath beaker at 60–80 °C for 1 h, and then certain amounts of EuCl3, TbCl3 and Ce(NO3)3 were added for 1 h. After that, the reaction solution was cooled to room temperature, filtered at normal pressure, washed three times with absolute ethanol, dried at room temperature, and thoroughly ground in an agate mortar to obtain a white powder solid. The solid was then annealed at 200, 300, 400, 500, 600, 700 and 800 °C for 2 h to obtain materials at different annealing temperatures, and the phase analysis was carried out to determine the optimum annealing temperature of the material. Finally, the material at the optimum annealing temperature was further selected for various optical and spectral characterizations.
3 Results and discussion
3.1 Structural analysis of Na2SiF6:xRe3+ (Re3+ = Eu3+, Tb3+, Ce3+) materials
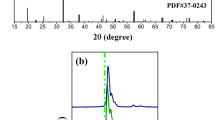
Figure 1a shows XRD patterns of the Na2SiF6 materials at different annealing temperatures. It can be seen that all diffraction peaks of the Na2SiF6 materials after annealing at 200 °C, 300 °C and 400 °C can be indexed to the Na2SiF6 [11, 12] (JCPDS no. 33–1280), corresponding to the hexagonal phase (the space group is P321, the lattice constants are a = b = 8.866 Å, c = 5.043 Å). In the structure, each Si4+ is connected with six F−, forming a regular SiF62− octahedron, and two Na+ are, respectively, located on both sides of the octahedral structure, and the crystal structure is highly symmetrical. However, as the annealing temperature increases again, the crystal structure of the Na2SiF6 material gradually decomposes. When annealing at 500 °C, a part of diffraction peaks of the material can correspond to the NaF (JCPDS no. 36–1455). That is, the matrix Na2SiF6 begins to decompose at this temperature (Na2SiF6\(\underline{\underline {\Delta }}\)2NaF + SiF4\(\uparrow\)) [13]. This phenomenon can be confirmed in the TG–DSC test of the Na2SiF6 material. Therefore, the optimum annealing temperature of the Na2SiF6 material is 400 °C, at this time, the material has good crystallinity and few miscellaneous peaks.
Figure 1b shows TG–DSC patterns of the Na2SiF6 material. In the temperature range of 35–150 °C, the obvious weight loss process of the TG curve and the distinct endotherm peak of the DSC curve can be seen in the picture, which are related to the loss of crystal water [14]. In the temperature range of 150–400 °C, there are no significant change in the TG and DSC curves, corresponding to the hexagonal Na2SiF6 crystal (JCPDS no. 33–1280). That is, the system is basically in a stable state. In the temperature range of 500–600 °C, the DSC curve shows an obvious endothermic peak, and the corresponding TG curve also shows an obvious phenomenon of weight loss, which are related to the decomposition of the Na2SiF6 crystal. Combined with Fig. 1a, we know that Na2SiF6 decomposes to form NaF (JCPDS no. 36–1455) [13], producing SiF4 gas. When the temperature continues to rise, in the temperature range of 600–700 °C, the DSC curve shows an obvious endothermic peak. Combined with Fig. S1, we speculate that this is related to the change in the crystal structure of NaF. That is NaF (JCPDS no. 36–1455) is further converted to NaF (JCPDS no. 01–1184). Since the synthesized Na2SiF6 material starts to decompose from 500 °C, the materials are all annealed at 400 °C for subsequent investigation.
Figure 2 shows representative XRD patterns of the Na2SiF6:xRe3+ materials (i.e., Na2SiF6, Na2SiF6:0.07Eu3+, Na2SiF6:0.09Tb3+, Na2SiF6:0.07Ce3+ and Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ materials) annealed at 400 °C for 2 h. In the figure, all the diffraction peaks of the representative materials correspond well to the hexagonal phase of Na2SiF6 (JCPDS no. 33–1280). There are no other impurity peaks detected from the secondary phase. The result clearly shows that a small amounts of Eu3+, Tb3+, Ce3+ doping does not cause significant changes in the matrix lattice in this experiment, and the Eu3+, Tb3+, Ce3+ were completely doped into the Na2SiF6 main matrix by replacing the Na+.
According to previous reports, to form a new solid solution, the difference in radius percentage (Dr) between the dopant and the possible substituted ion should not exceed 15%. It can be expressed as [15, 16]: \({\text{Dr}} =\left| {\frac{{{R_1} - {R_2}}}{{{R_1}}}} \right| \times 100\%\) (where R1 is the ionic radius of the bulk cation and R2 is the ionic radius of the dopant). The ionic radius of R1 (Na+) in the main matrix is 0.97 Å, and the ionic radii of the dopants R2 (Eu3+), R2 (Tb3+) and R2 (Ce3+) are 0.92 Å, 0.95 Å and 1.03 Å, respectively. The calculated Dr (Na+/Eu3+) is about 5.15%, Dr (Na+/Tb3+) is about 2.06%, and Dr (Na+/Ce3+) is about 6.19%, both less than 15%. Compared with Eu3+ and Tb3+, Ce3+ is more difficult to replace Na+. But from the perspective of the electronegativity of the element, the electronegativity of Na is 0.9, and the electronegativity of Ce is 1.12, which are relatively close. Therefore, these are all confirmed that Eu3+, Tb3+ and Ce3+ can occupy the Na+ position in the Na2SiF6 host lattice.
Due to the difference in the radii of Eu3+, Tb3+, Ce3+ and Na+, the diffraction peaks of the XRD patterns shift with the increase of the contents of Eu3+, Tb3+ and Ce3+. The shift can be explained by the Bragg diffraction equation [17]: 2dsin (θ) = n λ. When Eu3+ (0.92 Å) replaces Na+ (0.97 Å), the d value of the interplanar spacing decreases. To keep n λ constant, the diffraction peaks shift toward the high angle. As the concentration of Eu3+ increases, the angle of the offset increases. The phenomenon can be seen in Fig. S2. When Tb3+ (0.95 Å) replaces Na+ (0.97 Å), the same phenomenon can be discovered in Fig. S3. When Ce3+ (1.03 Å) replaces Na+ (0.97 Å), the d value of the interplanar spacing increases, and the diffraction peaks shift toward the low angle. As the concentration of Ce3+ increases, the angle of the offset increases (Fig. S4).
Figure S5 shows representative IR spectra of the Na2SiF6:xRe3+ materials (i.e., Na2SiF6:0.07Eu3+, Na2SiF6:0.09Tb3+, Na2SiF6:0.07Ce3+ and Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ materials) annealed at 400 °C for 2 h. In the figure, the absorption peak at 475 cm−1 corresponds to the bending vibration of SiF62−. The absorption peaks at 730 cm−1 and 495 cm−1 correspond to the tensile vibration of SiF62− [18]. This is consistent with XRD analysis, so the materials are pure hexagonal Na2SiF6. In addition, according to previous reports [19], the characteristic peak appearing at 1102 cm−1 is caused by the exchange of bromide ion with fluoride ion in sodium fluorosilicate.
Figure 3a shows representative TEM image of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material. It can be seen that the material is mainly made up of circular particles, which are prone to agglomeration. And the size of the crystal particle obtained by particle size measurement is about 119.15 nm. Figure 3b, c shows the electron diffraction pattern and HRTEM image of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material. The figures show diffraction spots or rings and clear lattice fringes, from which the (210), (201) and other facets of hexagonal phase Na2SiF6 could be clearly discerned. These lattice spacings are consistent with the Na2SiF6 (JCPDS no. 33–1280) measured by XRD. They further evidence that the material has good crystallinity.
Figure 4 shows the representative EDS energy spectrum of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material. O, F, Na, Si, S, Eu, Tb, and Ce elements can be observed in the energy spectrum. And the elemental ratios of Eu, Tb and Ce show that the three ions are uniformly distributed in the whole particle range according to the known quantity, indicating the formation of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material.
Figure S6 shows the representative Raman spectroscopy of the Na2SiF6 and Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ materials. According to previous reports [20,21,22], Raman scattering is sensitive to even symmetry (gerade) modes of vibration, while infrared radiation is sensitive to odd (ungerade) modes. There are a total of six phonon modes in the octahedral crystal field. Three gerade modes (v1, v2 and v5) are Raman active, while the remaining ungerade modes are IR active (v3 and v4) and silent modes (v6). However, in highly defective crystalline materials, the parity selection rules for Raman scattering may be partially relaxed or significantly changed. As this time, some inhibition modes can become Raman active, and even new local vibration modes can be observed. Therefore, if Eu3+, Tb3+, Ce3+ doped Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material presences highly defective, then some of the original inhibition modes and the new local vibration modes should be observed in the figure. However, it can be seen from the figure that the vibration peak positions of the two materials are identical. The result clearly shows that the as-synthesized Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ materials have pure hexagonal phase.
3.2 Luminescence performance analysis of the Na2SiF6:xRe3+ (Re3+ = Eu3+, Tb3+, Ce3+) materials
3.2.1 Luminescence performance of the Na2SiF6:xEu3+ materials
Figure 5a shows excitation and emission spectra of a representative material Na2SiF6:0.07Eu3+ in the Na2SiF6:xEu3+ materials. In the excitation spectrum, a series of the sharp absorption peaks in the range of 300–500 nm are caused by the 4f internal transitions of Eu3+. That is, the excitation peaks at 318, 362, 381, 395, 415, and 465 nm are attributed to 7F0 → 5H6, 7F0 → 5D4, 7F0 → 5G2, 7F0 → 5L6, 7F0 → 5D3 and 7F0 → 5D2 transitions of Eu3+ [23, 24], respectively. Among them, the shape of the excitation peak located at 395 nm in the ultraviolet region is the sharpest and the peak intensity is the strongest. That is, the excitation efficiency of this wavelength is high, so it is selected as the optimal excitation wavelength. In addition, the Na2SiF6:0.07Eu3+ material has a very weak single absorption broadband in the ultraviolet short-wave region (200–290 nm), which is caused by the charge transfer band of Eu3+–O2−. When Eu3+ is substituted for Na+, a positively charged \({\text{E}}{{\text{u}}^{\cdot \cdot }}{\text{Na}}\) vacancy defect is generated. It requires charge compensation. In atmospheric and non-reducing atmospheres, the two F− may enter the gap in a gap-filled manner to achieve charge balance. At the same time, trace oxygen in the atmosphere or other sources of oxygen in the system may also replace F−, thereby achieving charge compensation. That is, in the Na2SiF6 lattice, Eu3+ combines with the nearest trace O2− and the next adjacent Na+ to form Eu3+–O2−–Na+. And the electrons of O2− migrate from its full 2p orbit to the partially filled 4f6 shell of Eu3+, resulting in a weak charge transfer band [25].
In the emission spectrum, the Na2SiF6:0.07Eu3+ material under the excitation of 395 nm shows characteristic emission peaks of Eu3+ at 588 nm (5D0 → 7F1) and 612 nm (5D0 → 7F2), respectively. The strongest radiation of the Eu3+ in the emission spectrum mainly depends on its symmetry of the occupied position in the lattice of the matrix. When the symmetry is low, the strongest radiation appears as an electric dipole transition (5D0 → 7F2), otherwise it appears as a magnetic dipole transition (5D0 → 7F1) [26]. In the Na2SiF6 matrix, the emission of Eu3+ mainly manifests as a magnetic dipole transition (5D0 → 7F1) at 588 nm. This is because in the Na2SiF6 crystal, Eu3+ mainly replaces the position of Na+. And Na+ occupies a highly symmetry position in the Na2SiF6 lattice. So the emission of Eu3+ is dominated by the magnetic dipole transition (5D0 → 7F1) at 588 nm [27]. The characteristic emission peak of Eu3+ at 612 nm is the electric dipole transition (5D0 → 7F2). According to the selection rule, this electric dipole transition of Δl = 0 was originally forbidden [28]. However, as for the Na2SiF6:xEu3+ (x = 6–8 mol%) materials in Fig. 5b, when the doping amount of Eu3+ is 6 mol%, the original forbidden transition is relatively strong. This is mainly due to the fact that the 4f configuration is mixed with the oppositely symmetric configuration g or d, or the symmetry deviates from the inversion center, so that the original forbidden f–f transition becomes permissible. It indicates that Eu3+ is located in the center of the asymmetric field in the Na2SiF6 lattice at this time, presumably due to the partial Eu3+ entering the lattice gap. But as the concentration of Eu3+ increases, this transition gradually diminishes. This is because in the Na2SiF6 lattice, Eu3+ mainly replaces the Na+ position, and the Eu3+ (0.92 Å) radius is smaller than the Na+ (0.97 Å) radius. Therefore, as the concentration of Eu3+ increases, the Na2SiF6 unit cell shrinks and the crystal field effect is enhanced [27], making it more difficult for Eu3+ to enter the lattice gap.
Figure 5c shows emission intensity spectra of Na2SiF6:xEu3+ materials with different Eu3+ concentrations. It can be seen that as the Eu3+ doping amount increases, the luminescence intensity of the material increases, reaching a maximum at 7 mol% Eu3+. At this time, the energy transfer rate between the luminescent centers of Eu3+ is equal to the emission rate, so the luminescence reaches the strongest. As the doping amount continues to increase, the luminescence is weakened. This is because the positions between Eu3+ are close to each other, and the activator ions in the excited state interact with each other, thereby increasing the new energy loss mechanism, and resulting in concentration quenching phenomenon [29].
3.2.2 Luminescence performance of the Na2SiF6:yTb3+ materials
Figure 6a shows excitation and emission spectra of a representative material Na2SiF6:0.09Tb3+ in the Na2SiF6:yTb3+ materials. In the excitation spectrum, with the wavelength range of 220–250 nm, the broadband centered at 230, 265 nm all belong to the 4f8 → 4f75d1 transition of Tb3+. The narrow peaks at 285, 303, 318, 342, and 352 nm correspond to 7F6 → 3H3, 7F6 → 5H6, 7F6 → 5H7, 7F6 → 5L7 and 7F6 → 5D2 transitions of Tb3+ [30, 31], respectively. The characteristic excitation peaks at 370 and 378 nm correspond to the 7F6 → 5L10 transition of Tb3+. The reason why the excitation peak generated by this transition splits into two peaks is as follows: the 5d electrons are in the outer electron orbit, which are greatly affected by environmental factors, and the 7F6 energy level degenerate releases, causing the stokes splitting and forming two lines [27, 28]. Among these excitation bands, the excitation band at 378 nm in the ultraviolet region has the highest intensity, so it is selected as the optimum excitation wavelength. In the emission spectrum, the Na2SiF6:0.09Tb3+ material under the excitation of 378 nm has a series of sharp emission peaks at 492, 544, 592 and 612 nm, corresponding to 5D4 → 7FJ (J = 6, 5, 4, 3) transitions of Tb3+ [32, 33].
Excitation and emission spectra of the Na2SiF6:0.09Tb3+ material (a) emission spectra of the Na2SiF6:yTb3+(y = 6–10 mol%) materials (b) emission intensity spectra of the Na2SiF6:yTb3+ materials with different Tb3+ concentrations (c) simplified energy levels image of Tb3+ and emission spectra of the Na2SiF6:0.09Tb3+ material under the excitations of 352, 370 and 378 nm (A)
Figure 6a shows the simplified energy levels image of Tb3+ and emission spectra of the Na2SiF6:0.09Tb3+ material under the excitations of 352, 370 and 378 nm. Under the excitation of 378 nm, electrons first are excited from the ground state of 7F6 to the excited state of 5L10. Then, a non-radiative transition occurs, transitioning to a lower excited state at the 5D3 level. Subsequently, a second non-radiative transition occurs, causing the electrons decay from the 5D3 level to the 5D4 level. Finally, a radiation transition occurs, resulting in a series of characteristic emissions of Tb3+, namely 5D4 → 7F6 at 492 nm, 5D4 → 7F5 at 544 nm, 5D4 → 7F4 at 592 nm, and 5D4 → 7F3 at 612 nm [34]. Further, using excitations of 352 nm and 370 nm as excitation wavelengths respectively, almost the same emission spectrums are obtained. And the energy level transitions of the emission process are also almost same. That means, the illumination mechanisms are the same.
Figure 6b, c is emission spectrum and emission intensity spectra of the Na2SiF6:yTb3+ materials with different Tb3+ concentrations, respectively. The combination of the two graphs shows that the different Tb3+ doping amounts have little effect on the distribution position of the emission peaks, but the intensity of each emission peak greatly changes with the increase of Tb3+ doping amount. It is obvious that as the Tb3+ doping amount increases, the luminescence intensity of the material increases, reaching a maximum at 9 mol% Tb3+. However, since the non-radiative energy transfer between adjacent Tb3+ causes concentration quenching, the luminescence intensity of the material begins to decrease as the increasing of the concentration of Tb3+. Non-radiative energy transfer between activators is achieved primarily through two mechanisms: exchange interaction and multipole–multipole interaction. In general, when the critical distance between activator ions is less than 5 Å (ie, Rc < 5 Å), the non-radiative energy transfer between activators is dominated by exchange interactions. When Rc > 5 Å, the multipole–multipole interaction is dominant. Therefore, to explore the concentration quenching mechanism of Tb3+ in this system, the critical distance Rc between Tb3+ in the Na2SiF6 matrix is calculated by the given formula [35, 36]: \({R_{\text{c}}} =2{\left( {\frac{{3V}}{{4\pi Z{X_{\text{c}}}}}} \right)^{1/3}}\) (where V is the volume of the unit cell, Z is the number of cations per unit cell, and Xc is the critical doping concentration of the activator ion). For the Na2SiF6:yTb3+ materials, V = 195.41Å3, Z = 3, Xc (Tb3+) = 9 mol%. So the calculated Rc (Tb3+) is about 11.14 Å, which is much larger than 5 Å. Therefore, the multipole–multipole interaction is the main mechanism for the concentration quenching of Tb3+ in this system.
3.2.3 Luminescence performance of the Na2SiF6:zCe3+ materials
Figure 7a shows excitation and emission spectra of a representative material Na2SiF6:0.07Ce3+ in the Na2SiF6:zCe3+ materials. In the excitation spectrum, there is a broad and strong excitation band from 210 to 310 nm, centered at about 290 nm, so selecting 290 nm of the ultraviolet light as the optimal excitation wavelength. The peak is assigned to the 4f → 5d transition of Ce3+. In the emission spectrum, it has a single-peak broadband in the range of 380–600 nm, centered at about 465 nm, due to the 5d → 4f transition of Ce3+. Its ground state is 4f15d0 and the excited state is 4f05d1. When 4f loses electrons, it leaves two energy levels of 2F7/2 and 2F5/2, and the energy difference between the two levels is about 2300 cm−1. At this time, the 5d electrons in the excited state will form a 2D energy level, and the 2D energy level will further split into two energy levels of 2D3/2 and 2D5/2 due to spin–orbit coupling. The energy difference between the two energy levels is about 1890 cm−1. The 5d orbit of Ce3+ is exposed to the outer layer. When it is subjected to the crystal field, the original discrete energy levels will form continuous energy bands. Further, when a 5d → 4f energy level transition occurs, a luminescence phenomenon occurs in the visible light region [37].
Figure 7b shows emission intensity spectra of Na2SiF6:zCe3+ materials with different Ce3+ concentrations. It is obvious that as the Ce3+ doping amount increases, the luminescence intensity of the material increases, reaching a maximum at 7 mol% Ce3+. And when the doping amount continues to increase, the luminescence intensity begins to decrease, and the concentration quenching phenomenon also occurs.
Based on the above analyses, it can be clearly seen that the Eu3+, Tb3+ and Ce3+ single-doped Na2SiF6:xRe3+ materials can illuminate red, green and blue primary colors under specific UV light excitation. Figure 8 shows the CIE coordinates of Na2SiF6:0.07Eu3+, Na2SiF6:0.09Tb3+ and Na2SiF6:0.07Ce3+ materials, and their color coordinates are listed in Table 1. In the figure, serial numbers 1, 2 and 3 correspond to the red, green and blue CIE coordinates of Na2SiF6:0.07Eu3+ (λex = 395 nm), Na2SiF6:0.09Tb3+ (λex = 378 nm) and Na2SiF6:0.07Ce3+ (λex = 290 nm), respectively. The triangle in the figure depicts the tunable regions of the Eu3+, Tb3+ and Ce3+ single-doping materials. To obtain a single-component white phosphor, Eu3+, Tb3+ and Ce3+ are further co-doped in the Na2SiF6 matrix. The following is a detailed discussion of the luminescence performance of the three-doped system, namely the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material.
3.2.4 Luminescence performance of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material
Selecting the red emission wavelength (612 nm) of Eu3+ as the monitoring wavelength, the measured excitation spectra of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material are shown in Fig. 9a. The broadband at 290 nm belongs to the 4f → 5d electronic transition of Ce3+. The sharp excitation peaks at 318, 352, 378, and 395 nm correspond to 7F0 → 5H6 of Eu3+ or 7F6 → 5H7 of Tb3+, 7F6 → 5D2 of Tb3+, 7F6 → 5L10 of Tb3+ and 7F0 → 5L6 of Eu3+ [38, 39]. According to various excitation characteristics, the measured material exhibits multi-peak emission in the visible light region by selecting the above-monitored different excitation wavelengths as excited wavelengths in turn. Figure 9b shows emission spectrum of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material excited by 290 nm. It can be seen that the main emission peaks are located at 465, 490, 544, 588 and 612 nm, respectively. The spectrum represents full-color emission, including red, green and blue components that allow the realization of white light emitting. Among them, the emission peak at 465 nm corresponds to the 5d → 4f transition of Ce3+ and the 5D2 → 7F0 transition of Eu3+. Several peaks at 490, 544, and 588 nm are attributed to the 5D4→7FJ (J = 6, 5, 4) transitions of Tb3+, respectively. In addition, Tb3+ can also produce weak red emission at 612 nm (5D4 → 7F3), while red emission at 588 and 612 nm is attributed to the 5D0 → 7FJ (J = 1, 2) transitions of Eu3+, respectively [40]. Therefore, there is a superposition of the emission spectra of Tb3+ and Eu3+ in the red light-emitting region of 590–620 nm. Figure S7 is the quantum yield diagram of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material excited by 290 nm, and the result is 4.92%.
The Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material’s excitation spectra (a) emission spectra under the excitation of 290 nm (b) emission spectra under the excitation of different excitation wavelengths (c) and emission intensity spectra of main characteristic peaks corresponding to red, green and blue (d)
Figure 9c shows emission spectra of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material excited by different excitation wavelengths. It can be seen that the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material can all detect the red characteristic luminescence of Eu3+, the green characteristic luminescence of Tb3+ and the blue characteristic luminescence from Ce3+ under the excitation of UV light with different excitation wavelengths. However, the luminescence intensity of each emission peak varies depending on the energy of the excitation wavelength [41, 42]. As the excitation wavelength energy decreases, the emission peak intensity of Ce3+ at 465 nm decreases, and the emission peak intensity of Tb3+ at 544 nm also decreases slightly, but the emission peak intensity of Eu3+ at 612 nm is rapidly enhanced. The change in their energy is shown in Fig. 9d. From these adjustable emission spectra, it can know that the intensity of emission of each characteristic emitted light from the same phosphor can be adjusted by changing the excitation wavelength of the UV light.
Figure 10 shows simplified energy level diagram of Ce3+, Tb3+ and Eu3+ of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material to explain possible energy transfer during excitation and emission. In the Na2SiF6:zCe3+ materials, Ce3+ replaces the Na+ level. So it is speculated that in the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material, Ce3+ still replaces the Na+ level. Since Na+ is in the six-coordinate field of the Na2SiF6 matrix [22], the 5d orbit of Ce3+ is still in the six-coordinate field after replacing Na+. In addition, since the multi-doping is easy to cause lattice distortion, it is speculated that the 5d orbit of Ce3+ is in the twisted six-coordinate field. At this time, the 5d orbit is prone to splitting. So the two d-orbits of the energy reduction of Ce3+ calculated by combing the excitation and emission spectrum are located at 34,040 and 22,499 cm−1, respectively [43].
Based on the above experimental results, it is considered that the following luminescence processes exist in the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material. Under the excitation of 290 nm ultraviolet light, electrons on Ce3+ are transited from the ground state level to the splitting higher 5d energy level (34,040 cm−1). Then these electrons relax to a lower 5d energy level (22,499 cm−1) through multi-phonon relaxation, and further produce the characteristic emissions of Ce3+. Or these electrons transfer their excitation energy from the lower 5d energy level (22,499 cm−1) of Ce3+ to the excited 5D4 energy level of Tb3+ by cross relaxation, and further generate the characteristic emissions of Tb3+. Or these electrons transfer their excitation energy to the 5D1 level of Eu3+ via Tb3+ bridging, then relax to the 5D1 level of Eu3+ through multi-phonon relaxation, and further yield the characteristic emissions of Eu3+ [44,45,46].
Since the energy difference between 5D4 → 7F5 of Tb3+ is similar to that of 7F0 → 5D1 of Eu3+, the energy released by 5D4 → 7F5 of Tb3+ in the system is transmitted to the 7F0 → 5D1 of Eu3+ in the form of non-radiative resonance transmission. Then these electrons relax to the lowest excited 5D0 level of Eu3+ by multi-phonon relaxation, and further the characteristic emissions of Eu3+ occur [8, 47]. As the excitation wavelength increases and the excitation energy decreases, the characteristic emissions corresponding to Ce3+ and Tb3+ requiring higher excitation energy are gradually weakened, while the characteristic emissions for Eu3+ requiring lower excitation energy is continuously enhanced. When the optimal excitation wavelength (395 nm) of Eu3+ is selected for excitation, emission spectrum of the material reflects the strongest characteristic emission of Eu3+.
Figure 11a shows the emission spectrum of Na2SiF6:0.07Ce3+ (λex = 290 nm) material and the excitation spectrum of Na2SiF6:0.07Tb3+ (λem = 542 nm) material. The spectral overlap is observed between the emission spectrum of Ce3+ and the excitation spectrum of Tb3+. It is known from Dexter’s energy transfer theory [48, 49] that there is a possibility of the energy transfer from Ce3+ to Tb3+. The energy levels and processes involved can be described by 5d (Ce3+) + 7F5 (Tb3+) → 4f (Ce3) + 5D4 (Tb3+). Figure 11b shows the emission spectrum of the Na2SiF6:0.07Tb3+ material and the excitation spectrum of the Na2SiF6:0.03Eu3+ material. The spectral overlap is also observed between the emission spectrum of Tb3+ and the excitation spectrum of Eu3+, indicating that there is also the possibility of the energy transfer from Tb3+ to Eu3+. The energy levels and processes involved can be described as 5D4 (Tb3+) +7F2 (Eu3+) →7F5 (Tb3+) +5D0 (Eu3+). These two phenomena can further support the energy transfer process involved above.
Figure 12 shows CIE coordinates of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material excited by UV light at different excitation wavelengths, and their color coordinates are listed in Table 2. To visually understand the emission color of the material, the chromaticity coordinates of the material under UV light excitation of different excitation wavelengths are marked by numerical serial number in the CIE chromaticity diagram. In the diagram, the elliptical region in the middle belongs to the white light emitting region. And the serial numbers 1, 2, 3, 4, and 5 represent the chromaticity coordinates of the material excited by UV light at 290, 318, 352, 378 and 395 nm, respectively. It can be seen that the emission color of the Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material changes from white light to cold white light, warm white light and red orange light as the increase of excitation wavelength. Among them, the luminescence of the material under the excitation of 290 nm is the closest to the ideal white light. The white light excited by 318 nm contains more blue components. The white light excited by 352 nm contains more red components, and the human eye feels more comfortable with this light. Under the excitation of 378 and 395 nm, the material mainly emits red–orange light. It is concluded that the emission color from the same phosphor can be adjusted by changing the excitation wavelength of the UV light.
4 Conclusions
In this paper, Eu, Tb and Ce mono-doped and ternary co-doped Na2SiF6:xRe3+ luminescent materials were synthesized by co-precipitation method, and the structure and luminescence properties of the materials were investigated. The results show that the Eu, Tb and Ce single-doped Na2SiF6:xRe3+ materials exhibit characteristic red, green and blue luminescence, respectively, under appropriate UV light excitation. The ternary co-doped material exhibits full-color emission represented by Na2SiF6:0.03Eu3+–0.07Tb3+–0.07Ce3+ material and it contains the following luminescence phenomenon. As the excitation wavelength increases and the excitation energy decreases, the characteristic emissions corresponding to Ce3+ and Tb3+ are gradually weakened, and the characteristic emission corresponding to Eu3+ is continuously enhanced. The emission color changes from white light to cold white light, warm white light and red orange light as the increase of excitation wavelength. Among them, the luminescence of the material excited by 290 nm is the closest to the ideal white light. Under the excitation of 395 nm, the luminescence of the material is the strongest characteristic emission of Eu3+. This phosphor can be used as a single-component tunable white phosphor in white LEDs.
References
D.Q. Chen, W.D. Xiang, X.J. Liang, J.S. Zhong, H. Yu, M.Y. Ding, H.W. Lu, Z.G. Ji, J. Eur. Ceram. Soc. 35(3), 859–869 (2015)
H.C. Cheng, J.Y. Lin, W.H. Chen, Appl. Therm. Eng. 38(1), 105–116 (2012)
C.C. Lin, R.S. Liu, J. Phys. Chem. Lett. 2(11), 1268 (2011)
S. Pimputkar, J.S. Speck, S.P. DenBaars, S. Nakamura, Nat. Photon. 3(4), 180–182 (2009)
X.H. He, J. Zhou, N. Lian, M.Y. Guan, Silicon Bull. 28(4), 741–750 (2009)
D.R. Cooper, J.A. Capobianco, J. Seuntjens, Nanoscale 10(16), 7821–7831 (2018)
J. Cheng, J. Zhang, H.C. Zhang, S. Maryam, X.T. Bian, Z.H. Shen, X.W. Ni, J. Lu, Chin. Opt. Lett. 15(12), 121602 (2017)
L.J. Ren, X.H. L, X.Q. Du, L. Jin, W.M. Chen, Y.A. Feng, J. Lumin. 142(8), 150–154 (2013)
C.F. Zhu, Y.X. Yang, X.L. Liang, S.L. Yuan, G.R. Chen, J. Lumin. 126(2), 707–710 (2007)
D. Ghosh, K. Biswas, S. Balaji, K. Annapurna, J. Alloy. Compd. 747, 242–249 (2018)
T.C. Lang, T. Han, L.L. Peng, M.J. Tu, Mater. Chem. Front. 1, 928–932 (2017)
H.D. Nguyen, C. Lin, M.H. Fang, R.S. Liu, J. Mater. Chem. C 2(48), 10268–10272 (2014)
P.G. Sennikov, S.K. Ignatov, A.E. Sadov, A.G. Razuvaev, O. Schrems, Russ. J. Inorg. Chem. 54(2), 252–259 (2009)
J.J. Cao, B. Li, P.Y. Jia, Funct. Mater. 12, 12097–12101 (2014)
K. Li, H. Lian, M. Shang, J. Lin, Dalton Trans. 44(47), 20542–20550 (2015)
J.L. Zhang, G.Y. Hong, Chin. J. Lumin. 12(3), 224–229 (1991)
D.Z. Xu, J. Feng, X.Y. Yang, E.D. Zu, X.Y. Cui, J.C. Lin, W. Dong, Spectrosc. Spectr. Anal. 37(06), 1804–1808 (2017)
W.Y. Zhang, Q. Jing, Y. Fang, Z.H. Chen, Z. Für. Anorg. Und Allg. Chem. 643(22), 1739–1743 (2017)
D.J. Wei, A.J. Tang, P. Shen, B. Liao, Guizhou Chem. Ind. 36(05), 32–34 (2011)
R. Hoshino, S. Adachi, J. Appl. Phys. 114, 213502 (2013)
J.Q. Ning, C.C. Zheng, L.X. Zheng, S.J. Xu, J. Appl. Phys. 118, 073101 (2015)
J.Q. Ning, S.J. Xu, D.P. Yu, Y.Y. Shan, S.T. Lee, Appl. Phys. Lett. 91, 103117 (2007)
X. Qiao, H.J. Seo, Mater. Res. Bull. 49(1), 76–82 (2014)
P.C. Korir, F.B. Dejene, Appl. Phys. A 124(5), 356 (2018)
C.S. Shi, S.Q. Man, J.Y. Sun, J. Rare Earths 1, 35–38 (1991)
D.K. Singh, J. Manam, Appl. Phys. A 124(3), 261 (2018)
X.F. Wang, C. Zhang, C.Y. Deng, Chin. J. Lumin. 37(9), 1037–1042 (2016)
X.G. Wang, Z.Y. Yu, M. Namira, S.L. Bo, Chin. J. Inorg. Chem. 24(4), 571–575 (2008)
Z.P. Yang, Z. Guo, S.C. Zhu, W.J. Wang, Spectrosc. Spectr. Anal. 24(12), 1506–1510 (2004)
W.N. Zhao, Z.M. Xu, T.Y. Sun, X.H. Wu, S.S. Liu, Z.C. Ma, Solid State Commun. 178(1), 42–45 (2014)
C.H. Zhang, H.B. Liang, S. Zhang, C.M. Liu, D.J. Hou, L. Zhou, G.B. Zhang, J.Y. Shi, J. Phys. Chem. A 116(30), 15932–15937 (2012)
X.G. Wang, Spectrosc. Spectr. Anal. 28(2), 260–264 (2008)
S.L. Ji, L.L. Yin, G.D. Liu, L.D. Zhang, C.H. Ye, J. Phys. Chem. C 113(37), 16439–16444 (2009)
X.Y. Huang, B. Li, H. Guo, J. Alloy. Compd. 695, 2773–2780 (2017)
X.G. Zhang, L.Y. Zhou, Q. Pang, J.X. Shi, M.L. Gong, J. Phys. Chem. C 118(14), 7591–7598 (2014)
K.L. Qiu, H.Z. Lian, Y.Q. Han, M.M. Shang, R.V. Deun, J. Lin, Dyes Pigments 139, 701–707 (2016)
Y.P. Tong, T. Chen, Spectrosc. Spectr. Anal. 33(11), 2930–2934 (2013)
B.L. Wang, L.Z. Sun, H.D. Ju, Solid State Commun. 150(31), 1460–1462 (2010)
F.P. Zhuo, W. Zhang, J.M. Huo, Y.L. Zhao, W. Wu, X. Ding, Y.R. Shi, Y.H. Wang, J. Lumin. 33(3), 238–242 (2012)
B.H. Li, J. Yang, J. Wang, M.M. Wu, Opt. Mater. 36(10), 1649–1654 (2014)
U. Balderas, S. Carmona, L. Mariscal, I. Martínez, C. Falcony, Chem. Phys. 511, 1–6 (2018)
J. Li, Z.Y. Mao, D.J. Wang, Y.C. Zhu, L. Gan, F.F. Xu, Ceram. Int. 39(8), 9809–9813 (2013)
J. Wu, J.L. Zhang, W.L. Zhou, C.Y. Rong, L.P. Yu, C.Z. Li, S.X. Lian, Chem. J. Chin. Univ. 34(2), 306–312 (2013)
S.Y. Kim, K. Woo, K. Lim, K. Lee, H.S. Jang, Nanoscale 5(19), 9255–9263 (2013)
D. Jia, W. Jia, X.J. Wang, W.M. Yen, Solid State Commun. 129(1), 1–4 (2004)
B. Li, X.Y. Huang, Ceram. Int. 44, 2915–4923 (2017)
M. Shang, G. Li, X. Kang, D. Yang, D. Geng, L. Jun, ACS Appl. Mater. Interfaces 3(7), 2738–2746 (2011)
L. Jin, X.Q. Du, X.H. Lei, L.J. Ren, Y.A. Feng, W.M. Chen, Appl. Phys. A 114(2), 631–636 (2014)
S.L. Zhao, F.X. Xin, S.Q. Xu, D. Deng, L.H. Huang, H.P. Wang, Y.J. Hua, J. Non Cryst. Solids 357(11), 2424–2427 (2011)
Acknowledgements
This work is supported by the National Natural Science Foundation of China (no. 21261010), the Natural Science Fund of Inner Mongolia Autonomous Region (no. 2015MS0227), the Second-Level Candidate (Disciplinary Leader) Research Fund (no. RCPY-2-2012-K-046) of the “Ten, Hundred, Thousand” Talents Project of Inner Mongolia Normal University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, JY., Wang, XG. Preparation, structure and luminescent performance of Na2SiF6:Re3+ (Re3+ = Eu3+, Tb3+, Ce3+) powders. Appl. Phys. A 125, 178 (2019). https://doi.org/10.1007/s00339-019-2468-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-2468-1